Abstract
Infant siblings of children with autism spectrum disorder display differences in early language and social communication skills beginning as early as the first year of life. While environmental influences on early language development are well documented in other infant populations, they have received relatively little attention inside of the infant sibling context.
In this study, we analyzed home video diaries collected prospectively as part of a longitudinal study of infant siblings. Infant vowel and consonant-vowel vocalizations and maternal language-promoting and non-promoting verbal responses were scored for 30 infant siblings and 30 low risk control infants at 9 months of age. Analyses evaluated whether infant siblings or their mothers exhibited differences from low risk dyads in vocalization frequency or distribution, and whether mothers’ responses were associated with other features of the high risk context. Analyses were conducted with respect to both initial risk group and preliminary outcome classification.
Overall, we found no differences in infants’ consonant-vowel vocalizations, the frequency of overall maternal utterances, or the distribution of mothers’ response types. Both groups of infants produced more vowel than consonant-vowel vocalizations, and both groups of mothers responded to consonant-vowel vocalizations with more language-promoting than non-promoting responses. These results indicate that as a group, mothers of high risk infants provide equally high quality linguistic input to their infants in the first year of life and suggest that impoverished maternal linguistic input does not contribute to high risk infants’ initial language difficulties. Implications for intervention strategies are also discussed.
Keywords: autism, infant siblings, language development, maternal responses, feedback loop
Introduction
Language Development in Infant Siblings of Children with Autism
Delays in early language and communication are one of the most striking early symptoms in children later diagnosed with autism spectrum disorder (ASD), and have been reported by 12 months of age in both retrospective and prospective samples (Mitchell et al., 2006; Osterling & Dawson, 1994; Ozonoff et al., 2010; Patten et al., 2014; Watson, Crais, & Baranek, 2013; Zwaigenbaum et al., 2005). Prospective investigations into the early development of ASD have typically followed the infant siblings of children already diagnosed, as these infants are at increased risk for ASD relative to the general population (Ozonoff et al., 2011). These investigations have identified atypical language trajectories across this group of high risk infants (hereafter, “infant siblings”), both those later diagnosed as well as a substantial minority of those who are not. Compared with low risk, typically developing infants, infant siblings show delays in the achievement of reduplicated babbling, produce fewer speech-like vocalizations and socially directed vocalizations during the first year of life, use proportionally fewer canonical syllables at 9 months, and produce fewer gestures in the first and second years of life (Iverson & Wozniak, 2007; Leezenbaum, Campbell, Butler, & Iverson, 2013; Mitchell et al., 2006; Ozonoff et al., 2010, 2014; Paul, Fuerst, Ramsay, Chawarska, & Klin, 2011; Talbott, Nelson, & Tager-Flusberg, 2015b).
The presence of language and communication delays amongst both diagnosed and nondiagnosed infant siblings is assumed to reflect shared familial risk factors, but the specific factors contributing to this risk remain unclear. Twin studies provide support for the heritability of ASD, but recent genetic analysis of non-twin siblings found limited overlap between diagnosed siblings in ASD-relevant mutations (Colvert et al., 2015; Yuen et al., 2015). These findings underscore the complexity of the genetic and environmental interactions that contribute to siblings’ increased risk and highlight the need to consider not only infants’ own predispositions and emerging symptoms, but the ways in which these factors may shape their environment.
The contribution of the early social environment to the language development of infant siblings has received relatively little attention, likely due to the historical misattribution of autism’s etiology to maternal behavior. However, there is a vast literature documenting the influence of linguistic input on the language development of both typically developing infants and those from wide-ranging at-risk contexts (e.g. infant prematurity, hearing loss, maternal depression, low familial socioeconomic status [SES]), and better understanding how these factors both affect and are affected by the familial autism context will have important implications for early intervention practices.
Environmental and Dyadic Influences on Early Language Development
The impact of environmental input on children’s language ability is illustrated clearly in the domain of vocabulary acquisition, where wide variation in the amount of speech children hear is associated with corresponding variation in children’s vocabulary size (Hart & Risley, 1995; Pan, Rowe, Singer, & Snow, 2005; Rowe, 2012). Verbal input is also associated with infants’ phonemic perception and production. Live, contingent interactions help infants to relearn non-native phonemic contrasts they have lost as a result of perceptual narrowing (Kuhl, Tsao, & Liu, 2003). Experimentally manipulating contingent maternal verbal responses to 9-month-old infants’ vocalizations to consist solely of either vowel or consonant-vowel responses results in specific increases in infants’ production of the same type of vocalizations they received as responses (Goldstein & Schwade, 2008). Mothers spontaneously provide language-promoting responses more frequently to infant vocalizations containing a consonant than those without (Gros-Louis, West, Goldstein, & King, 2006). It has been hypothesized that these kinds of contingent responses to infants’ early vocalizations are part of a naturally occurring social feedback loop wherein infants’ more developmentally advanced vocalizations are differentially reinforced, resulting in increasingly advanced vocal production (Goldstein, King, & West, 2003; Gros-Louis et al., 2006; Warlaumont, Richards, Gilkerson, & Oller, 2014).
Of relevance to this study, variations in input are also shaped by children’s own abilities and characteristics. This bidirectional influence of infant characteristics on maternal behavior and vice versa is a core feature social theories of language acquisition which emphasize the social context and transactional nature of early language learning (Bruner, 1981; Hoff, 2006; Sameroff, 1983). The delays in consonant production reported for infant siblings in the first year of life suggest that infant siblings may contribute to changes in the hypothesized vocal feedback loop by providing a different set of vocalizations for mothers to respond to and consequently, limiting the responses infants themselves are able to learn from. This pattern has been observed in the vocalizations and gesture production of young children with autism and infant siblings beginning in the second year of life but has not been examined amongst infant siblings in the first year of life (Leezenbaum et al., 2013; Warlaumont et al., 2014).
Changes in maternal communicative behavior during dyadic interactions with infant siblings have also been reported beginning near the end of the first year of life, supporting the need to examine the contributions of both infants and their mothers to early vocal dyadic interactions (Campbell, Leezenbaum, Mahoney, Day, & Schmidt, 2014; Leezenbaum et al., 2013; Talbott et al., 2015b; Wan et al., 2013). Diminished early exposure to language (e.g., for infants with chronic ear infections or from low SES backgrounds, both of whom hear less speech) has been associated with difficulties with speech perception and phonological awareness that persist well into childhood (Nittrouer & Burton, 2005; Nittrouer, 1996). Similar effects of early exposure to speech on later outcomes are observed in children’s vocabulary size (Hart & Risley, 1995; Pan et al., 2005; Rowe, 2012). The impact of varying environmental input on infants’ language abilities seems to be particularly significant for infants receiving relatively diminished environmental input, though the factors that predict such diminished environmental input vary considerably. These include maternal depression, low SES (including maternal education levels and knowledge of child development), and chronic ear infections or other issues that impede infants’ perceptual abilities (Bettes, 1988; Hart & Risley, 1995; Pan et al., 2005; Rowe, 2008; Warlaumont et al., 2014). Some of the factors associated with diminished environmental input also occur within the familial autism context and thus may influence the dyadic interactions and early language development of infant siblings. These include mothers’ broader autism phenotype characteristics and depressive symptoms, both of which are elevated in parents of children with ASD and are associated with differences in pragmatic language use and reduced linguistic input, respectively (Bailey, Golden, Roberts, & Ford, 2007; Ingersoll & Hambrick, 2011; Lindgren, Folstein, Tomblin, & Tager-Flusberg, 2010; Ruser et al., 2007). The elevated levels of concern consistently reported by mothers of high risk infants across the first year of life may also be associated with reduced linguistic input to the extent that those concerns reflect increased anxiety and a less sensitive pattern of responding (Hess & Landa, 2012; Ozonoff et al., 2009; Sacrey et al., 2015; Talbott, Nelson, & Tager-Flusberg, 2015a). Alternatively, elevated concerns driven by maternal hypervigilance may be associated with increased attention, prompting, and reinforcing of infants’ early communication. Increases in these facilitative maternal behaviors may also be supported by high risk mothers’ knowledge of early autism symptoms and exposure to intervention strategies through their experience with an older diagnosed child. Higher levels of maternal education have been associated with increased frequency of vocal exchanges and contingency of maternal responses for mothers of both typically developing children and those with ASD (Warlaumont et al., 2014).
The Current Study
Together, the previous literature indicates that changes in both vocal and gestural feedback loops are observed in children with autism and infant siblings in the second year of life and that these transactional effects are primarily driven by infants’ less sophisticated production patterns (Campbell et al., 2014; Leezenbaum et al., 2013; Warlaumont et al., 2014). Several studies have also reported delays in the consonant production of infant siblings and children with ASD in the first year of life, yet none have investigated mothers’ spontaneous responses to these early vocalizations (Iverson & Wozniak, 2007; Patten et al., 2014; Paul et al., 2011). The overarching goal of the current study was to understand better the factors that promote early language development, particularly within the everyday interactions of infants and their caregivers. Here, this was accomplished by analyzing the vocal production of 9-month-old high risk infant siblings, their mothers’ verbal responses to these pre-linguistic vocalizations, and associations with standardized measures of infant and maternal characteristics hypothesized to influence them. These behaviors were examined using a home-based video diary procedure in which dyadic interactions were filmed in the home and collected prospectively as part of an ongoing study of infant siblings of children with autism. We hypothesized that infant siblings would show decreased production of consonant-containing utterances compared to low risk controls (LRCs), and that high risk mothers’ contingent responses to infants’ vocalizations would be associated with measures of concurrent autism-related concerns about their infants, self-reported broader autism phenotype characteristics, and the symptom severity of the older diagnosed child.
Methods
Participants
Participants included 30 infant siblings of children with autism and 30 LRC infants and their mothers. These families were participating in a longitudinal study of infants at risk for autism conducted jointly at Boston University and Boston Children’s Hospital/Harvard Medical School. For the larger project, interested families were contacted by the study coordinator, who conducted a detailed telephone eligibility interview. All subjects were screened for exclusionary criteria (prematurity, extended stays in the neonatal intensive care unit, maternal drug or alcohol use during pregnancy, family history of genetic disorders associated with ASD, and primary languages other than English). Infants were enrolled into the high risk autism group (HRA) if they had an older sibling with a diagnosis of Autism, Asperger’s Syndrome, or Pervasive Developmental Disorder-Not Otherwise Specified, confirmed by expert community diagnosis. Infants were enrolled into the LRC group if they had at least one older sibling who was typically developing and no first-degree relatives diagnosed with an ASD or other neurodevelopmental disorder. The sample was well matched for gender (52% male) and was primarily Caucasian (13% non-Caucasian) and high SES, with the majority of mothers in each group having at least a college degree (8.8% had less than a college degree) and an income over $75,000 (20.5% had less than $75,000). There were no significant group differences in the gender ratio, infant race, maternal education or family income. Informed consent was obtained from parents prior to participation.
Procedure
As part of the larger longitudinal study, infants were seen in the laboratory several times from 3 to 36 months of age where they participated in a range of standardized behavioral assessments, eye-tracking, fNIRS, and neurophysiological paradigms. A substantial subset of families also contributed genetic material for additional analyses. Families were also asked to provide both written and home diaries from 6 to 18 months of age. Because these diary measures were completed primarily by mothers, all parent measures are hereafter referred to as maternal measures. Video diaries were filmed monthly and consisted of semistructured interactions between infants’ and their mothers which lasted approximately 20 min. Mothers were instructed to present infants with a series of toys, play social games, elicit vocal imitation and smiles, read a picture book, and play for several minutes. Written diaries were completed weekly and consisted of eight items. Parents were asked to report on infants’ new sounds, words, or gestures, to describe infants’ play, and describe any concerns about their infants’ development (see Talbott et al., 2015a for a full description of the written diary collection and coding procedure.) Video and Written diary measures were scored by coders blind to group membership and trained extensively on the coding schemes (described below).
The focus of the current study involves a subset of laboratory and home-based measures collected at 9 months of age.
Laboratory-Based Measures
Autism observation scale for infants
The Autism observation scale for infants (AOSI; Bryson, Zwaigenbaum, McDermott, Rombough, & Brian, 2008) is an 18-item assessment that measures a range of autism-related behaviors (visual attention and tracking, social interest and reciprocity, affect, atypical sensory and motor behaviors, etc.) during a brief semistructured interaction between a trained examiner and the infant, who is seated on their parents’ lap. Individual items are scored from 0 to 2 or 3, with higher scores indicating greater atypicality. The scale yields two final scores: the total number of items endorsed, and the total raw score (out of a possible 50). AOSI total raw scores were used here as a measure of autism symptoms.
Autism diagnostic observation schedule-generic
The Autism Diagnostic Observation Schedule-Generic (Lord et al., 2000) is a semi-structured play-based interaction designed to assess participants’ social and communicative abilities across a range of contexts which vary according to language ability. The presence of repetitive behaviors and restricted interests are also noted. Individual items are scored from 0 to 3, with higher scores indicating more profound impairment. The items in the scoring algorithm map onto DSM-IV criteria for ASD, and empirically derived cutoffs can be used to categorize scores into those meeting criteria for Autism, Autism Spectrum, or non-spectrum. For the current study, ADOS scores were used to classify infants into diagnostic groups in combination with a clinical best estimate judgment. The ADOS was administered at 18, 24, and 36 months of age.
Questionnaire Measures
Family SES information
Basic demographic information was collected upon entry to the study and includes: race and ethnicity for each parent, proband, and infant, maternal and paternal education, and family income.
Maternal broader phenotype characteristics
The presence of broader autism phenotype characteristics in mothers was assessed using the Broad Autism Phenotype Questionnaire (BAP-Q; Hurley, Losh, Parlier, Reznick, & Piven, 2007). The BAP-Q is a 36-item self-report questionnaire that assesses behavior across three subscales: aloof, pragmatic language, and rigidity. It was collected once from mothers upon entry to the study. BAP-Q Pragmatic Language subscale scores were used here as a measure of relevant broader phenotype features in mothers.
Proband autism symptoms
Proband ASD symptoms were measured during the telephone screen using the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003). The SCQ is a 40-item parent report screening measure that covers communication, social interactions, and restricted and repetitive behaviors. There are two different versions of the SCQ: a “current” version for children under the age of 5, and a second “lifetime” version for children 5 years or older. Total score is out of 39, with higher scores indicating greater impairment.
Video Diary Measures
Of the total video diary session, maternal and infant vocalizations were scored from the toy and book reading sections of the home video diaries. These sections were selected because they provided a more consistent context across families (rather than the free play section, which varied in terms of both the activities and presence of siblings or other family members). For each infant, the diary closest in age to 9 months but within the range of 8–10 months was selected for coding. The coding scheme used to analyze infant and maternal vocalizations was adapted from Gros-Louis et al. (2006), who scored infant and maternal vocalizations at the same age, but in the laboratory.
Infant vocalizations
Infant vocalizations that occurred during the toy and book sections of each diary were scored. Vegetative sounds, laughter, crying, and other nonspeech sounds were excluded. Infants’ vocalizations were further classified as either vowel-only (VV) or consonant-vowel (CV) utterances. Utterances were defined as segments of speech produced without readily discernable pauses between them. Utterances were classified as CV if they included at least one consonant, an approach used in previous studies of early vocal production (Gros-Louis et al., 2006; Ozonoff et al., 2010). In order to control for differences in session length, infant vowel, and consonant-vowel data are expressed as the number of vocalizations of each type occurring per minute.
Maternal contingent responses
Maternal vocalizations that occurred during the toy and book sections of each diary were classified as either noncontingent or contingent. Vocalizations were categorized as contingent if they occurred within 2 sec of an infant vocalization and were directed at the same object, involved imitation of the same sound, provided the label for the infants’ object of focus, etc.). These contingent vocalizations were scored across the following categories (adapted from Gros-Louis et al., 2006): Language Promoting (Acknowledgement, Imitation, Label, and Question) and Non-Promoting (Attribute, Directive, and Play). Descriptions and examples of each of these responses are included in Table 1. Because the rate of each of these maternal responses depends on the number of vocalizations produced by the infant, scores for the 7 individual response types and 2 summary codes were calculated as the proportion of infant vocalizations receiving each type of response. The total number of maternal vocalizations (both contingent and non-contingent) was also scored to provide a measure of overall talk. This Maternal Total Utterance score is expressed as the rate per minute to control for differences in session length.
Table 1.
Definitions and Examples of Maternal Contingent Response Codes
| Response Category | Response Type | Definition | Examples |
|---|---|---|---|
| Acknowledgement | Conversational fillers | “oh, really” “mm hmm” | |
| Language-promoting | Imitation | An approximate imitation of an infant vocalization or an expansion based on the sounds of the vocalization | “ta-ta” “bottle” |
| Label | Providing the name of an object | “That’s a wand” | |
| Question | Any question | “you want more?” | |
| Attribute | Responses describing object characteristics or values any instructions sound effects or singing | “It’s the same color” “that one’s boring” | |
| Non-promoting | Directive Play | “shake the rattle” “bam, bam!” |
Note: coding scheme adapted from Gros-Louis et al. (2006).
Reliability procedures
An undergraduate student with training in early speech development and blind to the specific study hypotheses was trained on the coding scheme. 15% of data files (10 dyads) were double scored by the first author to maintain and assess ongoing reliability. Inter-rater reliability was assessed using an intra-class correlation coefficient, which was in the excellent range for both infant vocalization variables (VV = 0.80, CV = 0.96), and the good to excellent range for the maternal variables (maternal total utterances = 0.94, acknowledgement = 0.84, imitation = 0.87, label = 0.96, question = 0.72, attribute = .67, directive = 0.64, play = 0.92).
Written Diary Measures
Maternal concerns
Concerns reported in weekly home-based written diaries were scored across the following categories: General/Medical, Language, Social Communication, and Restricted and Repetitive Behaviors. The Language, Social Communication, and Restricted and Repetitive Behavior scores were collapsed into a single Total Autism Concerns Score. The coding scheme and procedures are described in detail in Talbott et al. 2015a. For the current study, mothers’ Total Autism Concerns reported between 9 and 10 months are used as a measure of concurrent maternal concerns. Due to significant positive skew, this variable was transformed using a logarithmic transformation prior to analysis.
Results
Infants were on average 9 months of age at the time of filming for the diaries included in this analysis, which did not differ by group (HRA: Mean= 8.93, SD=.78, LRC: Mean=9.10, SD=.66), t(58) = −.889, p = .38. There were no group differences in the total video diary session duration, which were an average of 9.8 min, t(58)= .795, p = .43.
Six infants met criteria for ASD on the ADOS at their most recent study visit. Five of these were at 36 months and one at 18 months, all of whom also received expert clinical judgments of ASD. Although limited by the small number of these outcome infants (hereafter referred to as ASD), analyses reported below consider them separately from the high risk infants who were not classified as ASD (N = 24); these non-diagnosed infants are referred to as the high risk negative (HRA-N) group.
To address our specific study goals, we first analyzed infant data to determine whether infants differed in their overall vocalization rate or by utterance type. These analyses were followed up with more detailed analysis of mothers’ responses to these vocalizations to better characterize the distribution of response types across the three groups and to determine whether mothers differed in their pattern of responding to different infant vocalization types. Finally, within the high risk group, associations between maternal vocalizations and maternal and family characteristics were analyzed.
Infant Language and Communication
9 month video diary data
Descriptive information on infant vocalization rates are presented in Table 2. To determine whether the three groups of infants differed in the rate of vocalizations or the relative frequency of each type, a 2 × 3 repeated-measures analysis of variance (ANOVA) with vocalization type (VV, CV) as within-subjects and group (ASD, HRA-N, LRC) as between-subjects factors was performed. There were no significant main effects of Group or a Vocalization Type x Group Interaction (both p’s >.40). There was a significant main effect of Vocalization Type, F(1, 57) = 62.11, p<.001, indicating that infants in all groups produced significantly more Vowel than Consonant-Vowel vocalizations.
Table 2.
Infant Language Production at 9 Months, by Group
| Language Measure (mean, SD) | Group
|
||
|---|---|---|---|
| LRC n = 30 | HRA-N n = 24 | ASD n = 6 | |
| Vowel | 2.80 (1.3) | 3.38 (2.0) | 2.40 (2.0) |
| Consonant-vowel | .68 (.70) | .76 (.84) | .65 (.55) |
Note: No significant group differences
Chi-square analyses were also used to examine the relative percentages of infants in each group who did not produce any consonants. There were no significant differences between the groups, with 22% of the total sample (6 LRC, 6 HRA-N, 1 ASD) producing no consonants, χ2 (1, N = 60) = .295, p=.86.
Maternal Vocalizations and Responses
Mothers produced an average of 12.22 utterances per minute, which did not differ between the groups (LRC: M = 11.56, SD = 4.4; HRA-N: M = 12.80, SD = 4.7, ASD: M =13.20, SD = 5.50), F(2,59) =.624, p = .54. The three groups also did not differ in the overall proportion of infant vocalizations they responded contingently to, with LRC mothers responding to 46%, HRA-N mothers responding to 40%, and ASD mothers responding to 35% of infants’ total utterances, F(2, 59) = .92, p = .41.
Distribution of maternal response types
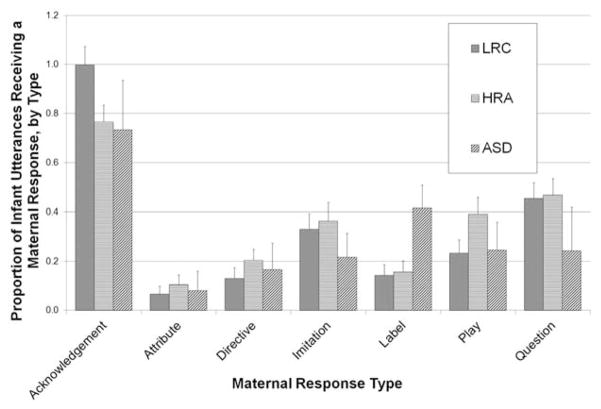
A 3 × 7 repeated-measures ANOVA, with group as between-subjects (ASD, HRA-N, LRC) and maternal response type (acknowledgements, imitation, label, question, play, directive, descriptive) as within-subjects factors, was utilized to analyze the distribution of individual maternal response types between the two groups. Due to significant positive skew within the individual response types, data were arcsine transformed prior to analysis. Descriptive information on the distribution of these individual response types (transformed data) is presented in Figure 1. There was a significant main effect of Response, F (4.93, 348) = 48.64, p <.001. Contrasts revealed that overall, mothers produced significantly more Acknowledgements than any other response type (all p’s <.001.), higher Imitation than Attributes (p = .03), Play than Attributes (p = .02) and Questions than either Attributes or Directives (both p’s< .01). These main effects were qualified by a significant Group × Maternal Response Type Interaction, F(9.78, 342) = 1.88, p = .05, indicating some differences in the pattern of responses between the groups. Simple effects analyses revealed significant group differences only for mothers’ rate of Label responses, F(2,59) = 3.74, p = .03. Post hoc tests (Tamhane’s) revealed no robust differences between the groups, but a trend level difference for mothers of ASD infants to use more labels than mothers of LRC infants (p = .09).
Figure 1.
Distribution of maternal contingent response types, by group. Error bars represent standard errors.
Maternal responses to infant vowel and consonant-vowel vocalizations
We were next interested in determining whether mothers’ responses differed to each of the two infant vocalization types. Because of this interest in examining differential response patterns, these analyses were conducted using dyads whose infants had produced both vocalization types (24 LRC, 18 HRA, 5 ASD). Rather than investigating responses to infant vocalizations across all seven individual maternal response types, we were primarily interested in determining whether mothers differed in their use of Language Promoting and Non-Promoting responses to infants’ vocalizations. To address this question, differences in maternal responses to vowel and consonant-vowel vocalizations between the risk groups were examined using a 2 × 2 × 2 repeated-measures ANOVA, with Infant Vocalization Type (VV and CV) and Maternal Response Type (Language Promoting and Non-Promoting) as the within-subjects factors and Group (HRA and LRC) as the between-subjects factors. Of these summary maternal response variables, only Maternal Non-Promoting Responses to Consonant-Vowels demonstrated significant positive skew, due to a large number of zeroes. Arcsine transformations were conducted to improve the normalization of these summary variables, but did not significantly improve the distribution shape. We proceeded with using the non-transformed values for ease of interpretation, and the pattern of results was unchanged when the analyses were conducted using the transformed variables. Descriptive information on means and standard deviations for these summary response variables are presented in Table 3.
Table 3.
Maternal Language Promoting and Non-Promoting Responses, by Group
| Vocalization Type (mean, SD) | Group
|
||
|---|---|---|---|
| LRC n = 24 | HRA-N n =18 | ASD n = 5 | |
| Promoting, vowels | .36 (.18) | .31 (.16) | .16 (.21) |
| Non-Promoting, vowels | .13 (.11) | .15 (.10) | .07 (.11) |
| Promoting, consonant-vowels | .53 (.27) | .50 (.36) | .50 (.24) |
| Non-Promoting, consonant-vowels | .10 (.14) | .05 (.06) | .00 (.00) |
Note: no significant group differences. Main effect of response type, F(1,44) = 61.51, p<.01, and infant vocalization by response type interaction.
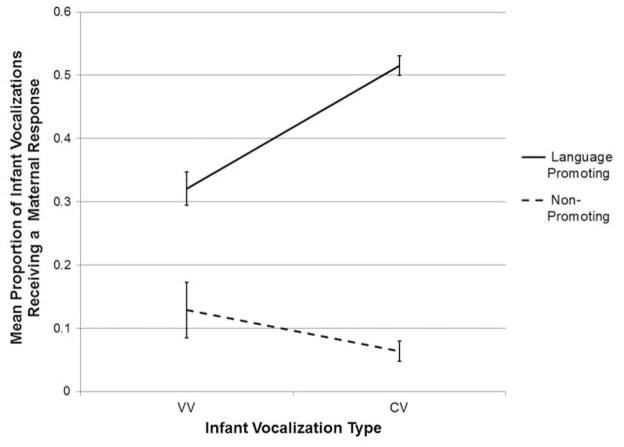
For this ANOVA, there were significant main effects of both child vocalization type, F(1,44) = 9.61, p = .003, and maternal response type, F(1,44) = 61.51, p <.001, with infants overall producing more Vowel than Consonant-Vowel utterances, and mothers overall producing more Language Promoting than Non-Promoting Responses. These main effects were modulated by a significant Infant Vocal Type X Maternal Response Type interaction, F(1,44) = 22.45, p <.001. There were no significant main or interaction effects involving group. Simple main effects analyses were conducted to determine the source of this significant interaction and revealed that Language Promoting responses occurred significantly more frequently in response to Consonant-Vowel than Vowel vocalizations (F(1, 46) = 21.57, p <.001), while the opposite pattern was observed for Non-Promoting responses, which occurred significantly more in response to Vowels than Consonant-Vowels (F(1,46) = 10.43, p = .002). This interaction between Language- and Non-Promoting responses and infant vocalizations is displayed in Figure 2.
Figure 2.
Mean proportion of infant vocalizations receiving a maternal response, by maternal response type. Error bars represent standard errors.
Interrelations Amongst Maternal Language and Background Characteristics
Finally, to examine relationships between maternal vocalizations and maternal and family characteristics hypothesized to influence their vocalization patterns, Pearson correlations were calculated to assess the relations between Maternal Total Utterances and Total Contingent Responses and maternal and family background characteristics of interest: concurrent infant autism symptoms and mothers’ ASD-related concerns, maternal self-reported broader phenotype characteristics, and the older diagnosed child’s symptom severity. Zero-order Pearson’s correlation coefficients are presented in Table 4. None of these associations were significant.
Table 4.
Zero-order Pearson Correlations Coefficents between Maternal Vocalizations and Responses and Family Background Factors, for High Risk Families
| BAP-Q Pragmatic Language | ASD Concerns | AOSI Score | Proband SCQ | |
|---|---|---|---|---|
| Maternal total utterance rate | .01 | .04 | −.21 | .01 |
| Maternal total contingent response rate | −.12 | .07 | −.16 | −.29 |
Note: These correlations were conducted on the subset of HRA infants with available data; for BAP, n =21;Concerns: n = 24; AOSI, n =17; SCQ n = 28.
BAP-Q = Broader Autism Phenotype Questionnaire, AOSI = Autism Observation Scale for Infants, SCQ = Social Communication Questionnaire.
Discussion
In this study, we examined infant vocalizations and maternal responses to those vocalizations at 9 months of age, and relations between high risk mothers’ responses to their infants’ vocalizations and maternal characteristics hypothesized to contribute to their behavioral responses. We found no differences in infants’ vowel and consonant-vowel production rates at 9 months of age between low risk typically developing infants, high risk infants who were not diagnosed with ASD, and in the small subset of infants who later were classified as meeting criteria for ASD. In general, mothers in all three groups responded similarly to their infants’ early vocalizations, though mothers of infants’ later diagnosed tended to respond by labeling objects more frequently than the other two groups. The general pattern of maternal responses reported here closely replicates the findings of Gros-Louis et al (2006), who also reported that mothers’ responses to their infants’ vocalizations were most frequently acknowledgements.
All three groups of mothers demonstrated significant differentiation in their responses to infants’ early vocalizations, responding with feedback previously hypothesized to promote langauge development significantly more frequently when infants produced consonant-vowel utterances rather than vowel-only utterances. This differential responding to consonants with higher quality maternal feedback is consistent with previous laboratory-based analyses of maternal contingent responses to typically developing infants of the same age (Gros-Louis et al., 2006). Our results extend these laboratory-based findings to the home, and suggest that for this specific feature of dyadic interactions, laboratory-based interactions largely reflect the daily interactions of 9-month-old infants and their mothers.
Many of the reported effects of environmental (maternal) input on infants’ language development have been most striking in cases of relatively impovershed input, as in the case of families from low SES, or in infants with physical hearing issues (i.e.chronic ear infections) (Nittrouer & Burton, 2005). The current investigation was not designed to answer questions about differences in maternal input related to SES, and the majority of families participating in this study were from high SES backgrounds and thus unlikely to provide relatively impovershed linguistic input associated with SES. The current study was designed to assess whether the vocalization patterns mothers of high risk infants were associated with other features of the familial autism context, including increased social communication difficulties and consonant production amongst infant siblings and differences in pragmatic language use, increased frequecy and level of concern, and increased exposure to autism symptoms and related behavioral adaptations amongst mothers. If any of these factors contibuted to less frequent or lower quality feedback to high risk infants’ early vocal production, it may have helped to explain some of the delays in language ability amongst high risk infant siblings.
Our results clearly demonstrate this is not the case. Mothers of high risk infants are talking to their babies, and critically, contingently responding, and thus reinforcing, infants’ early language production. The feedback they provide to their infants’ 9-month vocalizations is no different in terms of both frequency and content as mothers of low risk infants. These results suggest that risk status does not negatively influence maternal behavior in this domain. This is now one of several studies demonstrating that on the whole, mothers of high risk infants show little differences in the lingustic and communicative input they provide to their infants (Campbell et al., 2014; Leezenbaum et al., 2013; Talbott et al., 2015b; Wan et al., 2012, 2013). It is important to note that the infants in our sample did not demonstrate differences in consonant production that have been reported previously. Thus, our data do not eliminate the possibility that differences in maternal behavior emerge as a consequence of the language delays observed more frequently in the second year of life amongst both later diagnosed and nondiagnosed high risk infants (Iverson & Wozniak, 2007; Mitchell et al., 2006; Ozonoff et al., 2014; Paul et al., 2011). Additionally, while our data suggest there are minimal differences in the type of linguistic input mothers of high risk infants provide, differences in dyadic features of early interactions have also been reported, and these interactive qualities may also contribute to meaningful differences in infants’ language learning opportunities (Northrup & Iverson, 2015; Wan et al., 2013).
The fact that we did not observe differences between the groups, particuarly in infants’ consonant production, was contrary to our initial hypotheses, but likely reflects the heterogeneity of language ability amongst high risk infants and the fact that atypical language and social behaviors are just beginning to emerge during this period of development (Ozonoff et al., 2010, 2014). While delays in prelinguistic phonemic development can be an early risk marker for ASD, they are not observed amongst all children with ASD. Contingent interactions do influence the development of infants’ phonemic perception, but the amount of input required for typical acquisition is fairly minimal and most children will learn to distinguish the phonemes of their native language at roughly the same ages (Elsabbagh et al., 2013; Kuhl et al., 2003; Nittrouer & Burton, 2005). Previous work from our group has demonstrated that infant siblings, drawn from the same cohort examined here, exhibit a typical trajectory of perceptual narrowing over the first year of life (Seery, Vogel-Farley, Tager-Flusberg, & Nelson, 2013). It is important to note that definitive interpretation of our data is limited by the small sample size, particularly with regards to the ASD group, which limits our ability to detect small but meaningful differences in this group.
Our findings have high relevance for early intervention programs and policies, as they suggest that it may be more appropriate for parent coaching practices to target the families of infant siblings who are demonstrating early symptoms or communication delays, rather than across the group as a whole. On the other hand, mothers of high risk infants report significantly elevated levels of concern regarding their infants’ development across the entire first year of life, as well as more elevated depressive symptoms—so it seems appropriate to offer education, monitoring, or coaching practices that provide mothers with support in these areas and provide optimally rich language-learning environments for high risk infants (Sacrey et al., 2015; Talbott et al., 2015a).
The rich, high-quality linguistic input provided to high risk infant siblings by their mothers in the first year of life is almost certainly a protective factor in their language development. The extent to which this input is characteristic of families who are not participating in intensive, university-based longitudinal investigations (families both with and without children already diagnosed) is not clear but warrants further investigation. Future investigations should also determine whether intervening to increase high risk mothers’ frequency of contingent responses results in increased frequency of concurrent infant vocalizations or more rapid language development. Such studies would have clear and important implications for early intervention practices and are of particular interest for high risk infant siblings who are exhibiting overt delays in early language, as has been reported in other samples.
Acknowledgments
Grant sponsor: NIH; Grant number: R01-DC010290, R21 DC 08637; Grant sponsor: Simons Foundation; Grant number: 137186; Grant sponsor: Autism Speaks; Grant number: 1323.
We are extremely grateful to the many children and families who have participated in this study, and to the project staff and interns who have assisted in the data collection. This work was supported by grants from the NIH (R01-DC010290 and R21 DC 08637), Autism Speaks, and the Simons Foundation (137186).
References
- Bailey DB, Golden RN, Roberts J, Ford A. Maternal depression and developmental disability: Research critique. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:321–329. doi: 10.1002/mrdd. [DOI] [PubMed] [Google Scholar]
- Bettes BA. Maternal depression and motherese: Temporal and intonational features. Child Development. 1988;59:1089–1096. doi: 10.1111/j.1467-8624.1988.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Bruner J. The social context of language acquisition. Language & Communication. 1981;1:155–178. [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The autism observation scale for infants: Scale development and reliability data. Journal of Autism and Developmental Disorders. 2008;38:731–738. doi: 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Leezenbaum NB, Mahoney AS, Day TN, Schmidt EN. Social engagement with parents in 11-month-old siblings at high and low genetic risk for autism spectrum disorder. Autism. 2014 doi: 10.1177/1362361314555146. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E, … Bolton P. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry. 2015;72:415–423. doi: 10.1001/jamapsychiatry.2014.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Hohenberger A, Campos R, Van Herwegen J, Serres J, de Schonen S, … Karmiloff-Smith A. Narrowing perceptual sensitivity to the native language in infancy: Exogenous influences on developmental timing. Behavioral Sciences. 2013;3:120–132. doi: 10.3390/bs3010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MH, King AP, West MJ. Social interaction shapes babbling: Testing parallels between birdsong and speech. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8030–8035. doi: 10.1073/pnas.1332441100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MH, Schwade JA. Social feedback to infants’ babbling facilitates rapid phonological learning. Psychological Science. 2008;19:515–523. doi: 10.1111/j.1467-9280.2008.02117.x. [DOI] [PubMed] [Google Scholar]
- Gros-Louis J, West MJ, Goldstein MH, King AP. Mothers provide differential feedback to infants’ prelinguistic sounds. International Journal of Behavioral Development. 2006;30:509–516. doi: 10.1177/0165025406071914. [DOI] [Google Scholar]
- Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. European Journal of Pediatrics. 1995;1995:xxiii–268. doi: 10.1007/s00431-005-0010-2. [DOI] [Google Scholar]
- Hess CR, Landa RJ. Predictive and concurrent validity of parent concern about young children at risk for autism. Journal of Autism and Developmental Disorders. 2012;42:575–584. doi: 10.1007/s10803-011-1282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff E. How social contexts support and shape language development. Developmental Review. 2006;26:55–88. doi: 10.1016/j.dr.2005.11.002. [DOI] [Google Scholar]
- Hurley RSE, Losh M, Parlier M, Reznick JS, Piven J. The broad autism phenotype questionnaire. Journal of Autism and Developmental Disorders. 2007;37:1679–1690. doi: 10.1007/s10803-006-0299-3. [DOI] [PubMed] [Google Scholar]
- Ingersoll B, Hambrick DZ. The relationship between the broader autism phenotype, child severity, and stress and depression in parents of children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2011;5:337–344. doi: 10.1016/j.rasd.2010.04.017. [DOI] [Google Scholar]
- Iverson JM, Wozniak RH. Variation in vocal-motor development in infant siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37:158–170. doi: 10.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM. Foreign-language experience in infancy: Effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9096–9101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leezenbaum NB, Campbell SB, Butler D, Iverson JM. Maternal verbal responses to communication of infants at low and heightened risk of autism. Autism: The International Journal of Research and Practice. 2013;18:694–703. doi: 10.1177/1362361313491327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren KA, Folstein S, Tomblin B, Tager-Flusberg H. Language and reading abilities of children with autism spectrum disorder and their first-degree relatives. 2010;2:22–38. doi: 10.1002/aur.63.Language. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, … Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, Bryson S. Early language and communication development of infants later diagnosed with autism spectrum disorder. Journal of Developmental and Behavioral Pediatrics. 2006;27:S69–S78. doi: 10.1097/00004703-200604002-00004. [DOI] [PubMed] [Google Scholar]
- Nittrouer S. The relation between speech perception and phonemic awareness: Evidence from low-SES children and children With chronic OM. J Speech Hear Res. 1996;39:1059–1070. doi: 10.1044/jshr.3905.1059. [DOI] [PubMed] [Google Scholar]
- Nittrouer S, Burton LT. The role of early language experience in the development of speech perception and phonological processing abilities: evidence from 5-year-olds with histories of otitis media with effusion and low socioeconomic status. Journal of Communication Disorders. 2005;38:29–63. doi: 10.1016/j.jcomdis.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Northrup JB, Iverson JM. Vocal coordination during early parent—infant interactions predicts language outcome in infant siblings of children with autism spectrum disorder. Infancy. 2015 doi: 10.1111/infa.12090. Advance Online Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: A study of first birthday home videotapes. Journal of Autism and Developmental Disorders. 1994;24:247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Losif AM, Baguio F, Cook IC, Hill MMM, Hutman T, … Young GS. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:256–266. doi: 10.1097/00004583-201003000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, … Iosif AM. The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:398–407.e2. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young G, Steinfeld M, Hill MM, Cook I, Hutman T, … Sigman M. How early do parent concerns predict later autism diagnosis? Journal of Developmental and Behavioral Pediatrics. 2009;30:367–375. doi: 10.1097/dbp.0b013e3181ba0fcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BA, Rowe ML, Singer JD, Snow CE. Maternal correlates of growth in toddler vocabulary production in low-income families. Child Development. 2005;76:763–782. doi: 10.1111/j.1467-8624.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Patten E, Belardi K, Baranek GT, Watson LR, Labban JD, Oller DK. Vocal patterns in infants with autism spectrum disorder: Canonical babbling status and vocalization frequency. Journal of Autism and Developmental Disorders. 2014;44:2413–2428. doi: 10.1007/s10803-014-2047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: Vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry. 2011;52:588–598. doi: 10.1111/j.1469-7610.2010.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe ML. Child-directed speech: relation to socioeconomic status, knowledge of child development and child vocabulary skill. Journal of Child Language. 2008;35:185–205. doi: 10.1017/S0305000907008343. [DOI] [PubMed] [Google Scholar]
- Rowe ML. A longitudinal investigation of the role of quantity and quality of child-directed speech in vocabulary development. Child Development. 2012;83:1762–1774. doi: 10.1111/j.1467-8624.2012.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruser TF, Arin D, Dowd M, Putnam S, Winklosky B, Rosen-Sheidley B, … Folstein S. Communicative competence in parents of children with autism and parents of children with specific language impairment. Journal of Autism and Developmental Disorders. 2007;37:1323–1336. doi: 10.1007/s10803-006-0274-z. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social communication questionnaire. Los Angeles: CA: Western Psychological Services; 2003. [Google Scholar]
- Sacrey LR, Zwaigenbaum L, Bryson S, Brian J, Smith IM, Roberts W, … Armstrong V. Can parents’ concerns predict autism spectrum disorder? A prospective study of high-risk siblings from 6 to 36 months of age. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54:470–478. doi: 10.1016/j.jaac.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ. Developmental systems: contexts and evolution. In: Mussen P, editor. Handbook of child psychology. Vol. 1. New York: Wiley; 1983. pp. 237–294. [Google Scholar]
- Seery AM, Vogel-Farley V, Tager-Flusberg H, Nelson Ca. Atypical lateralization of ERP response to native and non-native speech in infants at risk for autism spectrum disorder. Developmental Cognitive Neuroscience. 2013;5:10–24. doi: 10.1016/j.dcn.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott MR, Nelson CA, Tager-Flusberg H. Diary reports of concerns in mothers of infant siblings of children with autism across the first year of life. Journal of Autism and Developmental Disorders. 2015a;45:2187–2199. doi: 10.1007/s10803-015-2383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott MR, Nelson CA, Tager-Flusberg H. Maternal gesture use and language development in infant siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015b;45(1):4–14. doi: 10.1007/s10803-013-1820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, Plummer F. Quality of interaction between at-risk infants and caregiver at 12–15 months is associated with 3-year autism outcome. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2013;54:763–771. doi: 10.1111/jcpp.12032. [DOI] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, Plummer F, … Tucker L. Parent-infant interaction in infant siblings at risk of autism. Research in Developmental Disabilities. 2012;33:924–932. doi: 10.1016/j.ridd.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Warlaumont AS, Richards Ja, Gilkerson J, Oller DK. A social feedback loop for speech development and its reduction in autism. Psychological Science. 2014;25:1314–1324. doi: 10.1177/0956797614531023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L, Crais E, Baranek G. Communicative gesture use in infants with and without autism: A retrospective home video study. American Journal of Speech-Language Pathology. 2013;22:25–40. doi: 10.1044/1058-0360(2012/11-0145). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen RKC, Thiruvahindrapuram B, Merico D, Walker S, Tammimies K, Hoang N, … Scherer SW. Whole-genome sequencing of quartet families with autism spectrum disorder. Nature Medicine. 2015;21:185–191. doi: 10.1038/nm.3792. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]