Abstract
Microiontophoresis is a drug delivery method in which an electric current is used to eject molecular species from a micropipette. It has been primarily utilized for neurochemical investigations, but is limited due to difficulty controlling and determining the ejected quantity. Consequently the concentration of an ejected species and the extent of the affected region are relegated to various methods of approximation. To address this, we investigated the principles underlying ejection rates and examined the concentration distribution in microiontophoresis using a combination of electrochemical, chromatographic, and fluorescence-based approaches. This involved a principal focus on how the iontophoretic barrel solution affects ejection characteristics. The ion ejection rate displayed a direct correspondence to the ionic mole fraction, regardless of the ejection current polarity. In contrast, neutral molecules are ejected by electroosmotic flow (EOF) at a rate proportional to the barrel solution concentration. Furthermore, the presence of EOF was observed from barrels containing high ionic strength solutions. In practice, use of a retaining current draws extracellular ions into the barrel and will alter the barrel solution composition. Even in the absence of a retaining current, diffusional exchange at the barrel tip will occur. Thus behavior of successive ejections may slightly differ. To account for this, electrochemical or fluorescence markers can be incorporated into the barrel solution in order to compare ejection quantities. These may also be used to provide an estimate of the ejected amount and distribution provided accurate use of calibration procedures.
Introduction
Iontophoresis describes delivery of molecular species by an electric field. Its use includes therapeutic treatment of the skin, eye, and nail, and has separately evolved into a tool for neurochemical investigations.1–3 The latter approach utilizes drug delivery from micropipettes and is termed microiontophoresis. Originally developed for studies at the neuromuscular junction, this technique has since been incorporated to administer drugs to local regions of the brain.4–8 As a delivery method, microiontophoresis is advantageous due to the low solution volume required, the ability to bypass the blood brain barrier, rapid application time, and confined delivery regions.9 Despite these benefits, general use has been limited because of difficulties verifying ejection progress and the inability to accurately determine ejected concentrations.
Microiontophoresis can be performed in either anodic or cathodic mode, which use positive and negative currents, respectively, to eject drugs from the pipette barrel. Anodic iontophoresis has traditionally been used to eject positive ions through electrostatic migration. More recently it has been demonstrated that this practice also generates an outward electroosmotic flow (EOF), which aids cation delivery and allows for the ejection of neutral molecules.10 In contrast, anodic iontophoresis is typically used for the delivery of anions.
Common practices in microiontophoresis involve scaling the ejection quantity by adjusting the magnitude of the ejection current, or simply using a current that results in a response.11–14 However ejection quantities can vary between probes, and barrels may become damaged or clogged during experiments which alters ejection behaviour.15, 16 To obtain more certainty in ejection status, several technical advances have been developed which also help to address the quantitative shortcomings. Most notably, ejected solute can be monitored in real time by incorporation of a voltammetric microelectrode adjacent to the iontophoresis barrel.17 Ejection of electroactive drugs or markers is detected by the microelectrode upon ejection, confirming delivery, and can provide an estimate of the average local concentration.18, 19 Other techniques involve placing independent electrodes near the ejecting barrel and the incorporation of fluorescent markers in the ejection solution.20–22 Theoretical approaches have also been applied in efforts to accurately determine ejection quantity.22–25 These largely focus on the transport number, defined as the fraction of the ejection current accounted for by the target ion. Unfortunately such predictions are approximate because transport numbers vary between barrels under similar ejection conditions, and predict no delivery of neutral species.26
Iontophoretic ejections typically involve application of a constant current through the barrel. If accurate, the transport number could be used determine the delivery rate and total quantity ejected for an ionic species. Importantly this concept only applies to ions, since neutral molecules do not contribute to the ejection current. Instead these species are more accurately represented by the transference number, defined as the amount of substance ejected per unit charge. Although this is the more complete term, distinction between transference and transport numbers is rarely reflected in current literature.27, 28
Here we examine how barrel composition determines ejection rates in microiontophoresis. This includes a distinction between the delivery of ions and neutral molecules, accounting for their different mass transport mechanisms. We also examine how retaining currents (opposite in polarity to the ejection) may impact the reliability of such predictions on subsequent ejections. Separately the spatial distribution of ejected species in cathodic iontophoresis is examined and compared to anodic iontophoresis. Lastly, we exhibit the use of iontophoresis in vivo to demonstrate the principles described.
Experimental
Chemicals and Solutions
Chemicals were received from Sigma Aldrich (St. Louis, MO) unless otherwise noted. Phosphate buffered saline (140 mM NaCl, 3 mM KCl, 10 mM NaH2PO4) was diluted from a 10× stock and adjusted to pH=7.4 with 5 M NaOH on the day of use. All iontophoresis solutions were made fresh from deionized water and filtered (0.45 µm Nylon, Nalgene, USA) prior to addition to the barrel. Their pH was measured to be between 5 and 7. For the purpose of mole fraction calculations, the following reagents were used to make iontophoretic solutions: dopamine hydrochloride (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), L-glutamic acid monosodium hydrate (glutamate), hydroquinone (HQ), and disodium fluorescein (fluorescein).
Probe Fabrication, Iontophoretic Ejection, and Electrochemical Detection
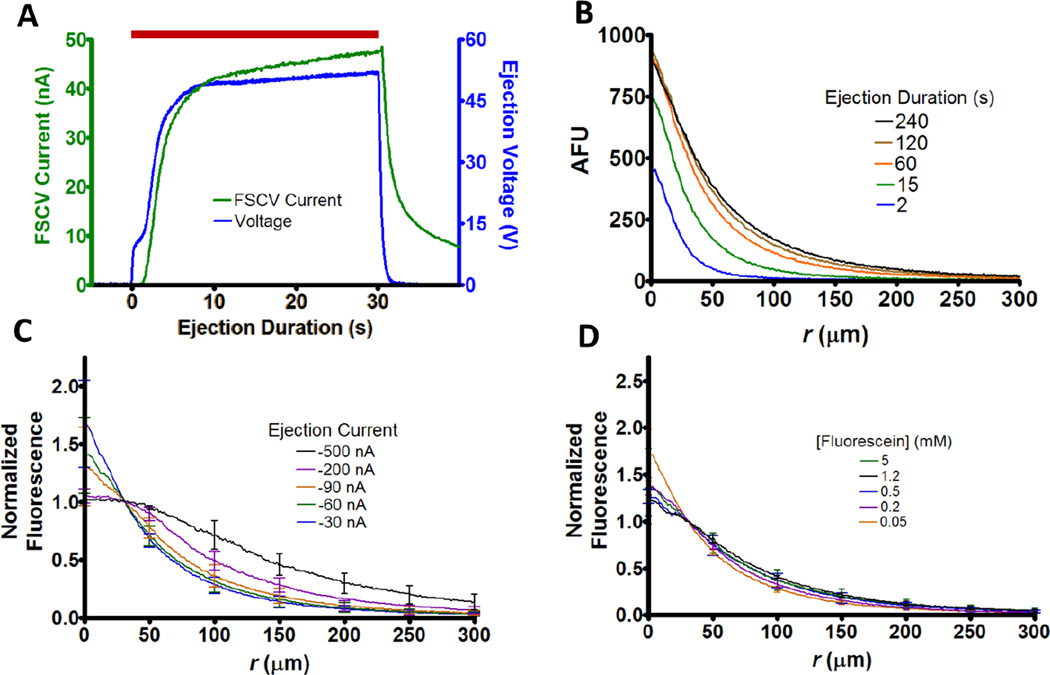
Four-barreled pre-fused glass capillaries (Friedrich & Dimmock, Millville, NJ) were used to fabricate iontophoresis probes as previously described.29 Once pulled, inner barrel diameters ranged from 0.5 to 1 µm. Ejection currents were administered with a locally constructed current source (UNC Electronics Facility, Chapel Hill, NC) which was controlled with LabVIEW code (National Instruments, Austin, TX) using a NI-USB-6343 DAQ card (National Instruments). Ejections were facilitated through specification of a constant ejection current and the corresponding voltage was monitored. The ejection voltages as reported represent the average for the last 5 s of the ejection, which typically plateaued within seconds of initiation (Figure 1A, blue). For probes utilizing electrochemical detection, a carbon fiber was inserted into one of the barrels and the exposed tip was cut between 75–125 µm. This allowed ejection monitoring via fast-scan cyclic voltammetry (FSCV) (Figure 1A, green). Unless otherwise noted, a triangular waveform was applied at 10 Hz from −0.4 to +1.0 V and back at 400 V/s versus a Ag/AgCl pellet reference (World Precision Instruments, Sarasota, FL). Between scans, the potential was held at −0.4 V. For electrical connection to the carbon fiber, a 4 M CH3COOK solution containing 0.15 M NaCl was placed in the microelectrode barrel and a stainless steel wire was inserted. Electrochemical data were collected using a locally built current transducer (UNC Chemistry Electronics Facility, Chapel Hill, NC), digitized (PCIe-6363, National Instruments), and analyzed after background subtraction, signal averaging, and filtering (2–10 kHz) with HDCV software.30
Figure 1.
Quantitative methods and spatial characterization. (A) Temporal profile for 30 nA ejection of 5 mM hydroquinone in 5 mM NaCl. The background subtracted FSCV current (green) at a carbon-fiber microelectrode and the iontophoretic pump voltage (blue) both reach steady state shortly after initiation. The red bar represents the time of the ejection. (B) Representative temporal fluorescent profiles for an ejection of 5 mM fluorescein in 0.5 mM NaCl. The ejection was performed into the cortex of a rat brain slice at −90 nA. In all cases, r=0 represents the tip of the barrel. (C) Average normalized fluorescence distribution for ejections of 5 mM fluorescein in 0.5 mM NaCl (n=6). Here and elsewhere, profiles were recorded after 4 min of continuous ejection and n represents the number of probes used to perform ejections. Unless otherwise noted error bars represent ±1 SD. (D) Average normalized fluorescence distributions for ejected fluorescein solutions at −90 nA (n=6) from a 0.5 mM NaCl solvent.
Liquid Chromatography Sample Collection and Analysis
Samples were collected for liquid chromatography by microiontophoretic ejection into 100 µL aliquots of PBS contained in a 0.5 mL Eppendorf polyethylene tube as previously described.15 A chloridized Ag wire served as a pseudo-reference electrode. A liquid chromatograph (HP Series 1050, Hewlett Packard, Palo Alto, CA) was used to separate analytes within each aliquot. The column consisted of a C18 reverse phase (5 µM particle diameter, 4.6 × 250 mm, Phenomenex, Torrance, CA), and 20 µL samples were injected. The mobile phase contained 100 mM citric acid, 1 mM sodium hexyl sulfate (Research Plus, Barnegat, NJ), and 0.1 mM EDTA adjusted to pH=3 and was 8/92% (v/v) methanol/H2O. Amperometric detection employed a thin layer glassy carbon electrode (Bioanalytical Systems, West Lafayette, IN) with an applied potential of +0.8 V vs. a Ag/AgCl reference electrode. The detector was controlled by homebuilt electronics and customized LabVIEW software (Jorgenson Lab, UNC). Aliquot concentrations were determined by comparison to calibration curves prepared from integrated peak areas.
Animal Care and Use
Adult male Sprague-Dawley rats (Charles River, Wilmington, MA) were used for the duration of the study. Animals were dually housed with food and water provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. Care was given to minimize the number of animals used and to reduce any suffering.
Brain Slice Protocol
Rats were anesthetized with urethane (1.5 g/kg) and brains were quickly removed after decapitation and submerged into oxygenated (95% O2, 5% CO2) chilled artificial cerebral spinal fluid (aCSF) consisting of 126 mM NaCl, 2.5 mM KCl, 1 mM NaH2PO4, 26 mM NaHCO3, 2 mM MgSO4, 2 mM CaCl2, and 11 mM glucose (pH=7.4). Coronal slices (300 µM thick) were made containing the striatum and cortex using a vibratome (VF-200, Precisionary Instruments, San Jose, CA) with stainless steel feather blades (Fendrihan, USA). Slices were immediately placed in room temperature (20 °C) aCSF and allowed to recover for at least 1 hour. For measurements slices were moved into a perfusion chamber (RC-22, Warner Instruments, Hamden, CT), anchored (SHD-22KIT, Warner Instruments) and perfused with 37 °C oxygenated aCSF at 2 mL/min on the stage of an Eclipse FN1 microscope (Nikon Instruments). Slices were allowed a 30 min equilibration period before investigation.
Fluorescence
The microscope was equipped for epifluorescence measurements with a xenon excitation source (X-Cite 120, EXFO) and a GFP bandpass filter cube (Ex: 450–490 nm, DCM: 495 nm, Em: 500–550 nm, Nikon Instruments) to select wavelengths for fluorescein. The iontophoresis barrel tip was inserted ~30 µm under the slice surface using a micromanipulator (MPC-200-ROE, Sutter Instruments, Novato CA). To ensure steady state, ejections were performed for 4 min before imaging (Figure 1B). The voltage was monitored at all times to ensure clogging did not occur, signified by the absence of abrupt increases in potential. During imaging aCSF perfusion was temporarily suspended so that profiles were free from convection. Fluorescence images were recorded using a Retiga Exi camera (QImaging, Surrey, BC, Canada) with QCapture software (QImaging) at a pixel resolution of 1 µm. Radial profiles were evaluated using a Matlab (Mathworks, Natick, MA) script.15 This was done by averaging 11 background subtracted cross-sectional radial profiles in the image plane. Due to the proximity of fluorescein’s pKa (6.4) to the barrel solution pH (5–6), only ejections performed on the same day and prepared from the same stock were compared.
In Vivo Single Unit Recording
Rats were anesthetized with urethane (1.5 g/kg). After placement in a stereotaxic frame, an iontophoretic probe with a carbon-fiber electrode was surgically implanted in the dorsal striatum using positioning from a stereotaxic atlas (coordinates relative to bregma were anterior-posterior (AP) +1.5 mm, medial-lateral (ML) +1.8 mm, AP +0.2 mm, ML +2.5 mm, each dorsal-ventral −4.0 to −6.0 mm).31 Following insertion into the brain the potential of the microelectrode was cycled for 30 minutes to achieve a stable voltammetric response. The voltage was scanned from −0.4 V to +0.8 V and down to −1.4 V to detect oxygen at 400 V/s. Cyclic voltammograms were generated at a repetition rate of 5 Hz. The three iontophoretic barrels contained 100, 10, and 0.5 mM glutamate (drug) with equimolar 3,4-dihydroxyphenylacetic acid (DOPAC). Solutions also contained 5, 0.5, 0.025 mM NaCl, respectively. DOPAC served as an electroactive marker for ejections and allowed estimation of average, local concentrations.32 Barrels were primed by ejecting for ~30 s above the targeted location. Electrochemical measurements employed a homemade current transducer (UNC Chemistry Electronics Facility, Chapel Hill, NC) and HDCV.30 The electrode alternated between voltammetric mode and voltage measurements of single unit spikes as previously described.33 Between waveforms, the electrophysiological signal was amplified (×10,000), bandpass filtered (0.3–3 kHz), and digitized (Digitizer, Plexon, Dallas, TX). Single units were sorted off-line (Offline Sorter, Plexon) and activity that was time-locked to the iontophoretic ejections was evaluated (Neuroexplorer, Plexon). For each ejection, the glutamate concentration was calculated from the DOPAC electrochemical signal. To accomplish this, the average DOPAC concentration upon ejection was determined by comparison of the oxidation current to a flow analysis calibration.17 Since glutamate was equimolar with DOPAC in the barrel and has a similar charge and mobility, this value approximates the average ejected glutamate concentration.
Results and discussion
Spatial Characterization
In a prior paper, we demonstrated a variety of methods to quantitate microiontophoretic ejections.15 Fast scan cyclic voltammetry (FSCV) with a carbon-fiber electrode adjacent to the iontophoretic barrel allows determination of the time course of ejections for electroactive species (Figure 1A, green). Additionally, the total amount ejected over a time period can be quantitated by ejections into small volumes (~100 µL) that are analyzed by liquid chromatography with electrochemical detection. Lastly, the spatial distribution of ejected species can be visualized by fluorescence microscopy. Using this approach, we showed that ejected species have a spherical concentration distribution centered at the ejecting barrel. The radial profiles obtained during a prolonged ejection reveal the limit of the spatial range obtained with microiontophoresis (Figure 1B). The fluorescence temporal profiles reveal that a steady-state condition is achieved within 2 min of the start of ejection.
To evaluate the spatial distribution of cathodic iontophoresis, we examined the radial distribution of the monoanion fluorescein (pH=5) using different ejection currents. All profiles were examined at steady-state, which was chosen to be 4 min after continuous ejection to ensure complete formation. Because the receiving medium can influence the ejected distribution, all ejections were made into the cortex of rat brain slices.34, 35 To account for differences in ejection quantity, profiles were normalized by the intensity 30 µm from the barrel tip. The normalized spatial distributions for ejections using currents < 200 nA were nearly identical (Figure 1C). This was also the case in our prior studies for ejections with anodic current for positive and neutral species, owing to diffusion as the dominant mass transport mechanism.15 Likewise, the fluorescein profiles follow a 1/r distribution at distances beyond 100 µm from the barrel tip for currents up to 500 nA, indicating diffusion is the dominant mass transport mechanism beyond this distance regardless of ejection current magnitude.
To determine if the barrel concentration impacted the distribution, steady states were compared for −90 nA ejections of different fluorescein solutions (Figure 1D). When normalized to account for concentration differences, the responses were not statistically different (2-Way ANOVA, p=0.504). Taken together with our prior published work, these results indicate that distributions for ejections are identical regardless of current polarity or barrel concentration.
Modulating Transport Number with Ionic Mole Fraction
Ejection quantities were then examined to determine how well predicted transport numbers matched observed values. A theoretical value for the transport number (ti) can be calculated for an ion from Equation 1, where zi is the unit charge, ui is the mobility in an electric field, Ci is concentration, and the sum is over all ions in solution, j.
| (Equation 1) |
Importantly this method assumes only transport through migration and does not account for electroosmotic flow (EOF). According to this equation, if ionic species have similar mobilities then the transport number for a given ion scales with the percentage of charge it makes up in the barrel solution. We denote this term the ionic mole fraction, which accounts for both concentration and charge of an ionic species relative to the whole. The ionic mole fraction should provide a means to predictably modulate the transport number by adjusting the relative percentage of ions in the barrel solution. To examine this, ejections of 5 mM dopamine (DA), a monocation at pH=6, were performed from solutions with different NaCl concentrations. Barrels also contained the neutral species hydroquinone (HQ) at the same concentration of DA. The mobility of an ionic species, ui, can be determined from the Nernst-Einstein equation (Equation 2) where Di is the diffusion coefficient, qe is the elementary charge (1.6 × 10−19 C), kB is Boltzmann’s constant (1.38 × 10−23 J · K−1),
| (Equation 2) |
and T is temperature. For DA this value, 2.3 × 10−4 cm2V−1 s−1, is similar in magnitude with reported mobility values for Na+ and Cl− (5.9 × 10−4 and 7.9 × 10−4cm2V−1 s−1, respectively), which should allow modulation of the transport number via the concentration.36 To test this, the observed transport number was calculated from the fraction of the ejection current accounted for by DA. This was done using Equation 3, where nDA is the ejected DA quantity determined from LC analysis, tej is the ejection time, F is Faraday’s constant (96,485 C/mol) and iej is the ejection current. As predicted, the observed transport number yielded a direct relationship (r2 = 0.991) with the DA ionic mole
| (Equation 3) |
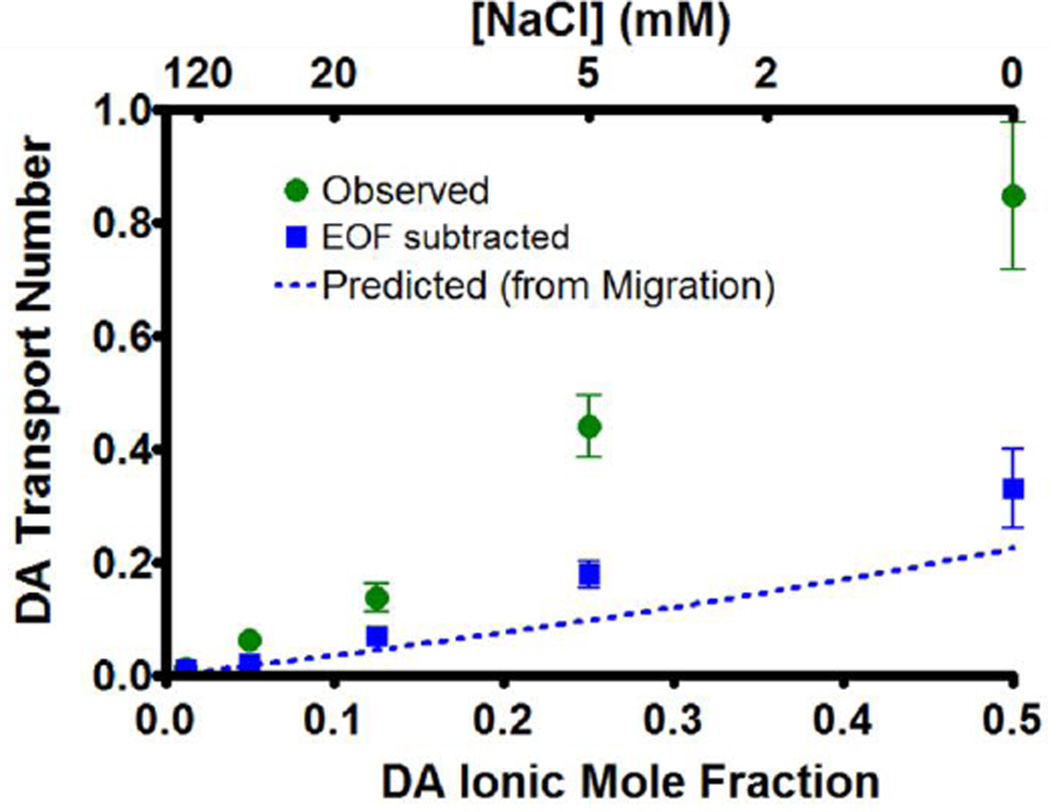
fraction. The data (Figure 2, green) was then compared with the predicted transport number (dotted blue line), calculated from Equation 1. For all solutions, the observed transport number was greater than the predicted value. To determine whether this was due to EOF, the amount of HQ ejected was subtracted from that of DA for each ejection. Recall HQ is neutral and can thus be used to quantitate EOF, so the DA amount ejected after HQ subtraction was due only to migration. The transport number for this new DA amount was then recalculated using Equation 3 (blue squares) and displayed better agreement with the predicted values. Thus most of the discrepancy between the observed and predicted DA transport numbers is accounted for by EOF. Additionally the visible reduction of the transport number variance for the migration quantity indicates that much of the difference in ejection quantity between barrels containing identical solutions was attributable to different rates of EOF.
Figure 2.
Transport number dependence on ionic mole fraction. Ejections of 5 mM DA and 5 mM HQ in NaCl (n=6) were performed at 150–2000 nA and ejection quantities were determined by liquid chromatography. The observed transport number was calculated by the fraction of the ejection current accounted for by the ejection of DA (green circles). After subtracting the DA ejected due to EOF, transport numbers were recalculated (blue squares). The predicted transport number due to migration (Equation 1) is represented by the dotted blue line.
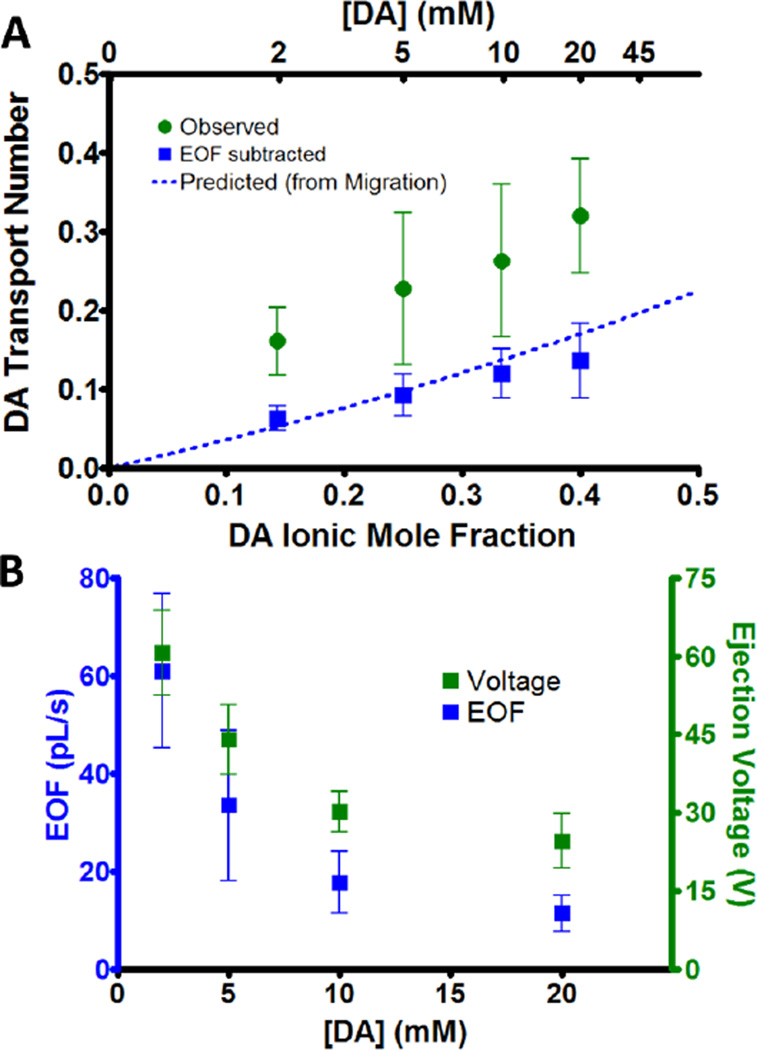
Next, ejections of solutions with different DA concentration in 5 mM NaCl were performed. To monitor EOF, solutions contained HQ at the same concentration as DA. Again, the observed transport number was strongly correlated with the ionic mole fraction (Figure 3A, green, r2 = 0.987) and the predicted transport number underestimated experimental values. Once the amount of DA ejected due to EOF was subtracted (blue squares), the transport numbers matched those predicted by migration (dotted blue line). Just as in Figure 2, the discrepancy in the variance between the two cases suggests that barrels displayed a greater inconsistency in the EOF rate compared to migration.
Figure 3.
Effect of ion concentration on transport number and EOF. (A) DA observed transport numbers (green circles) for 120 nA ejections of equimolar DA and HQ in 5 mM NaCl (n=6). The transport numbers were recalculated after subtracting the DA amount ejected due to EOF (blue squares). The predicted transport number (dotted blue line) from Equation 1 is shown for comparison. (B) The iontophoretic pump voltage (green) and EOF (blue) for ejections in part A.
These cases demonstrate several important factors regarding the transport number in microiontophoresis. First, it is predictably altered by the ionic mole fraction, observations which have also been reported in transdermal iontophoresis.37, 38 Importantly this provides a way to modify or predict changes in the ejection rate of a target ion. Secondly, the calculated transport number systematically underestimated the observed values, as larger quantities of DA were ejected than predicted from migration. This can mostly be explained by EOF, which is not accounted for in the prediction of transport numbers.
EOF Velocity Corresponds with Ejection Voltage
The EOF in the experiments of Figure 3A was calculated using the ejected HQ quantity and is shown in Figure 3B (blue).
Barrels higher in DA concentration displayed reduced EOF, which coincided with a drop in the iontophoresis pump voltage required to facilitate the ejections (green). This drop in voltage can be precisely attributed to the increase in solution conductivity at higher concentrations (r2 = 0.98 between voltage and solution resistivity, calculated from mobility and concentration).
As in capillary electrophoresis, the EOF velocity (vep) appears related to the electric field (E) by the EOF mobility (μep), where vep=μepE. However, the electric field, proportional with the applied potential, does not decrease quite as rapidly as the EOF. This suggests that part of the decrease in the EOF may be independent of the voltage. This could be due to a reduced double layer around the barrel wall with increased ionic strength, a phenomenon that is well established in capillary electrophoresis.
Ejection Rates of Neutral Molecules
Next we studied the ejection rate of neutral molecules ejected at a constant ionic strength. Neutral substances do not contribute to the ejection current so the transport number and ionic mole fraction do not apply. Instead the transference number, amount ejected per unit charge, provides a better comparison for ejection quantities. Here we examined the molar ejection rate of neutral substances, which is proportional to the transference number when ejections are performed at a constant current.
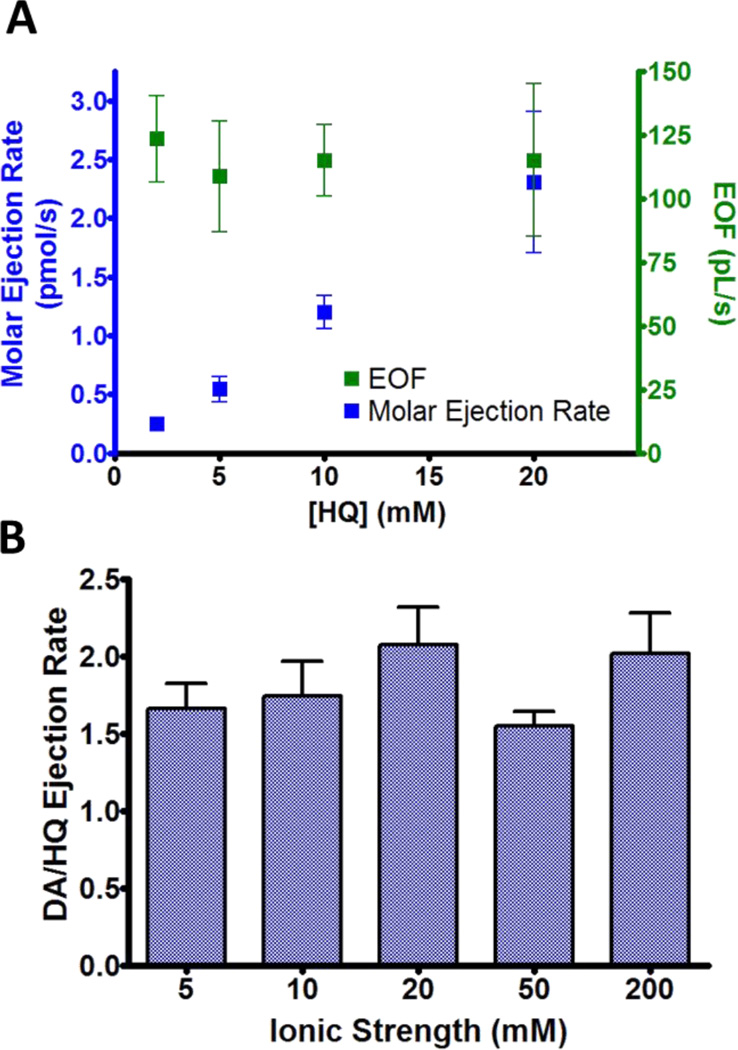
The molar ejection rate should correspond directly to the barrel concentration if EOF is the dominant ejection mechanism. To determine if this was the case, 120 nA ejections of different HQ concentrations in 5 mM NaCl were performed and quantities were once again analyzed by LC. A linear relationship (r2 = 0.999) was found between the barrel concentration and the molar ejection rate (Figure 4A, blue). Additionally the EOF (green) and ejection voltage (not shown) did not differ between barrels (P=0.673, P=0.120, respectively). Thus in contrast to ions, the ejection rate for a neutral species corresponds to barrel concentration rather than the mole fraction.
Figure 4.
EOF and ejection rates for neutral molecules. (A) Molar ejection rate (blue) and EOF (green) for 120 nA ejections of HQ in 5 mM NaCl (n=6). (B) Relative ejection rates of DA and HQ at different ionic strengths (n=6). Barrels contained 5 mM DA and HQ and the remaining ionic strength was due to NaCl. Ejections were performed at a near constant voltage (100 V) for which currents ranged from 150–2500 nA.
The previous results illustrate EOF in microiontophoresis at low ionic strength (~5 mM). However the double layer thickness adjacent to the glass wall is reduced at higher ion concentrations, which could ultimately inhibit EOF and prevent the delivery of neutral molecules. Additionally, if ejecting multiple substances from the same barrel, a change in the ejection mechanism could lead to a different ejection ratio dependent on the barrel composition.
To examine if this occurred, ejections of 5 mM DA and HQ in different NaCl solutions were performed. Since DA is transported by migration and EOF, an increase in its ejection rate compared to HQ would indicate an increased migration component compared to EOF. However, for ionic strengths up to 200 mM, this was not observed (Figure 4B). Similar observations of significant EOF at high ionic strength in capillary electrophoresis and transdermal iontophoresis have also been reported.39, 40 Thus EOF occurs to an appreciable extent in microiontophoresis even under highly ionic conditions, facilitating the ejection of bulk solution and permitting the delivery of neutral molecules.
Cathodic Iontophoresis Ejection Rate Characterization
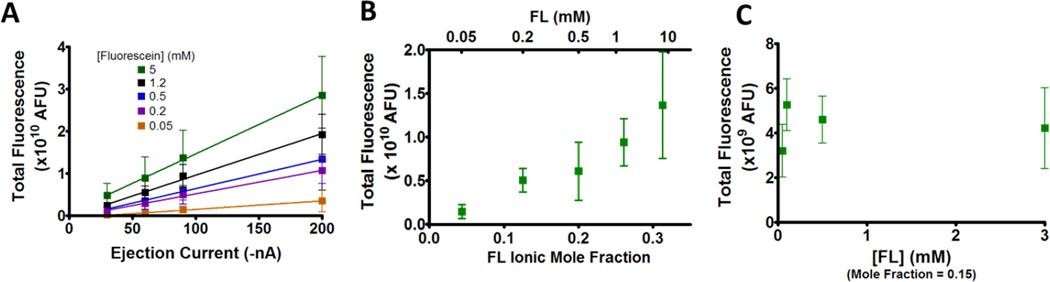
To determine if similar principles concerning ejection rates extend to cathodic iontophoresis, ejections of the monoanion fluorescein (pH=5) were performed from 0.5 mM NaCl using current of a negative polarity. Ejection rates were compared by the total fluorescence at steady state, determined by spherical integration of the radial fluorescence profile taken after 4 min of applying the ejection current. Just as we have previously demonstrated in anodic iontophoresis, cathodic ejections also show a linear relationship between the ejection rate and current (Figure 5A). To determine if the ionic mole fraction likewise determined the ejection rate, average intensities for −90 nA ejections were plotted versus the fluorescein ionic mole fraction (Figure 5B). As in anodic iontophoresis, this resulted in the predicted linear (r2 = 0.965) relationship.
Figure 5.
Cathodic iontophoresis ejection rate characterization for ejections into brain tissue. (A) Average total fluorescence at steady state for ejections of different fluorescein solutions (n=6). All barrels contained 0.5 mM NaCl. (B) Total fluorescence for −90 nA ejections. (C) Total fluorescence for ejections of fluorescein solutions at a fixed ionic mole fraction of 0.15 (n=5). The remainder of the charge was due to NaCl.
As additional confirmation, ejections from barrels of a fixed fluorescein mole fraction (0.15) were performed to determine if they displayed similar delivery rates when ejected at a constant current (−90 nA). These barrels had different fluorescein concentrations while the NaCl concentration was adjusted to maintain a constant ionic mole fraction. Unlike ejections of different fluorescein mole fractions (5B), these barrels resulted in similar fluorescence intensities (P=0.160) after ejection (Figure 5C). Thus we conclude that ion ejection rates for both cathodic and anodic microiontophoresis are similarly governed by the ionic mole fraction.
Retaining Currents Compromise Ejection Integrity
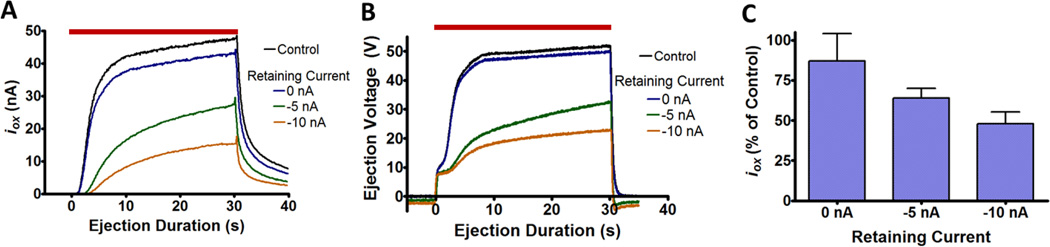
We have thus far demonstrated how changes to ejection rates can be predicted from initial barrel concentrations. Next we examine a common practice that may compromise such predictions. Some experiments utilize retaining currents to reduce leakage from the barrel.41, 42 However, it also draws some ions into the barrel from the ejection medium and drives out oppositely charged ions, all of which will alter the barrel solution composition. To examine this, 30 s ejections of 5 mM HQ in 5 mM NaCl, which were monitored electrochemically with a carbon-fiber microelectrode in an adjacent barrel, were performed after applying different retaining currents over 5 min intervals. Controls (30 s ejections initiated 15 s after a 1 min priming ejection) were taken before each trial for comparison. Ejections following no retaining current displayed slightly less HQ current compared to the controls, likely due to diffusional exchange at the tip interface (Figures 6A, 6C). Following retaining currents, ejections resulted in a significant reduction of the HQ current, iox (P=0.0038). Additionally, the voltage required to facilitate the ejection also decreased, indicating greater solution conductivity (Figure 6B).
Figure 6.
Effects of retaining currents on subsequent ejections. (A) Oxidation current at a carbon-fiber electrode for ejections of 5 mM HQ and NaCl. +30 nA ejections were performed following a 5 min waiting period at the designated retaining current. This was repeated four times with characterization occurring on the last ejection of the series. (B) Iontophoretic pump voltage for the ejections in part A. (C) Decline in HQ oxidation current of HQ after waiting periods (n=6). Control ejections were performed prior to the start of the series described above. This was compared to the HQ oxidation current of the final ejection. The current value was recorded as the average oxidation current during the last 5 s of the ejection.
Although a retaining current mitigates diffusion from the barrel, it also compromises subsequent ejections. The change in the barrel solution composition alters the transport and transference numbers between ejections. Rather than applying a retaining current, alternative approaches to reduce these problems such as smaller barrel diameters or less concentrated solutions could be adopted. Electrochemical monitoring as used in this work enables detection of these problems in experimental systems.
Comparison of Ejection Quantity with Markers
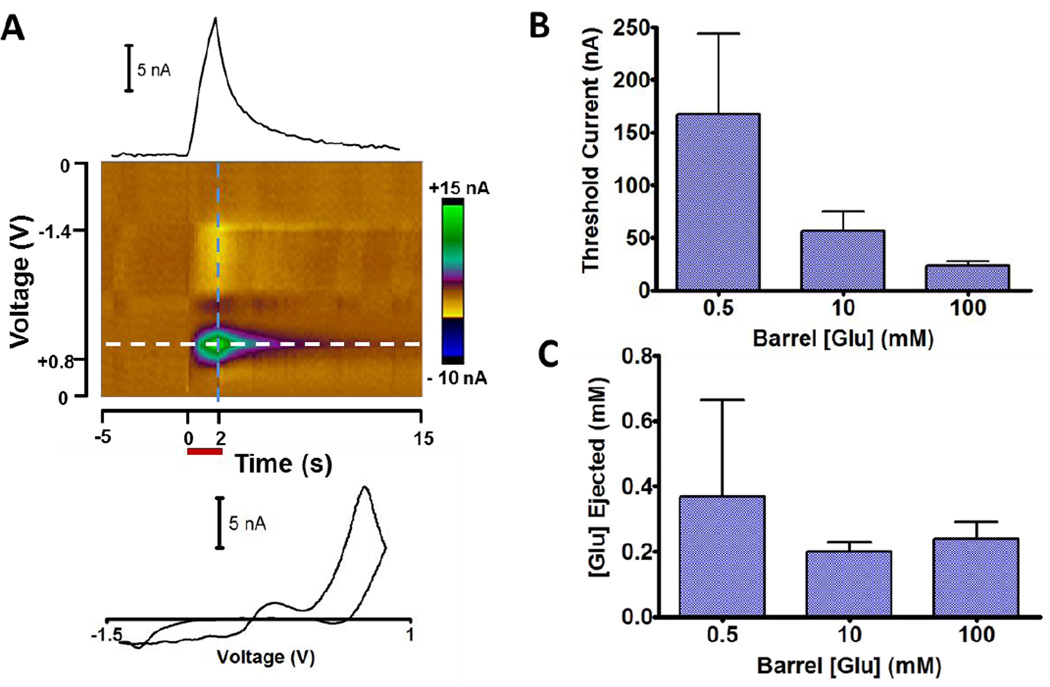
The microiontophoretic principles demonstrated above were determined under ideal conditions. In practice, factors such as variable ejection duration and current magnitude, along with barrel obstruction from biological media may cause deviations. To evaluate practical application in a physiological process, glutamate was ejected in anesthetized rats into the dorsal striatum. Glutamate is an excitatory neurotransmitter that induces striatal cell firing and is heavily involved in modulating activity.43 It is a monoanion and was iontophoretically introduced from barrels that also contained 3,4-dihydroxyphenylacetic acid (DOPAC) as an monoanionic electroactive marker. DOPAC was separately determined to have no impact on cell firing in the region. Its ejection was monitored electrochemically by FSCV repeated at 5 Hz with an attached carbon-fiber electrode. Cell firing rate was monitored in the 180 ms interval between scans at the same carbon fiber.33 The three iontophoretic barrels contained 0.5, 10, and 100 mM glutamate with an equimolar concentration of DOPAC and a proportional amount of NaCl. For each barrel, 2 s ejections at 3 min intervals were used while increasing the ejection current until the threshold for cell firing was found. During ejection, the FSCV response for DOPAC was clearly observed (Figure 7A).
Figure 7.
Deviation from ideal behavior in vivo. Barrels containing equimolar glutamate and DOPAC were used to evoke activity from medium spiny neurons in the dorsal striatum of an anesthetized rat. The iontophoresis probe contained 3 barrels of different concentrations and a carbon-fiber electrode to simultaneously monitor ejections through FSCV and record electrophysiology. (A) Electrochemical response for a 2 s ejection of 100 mM glutamate/DOPAC. The false color plot shows the background subtracted current. A horizontal cross-section (white dash) results in a current versus time trace (above) and the vertical cross section (blue dash) returns the DOPAC cyclic voltammogram (below). (B) Average ejection current magnitude required of each solution to initiate cell firing. Error bars represent the SEM (n=5). (C) Average local glutamate concentration for ejections in B. Concentrations were determined from the DOPAC signal with comparison to flow injection analysis calibration.
The minimum ejection current required to evoke cell firing for each of the barrels is shown in Figure 7B. Higher ejection currents were required for less concentrated solutions despite each solution having the same ionic mole fraction for glutamate. However, the glutamate concentration in the extracellular fluid that evoked firing, as assessed by the electrochemical marker (DOPAC), was equal across each of the barrel concentrations examined (Figure 7C). The apparent discrepancy between these results is explained by recalling that ions can diffuse into the barrel even without a retaining current (Figure 6), altering the true mole fraction at the end of the barrel. Since approximately equal quantities would be expected to diffuse into all barrels, this would most dramatically lower the fraction for the lowest concentration. In turn, this requires a larger current to eject the same quantity of the drug. Slight differences in EOF due to different applied voltages may also occur. Applications with short ejection times and long intervals between ejections are particularly susceptible to errors due to mixing. These experiments demonstrate the crucial role of electrochemical monitoring and its ability to correct for this effect.
Although the threshold extracellular glutamate concentration estimated from the electroactive marker was similar for all barrels, this estimate may not be precise. The NMDA, AMPA, and kainite receptors which bind glutamate are activated at low µM levels, while excitotoxicity in striatal cells occurs around 100 µM.44–46 The likely source of error is the calibration procedure for the electroactive marker, DOPAC. Calibration was done with flow injection analysis that results in a steady-state response to concentration. However, with 2 s ejections a steep concentration gradient exists across the electrode (Figure 1B). Thus, while the DOPAC response during these short ejections represents the average concentration across the electrode, it does not report the concentration at the microscopic site where the response was evoked by glutamate. Despite this, the ejected amounts which initiated cell firing resulted in similar responses to the electrochemical marker. Therefore, the oxidation current provided a reliable determination of the relative glutamate concentration at sites near iontophoresis probes, which demonstrates it can be an effective procedure for comparing relative ejection quantities. This is preferable to reliance on the ejection current and a theoretical transport number, which as we have shown, are susceptible to experimental conditions.
Conclusions
We have demonstrated principles enabling greater control of microiontophoretic ejections. The concentration distribution of ejected species is diffusion limited 100 µm from the barrel tip for ejections <500 nA, and the ejection rate increases proportionally with the current. Both properties are independent of the ejection current polarity. Concerning ejection rates of molecular species, ions are ejected in proportion to ionic mole fraction of the barrel solution. For neutral substances, the transference number scales with the barrel concentration. To best maintain barrel composition between ejections, retaining currents should be avoided. In practice, diffusional exchange between the barrel solution and the ejection medium will occur. Electrochemical or fluorescent markers can be incorporated into the barrel solution to monitor ejection progress and correct for variability in ejected quantities.
Supplementary Material
Acknowledgments
Collin McKinney and The University of North Carolina Department of Chemistry Electronics Facility constructed the iontophoresis and electrochemical instrumentation. Nick Boustead assisted with in vivo glutamate quantification. Funding for this work was provided by the NIH (DA010900).
References
- 1.Delgado-Charro MB. Expert Opinion on Drug Delivery. 2012;9:91–103. doi: 10.1517/17425247.2012.642364. [DOI] [PubMed] [Google Scholar]
- 2.Eljarrat-Binstock E, Domb AJ. Journal of controlled release : official journal of the Controlled Release Society. 2006;110:479–489. doi: 10.1016/j.jconrel.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 3.Priya B, Rashmi T, Bozena M. Expert Opinion on Drug Delivery. 2006;3:127–138. [Google Scholar]
- 4.Rutherford A, Garcia-Munoz M, Arbuthnott GW. Exp Brain Res. 1988;71:399–405. doi: 10.1007/BF00247499. [DOI] [PubMed] [Google Scholar]
- 5.Bucher ES, Fox ME, Kim L, Kirkpatrick DC, Rodeberg NT, Belle AM, Wightman RM. J. Cereb. Blood Flow Metab. 2014;34:1128–1137. doi: 10.1038/jcbfm.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nastuk WL. Fed. Proc. 1953:12. [PubMed] [Google Scholar]
- 7.Suh TH, Wang CH, Lim RKS. Chin J Physiol. 1936;10:61–78. [Google Scholar]
- 8.Windhorst U, Johansson H. Modern Techniques in Neuroscience Research. 1. Berlin Heidelberg: Springer; 1999. [Google Scholar]
- 9.Kovács P, Dénes V, Kellényi L, Hernádi I. Journal of Pharmacological and Toxicological Methods. 2005;51:147–151. doi: 10.1016/j.vascn.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Herr NR, Kile BM, Carelli RM, Wightman RM. Anal. Chem. 2008;80:8635–8641. doi: 10.1021/ac801547a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buffalari DM, Grace AA. The Journal of Neuroscience. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronzi D, Licata F, Li Volsi G. Neuroscience. 2015;300:360–369. doi: 10.1016/j.neuroscience.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Liu J, Gui ZH, Ali U, Fan LL, Hou C, Wang T, Chen L, Li Q. Neuroscience. 2011;182:193–202. doi: 10.1016/j.neuroscience.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Invernizzi RW, Pierucci M, Calcagno E, Di Giovanni G, Di Matteo V, Benigno A, Esposito E. Neuroscience. 2007;144:1523–1535. doi: 10.1016/j.neuroscience.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Kirkpatrick DC, Edwards MA, Flowers PA, Wightman RM. Analytical Chemistry. 2014;86:9909–9916. doi: 10.1021/ac5026072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purves R. Trends Neurosci. 1980;3:245–247. [Google Scholar]
- 17.Herr NR, Daniel KB, Belle AM, Carelli RM, Wightman RM. ACS Chem. Neurosci. 2010;1:627–638. doi: 10.1021/cn100056r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong-James M, Millar J. Nature. 1980;288:181–183. doi: 10.1038/288181a0. [DOI] [PubMed] [Google Scholar]
- 19.Kruk ZL, Armstrong-James M, Millar J. Life Sci. 1980;27:2093–2098. doi: 10.1016/0024-3205(80)90490-7. [DOI] [PubMed] [Google Scholar]
- 20.Ford CP, Gantz SC, Phillips PEM, Williams JT. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6975–6983. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaur G, Hrabetova S, Guilfoyle DN, Nicholson C, Hrabe J. Journal of neuroscience methods. 2008;171:218–225. doi: 10.1016/j.jneumeth.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purves R. J. Neurosci. Meth. 1979;1:165–178. doi: 10.1016/0165-0270(79)90014-1. [DOI] [PubMed] [Google Scholar]
- 23.Lalley P. In: Modern Techniques in Neuroscience Research. Windhorst U, Johansson H, editors. Berlin Heidelberg: Springer; 1999. pp. 193–212. ch. 7. [Google Scholar]
- 24.Bevan P, Bradshaw CM, Pun RY, Slater NT, Szabadi E. Experientia. 1981;37:296–297. doi: 10.1007/BF01991665. [DOI] [PubMed] [Google Scholar]
- 25.Kasting GB. Advanced drug delivery reviews. 1992;9:177–199. [Google Scholar]
- 26.Bradley PB, Candy JM. British Journal of Pharmacology. 1970;40:194–201. doi: 10.1111/j.1476-5381.1970.tb09913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helfferich FG. Ion Exchange. McGraw-Hill; 1962. [Google Scholar]
- 28.Mudry B, Carrupt PA, Guy RH, Delgado-Charro MB. Journal of controlled release : official journal of the Controlled Release Society. 2007;122:165–172. doi: 10.1016/j.jconrel.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belle AM, Owesson-White C, Herr NR, Carelli RM, Wightman RM. ACS Chem. Neurosci. 2013;4:761–771. doi: 10.1021/cn400031v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucher ES, Brooks K, Verber MD, Keithley RB, Owesson-White C, Carroll S, Takmakov P, McKinney CJ, Wightman RM. Analytical Chemistry. 2013;85:10344–10353. doi: 10.1021/ac402263x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson C, Paxinos G. The Rat Brain in Stereotaxic Coordinates. 6. London: Academic Press; 2006. [Google Scholar]
- 32.Zare HR, Namazian M, Coote ML. Electrochimica Acta. 2009;54:5353–5357. [Google Scholar]
- 33.Takmakov P, McKinney CJ, Carelli RM, Wightman RM. The Review of scientific instruments. 2011;82:074302. doi: 10.1063/1.3610651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guy Y, Faraji AH, Gavigan CA, Strein TG, Weber SG. Anal. Chem. 2012;84:2179–2187. doi: 10.1021/ac202434c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sykova E, Nicholson C. Physiol. Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacInnes DA. The Principles of Electrochemistry. New York: Dover; 1961. [Google Scholar]
- 37.Mudry B, Guy RH, Delgado-Charro MB. Biophysical journal. 2006;90:2822–2830. doi: 10.1529/biophysj.105.074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marro D, Kalia YN, Delgado-Charro MB, Guy RH. Pharmaceutical Research. 2001;18:1709–1713. doi: 10.1023/a:1013370529366. [DOI] [PubMed] [Google Scholar]
- 39.VanOrman BB, Liversidge GG, McIntire GL, Olefirowicz TM, Ewing AG. Journal of Microcolumn Separations. 1990;2:176–180. [Google Scholar]
- 40.Pikal MJ. Pharmaceutical Research. 1990;7:118–126. doi: 10.1023/a:1015816532532. [DOI] [PubMed] [Google Scholar]
- 41.Gerhardt GA, Palmer MR. Journal of Neuroscience Methods. 1987;22:147–159. doi: 10.1016/0165-0270(87)90009-4. [DOI] [PubMed] [Google Scholar]
- 42.Harding JW, Felix D. Journal of Neuroscience Methods. 1987;19:209–215. doi: 10.1016/s0165-0270(87)80004-3. [DOI] [PubMed] [Google Scholar]
- 43.Gerfen CR, Surmeier DJ. Annual review of neuroscience. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patneau DK, Mayer ML. The Journal of Neuroscience. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q, Harris C, Brown CS, Howe A, Surmeier DJ, Reiner A. Experimental Neurology. 1995;136:212–224. doi: 10.1006/exnr.1995.1098. [DOI] [PubMed] [Google Scholar]
- 46.Kew JNC, Davies CH. Ion channels from structure to function. 2nd. Oxford: Oxford University Press; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.