Abstract
Information-processing biases may contribute to the intergenerational transmission of depression. There is growing evidence that children of depressed mothers exhibit attentional biases for sad faces. However, findings are mixed as to whether this bias reflects preferential attention toward, versus attentional avoidance of, sad faces suggesting the presence of unmeasured moderators. To address these mixed findings, we focused on the potential moderating role of genes associated with HPA-axis reactivity. Participants included children (8–14 years old) of mothers with (n = 81) and without (n = 81) a history of depression. Eye movements were recorded while children passively viewed arrays of angry, happy, sad, and neutral faces. DNA was obtained from buccal cells. Children of depressed mothers exhibited more sustained attention to sad faces than did children of nondepressed mothers. Importantly, however, this relation was moderated by children’s genotype. Specifically, children of depressed mothers who carried reactive genotypes across the CHRH1 TAT haplotype and FKBP5 rs1360780 (but not SLC6A4 5-HTTLPR) exhibited less sustained attention to sad faces and more sustained attention to happy faces. These findings highlight the role played by specific genetic influences and suggest that previous mixed findings may have been due to genetic heterogeneity across the samples.
Keywords: attentional bias, depression, intergenerational transmission, polygenic
One of the strongest single risk factors for depression is a positive family history of depression. Children of depressed mothers are 3–4 times more likely to meet criteria for a major depressive disorder (MDD) by early adulthood than are individuals in the general population (for reviews, see Goodman, 2007; Hammen, 2009). Indeed, approximately 40% of these children are expected to meet criteria for MDD by age 20 and 60% by age 25 (Beardslee et al., 1998), compared to only approximately 16% of individuals under age 25 in the general population (Blazer et al., 1994). There is also evidence that children of depressed, compared to non-depressed, mothers have an earlier age of onset and more chronic course of MDD (for reviews, see Goodman, 2007; Hammen, 2009). Clearly, then, it is important to understand why children of depressed mothers are at such an increased risk for depression themselves.
The specific mechanisms involved in the intergenerational transmission of depression likely involve a combination of cognitive, environmental and genetic factors, with theorists suggesting that information-processing biases may serve as a key pathway of risk in the intergenerational transmission of depression (Goodman, 2007; Gotlib & Colich, 2014). According to cognitive models (e.g., Clark, Beck & Alford, 1999), depression and depression risk are characterized by biases in attention to, interpretation of, and memory for stimuli reflecting themes of hopelessness and loss. There has been growing interest in the role of attentional biases in depression, with research showing that currently and formerly depressed adults (Joormann & Gotlib, 2007) and adolescents (Hankin, Gibb, Abela, & Flory, 2010) exhibit preferential attention specifically to sad faces but not to other facial displays of emotion. Because the bias is primarily observed when using longer stimulus presentation durations, attentional biases in depression are thought to reflect difficulty disengaging attention from depression-relevant stimuli once it has been captured rather than preferential initial orienting of attention (Gotlib & Joormann, 2010; Joormann, 2009).
More recently, researchers have begun to examine attentional biases in children of depressed mothers to determine whether they may serve as a mechanism of risk of the intergenerational transmission of depression. Consistent with studies in depressed adults, these studies have shown that children of depressed mothers exhibit attentional biases specifically for sad, but not happy or angry faces (Gibb, Benas, Grassia, & McGeary, 2009; Joormann, Talbot, & Gotlib, 2007; Kujawa et al., 2011). However, the direction of bias observed in these children was different across studies. Specifically, whereas two of the studies (Joormann et al., 2007; Kujawa et al., 2011) observed preferential attention toward sad faces in children of mothers with a history of MDD, the other (Gibb et al., 2009) found evidence of attentional avoidance of sad facial stimuli. This latter finding is consistent with what is observed in the infant literature in which there is clear evidence that infants look less at sad faces and more at happy faces (Montague & Walker-Andrews, 2001; Soken & Pick, 1999; Termine & Izard, 1988; but also see Hunnius, de Wit, Vrins, & von Hofsten, 2011), a tendency that is even stronger among infants of depressed mothers (Hernandez-Reif, Field, Diego, Vera, & Pickens, 2006). Indeed, infants of depressed mothers spend less time looking at their mothers’ faces than do infants of nondepressed mothers (Boyd, Zayas, & McKee, 2006; Field, 1995). Consistent with Gross’s (2014) theory regarding the role of attentional allocation as an early emotion regulation strategy, theorists have suggested that infants become upset following exposure to sad facial stimuli and then avert their gaze in an effort to regulate their negative affect (e.g., Bistricky, Ingram, & Atchley, 2011; Termine & Izard, 1988). To date, however, no studies of which we are aware have examined the link between emotion regulation strategies and attentional biases in children. Consistent with the hypothesis that children of depressed mothers are more reactive to the presentation of sad faces, there is evidence that children of depressed mothers exhibit heightened cognitive-affective reactivity specifically to the presentation of sad faces, but not other facial displays of emotion, in the form of increased pupil dilation (Burkhouse, Siegle, & Gibb, 2014).
When seeking to understand the mixed findings in the literature on children of depressed mothers, it is important to note how attentional biases were assessed in these studies. Historically, attentional biases have been examined using a probe detection task (cf. MacLeod, Mathews, & Tata, 1986), in which preferential attention for emotional faces (e.g., sad versus neutral) is inferred when participants’ reaction times to the probe are faster when the probe appears in the location of the emotional face than in the location of the neutral face. However, recently research has moved towards eye movement paradigms that have the benefit of providing a continuous index of participants’ gaze patterns, and which is less prone to variation due to non-attentional factors such as motor responsiveness (Eizenman et al., 2003). The typical structure of these paradigms are that participants simply view an array of emotional faces (angry, happy, sad, neutral) presented simultaneously on the screen for relatively long trials (e.g., 20 seconds). Individuals’ attention to each face is estimated from the duration of participants’ gaze position, where shifts in gaze position are thought to closely follow shifts in attentional focus (cf. Corbetta et al., 1998). Relative differences in gaze durations across different facial expressions of emotion are interpreted as indicating an attentional bias for the emotion represented (cf. Eizenman et al., 2003; Kellough, Beevers, Ellis, & Wells, 2008). Consistent with the dot-probe literature, findings from these studies have shown that depressed adults exhibit greater sustained attention to depression-relevant stimuli than non-depressed controls (Eizenman et al., 2003; Kellough et al., 2008), with one study suggesting that depressed adults also exhibited less sustained attention to positive stimuli, than did a never depressed group (Kellough et al., 2008). However, the one study that has focused on depressed children found the opposite pattern (Harrison & Gibb, in press). Specifically, currently depressed children looked more at happy and less at sad faces than did children with no history of depression, which the authors suggested could reflect an (unsuccessful) attempt to use attentional deployment as an emotion regulation strategy as described above (cf. Gross, 2014). No study of which we are aware has directly assessed patterns of attentional deployment using an eye-tracker in this type of passive viewing task among children of depressed mothers, leaving unanswered the question of whether preferential attention versus attentional avoidance would be observed in this at-risk population.
As noted above, research on attentional biases in children of depressed mothers is consistent in suggesting the presence of biased attention to sad faces. This research is also notable for the strongly mixed findings regarding the direction of this bias: preferential attention toward sad faces versus attentional avoidance of sad faces. Whenever there are mixed findings across studies, it suggests the presence of unmeasured moderating influences. With regard to information-processing biases, one influence that has been highlighted by theorists and researchers is genetic variation. Approximately 40% of the variance in depression risk is due to genetic factors (Sullivan, Neale, & Kendler, 2000) and there is growing interest in integrating cognitive and genetic models of depression risk to determine how variation in specific genes may increase risk for the development of information-processing biases (e.g., Beck, 2008; Gibb et al., 2013). Most of the research to date has focused on a functional polymorphism (5-HTTLPR) in the serotonin transporter gene (SLC6A4) and there is growing evidence that carriers of the lower expressing short (S) or LG alleles, compared to those homozygous for the LA allele, exhibit attentional biases for affectively relevant stimuli, perhaps particularly if they have a history of early life stress (for reviews, see Gibb et al., 2013; Pergamin-Hight, Bakermans-Kranenburg, van IJzendoorn, & Bar-Haim, 2012). Of particular relevance, there is evidence from one study that children’s 5-HTTLPR genotype may moderate the link between maternal depression and children’s attentional biases for sad faces such that children carrying the 5-HTTLPR S or LG allele, but not those homozygous for the LA allele, exhibit attentional avoidance of sad faces (Gibb et al., 2009).
Despite the promising results for 5-HTTLPR, it is clear that depression risk is not limited to variation in any single gene, even when focused on potential endophenotypes like attentional biases, and there is growing interest in examining aggregate levels of influence across multiple genes within a given biological system. Indeed, there is evidence for polygenic influences in psychiatric diseases such as schizophrenia and bipolar disorder from genome-wide association studies (e.g., Patel et al., 2010; Purcell et al., 2009) as well as more focused candidate gene approaches to studying narrowly defined biological pathways such as dopamine-related reward signaling in ventral striatum (Nikolova, Ferrell, Manuck, & Hariri, 2011). For the current study, we focused on genes associated with altered activity in the hypothalamic-pituitary-adrenal (HPA) axis based on evidence that 5-HTTLPR acts, in part, by increasing HPA axis reactivity to stress (for a review, see Miller, Wankerl, Stalder, Kirschbaum, & Alexander, 2012). Specifically, in addition to 5-HTTLPR, we focused on variation in two additional genes closely associated with HPA axis function: a three-SNP haplotype (TAT) in the corticotropin-releasing hormone type 1 receptor (CRHR1) gene and a single nucleotide polymorphism (SNP) in the gene encoding FK506 binding protein 51 (FKBP5 rs1360780).
Following a stressor, corticotropin releasing hormone (CRH) is released from the hypothalamus, causing adrenocoricotropin (ACTH) to be released by the pituitary, which then causes glucocorticoids including cortisol to be synthesized and released by the adrenal cortex. The CRHR1 gene codes for the CRH receptor and variation in CRHR1 genotype has been shown to affect the level of cortisol released in response to a laboratory-based stressor (Sheikh, Kryski, Smith, Hayden, & Singh, 2013). There is evidence that three CRHR1 SNPs – rs7209436, rs110402, and rs242924 – form a protective TAT haplotype. Specifically, among individuals reporting a history of childhood abuse, those with no copies of the protective TAT haplotype, compared to those carrying one or two copies of the haplotype, reported significantly higher current depressive symptoms and greater risk for depression, with risk appearing to decrease with each additional copy of the TAT haplotype present (Bradley et al., 2008; Laucht et al., 2013; Polanczyk et al., 2009). To our knowledge, however, no studies have examined the impact of the CRHR1 TAT haplotype on children’s attentional biases.
The FKBP5 protein plays a key role in HPA axis activity by regulating the sensitivity of the glucocorticoid receptor, with higher levels of FKPB5 expression associated with lower glucocorticoid activity (for a review, see Zannas & Binder, 2014). The FKBP5 rs1360780 T allele is associated with greater FKBP5 expression and a prolonged cortisol response to stress (Zannas & Binder, 2014). Because of these effects, there has been growing interest in how FKBP5 rs1360780 genotype may moderate the impact of stress, particularly early life stress, on later risk for psychiatric disorders. A number of studies have now shown that, among individuals with a history of childhood abuse, carriers of the FKBP5 rs1360780 T allele, compared to those homozygous for the C allele, are at increased risk for MDD (Zannas & Binder, 2014). Importantly for the current study, there is evidence from one study that carriers of the T allele also exhibit a stronger attentional bias to threat-relevant stimuli than those homozygous for the C allele in a dot probe task (Fani et al., 2013). It should be noted, however, that this study focused only on the main effect of genotype and did not examine the potential moderating role of family influences and also did not include sad faces within the dot probe task.
Our goal in the current study, therefore, was to examine attentional biases in children of mothers with a history of MDD. Seeking to address previous mixed findings in the literature, we first examined the impact of mother MDD on children’s attentional biases independent of genotype. Consistent with eye-tracking research demonstrating attentional avoidance in currently depressed children (Harrison & Gibb, in press) we predicted that children of mothers with a history of MDD during the children’s lives would exhibit attentional avoidance of sad faces during a passive viewing task. Next, building from previous research suggesting that variation in genes linked to HPA axis reactivity may help to determine which children of depressed mothers exhibit the strongest attentional biases (Gibb et al., 2009), we examined the potential influence of genes known to influence HPA axis reactivity to stress: 5-HTTLPR, FKBP5 rs1360780, and CRHR1 TAT haplotype. We predicted that children of depressed mothers carrying more reactive genotypes (i.e., a greater number of 5-HTTLPR S or LG alleles, a greater number of FKBP5 rs1360780 T alleles, and a fewer number of copies of the “protective” CRHR1 TAT haplotype) would exhibit greater attentional avoidance of sad faces. In addition to examining variation in these genes individually, we also examined aggregate levels of influence across the genes. There is growing recognition that psychiatrically-relevant phenotypes, including information-processing biases, are likely impacted by the combined influence of multiple genes acting together. One method for examining these polygenic influences is to look at aggregate levels of influence across the genes, summing the number of “reactive” polymorphisms present (for a review, see Gibb et al., 2013). We predicted that this polygenic reactivity score (PGRS) would explain a greater amount of the variance in children’s attentional biases than would variation in any single gene.
Method
Participants
A total of 239 mother-child dyads were recruited for this study. Seventy-seven of these were excluded from analyses due to insufficient data (i.e. completed less than eight trials of the attention task), leaving us with a total of 162 mothers-child dyads for analysis. Included families did not differ significantly from excluded families based on mother or child depression (lifetime or current diagnoses of MDD or current depressive symptom levels) or demographic variables (child age or sex) (all ps > .25). To qualify for the study, mothers were required to either meet criteria for MDD during the child’s lifetime according to the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV; American Psychiatric Association, 1994) (n = 81) or have no lifetime diagnosis of any DSM-IV mood disorder and no current Axis I diagnosis (n = 81). Exclusion criteria for both groups included a history of schizophrenia or other psychotic disorders, a history of bipolar disorder, or alcohol or substance dependence within the last six months. To participate in the study, children had to be between the ages of 8–14 years and only one child per mother could participate. If more than one child in this age range was available, one child was chosen at random to participate. There were no diagnostic exclusion criteria for children. For the remaining children in our sample, the average age was 10.99 years (SD = 1.92), 49.7% were female, and 85.5% were Caucasian. The average age of mothers in our sample was 40.95 years (SD = 6.97) and 92.4% were Caucasian. The median annual family income was $50,001–55,000. Participant characteristics are presented separately for each mother MDD group in Table 1.
Table 1.
Demographic characteristics
| Children of Depressed Mothers (n = 81) | Children of Nondepressed Mothers (n = 81) | |
|---|---|---|
| Sex (% female) | 49.38 | 50.62 |
| Age (M, SD) | 10.89 (1.94) | 11.11 (1.88) |
| SLC6A4 (5-HTTLPR) | ||
| S′S′ | 13 | 16 |
| S′L′ | 49 | 48 |
| L′L′ | 19 | 17 |
| CRHR1 (copies of TAT) | ||
| 0 | 34 | 32 |
| 1 | 39 | 37 |
| 2 | 8 | 12 |
| FKBP5 (rs1360780) | ||
| TT | 10 | 11 |
| CT | 26 | 32 |
| CC | 45 | 38 |
Measures
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1995) and the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL; Kaufman, Birmaher, Brent, & Rao, 1997) were used to assess for current DSM-IV Axis I disorders in mothers and their children, respectively. The SCID-I and K-SADS-PL were administered by separate interviewers. For the K-SADS-PL, mothers and children were interviewed separately. As noted above, 81 mothers met criteria for MDD during their child’s life (15 met criteria for current MDD). In terms of children’s diagnoses, ten met criteria for a lifetime diagnosis of MDD (9 of whom had a mother with a history of MDD). To assess inter-rater reliability, a subset of 20 SCID and K-SADS interviews from this project were coded by a second interviewer and kappa coefficients for MDD diagnoses in mothers and children were excellent (all κs = 1.00).
Mothers’ and children’s symptoms of depression were assessed using the Beck Depression Inventory-II (Beck, Steer, & Brown, 1996) and Children’s Depression Inventory (Kovacs, 1981), respectively. Numerous studies have supported the reliability and validity of both measures (Beck et al., 1996; Kovacs, 1981, 1985; Smucker, Craighead, Craighead, & Green, 1986). In the current study, both the BDI-II and the CDI exhibited good internal consistency (αs = .94 and 85, respectively).
Children’s attentional biases for facial displays of emotion were assessed using a passive viewing task that quantified continuous gaze patterns over a relatively long (20s) free-viewing trial. The passive viewing task was programmed and run using E-prime (version 2). A 24 inch Tobii T60XL eye tracking monitor (60Hz data rate; 1920 × 1200 pixels) was used to display stimuli and record eye movements using infrared Pupil Centre Corneal Reflection, which illuminates the eye and calculates gaze direction in relation to the monitor location, with gaze data accurate to .5 degrees with an error (drift) of .1 degree. Each participant sat 65cm from the monitor during the passive viewing task. Before each session, participants completed a five-point calibration of the eye tracker where they are asked to look at specific points at the center and corners of the monitor. Accuracy was confirmed by visual inspection of fixations recorded during the calibration procedure. The task consisted of four faces, sized 13cm high (17° visual angle) × 12cm wide (8° visual angle), and arrayed in a 2 × 2 grid on screen (i.e. upper left, upper right, lower left, lower right). There was 20cm (19° visual angle) between the center of each stimulus horizontally and 16cm vertically (25° visual angle). Facial stimuli for this task were drawn from the Karolinska Directed Emotional Faces (K-DEF; Calvo & Lundqvist, 2008; Lundqvist, Flykt, & Ohman, 1998) stimulus set consisting of adult actors exhibiting angry, happy, sad, and neutral facial expressions. Stimuli were presented in their original color and were cropped of non-facial features (e.g. hair, neck and shoulders) using an ovaled mask. Each trial started with a fixation cross presented for 1s, followed by the appearance of four faces from the same actor representing angry happy, sad, and neutral expressions that were presented for 20s. In each trial, faces depicting each emotion were presented randomly in each of the four different quadrants and each emotional expression occurred with equal frequency in all four quadrants. The task consisted of a total of 16 trials (8 with female actors and 8 with male actors). Participants were asked to freely view the stimuli and maintain their attention on the screen for the entire time. Attentional allocation during each trial was measured as gaze durations lasting greater than or equal 100ms in each of the face locations. In line with previous studies (Kellough et al., 2008; Harrison & Gibb, in press), gaze data was divided into 4-second epochs (5 total) in each trial and the proportion of time (i.e. gaze duration) participants looked at each face within each epoch was calculated, which allowed us to test for potential changes in the trajectories of attentional allocation across the duration of each trial. Gaze proportions in each epoch were calculated by dividing participants’ gaze duration for each facial emotion type by the gaze duration for all faces and then averaged across trials.
Finally, children’s DNA was collected and isolated from buccal cells using established methods (Freeman et al., 1997; Lench, Stanier, & Williamson, 1988). For 5-HTTLPR, the S alleles were assayed using previously reported methods (Pooley, Houston, Hawton, & Harrison, 2003) and the rs25531 SNP genotypes (LA vs. LG) were obtained using a combination of published methods. Specifically, the primers used for PCR were those reported in Hu et al., (2005) and the MspI restriction site protocol follows Wendland et al. (2006). Samples were analyzed on an ABI PRISM® 3130xl Sequencer. Coding for the number of lower expressing (S or LG) alleles present yielded three groups: S′S′ (children with 2 copies of the lower expressing alleles; n = 29), S′L′ (children with 1 copy of the lower expressing allele; n = 97), and L′L′ (children homozygous for the LA allele; n = 36). Results of an exact test for Hardy Weinberg proportions using Markov chain–Monte Carlo implementation (Engels, 2009; Guo & Thompson, 1992) indicate that our observed genotype frequencies do not differ from Hardy Weinberg equilibrium (p = .0647 +/− .0003).
For CRHR1, 3 SNPs were genotyped – rs7209436, rs110402, and rs242924 – that form a T-A-T haplotype (Bradley et al., 2008; Polanczyk et al., 2009). The Taqman assay IDs for rs7209436, rs110402, and rs242924 were C___1570087_10, C___2544843_10, and C___2257689_10, respectively. The three CRHR1 polymorphisms were genotyped using fluorogenic 5′ nuclease (Taqman, Applied Biosystems, Foster City, CA) method involving reagents (VIC(tm) and FAM(tm), labeled probes, and TaqMan® Universal PCR Master Mix without AMPerase® UNG) obtained from Applied Biosystems (ABI). Genotype determination was performed using primers purchased from ABI or Integrated DNA Technologies (Coralville, IA). Genotypes were obtained using an ABI Prism 7300 Sequence Detection System using both absolute quantification and allelic discrimination modes (Livak, Flood, Marmaro, Giusti, & Deetz, 1995). All markers were found to be in Hardy-Weinberg equilibrium using default parameters in Haploview (p < .001; Barrett, 2009). In order to (a) maximize the amount of information provided by the multiple markers, and (b) circumvent loss of power due to multiple testing, we utilized all of the available SNP data to identify haplotype blocks (i.e., the combinations of SNP markers that are statistically associated). Haploview was used to visualize haplotype blocks (Barrett, 2009; Barrett et al., 2005). Marker to marker D′ correlations were as follows: rs7209436 – rs110402 = 0.95, rs7209436 – rs242924 = 0.88, rs110402 – rs242924 = 0.93. Haplotypes for both chromosomes were then confirmed and extracted using PHASE (Version 2.1, Stephens & Donnelly, 2003; Stephens et al., 2001), requiring that the probability of a haplotype be greater than or equal to 0.80. PHASE haplotypes were used to construct diplotypes (i.e., combination of haplotypes across the pair of homologous chromosomes) that were used in the regression analyses. CRHR1 was coded as the number of copies of the protective TAT haplotype that were present: 0 (n = 66), 1 (n = 76) and 2 (n = 20).
Finally, FKBP5 (rs1360780) was genotyped following the procedures used for CRHR1 SNPs above. The Taqman assay ID for rs1360780 was C___8852038_10. Coding for the number of T alleles present yielded the following three groups: TT (n = 21), CT (n = 58), CC (n = 83). Results of an exact test for Hardy Weinberg proportions using Markov chain–Monte Carlo implementation (Engels, 2009; Guo & Thompson, 1992) yielded a p value of .0436 +/−.0002, suggesting the presence of excess homozygosity in our sample.
Procedure
Potential participants were recruited from the community through a variety of means (e.g., television, newspaper and bus ads, and flyers). Mothers responding to the recruitment advertisements were initially screened over the phone to determine potential eligibility. Upon arrival at the laboratory, mothers were asked to provide informed consent and children were asked to provide assent to be in the study. Next, children completed the passive viewing task. During this time, the mother was administered the K-SADS-PL by a trained interviewer. After completing the K-SADS-PL with the mother, the same interviewer then administered the K-SADS-PL to the child. While children were being administered the K-SADS-PL, the mother was then administered the SCID-I by a separate interviewer. Finally, mothers completed the BDI-II and children completed the CDI. Families were compensated a total of $75 for their participation in this part of the study. The project was approved by the university’s internal review board.
Analysis Plan
Analyses proceeded in three stages. In the first stage, we examined the effects of mother MDD group independent of child genotype to provide a more direct comparison with other studies that have not considered specific genetic influences (e.g., Joormann et al., 2007; Kujawa et al., 2011). In the second stage, we examined the moderating impact of each gene separately. Because we were primarily focused on evaluating the PGRS (sum of reactive alleles/haplotypes present across multiple polymorphisms) models were tested using a linear, additive model of genetic influence. Third, any single genes exhibiting the predicted effects in stage two were then combined into a single polygenic reactivity score (PGRS) to evaluate their combined influence. The PGRS was created by summing the number of copies of reactive alleles/haplotypes present across each of the genes. For each analysis stage, the dependent variable was the proportion of gaze duration for each of the four facial expressions calculated separately for each epoch.
Results
Descriptive statistics for the proportion of gaze duration are presented in Table 2, and are presented separately by emotion, epoch, and mother MDD group. Next, focusing on the role of maternal MDD history independent of child genotype, we examined patterns of attentional allocation during the passive viewing task using a 2 (Mother MDD: yes, no) × 4 (Emotion: angry, happy, sad, neutral) × 5 (Epoch: 1–5) linear mixed model with proportion of gaze duration scores serving as the dependent variable. There was a significant main effect of Emotion, F(3, 1117.09) = 75.79, p < .001, which was qualified by a significant Mother MDD × Emotion interaction, F(3, 1117.09) = 5.56, p = .001. Neither the main effect of Mother MDD nor any of the effects involving Epoch were significant (lowest p = .19). Examining the form of the Mother MDD × Emotion interaction, we tested for Mother MDD differences within each emotion separately. We found significant Mother MDD differences in children’s sustained attention to sad, F(1, 156.49) = 4.22, p = .04, but not angry, F(1, 154.28) = 1.70, p = .20, happy, F(1, 156.49) = 2.72, p = .10, or neutral, F(1, 155.81) = 0.05, p = .82, faces. However, contrary to our predictions, children of depressed mothers showed greater attention to sad faces (Mproportion = .23, SE = .01) than did children of nondepressed mothers (Mproportion = .21, SE = .01).
Table 2.
Means and Standard Deviations for Children’s Attentional Allocation (Raw and Proportion Gaze Duration) by Emotion, Epoch, and Maternal MDD history
| Emotion | Epoch | Children of Depressed Mothers
|
Children of Nondepressed Mothers
|
||
|---|---|---|---|---|---|
| Raw Gaze | Proportion Gaze | Raw Gaze | Proportion Gaze | ||
| Angry | 1 | .83 (.28) | .27 (.09) | .80 (.29) | .26 (.08) |
| 2 | .96 (.36) | .25 (.09) | .98 (.34) | .25 (.08) | |
| 3 | .97 (.36) | .24 (.10) | .98 (.39) | .25 (.11) | |
| 4 | 1.03 (.40) | .26 (.11) | 1.00 (.40) | .25 (.11) | |
| 5 | 1.02 (.36) | .26 (.10) | .93 (.40) | .23 (.11) | |
| Happy | 1 | .84 (.31) | .27 (.10) | .93 (.35) | .30 (.13) |
| 2 | 1.09 (.43) | .28 (.11) | 1.20 (.53) | .30 (.14) | |
| 3 | 1.18 (.45) | .30 (.11) | 1.24 (.55) | .31 (.14) | |
| 4 | 1.11 (.42) | .28 (.12) | 1.25 (.61) | .31 (.16) | |
| 5 | 1.09 (.43) | .27 (.13) | 1.24 (.60) | .31 (.15) | |
| Sad | 1 | .71 (.22) | .23 (.08) | .71 (.27) | .22 (.08) |
| 2 | .96 (.32) | .25 (.10) | .86 (.35) | .22 (.09) | |
| 3 | .93 (.32) | .24 (.08) | .82 (.36) | .20 (.09) | |
| 4 | .90 (.35) | .23 (.09) | .82 (.32) | .20 (.08) | |
| 5 | .89 (.36) | .22 (.09) | .81 (.38) | .20 (.10) | |
| Neutral | 1 | .69 (.27) | .22 (.08) | .70 (.27) | .22 (.09) |
| 2 | .90 (.31) | .23 (.09) | .90 (.33) | .23 (.08) | |
| 3 | .88 (.34) | .22 (.10) | .94 (.44) | .24 (.11) | |
| 4 | .94 (.41) | .24 (.11) | .94 (.37) | .24 (.10) | |
| 5 | .97 (.38) | .24 (.10) | .99 (.51) | .25 (.13) | |
We then examined the impact of children’s 5-HTTLPR, CRHR1, and FKBP5 genotypes. Focusing first on 5-HTTLPR, we found a significant 5-HTTLPR × Mother MDD × Emotion interaction, F(3, 1146.40) = 6.06, p < .001. Examining the form of this interaction, we found that the 5-HTTLPR × Emotion interaction was significant among children of depressed mothers, F(3, 577.55) = 5.96, p = .001, but not among children of nondepressed mothers, F(3, 561.42) = 1.28, p = .28. Examining this further, however, 5-HTTLPR genotype was not significantly related to sustained attention for any specific emotion type among children of depressed mothers: angry, t(77.73) = −1.95, p = .06, reffect size = −.22, happy, t(75.62) = 1.34, p = .18, reffect size = .15, sad, t(76.88) = −0.05, p = .96, reffect size = −.006, or neutral, t(75.28) = −0.08, p = .94, reffect size = −.009.
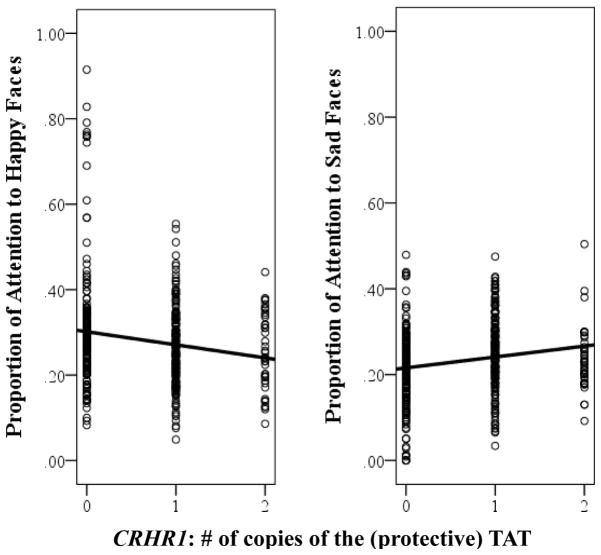
Focusing next on the CRHR1 TAT haplotype, we again found a significant interaction with children’s genotype: CRHR1 × Mother MDD × Emotion, F(3, 1151.10) = 4.03, p = .007. Examining the form of this interaction, we found that the CRHR1 × Emotion interaction was significant among children of depressed mothers, F(3, 583.29) = 6.10, p < .001, but not among children of nondepressed mothers, F(3, 561.53) = 1.90, p = .33. Examining this further, we found that among children of depressed mothers, the number of copies of the CRHR1 TAT haplotype present was associated with children’s sustained attention to happy, t(76.25) = −2.03, p = .04, reffect size = −.23, and sad, t(76.97) = 2.17, p = .03, reffect size = .24, faces but not angry, t(77.17) = 1.42, p = .16, reffect size = .16, or neutral, t(75.58) = −0.67, p = .51, reffect size = .08, faces. As can be seen in Figure 1, children of depressed mothers with fewer copies of the protective CRHR1 TAT attended more to happy faces and less to sad faces.
Figure 1.
Summary of analyses showing sustained attention to happy (left) and sad (right) faces among children of mothers with a history of MDD as a function of CRHR1 TAT haplotype.
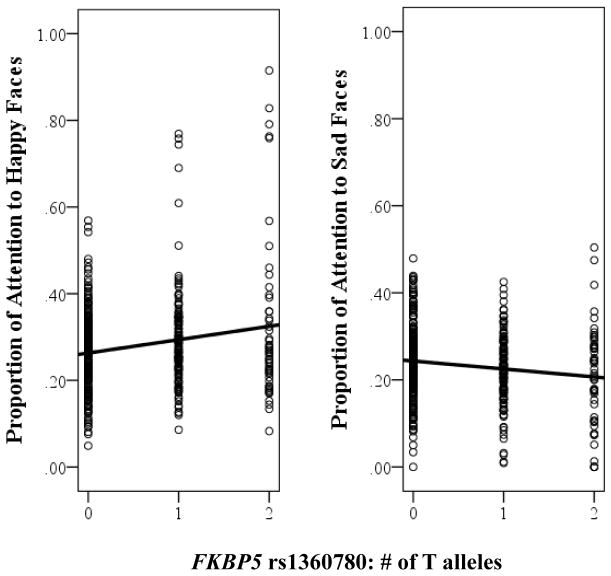
Similar results were obtained when we looked at FKBP5. Specifically, we found a significant FKBP5 × Mother MDD × Emotion interaction, F(3, 1148.25) = 5.41, p = .001. Examining the form of this interaction, we found that the FKBP5 × Emotion interaction was significant among children of depressed mothers, F(3, 580.39) = 7.66, p < .001, but not among children of nondepressed mothers, F(3, 560.77) = 0.92, p = .43. Examining this further, we found that among children of depressed mothers, the number of FKBP5 T alleles present was associated with children’s sustained attention to happy, t(74.81) = 2.17, p = .03, reffect size = .24, and sad, t(76.81) = −2.19, p = .03, reffect size = −.24, faces but not angry, t(77.05) = −1.06, p = .29, reffect size = .12, or neutral, t(75.30) = 0.10, p = .92, reffect size = .01, faces. As can be seen in Figure 2, children of depressed mothers with an increasing number of FKBP5 T alleles attended more to happy faces and less to sad faces.
Figure 2.
Summary of analyses showing sustained attention to happy (left) and sad (right) faces among children of mothers with a history of MDD as a function of FKBP5 rs1360780 genotype.
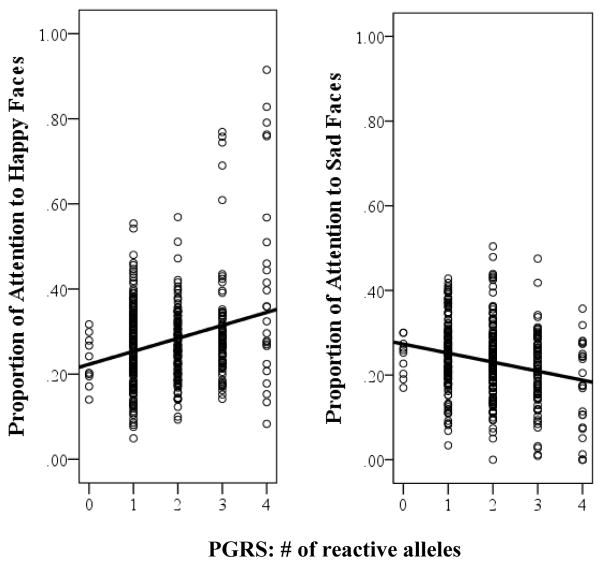
Given the significant results observed for CRHR1 and FKBP5 we then created a polygenic reactivity score (PGRS) by summing the number of reactive alleles across both polymorphisms to determine whether the PGRS could account for more variance in children’s attentional biases than either polymorphism alone. Because the CRHR1 TAT haplotype is protective, the number of copies of the TAT haplotype present was reversed scored prior to creating the PGRS. Scores on the PGRS ranged from 0 to 4 in both groups and were normally distributed for children of mothers with a history of MDD (skew: z = 1.82, kurtosis: z = −.87) and for children of mothers without a history of depression (skew: z = −0.08, kurtosis: z = −0.87) (please see Table 3 for additional details). In these analyses, we found a significant PGRS × Mother MDD × Emotion interaction, F(3, 1151.84) = 9.48, p < .001. Examining the form of this interaction, we found that the PGRS × Emotion interaction was significant among children of depressed mothers, F(3, 585.26) = 13.73, p < .001, but not among children of nondepressed mothers, F(3, 560.74) = 1.65, p = .18. Examining this further, we found that among children of depressed mothers, the PGRS (i.e., number of more reactive alleles present across CRHR1 and FKBP5) was associated with children’s sustained attention to happy, t(75.27) = 3.02, p = .003, reffect size = .33, and sad, t(76.95) = −3.05, p = .003, reffect size = −.33, faces but not angry, t(76.94) = −1.76, p = .08, reffect size = −.20, or neutral, t(75.45) = 0.52, p = .61, reffect size = .06, faces. As can be seen in Figure 3, children of depressed mothers with a higher PGRS attended more to happy faces and less to sad faces. 1
Table 3.
Polygenic Reactivity Score (PGRS; CRHR1 & FKBP5) Distribution by Maternal History of Depression
| Mother Lifetime MDD | |||
|---|---|---|---|
|
| |||
| Genotype | Allele/Haplotype | Yes | No |
| PGRS(CRHR1 & FKBP5) | 0 | 2 | 8 |
| 1 | 31 | 19 | |
| 2 | 27 | 31 | |
| 3 | 16 | 18 | |
| 4 | 5 | 5 | |
Figure 3.
Summary of analyses showing sustained attention to happy (left) and sad (right) faces among children of mothers with a history of MDD as a function of children’s polygenic reactivity score (PGRS; sum of reactive alleles/haplotypes across CRHR1 and FKBP5).
Although we also examined the link between children’s lifetime history of MDD and their attentional biases, none of the main effects or interactions was significant (lowest p = .30). However, children’s attentional bias scores for sad faces were significantly related to their current depressive symptom levels. Specifically, statistically controlling for the influence of mother MDD, we examined the influence of children’s current symptoms of depression (CDI) on sustained attention and found a significant three-way CDI × Emotion × Epoch interaction, F(12,575.88) = 2.13, p = .01. The CDI × Epoch interaction was significant for Sad faces, F(4, 278.47) = 4.10, p = .003, but not Happy, Angry or Neutral faces (lowest p = .11). Increased symptoms of depression was associated with decreased attention to Sad faces at relatively late time periods trials within Epoch 4, t(159) = −2.40, p = .01 reffect size = −.18, and Epoch 5, t(159) = −2.79, p = .006, reffect size = −.21, but not Epoch 1, t(159) = 0.76, p = .45, reffect size = .06, Epoch 2, t(159) = 1.74, p = .08, reffect size = .14, or Epoch 3, t(159) = −0.55, p = .58, reffect size = −.04. These results suggest that children exhibiting higher current symptoms of depression exhibited increased attentional avoidance of sad faces the longer the stimuli set was on the screen. None of the effects of children’s depression (diagnoses or symptoms) on attentional biases was significantly moderated by children’s genotype (lowest p = .17).
A series of follow-up analyses were then conducted to determine the robustness of these effects. First, to determine if the results were due simply to the presence of current depression in the mothers, we re-conducted all of the analyses excluding dyads in which the mother met criteria for current MDD and statistically controlling for mothers’ current depressive symptom levels. Excluding child genotype from the model, we found that although the mother MDD × Emotion interaction remained significant, F(3, 1031.68) = 4.71, p = .003, the follow up mother MDD group difference in sustained attention to sad faces was reduced to non-significant, F(1, 141.03) = 2.89, p = .09. In contrast, the PGRS results were maintained including the overall three-way PGRS × Mother MDD × Emotion interaction, F(3, 1032.76) = 10.21, p < .001, the two-way PGRS × Emotion interaction among children of depressed mothers, F(3, 464.64) = 13.95, p < .001, but not among children of nondepressed mothers, F(3, 557.75) = 1.65, p = .17, and, finally, the significant the main effect of PGRS among children of depressed mothers on children’s sustained attention to happy, t(60.82) = 2.86, p = .006, reffect size = .34, and sad, t(61.93) = −2.59, p = .01, reffect size = −.31, faces. Second, to determine whether current results would still be observed in at-risk children prior to them developing depression themselves, we re-conducted the analyses while both excluding children with current or past MDD and statistically controlling for children’s current depressive symptom levels. Excluding child genotype from the model, the mother MDD results were maintained including the Mother MDD × Emotion interaction, F(3, 1071.98) = 5.31, p = .001, and the main effect of mother MDD group on sustained attention to sad faces, F(1, 145.69) = 5.68, p = .02. In addition, all of the PGRS results were maintained including the overall three-way PGRS × Mother MDD × Emotion interaction, F(3, 1074.17) = 7.79, p < .001, the two-way PGRS × Emotion interaction among children of depressed mothers, F(3, 513.65) = 11.98, p < .001, but not among children of nondepressed mothers, F(3, 552.52) = 1.46, p = .22, and, finally, the significant the main effect of PGRS among children of depressed mothers on children’s sustained attention to happy, t(65.10) = 2.60, p = .01, reffect size = .31, and sad, t(66.75) = −3.29, p = .002, reffect size = −.37, faces. Third, given potential concerns about population stratification, we re-conducted the genetic analyses focusing only on Caucasians in the sample. All of the PGRS results were maintained including the overall three-way PGRS × Mother MDD × Emotion interaction, F(3, 969.67) = 9.01, p < .001, the two-way PGRS × Emotion interaction among children of depressed mothers, F(3, 453.00) = 11.72, p < .001, but not among children of nondepressed mothers, F(3, 511.63) = 1.70, p = .17, and, finally, the significant the main effect of PGRS among children of depressed mothers on children’s sustained attention to happy, t(59.35) = 2.74, p = .008, reffect size = .34, and sad, t(57.69) = −2.58, p = .01, reffect size = −.32, faces.
Discussion
The primary goal of this study was to clarify previous mixed findings regarding the nature of attentional biases in children of depressed mothers. Specifically, although research has consistently shown that children of depressed mothers exhibit attentional biases for sad facial stimuli, some studies have suggested that this bias reflects preferential attention toward sad faces (Joormann et al., 2007; Kujawa et al., 2011) whereas others have found evidence of attentional avoidance (Gibb et al., 2009). The current results suggest that children’s genotype makes an important contribution to the nature of the bias observed in children of depressed mothers. Specifically, ignoring genetic influences, we found that children of mothers with a history of MDD during the child’s life exhibited greater sustained attention specifically to sad, but not happy, angry, or neutral faces. This replicates what has been observed in previous research examining the effects of maternal depression independent of child genotype (Joormann et al., 2007; Kujawa et al., 2011). Importantly, however, this effect was moderated by variation in two genes associated with HPA axis reactivity. Children of depressed mothers who carried fewer copies of the protective CRHR1 TAT haplotype or more copies of the FKBP5 rs1360780 T allele exhibited less sustained attention to sad faces and more sustained attention to happy faces. In addition, supporting the utility of examining aggregate levels of influence across multiple genes, a polygenic reactivity score incorporating the number of reactive alleles across both genes accounted for approximately twice as much variance in children of depressed mothers’ attentional biases for both sad and happy faces (11%) compared to either gene alone (5% to 6%). These findings were maintained when we excluded dyads in which the mother met criteria for current MDD and statistically controlled for mothers’ current depressive symptom levels, suggesting that the effects are not simply a state-like correlate of mothers’ current depression. The findings were also maintained when we excluded children with a lifetime history of MDD and statistically controlled for children’s current depressive symptoms, suggesting the effects are present prior to the onset of depression in the children.
These results have a number of potentially important implications for understanding mechanisms of risk in the intergenerational transmission of depression and for cognitive models of depression more generally. According to current cognitive models of depression (e.g., Clark, Beck, & Alford, 1999), depressed and at-risk individuals are hypothesized to exhibit an attentional bias specifically for depression-relevant stimuli such as sad faces, particularly deficits in the ability to disengage attention from these stimuli. This hypothesis has been supported in a number of studies in adults using reaction-time and eye-tracking indices of attentional biases (for reviews, see Armstrong & Olatunji, 2012; Gotlib & Joormann, 2010). In contrast, a recent eye tracking study showed that currently depressed children tend to avoid looking at sad faces and look more at happy faces (Harrison & Gibb, in press). This latter result is more consistent with the infant literature in which sad and at-risk infants tend to avert their gaze from sad faces (Boyd, Zayas, & McKee, 2006; Field, 1995; Termine & Izard, 1988). Building from broader theories of emotion regulation (e.g., Gross, 2014) in which attentional deployment is highlighted as a key early mechanism of emotion regulation, one could propose a developmental progression in which (i) at-risk infants are able to avert their gaze from sad faces to effectively regulate their mood, (ii) depressed and at-risk children are able to avert their gaze from sad faces but this is no longer sufficient to effectively regulate their mood (perhaps because of more covert processes such as rumination that effectively keep them from fully disengaging), and (iii) depressed adults are no longer able to avert their gaze from sad faces leading to the consistent finding of increased sustained attention to sad faces in the adult literature. As such, attentional biases may serve different functions or may have different underlying mechanisms in children versus adults. Exactly how to integrate observations of attentional avoidance in at-risk within broader cognitive models of depression will necessitate further research. Specifically, studies are needed to examine children’s neural and physiological reactivity to the presentation of sad faces, how this reactivity may be modulated by different patterns of attentional allocation (e.g., engagement versus disengagement), and how this may change across development.
As noted previously, the direction of attentional biases in children of depressed mothers has remained unclear, with studies finding evidence of preferential attention (Joormann et al., 2007; Kujawa et al., 2011) and attentional avoidance (Gibb et al., 2009). One factor that has been proposed to account for this difference in findings is that a sad mood induction was used in Joormann et al. (2007) and Kujawa et al. (2011) but not in Gibb et al. (2009). However, the degree of attentional avoidance of sad faces was directly related to children’s current symptoms of depression in both Gibb et al. (2009) and in the current study, suggesting that current mood state is not driving the difference in findings. In addition, in the current study, preferential attention to sad faces was observed among children of depressed mothers – when we collapsed across all children and did not take into account the influence of CRHR1 or FKBP5 – despite the absence of any mood induction and the use of a passive viewing task rather than the dot probe. Therefore, a mood induction may not be necessary to observe attentional biases in at-risk children. To definitively test this hypothesis, studies are needed that assess children’s attentional biases before and after a negative mood induction. Pending this type of study, the current results suggest that previous mixed findings may have been due in part to genetic variation in the samples studied. That is, in the current study, we found that the degree of attention allocated to sad versus happy faces among children of depressed mothers varied as a function of their genotypes for the CRHR1 TAT haplotype and FKPB5 rs1360780 such that children with genotypes associated with greater HPA axis reactivity exhibited less attention to sad faces and more attention to happy faces. This pattern of findings is consistent with emotion regulation models of attentional deployment (Gross, 2014) in that more genetically reactive children may avert their gaze from emotionally aversive images (sad faces) and direct their gaze toward happy faces in an effort to regulate negative affect. Although this may be adaptive in the short term, there is evidence that the degree to which children exhibit attentional avoidance of sad faces predicts increased risk for depression in the future, particularly if they carry a reactive genotype (Gibb et al., 2009). In this light, we should note that one of the studies that has found evidence of preferential attention toward sad faces in children of depressed mothers focused on 8–14 year old daughters of mothers with a history of recurrent MDD but who had no history of psychopathology themselves (Joormann et al., 2009), raising the possibility that they may represent a particularly resilient sample.
The current results are also consistent with a growing literature indicating that atypical HPA axis functioning may precede the emergence of depression in children of depressed mothers (see Guerry & Hastings 2011; Lopez-Duran, 2009 for reviews). Functionally, the CRHR1 and FKBP5 genotypes are associated with the initiation and conclusion of the HPA axis stress response, respectively (Binder, 2009; Gunnar & Vazquez, 2006). This study is the first of which we are aware to examine the impact of CRHR1 and FKBP5 genotypes on attentional biases in children. Although our results are consistent with the hypothesis that HPA axis disruption plays an important role in the development of attentional biases in children of depressed mothers, additional research is needed to more definitively test this hypothesis. This research should focus on multi-wave longitudinal designs so that developmental trajectories in attentional bias can be examined. Research is also needed to provide a direct examination of HPA axis outputs (e.g., cortisol) on changes in children’s attentional biases. Future studies will need to be conducted to further examine the influence these genes may have in the intergenerational transmission of depression.
Despite the strong and consistent results obtained for CRHR1 and FKBP5, no significant results were observed for 5-HTTLPR, which fails to replicate previous findings (e.g. Gibb et al., 2009). The current study used similar inclusion/exclusion criteria as that used in Gibb et al. (2009) but used a different task (passive viewing versus dot probe), which may have contributed to the variation in results. In this light, we should note that a number of studies have now documented the effect of 5-HTTLPR genotype on attentional biases using the dot probe task (for a meta-analytic review, see Pergamin-Hight et al., 2012). However, ours is the first study of which we are aware to examine the impact of 5-HTTLPR using a passive viewing paradigm. It is possible that task-related differences (e.g., stimulus presentation time, free viewing versus probe detection) may have contributed to the variation in results. Additional research is needed, therefore, to determine the role that 5-HTTLPR genotype may play in the development of attentional biases, whether as a main effect, interacting with environmental influences, or in combination with other genetic influences (aggregate or epistatic).
The current study exhibited a number of strengths including the use of diagnostic interviews to characterize mothers and children, and the focus on aggregate levels of influence across multiple genes known to influence HPA axis reactivity. In particular, the use of eye tracking rather than reaction times was shown to be useful in clarifying the nature children’s attentional biases, further research exploring children’s attention allocation to emotional information using other eye movement measures, such as scanning patterns within face stimuli (e.g. eyes, mouth), and within affective attention control paradigms (e.g. McSorley & van Reekum, 2013) may help to further characterize attentional biases in at-risk children. There were also some limitations that highlight important areas of future research. First, as noted above, the study was cross sectional and longitudinal research is needed to determine if (and how) maternal depression contributes to the prospective development of attentional biases in children, and how this effect may be moderated by variation in specific genes. Second, additional research is needed to better understand how the nature and function of attentional biases may change across development, from patterns of attentional avoidance of emotionally aversive stimuli to difficulties disengaging attention from these stimuli. Third, our study is based on the hypothesis that attentional avoidance represents a mal-adaptive emotion regulation strategy in children of depressed mothers and future research is needed to more precisely examine children’s neural and/or physiological reactivity to sad facial stimuli and how this may be modulated by patterns of gaze allocation. Fourth, as with any genetic association study, there is always the possibility that unmeasured genetic or other influences may have accounted for the findings. This said, the similarity of findings across CRHR1 and FKBP5 lends some support to our pathway based model suggesting that variations in genes associated with HPA axis activity may moderate the impact of maternal depression on the development of attentional biases in children3. Finally, although we focused on polygenic influences, we only included variation in three genes in this study and limited our focus to additive influences across the variants (i.e., unweighted influence). Larger studies are needed to examine a broader collection of genes within a given biological pathway and also to examine more complex relations among genes examined (e.g., epistatic influences).
In conclusion, the results of the current study help to explain the mixed results obtained in previous studies examining attentional biases among children of depressed mothers. Specifically, although these studies have consistently shown that children of depressed mothers exhibit attentional biases for sad faces, there was disagreement about whether this bias reflected preferential attention toward sad faces (Joormann et al., 2007; Kujawa et al., 2011) versus attentional avoidance of these faces (Gibb et al., 2009). The current results suggest that children’s genotype has an important influence on the nature of bias present. Specifically, although preferential attention to sad faces was observed in children of mothers with a history of MDD during the children’s lives, this was due primarily to children with genotypes associated with low HPA axis reactivity. Among children with genotypes associated with heightened HPA axis reactivity, there was evidence for attentional avoidance of sad faces combined with preferential attention toward happy faces, which may reflect an attempt at emotion regulation. These findings highlight the importance of considering the potential influence of specific genetic factors and also suggest that current cognitive models of depression may need to be modified to address possible developmental differences in the expression and function of attentional biases.
Key Points.
Children of depressed mothers show attention biases for sad faces
There is evidence for biases towards and away from sad faces, limiting the understanding of the nature of the bias present.
The current study shows genetic factors associated with heightened HPA axis reactivity moderate the direction of attention biases.
Findings highlight child’s biases may vary due to genetic factors, and suggest developmental differences in cognitive risk factors for depression.
Acknowledgments
This project was supported by grants R01 HD057066 and R01 MH098060 awarded to B. E. Gibb, 1S10RR023457-01A1 and Shared equipment grants (ShEEP) from the Medical Research Service of the Department of Veteran Affairs to J. E. McGeary. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the National Institutes of Health or the Department of Veterans Affairs. We would like to thank Lindsey Stone, Andrea Hanley, Katie Burkhouse, Anastacia Kudinova, Sydney Meadows, Michael Van Wie, and Devra Alper for their help in conducting assessments for this project and Kayla Beaucage for her help with genotyping.
Footnotes
Analyses including 5-HTTLPR (number of S or LG alleles) in the PGRS yielded findings almost identical to those obtained with 5-HTTLPR omitted. Specifically, we found a significant PGRS × Mother MDD × Emotion interaction, F(3, 1152.88) = 10.85, p < .001. The PGRS × Emotion interaction was significant among children of depressed mothers, F(3, 586.10) = 16.60, p < .001, but not among children of nondepressed mothers, F(3, 560.89) = 1.01, p = .39. Among children of depressed mothers, a higher PGRS (i.e., higher number of more reactive alleles/haplotypes across CRHR1 TAT haplotype, FKBP5 rs1360780, and SCL6A4 5-HTTLPR) was associated with children’s increased sustained attention to happy faces, t(74.86) = 3.50, p = .001, reffect size = .38, and decreased attention to both sad, t(76.98) = −2.65, p = .01, reffect size = −.29, and angry, t(77.30) = −2.66, p = .01, reffect size = −.29, faces. There was no significant relation with attention to neutral faces, t(75.42) = 0.40, p = .69, reffect size = .05, faces.
Current and lifetime diagnoses of DSM-IV anxiety disorders were also assessed for mothers and children. Lifetime diagnoses of anxiety disorders in mothers or children were not significantly related to children’s attentional biases. In addition, the main effects of PGRS among children of depressed mothers on sustained attention for happy and sad faces were maintained when statistically controlling for the influence of lifetime anxiety diagnoses in mothers and children, and when excluding dyads in which the mother or child met criteria for a current anxiety disorder. Details of these analyses are available from the first author.
References
- Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review. 2012;32:704–723. doi: 10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC. Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harbor Protocols. 2009;10 doi: 10.1101/pdb.ip71. pdb ip71. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Beardslee WR, Versage EM, Gladstone TRG. Children of affectively ill parents: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:1134–1141. doi: 10.1097/00004583-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cogntive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and-II in psychiatric outpatients. Journal of personality assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34:S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Bistricky SL, Ingram RE, Atchley RA. Facial affect processing and depression susceptibility: Cognitive biases and cognitive neuroscience. Psychological Bulletin. 2011;137:998–1028. doi: 10.1037/a0025348. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: The National Comorbidity Survey. American Journal of Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- Boyd RC, Zayas LH, McKee MD. Mother-infant interaction, life events and prenatal and postnatal depressive symptoms among urban minority women in primary care. Maternal and Child Health Journal. 2006;10:139–148. doi: 10.1007/s10995-005-0042-2. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Siegle GJ, Gibb BE. Pupillary reactivity to emotional stimuli in children of depressed and anxious mothers: Evidence for experience-specific patterns of reactivity. Journal of Child Psychology and Psychiatry. 2014;55:1009–1016. doi: 10.1111/jcpp.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MG, Lundqvist D. Facial expressions of emotion (K-DEF): Identification under different display-duration conditions. Behavior Research Methods. 2008;40:109–115. doi: 10.3758/brm.40.1.109. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT, Alford B. Scientific foundations of cognitive theory and therapy of depression. New York: John Wiley and Sons; 1999. [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, … Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Eizenmann M, Yu LH, Grupp L, Eizenman E, Ellenbogen M, Gemar M, Levitan RD. A naturalistic visual scanning approach to assess selective attention in major depressive disorder. Psychiatry Research. 2003;118:117–128. doi: 10.1016/s0165-1781(03)00068-4. [DOI] [PubMed] [Google Scholar]
- Engels WR. Exact tests for Hardy-Weinberg proportions. Genetics. 2009;183:1431–41. doi: 10.1534/genetics.109.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Gutman D, Tone EB, Almli L, Mercer K, Davis J, … Ressler KJ. FKBP5 and attention bias for threat. JAMA Psychiatry. 2013;70:392–400. doi: 10.1001/2013.jamapsychiatry.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T. Infants of depressed mothers. Infant Behavior and Development. 1995;18:1–13. doi: 10.1016/j.infbeh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders – Patient Edition (SCID-I/P) New York: Biometrics Research Department, NY State Psychiatric Institute; 1995. [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavior Genetics. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Beevers CG, McGeary JE. Toward an integration of cognitive and genetic models of risk for depression. Cognition and Emotion. 2013;27:193–216. doi: 10.1080/02699931.2012.712950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Benas JS, Grassia M, McGeary J. Children’s attentional biases and 5-HTTLPR genotype: Potential mechanisms linking mother and child depression. Journal of Clinical Child and Adolescent Psychology. 2009;38:415– 426. doi: 10.1080/15374410902851705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annual Review of Clinical Psychology. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A development model for understanding mechanisms of transmission. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Colich NL. Children of parents with depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 3. New York: Guilford; 2014. pp. 240–258. [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Foland-Ross LC. Understanding Familial Risk for Depression: A 25-Year Perspective. Perspectives on Psychological Science. 2014;9(1):94–108. doi: 10.1177/1745691613513469. [DOI] [PubMed] [Google Scholar]
- Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–372. [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Conceptual and empirical foundations. In: Gross JJ, editor. Handbook of emotion regulation. 2. New York, NY: Guilford; 2014. pp. 3–20. [Google Scholar]
- Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clinical child and family psychology review. 2011;14:135–160. doi: 10.1007/s10567-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental Psychopathology. Developmental Neuroscience. 2. New York: Wiley; 2006. pp. 533–577. [Google Scholar]
- Hankin BL, Gibb BE, Abela JR, Flory K. Selective attention to affective stimuli and clinical depression among youths: role of anxiety and specificity of emotion. Journal of Abnormal Psychology. 2010;119:491–501. doi: 10.1037/a0019609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Children of depressed parents. In: Gotlib IH, Hammen C, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 275–297. [Google Scholar]
- Harrison AJ, Gibb BE. Attentional biases in currently depressed children: An eye-tracking study of biases in sustained attention to emotional stimuli. Journal of Clinical Child and Adolescent Psychology. doi: 10.1080/15374416.2014.930688. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Reif M, Field T, Diego M, Vera Y, Pickens J. Happy faces are habituated more slowly by infants of depressed mothers. Infant Behavior and Development. 2006;29:131–135. doi: 10.1016/j.infbeh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism: Clinical and Experimental Research. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hunnius S, de Wit TCJ, Vrins S, von Hofsten C. Facing threat: Infants’ and adults’ visual scanning of faces with neutral, happy, sad, angry, and fearful emotional expressions. Cognition and Emotion. 2011;25:193–205. doi: 10.1080/15298861003771189. [DOI] [PubMed] [Google Scholar]
- Joormann J. Cognitive aspects of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 298–321. [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116(1):135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children- Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kellough JL, Beevers CG, Ellis AJ, Wells TT. Time course of selective attention in clinically depressed young adults: An eye tracking study. Behaviour Research and Therapy. 2008;46:1238–1243. doi: 10.1016/j.brat.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica. 1981;46:305–315. [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory. Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Gotlib IH, Klein DN. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. Journal of Abnormal Child Psychology. 2011;39:125–135. doi: 10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmidt MH, Esser G, … Banaschewski T. Interactive effects of corticotropin-releasing hormone receptor 1 gene and childhood adversity on depressive symptoms in young adults: Findings from a longitudinal study. European Neuropsychopharmacology. 2013;23:358–367. doi: 10.1016/j.euroneuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Lench N, Stanier P, Williamson R. Simple non-invasive method to obtain DNA for gene analysis. The Lancet. 1988;331:1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Flood SJ, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. Genome Research. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. The Karolinska Directed Emotional Faces-KDEF, CD ROM from the Department of Clinical Neuroscience, Psychology section. Karolinska Institute; Stockholm: 1998. [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- McSorley E, van Reekum CM. The time course of implicit affective picture processing: An eye movement study. Emotion. 2013;13:769–773. doi: 10.1037/a0032185. [DOI] [PubMed] [Google Scholar]
- Miller R, Wankerl M, Stalder T, Kirschbaum C, Alexander N. The serotonin transporter gene-linked polymorphic region (5-HTTLPR) and cortisol reactivity: A meta-analysis. Molecular Psychiatry. 2012;18:1018–1024. doi: 10.1038/mp.2012.124. [DOI] [PubMed] [Google Scholar]
- Montague DPF, Walker-Andrews AS. Peekaboo: A new look at infants’ perception of emotion expressions. Developmental Psychology. 2001;37:826–838. [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SD, Le-Niculescu H, Koller DL, Green SD, Lahiri DK, McMahon FJ, Nurnberger JI, Jr, Niculescu AB., III Coming to grips with complex disorders: Genetic risk prediction in bipolar disorder using panels of genes identified through convergent functional genomics. American Journal of Medical Genetics Part B. 2010;153B:850–877. doi: 10.1002/ajmg.b.31087. [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Bakermans-Kranenburg MJ, van IJzendoorn MH, Bar-Haim Y. Serotonin transporter gene and biased attention for emotional information: A meta-analysis. Biological Psychiatry. 2012;71:373–379. doi: 10.1016/j.biopsych.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, … Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: Replication and extension. Archives of General Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley EC, Houston K, Hawton K, Harrison PJ. Deliberate self-harm is associated with allelic variation in the tryptophan hydroxylase gene (TPH A779C), but not with polymorphisms in five other serotonergic genes. Psychological Medicine. 2003;33:775–783. doi: 10.1017/s0033291703007463. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh HI, Kryski KR, Smith HJ, Hayden EP, Singh SM. Corticotropin-releasing hormone system polymorphisms are associated with children’s cortisol reactivity. Neuroscience. 2013;229:1–11. doi: 10.1016/j.neuroscience.2012.10.056. [DOI] [PubMed] [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children’s Depression Inventory. Journal of Abnormal Child Psychology. 1986;14:25–39. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- Soken NH, Pick AD. Infants’ perception of dynamic affective expressions: Do infants distinguish specific expressions? Child Development. 1999;70:1275–1282. doi: 10.1111/1467-8624.00093. [DOI] [PubMed] [Google Scholar]
- Stephens M, Donnelly PA. Comparison of Bayesian methods for haplotype reconstruction from population genotype data. American Journal of Human Genetics. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly PA. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Termine NT, Izard CE. Infants’ responses to their mothers’ expressions of joy and sadness. Developmental Psychology. 1988;24:223–229. [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Zannas AS, Binder EB. Gene-environment interactions at the FKBP5 locus: Sensitive periods, mechanisms and pleiotropism. Genes, Brain and Behavior. 2014;13:25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]