Abstract
Narcolepsy is a chronic sleep disorder linked to the loss of orexin-producing neurons in the hypothalamus. Cataplexy, a sudden loss of muscle tone during waking, is an important distinguishing symptom of narcolepsy and it is often triggered by strong emotions. The neural circuit underlying cataplexy attacks is not known, but is likely to involve the amygdala, a region implicated in regulating emotions. In mice models of narcolepsy, transfer of the orexin gene into surrogate neurons has been successful in ameliorating narcoleptic symptoms. However, it is not known whether this method also blocks cataplexy triggered by strong emotions. To examine this possibility the gene encoding mouse prepro-orexin was transferred into amygdala neurons of orexin knockout (KO) mice (rAAV-orexin; n=8). Orexin-KO mice that did not receive gene transfer (no-rAAV; n=7), or received only the reporter gene (rAAV-GFP; n=7) served as controls. Three weeks later, the animal’s sleep and behavior were recorded at night (no-odor control night), followed by another recording at night in the presence of predator odor (odor night). Orexin-KO mice given the orexin gene transfer into surrogate amygdala neurons had significantly less spontaneous bouts of cataplexy, and predator odor did not induce cataplexy compared to control mice. Moreover, the mice with orexin gene transfer were awake more during the odor night. These results demonstrate that orexin gene transfer into amygdala neurons can suppress both spontaneous and emotion-induced cataplexy attacks in narcoleptic mice. It suggests that manipulating amygdala pathways is a potential strategy for treating cataplexy in narcolepsy.
Keywords: Narcolepsy, sleep, emotion, predator odor, orexin, amygdala
INTRODUCTION
Narcolepsy is a chronic, life-long sleep disorder affecting almost 1 in 2000 Americans. The major symptoms of narcolepsy include excessive daytime sleepiness, cataplexy, sleep fragmentation, and hypnogogic hallucinations. Because narcolepsy diminishes the patient’s quality of life, many patients also develop various secondary psychological symptoms such as fatigue, stress, memory loss and depression (Aldrich, 1993; Mattarozzi et al., 2008). Orexin, a peptide localized in a small group of neurons in the hypothalamus area, has been linked to narcolepsy because there is a profound loss of orexin-producing neurons in humans with narcolepsy (Peyron et al., 2000; Thannickal et al., 2000; Nishino et al., 2000). Canines with narcolepsy have a mutation in the orexin-receptor-2 (Lin et al., 1999), and mice with deletion of the orexin gene (orexin knockout) (Chemelli et al., 1999) or with ablation of the orexin producing neurons (Hara et al., 2001) are valid animal models of the human disease.
Cataplexy, one of the symptoms of narcolepsy, is a sudden and transient attack of muscle weakness during waking, and often triggered by strong emotions including both positive (e.g. laughter, humor) and negative (e.g. anger, fear or sudden surprise) emotions (Aldrich, 1993; Mattarozzi et al., 2008). In narcoleptic mice and canines, cataplexy attacks can be triggered by emotions, such as food (Siegel et al., 1991, Burgess et al., 2013), wheel-running (Espana et al., 2007) or predator odor (Morawska et al., 2011). The mechanism responsible for emotion-induced cataplexy in humans and animals is still unclear, although the amygdala, a region important for emotion processing might play a pivotal role in triggering emotion-induced cataplexy (Brabec et al., 2011; Burgess et al., 2013; Meletti et al., 2015). Cataplexy-related neurons have been found in the amygdala of canines with narcolepsy (Gulyani et al., 2002). Moreover, bilateral lesions of the amygdala reduce cataplexy induced by stimuli associated with “positive emotions” (i.e. wheel running plus chocolate feeding) (Burgess et al., 2013). Although amygdala lesions were successful in reducing cataplexy, there are likely to be significant adverse consequences on affect and cognition as a result of lesioning this critical brain structure.
On the other hand, orexin gene transfer into specific neurons in the brains of mice models of narcolepsy has successfully decreased spontaneous bouts of cataplexy (Liu et al., 2008 and 2011; Blanco-Centurion et al., 2013). However, the effects of orexin gene transfer into the amygdala neurons have not been determined. Because of the link between emotion and cataplexy we hypothesize that orexin gene transfer into surrogate amygdala neurons can rescue both spontaneous and emotion-induced cataplexy in narcoleptic mice. In the present study, predator odor (coyote urine) was used to trigger emotion-induced cataplexy in orexin KO mice (Morawska et al., 2011). We found that transfer of the orexin gene into surrogate neurons in the amygdala of orexin KO mice blocked both spontaneous and emotion-induced cataplexy, and also increased waking. This is the first evidence that orexin gene transfer into a discrete neural circuit blocks spontaneous and emotion-induced cataplexy as well as increases waking in narcoleptic mice.
MATERIALS AND METHODS
Ethics statement
All manipulations done to the mice adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Medical University of South Carolina (protocol #3267) and the Ralph H. Johnson VA (protocol #537) Institutional Animal Care and Use Committee.
Construction and delivery of rAAV vector
Mouse prepro-orexin cDNA fragment (393bp from Dr. Yanagisawa’s laboratory; University of Texas Southwestern Medical Center, Dallas, TX, USA.) was cloned into a rAAV plasmid from Harvard Gene Therapy Initiative Laboratory (Boston, MA), to form rAAV-orexin, in which orexin expression was driven by the CMV (cytomegalovirus) promoter. The control virus, rAAV-GFP, was created by replacing orexin cDNA with green fluorescent protein (GFP) cDNA. Vector packaging and titration were done by University of North Carolina Gene Therapy Initiative (final titer at 2.7–3.0×1013 genomic copies/ml).
Orexin knockout mice
Homozygous orexin-KO mice derived from founders originally supplied by Dr. Yanagisawa (Southwestern Medical Center, Dallas, TX, USA) were used in this study. The mice have been backcrossed onto a C57BL/6J line for over 20 generations in our laboratory. Twenty-eight orexin-KO mice (male and female, 6–12 month old, 25–42 gram) were randomly assigned to the following groups: rAAV-orexin (n=10), rAAV-GFP (n=10) and no-rAAV (n=7).
Implanting sleep recording electrodes
Under deep anesthesia (isoflurane 2.0%) viral vectors were microinjected into the central nucleus of the amygdala (1.2 mm posterior to bregma, 2.9 mm lateral, and 4.6 mm ventral to brain surface; Hof et al., 2000) using a stereotaxic instrument (Kopf, Tujunga, CA). The experimental (rAAV-orexin) and control (rAAV-GFP) vectors were delivered in a volume of 500nl on each side of the brain using a 2.0μL Hamilton syringe coupled to a 33 gauge stainless steel injector (Plastics One, Roanoke, VA). Injections were done gradually over 15 min. After the microinjection, the injector needle was left in place for 10 min and then withdrawn slowly. At this time the mice were also implanted with electrodes to record the electroencephalogram (EEG) and electromyogram (EMG) as described previously (Liu et al., 2011). All electrode leads were then secured onto the skull with dental cement. After surgery the animals were housed singly in Plexiglas cages with food and water available ad libitum. The temperature in the sleep recording room was 25°C and a 12 h light/dark cycle (7:00 A.M. to 7:00 P.M. lights on) was maintained.
Two weeks after surgery, the animals were connected to lightweight recording cables and allowed one week of adaptation. On day 21 the EEG/EMG signal and behavior were continuously recorded during the 12 h lights-off period. Using a polygraph (AstroMed, Model 12; West Warwick, RI) EEG/EMG signals were amplified, filtered (0.3–100 Hz for EEG; 100-1K Hz for EMG), and then recorded onto a hard-disk with sleep data acquisition software (SleepSign; Kissei Comtec Co, Nagano, Japan). Behavioral data were also continuously recorded via night vision cameras. On day 23, 12 h sleep and behavior were again recorded in the presence of predator odor (coyote urine) during the 12 h lights-off period.
Delivery of predator odor
Predator odor test was on the night of day 23. 1.0 ml coyote urine (www.Predatorpee.com, Bangor, ME, USA) was placed in a 5 ml glass vial filled with cotton. There were holes in the vial’s plastic cap which allowed the mice to smell the predator odor. The vial was placed in the cage at the start of the animal’s active period (lights-off cycle) for 12 h. EEG/EMG and video recording started simultaneously when the test began. We chose to deliver the odor during the active period (night) because cataplexy is most prevalent at night in narcoleptic mice (Liu et al., 2011).
Identification of sleep–wake states, cataplexy
EEG, EMG and video recordings were scored manually with SleepSign software in 12 s epochs for wake, non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep and cataplexy. Wakefulness was identified by the presence of desynchronized EEG and high EMG activity. NREM sleep consisted of high-amplitude slow waves together with a low EMG tone relative to waking. REM sleep was identified by the presence of regular EEG theta activity coupled with low EMG relative to slow wave sleep.
Cataplexy was identified by a sudden loss of muscle tone when the mice were awake. The following criteria were used to identify cataplexy: the episodes had to occur when the mouse was awake (≥ 36 s) and engaged in an active behavior such as walking, running, grooming, eating, or drinking; the episode had to last at least 12 s; during the episode, theta activity had to be present, and delta activity diminished; and there had to be loss of muscle tone based on EMG and video data (Liu et al., 2011).
Immunostaining of transfected neurons
At the end of the study the mice were deeply anesthetized with isoflurane (5%) and were perfused transcardially with 0.9% saline (5–10 ml) followed by 10% buffered formalin in 0.1M PBS (50 ml). The brains were placed in 30% sucrose (0.1M PBS) and allowed to equilibrate. The brains were cut on a cryostat (40 um thick sections; coronal plane) and one-in-four series of sections were processed for visualization of orexin-A immunoreactivity. Briefly, the tissue was incubated overnight at room temperature in the goat anti-orexin-A antibody (Santa Cruz Biotechnology, 1:10,000), then incubated for 1 h with a biotinylated secondary antibody (donkey anti-goat IgG, Jackson Immunoresearch Laboratories, West Grove, PA, USA. 1:500). The antibodies were visualized using the avidin/biotin/diaminobenzidine-nickel staining method (Vector Laboratories Inc, Burlingame, CA, USA). Immunofluorescence was used to visualize two antigens. The free floating sections were incubated overnight in orexin-ir (goat anti-orexin-A; 1:500; Santa Cruz Biotechnology) in combination with vesicular GABA transporter (VGAT; rabbit anti-VGAT; 1:500; Millipore), tyrosine hydroxylase (TH; rabbit anti-TH;1:1000; Millipore), choline acetyltransferase (ChAT; rabbit anti-ChAT; 1:1000; Millipore), or serotonin (rat anti-serotonin;1:1000; Millipore) immunoreactive neurons. For immunofluorescence the following secondary antibodies were used: donkey anti-goat-Alexa Fluor 488; donkey anti rabbit-Alexa Fluor 568 or donkey anti-rat Alexa Fluor 594; 1:500; Invitrogen. Then the sections were washed and mounted onto gelatin-coated slides. Photomicrographs were obtained with a Nikon confocal microscope.
Statistical Analysis
Two-way repeated measures ANOVA with post-hoc tests (Bonferroni) compared the means among the three groups (Kirk, 1968). As there were unequal number of mice in each group (unbalanced design), the data were analyzed using the general linear model (SPSS). Statistical significance was evaluated at the P<0.05 (two-tailed) level.
RESULTS
Distribution of orexin-A-ir surrogate neurons and terminals
We intentionally selected the orexin knockout mice model of narcolepsy for this study because the gene for prepro-orexin has been genetically deleted and there is no evidence of the peptide in the tissue in these mice (Chemelli et al., 1999). We did not choose the orexin-ataxin-3 transgenic mice model of narcolepsy because not all of the orexin producing neurons die from the accumulation of the polyglutamine toxicity (Hara et al., 2001). As such, in the orexin-ataxin-3 transgenic mice the orexin peptide is evident in degenerating nerve terminals and a few surviving orexin somata, which could confound the results.
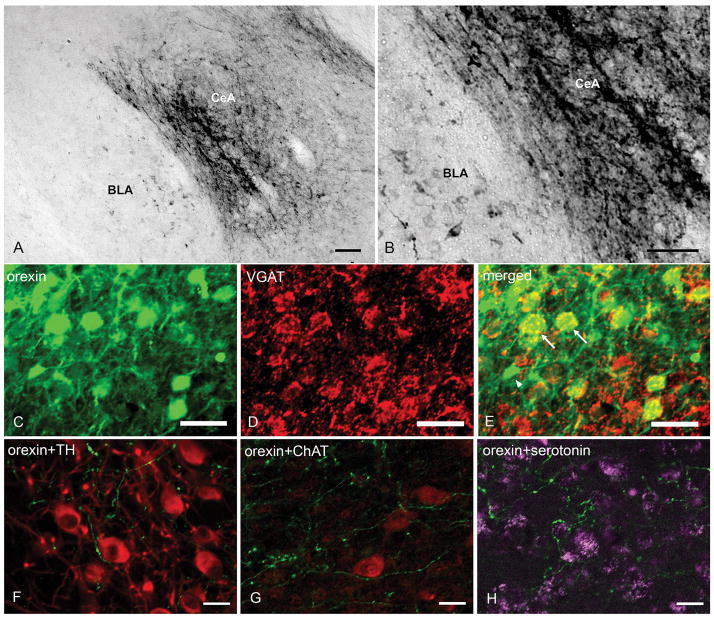
None of the mice in this study had orexin-A immunoreactive (orexin-A-ir) somata in the lateral hypothalamus, which confirms that these were orexin KO mice. rAAV-orexin was inserted into the CeA in ten orexin KO mice. In two mice orexin-A-ir neurons were not observed in the injection site, perhaps because the injection cannula was clogged. In the remaining eight orexin KO mice numerous orexin-A-ir somata and fibers were evident in the amygdala (6 mice with bilateral distribution, 2 mice with unilateral distribution, Figure 1). Most of the transfected somata were distributed in the CeA and BLA (Figure 2, A and B). Diffuse orexin-A-ir somata and terminals were also present in the basomedial amygdala (BMA) and adjacent areas in some mice. Double immunostaining showed co-localization of orexin and GABA in many transfected neurons (Figure 2, C, D and E). Distal orexin-A-ir terminals were found in the locus ceruleus (LC), ventral lateral periaqueductal grey (vlPAG) and the dorsal raphe (DR) (Figure 2, F, G and H). Orexin-A-ir was not found in the brains of control orexin-KO mice (no-rAAV and rAAV-GFP groups).
Figure 1.
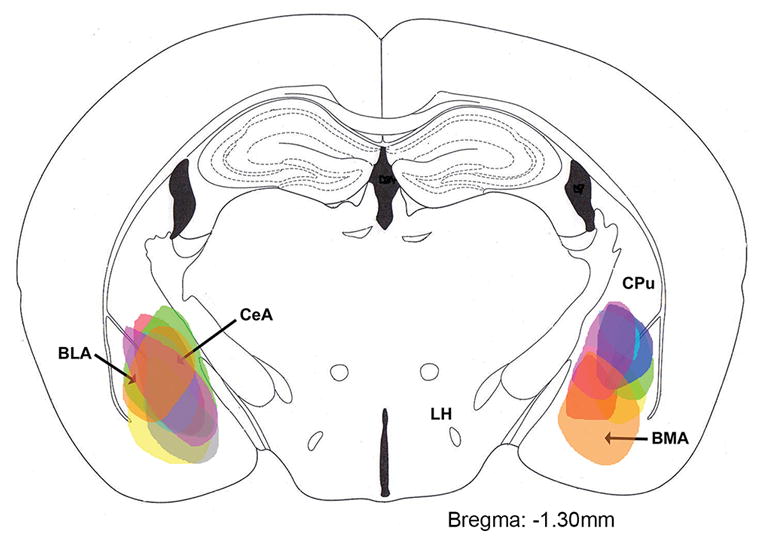
Schematic distribution of orexin-A-immunoreactive somata in the amygdala 29 days after rAAV-orexin gene transfer. Each color represents the distribution in one mouse (n=8). The drawing represents is a coronal section adapted from the mouse brain atlas (Hof et al., 2000).
Figure 2.
Distribution of orexin-A-ir in the amygdala and pons of orexin KO mice given rAAV-orexin in the amygdala. A and B: Orexin-A-ir was distributed in somata and proximal dendrites of CeA and BLA cells. C, D and E: Transfected prepro-orexin gene was expressed in GABA (arrows) and non-GABA (arrowhead) neurons in CeA. F, G and H: Orexin-A-ir was also observed in distal terminals in the pons in close proximity with noradrenergic neurons of the LC (F), cholinergic neurons in the vlPAG (G), and serotonin neurons of the DR (H). Scale bar in A and B: 100 μm. Scale bar in C–H: 25 μm.
rAAV-GFP was injected into the amygdala in ten orexin KO mice. In three mice GFP-positive somata were not evident in the injection site, perhaps because of clogged cannulae. In the remaining seven orexin KO mice numerous GFP positive somata were evident in the amygdala.
Effects on Cataplexy
Table 1 summarizes the average number and length of bouts of cataplexy in the mice. There was a significant effect between groups (F2, 19=92.01; P=0.001), exposure to odor (F1, 19=91.33; P=0.001) and interaction (F2, 19=19.7; P=0.001) on cataplexy number. Before exposure to the predator odor, there was no significant difference in the incidence of cataplexy between the two groups of control mice (no-rAAV vs. rAAV-GFP: t12=0.78, P=0.93). However, these control mice had three-fold more spontaneous bouts of cataplexy compared to the orexin KO mice given orexin gene transfer (no-rAAV vs. rAAV-orexin: t13=4.41, P=0.001; rAAV-GFP vs. rAAV-orexin: t13=5.14, P=0.001) (Table 1). When the mice were exposed to the predator odor, the control mice had twice as many bouts of cataplexy compared to the no-odor night (no-rAAV: t12=8.14, P=0.001: rAAV-GFP: t12=7.56, P=0.001; post-hoc versus no-odor) and five times as many bouts compared to the mice with the orexin gene transfer (no-rAAV vs. rAAV-orexin: t13=12.05, P=0.001; rAAV-GFP vs. rAAV-orexin: t13=12.20, P=0.001) (Table 1). In the mice with the orexin gene transfer, exposure to the odor did not significantly increase number of bouts of cataplexy (t14=0.54, P=0.60, Table 1). Thus, predatory odor did not trigger cataplexy in mice given orexin gene transfer into the amygdala.
Table 1. Number and average duration of cataplexy bout (±SEM).
Effect of orexin gene transfer on average (±SEM) duration and number of cataplexy bouts. The data were analyzed with a repeated measures ANOVA (GLM model) with post-hoc comparisons (Bonferroni). During the no odor night, the control groups (no-rAAV and rAAV-GFP) had three times more spontaneous bouts of cataplexy compared to the orexin KO mice with orexin gene transfer (*: P=0.001). Upon exposure to predator odor (coyote urine) the control groups had twice as many bouts of cataplexy compared to the no odor night (#: P=0.001) and five times as many bouts as the mice given orexin gene transfer (**: P=0.001). In orexin KO mice the predator odor did not significantly trigger cataplexy. There was no significant change in average length of cataplexy bouts among all three groups.
| Number | Duration (seconds) | |||
|---|---|---|---|---|
| no-odor night | odor night | no-odor night | odor night | |
| no-rAAV (n=7) | 19.62±0.89 | 43.79±1.32# | 21.57±1.87 | 20.47±1.69 |
| rAAV-GFP (n=7) | 21.71±.92 | 44.14±1.10# | 24.78±1.85 | 22.97±1.99 |
| rAAV-orexin (n=8) | 6.40±0.46* | 8.00±0.36** | 20.48±1.85 | 21.97±1.66 |
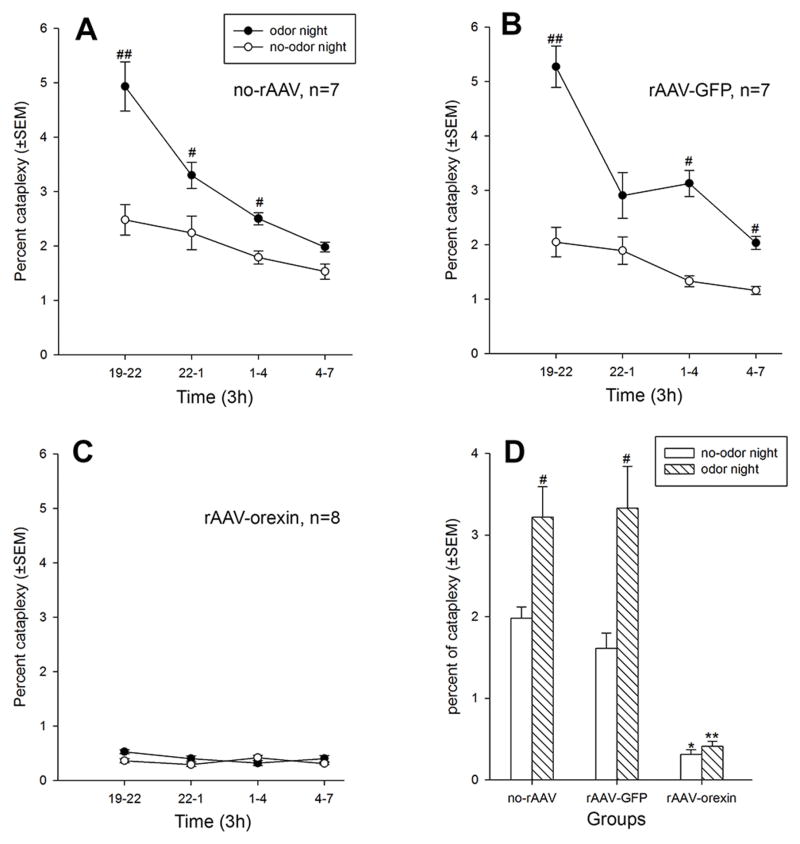
The percent of time spent in cataplexy over the 12h night period was also determined (Figure 3) and the data analyzed using a 2-way repeated measures ANOVA (three groups by two-factor repeated measures - time in 3h blocks nested within odor; Type split-plot factorial 3.42) (page 300; Kirk, 1968). There was a significant between group effect (F2, 19 =87.95; P=0.001) in that the orexin group had significantly less time in cataplexy compared to each of the two control groups (no-rAAV vs. rAAV-orexin: t13=7.82, P=0.001; rAAV-GFP vs. rAAV-orexin: t13=7.36, P=0.001). There was a significant effect of odor (F1, 19 =44.65; P=0.001) and a group by odor interaction effect (F2, 19 =10.76; P=0.001). There was a significant interaction effect (group x time x odor) (F6, 57 =4.23; P=0.001) with the two control groups having more percent cataplexy in the first three hours of the light-off cycle compared to the last quarter of the night (Figure 3A and 3B). This is consistent with published data (Chemelli et al., 1999) that in orexin-KO mice cataplexy attacks dominate during the beginning of the night cycle. Indeed, for the night cycle, before exposure to the odor control mice spent significantly more percent of time in cataplexy compared to the mice with the orexin gene transfer (no-rAAV vs. rAAV-orexin: t13=4.38, P=0.001; rAAV-GFP vs. rAAV-orexin: t13=4.40, P=0.001) (Figure 3D). In the two control groups exposure to the odor significantly increased percent cataplexy compared to the no-odor night (Figures 3A, B and D). When first exposed to the predator odor the percent of time spent in cataplexy in rAAV-orexin group did not increase, and across the 12h night period of odor exposure there was no significant difference in percent cataplexy when compared to the no-odor control night (t7=0.32, P=0.75; Figure 3, C and D). Thus, orexin gene transfer blocked emotion-induced cataplexy.
Figure 3.
Effects of orexin gene transfer into amygdala on percent (±SEM) cataplexy during the 12 h light-off period (night). Panels A–C summarize the time-course of percent cataplexy across the 12h night period, with each data point representing a 3 h average (±SEM). Data analyzed with repeated measures ANOVA (SPF-3.42 design; GLM model). Panel D summarizes the data over the 12h night period. Cataplexy was determined on the basis of both EEG/EMG and video behavioral data. The control groups are orexin KO mice given no virus (no-rAAV) or given the reporter gene, GFP (rAAV-GFP). The experimental group is orexin-KO mice given orexin gene transfer into the amygdala (rAAV-orexin). The experimental group spent significantly less time in spontaneous cataplexy, and exposure to predator odor did not increase cataplexy in these mice. On the other hand, predator odor (coyote urine) increased percent cataplexy in both control groups. #: P<0.05, versus no-odor control night; *: P=0.001, versus no-rAAV and rAAV-GFP groups at no-odor control night; **: P=0.001, versus no-rAAV and rAAV-GFP groups at odor night.
Effects on waking and NREM/REM sleep
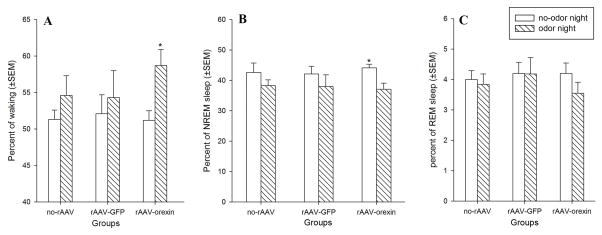
There was no significant difference in percent waking during the no-odor control night between the three groups. Thus, orexin gene transfer into the amygdala did not increase spontaneous levels of waking. However, when mice were exposed to the predator odor, orexin gene transfer into the amygdala made the mice significantly more awake compared to the no-odor night (t14=2.51, P=0.021). The no-rAAV and rAAV-GFP groups had slight increases in waking but these were not significant (Figure 4A). Total NREM sleep time in the rAAV-orexin group was significantly reduced in response to predator odor (t14=2.38, P=0.028, figure 4B). There was no significant change in total REM sleep time between control night and predator odor night in all three groups (Figure 4C).
Figure 4.
Effects of orexin gene transfer in amygdala on percent (±SEM) waking, NREM sleep and REM sleep during the 12 h light-off period (night). When exposed to predator odor, the orexin-KO mice with orexin gene transfer in amygdala had a significant increase in waking (panel A; *: P=0.021 versus no-odor control night) and a significant decrease in NREM sleep (panel B; *: P=0.028 versus no-odor control night). The other two groups also showed an increase in waking but this was not significant. Exposure to predator odor did not change percent REM sleep (panel C).
DISCUSSION
The primary result of this study was that orexin gene transfer into surrogate neurons of the amygdala (mostly CeA and BLA) suppressed not only spontaneous but also emotion-induced cataplexy behavior in the orexin-KO mice. Orexin-KO mice exhibit a behavioral phenotype that is strikingly similar to human narcolepsy patients (Chemelli et al., 1999). Like human narcolepsy, cataplexy in orexin-KO mice is triggered by strong positive (e.g. chocolate reward) and negative emotions (e.g. predator odor) (Morawska et al., 2011; Burgess et al., 2013). Previously, in orexin-KO mice spontaneous bouts of cataplexy were rescued by ectopic expression of the prepro-orexin gene in all cells (Willie et al., 2011). However, such a strategy does not identify a pathway involved in specific behaviors. On the other hand, site-directed orexin gene transfer limits the number of surrogate neurons expressing the orexin gene and also identifies a potential neural circuit. Our previous studies (Liu et al., 2008 and 2011; Blanco-Centurion et al., 2013) and other data (Hasegawa et al., 2014) determined that spontaneous bouts of cataplexy can be rescued by orexin gene transfer into specific brain neurons. However, those studies did not assess emotion induced cataplexy. The amygdala was selected as the primary site because of the evidence linking it to cataplexy (Gulyani et al., 2002; Burgess et al., 2013).
The behavior of the mice during the predator odor night was similar to a previous study that also utilized predator odor to induce cataplexy (Morawska et al., 2011). Upon odor delivery mice displayed various behavioral symptoms of mild fear responses including multiple direct examinations of the bottle (source of the odor), biting and burying the bottle, or running around to get away from the odor. During the no-odor night spontaneous bouts of cataplexy occurred when mice were eating, walking or grooming. However, during the odor night cataplexy in the two control groups was mostly triggered by odor-induced behaviors such as bottle biting or running (no-rAAV and rAAV-GFP). In the two control groups, the increase in cataplexy with activity such as running is consistent with previous study (Espana et al., 2007) where wheel-running behavior increased cataplexy. Mice in the rAAV-orexin group exhibited similar responses during the odor night, but it did not induce cataplexy. Unfortunately, we did not directly measure locomotor activity levels, but the rAAV-orexin group was awake more in response to the predator odor (Figure 4A). However, the increased waking did not induce cataplexy, indicating that emotion-induced cataplexy was blocked with orexin gene insertion into amygdala neurons.
The amygdala plays an important role in emotion processing (Kim et al., 2011; LeDoux JE et al., 2005). Predator odor-induced fear responses have been found to trigger c-Fos expression in the central (CeA) and basolateral (BLA) nucleus of the amygdala (Ryan et al., 2011). Some of these activated neurons might regulate muscle tone by projecting directly to the pons. In wild-type mice, pontine neurons receive not only GABA inhibitory control from the amygdala, but also orexin excitatory innervations from the lateral hypothalamus. These innervations maintain muscle tone during emotional stress, allowing the animal to escape. There is anatomical support for such a circuit. For instance, there are dense reciprocal connections between orexin neurons and the amygdala (Marcus et al., 2001; Cluderay et al., 2002). Orexin excites both GABA neurons and corticotropin-releasing factor (CRF) neurons in the central nucleus of amygdala (CeA) via orexin receptors (Bisetti et al., 2006). The amygdala projects to pontine structures controlling muscle tone such as the ventrolateral periaqueductal gray/lateral pontine tegmentum (vlPAG/LPT) and dorsal raphe (DR) (Boissard et al., 2003; Fung et al., 2011).
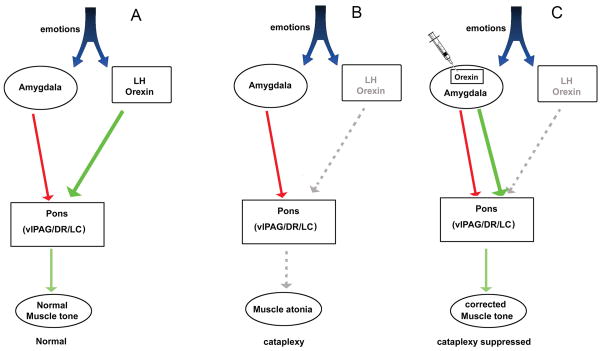
When orexin signaling is absent, as in narcolepsy, the balance between inhibitory and excitatory control on muscle tone is weakened, resulting in muscle atonia during waking. By reintroducing orexin into the amygdala-pons circuit, the balance was restored and cataplexy was significantly reduced (Figure 5). We suggest that in the present study during emotional distress the amygdala GABA neurons also released orexin, in addition to GABA, at pontine target sites. However, the presence of orexin stabilized muscle tone and blocked both spontaneous and emotion-induce cataplexy.
Figure 5.
A neural circuit model summarizes how orexin gene transfer into amygdala may rescue cataplexy. Evidence from many studies indicates that muscle tone is regulated by the activity of pontine neurons (Fraigne, et al., 2015). During emotions the orexin excitatory signal from the lateral hypothalamus neutralizes the inhibitory signal from the amygdala and maintains the activity of pontine neurons (panel A). In narcoleptic mice the orexin excitatory signal from the lateral hypothalamus is absent. Therefore, the inhibitory signal from the amygdala overwhelms and inhibits pontine neurons controlling muscle tone, resulting in muscle atonia during waking (i.e., cataplexy) (panel B). When the orexin gene is inserted into surrogate neurons in the amygdala, the excitatory orexin signal is re-introduced into the amygdala-pons circuit that maintains muscle tone, and cataplexy is suppressed (panel C).
We found that some of the transfected orexin-expressing neurons in amygdala were GABAergic and orexin-A-ir terminals were observed in pons in close proximity to neurons in the vlPAG, LC and DR, which are the critical downstream targets of amygdala for regulating muscle tone (Figure 2). We also noted that many non-GABA neurons were also transfected and there are some excitatory projections from amygdala such as CRF projection, which might also participate in cataplexy behavior during emotions. Indeed, CRF neurons in CeA are involved in fear response (Ryan et al., 2011). Since the rAAV vector used in this study transferred the orexin gene into many neurons in the amygdala the specific phenotype of the projection responsible for regulating cataplexy attacks cannot be identified. Phenotype-specific or projection-specific orexin gene transfer should be done in future studies to delineate the nerual circuit regulating cataplexy.
Our use of the orexin gene transfer method has been very useful in identifying surrogate neurons that ameliorate cataplexy. Areas in the brain where orexin gene transfer has decreased spontaneous bouts of cataplexy in orexin-KO and orexin/ataxin-3 mice models of narcolepsy include the lateral hypothalamus, the zona incerta, and the dorsolateral pons (Liu et al., 2008 and 2011; Blanco-Centurion et al., 2013). We have now determined that orexin can be placed into amygdala neurons and it blocks both spontaneous and emotion-induced cataplexy. These studies indicate that to decrease cataplexy orexin must be placed in surrogate neurons that project directly to the pontine neuronal circuitry regulating muscle tone. Orexin gene transfer into the striatum has no effect on cataplexy (Liu et al., 2011) even though the striatum regulates motor behavior. This is because the orexin is in local striatal interneurons which do not directly release orexin onto pontine targets. We have also determined that along with the direct innervation of the pons the surrogate neurons must also be active in waking. The activity in waking would release orexin to stabilize the motor circuit. For instance, cataplexy is not decreased when orexin is placed in melanin-concentrating hormone (MCH) containing neurons of the posterior hypothalamus (Liu et al., 2011). The MCH neurons innervate the pons (Yoon et al., 2013) but they are silent during waking (Hassani, et al., 2009). As such, when cataplexy is triggered these neurons are silent, indicating that orexin is not released from these transfected MCH neurons.
In conclusion, we have provided the first evidence that orexin gene transfer into surrogate neurons in the amygdala suppresses both spontaneous and emotion-induced cataplexy attacks. Further studies will identify the phenotype of amygdala neurons and their downstream targets regulating muscle tone during emotions. The gene transfer approach can serve as a tool to repair neural circuits regulating specific behaviors, such as muscle tone in narcolepsy.
Acknowledgments
Supported by Medical Research Service of the Department of Veterans Affairs (I01 BX000798) and NIH grants 1K01AG041520, NS052287, NS084477, NS079940 and HL091363. We thank Dr. Patrick K. Randall for support with the SPSS statistical analysis program.
ABBREVIATIONS
- Aq
aqueduct
- BLA
basolateral amygdala
- BMA
basomedial nucleus of amygdala
- CeA
central nucleus of amygdala
- ChAT
choline acetyltransferase
- CMV
cytomegalovirus
- CRF
corticotropin releasing factor
- DR
dorsal raphe
- EEG
electroencephalogram
- EMG
electromyogram
- GFP
green fluorescent protein
- LC
locus coeruleus
- LPT
lateral pontine tegmentum
- NREM
non-rapid eye movement
- OXR2
orexin receptor-2
- rAAV
recombinant adeno-associated virus
- REM
rapid eye movement
- TH
tyrosine hydroxylase
- VGAT
vesicular GABA transporter
- vlPAG
ventrolateral periaqueductal gray
References
- Aldrich MS. The neurobiology of narcolepsy-cataplexy. Progress in Neurobiology. 1993;41:533–41. doi: 10.1016/0301-0082(93)90042-q. [DOI] [PubMed] [Google Scholar]
- Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, Mühlethaler M. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Blanco-Centurion C, Liu M, Konadhode R, Pelluru D, Shiromani PJ. Effects of orexin gene transfer in the dorsolateral pons in orexin knockout mice. Sleep. 2013;36:31–40. doi: 10.5665/sleep.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. European Journal of Neuroscience. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- Brabec J, Rulseh A, Horinek D, Pala A, Guerreiro H, Buskova J, Petrovicky P, Nemcova V, Krasensky J, Seidl Z, Nimsky C, Sonka K. Volume of the amygdala is reduced in patients with narcolepsy - a structural MRI study. Neuroendocrinology Letters. 2011;32:652–656. [PubMed] [Google Scholar]
- Burgess CR, Oishi Y, Mochizuki T, Peever JH, Scammell TE. Amygdala Lesions Reduce Cataplexy in Orexin Knock-Out Mice. Journal of Neuroscience. 2013;33:9734–9742. doi: 10.1523/JNEUROSCI.5632-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regulatory Peptides. 2002;15:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Espana RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 2007;30:1417–1425. doi: 10.1093/sleep/30.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraigne JJ, Torontali ZA, Snow MB, Peever JH. REM Sleep at its Core - Circuits, Neurotransmitters, and Pathophysiology. Frontiers in Neurology. 2015;29:123. doi: 10.3389/fneur.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Xi M, Zhang J, Torterolo P, Sampogna S, Morales FR, Chase MH. Projection neurons from the central nucleus of the amygdala to the nucleus pontis oralis. Journal of Neuroscience Research. 2011;89:429–436. doi: 10.1002/jnr.22554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyani S, Wu MF, Nienhuis R, John J, Siegel JM. Cataplexy-related neurons in the amygdala of the narcoleptic dog. Neuroscience. 2002;112:355–365. doi: 10.1016/s0306-4522(02)00089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa E, Yanagisawa M, Sakurai T, Mieda M. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. Journal of Clinical Investigation. 2014;124:604–16. doi: 10.1172/JCI71017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proceedings of the National Academy of Sciences of USA. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE. Comparative cytoarchitectonic atlas of the C57BL/6 and 129/SV mouse brains. Elsevier; Amsterdam, Netherlands: 2000. [Google Scholar]
- Kim MJ1, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioral Brain Research. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Brooks/Cole; Belmont, CA: 1968. [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu M, Blanco-Centurion C, Konadhode R, Begum S, Pelluru D, Gerashchenko D, Sakurai T, Yanagisawa M, van den Pol AN, Shiromani PJ. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. Journal of Neuroscience. 2011;31:6028–6040. doi: 10.1523/JNEUROSCI.6069-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Thankachan S, Kaur S, Begum S, Blanco-Centurion C, Sakurai T, Yanagisawa M, Neve R, Shiromani PJ. Orexin (hypocretin) gene transfer diminishes narcoleptic sleep behavior in mice. European Journal of Neuroscience. 2008;28:1382–1393. doi: 10.1111/j.1460-9568.2008.06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. Journal of Comparative Neurology. 2001;18:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Mattarozzi K, Bellucci C, Campi C, Cipolli C, Ferri R, Franceschini C, Mazzetti M, Russo PM, Vandi S, Vignatelli L, Plazzi G. Clinical, behavioural and polysomnographic correlates of cataplexy in patients with narcolepsy/cataplexy. Sleep Medicine. 2008;9:425–433. doi: 10.1016/j.sleep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Meletti S, Vaudano AE, Pizza F, Ruggieri A, Vandi S, Teggi A, Franceschini C, Benuzzi F, Nichelli PF, Plazzi G. The Brain Correlates of Laugh and Cataplexy in Childhood Narcolepsy. Journal of Neuroscience. 2015;35:11583–11594. doi: 10.1523/JNEUROSCI.0840-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska M, Buchia M, Fendta M. Narcoleptic episodes in orexin-deficient mice are increased by both attractive and aversive odors. Behavioral Brain Research. 2011;222:397–400. doi: 10.1016/j.bbr.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nature Medicine. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Butler Ryan K, Sharko Amanda C, Oliver Elisabeth M, Brito-Vargas Paul. Activation of phenotypically-distinct neuronal subpopulations of the rat amygdala following exposure to predator odor. Neuroscience. 2011;175:133–144. doi: 10.1016/j.neuroscience.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Willie JT, Guilleminault C, Siegel JM. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–116. [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science. 1991;252:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Takahira H, Shibahara M, Hara J, Nomiyama M, Yanagisawa M, Sakurai T. Ectopic overexpression of orexin alters sleep/wakefulness states and muscle tone regulation during REM sleep in mice. Journal of Molecular Neuroscience. 2011;43:155–161. doi: 10.1007/s12031-010-9437-7. [DOI] [PubMed] [Google Scholar]
- Yoon YS, Lee HS. Projections from melanin-concentrating hormone (MCH) neurons to the dorsal raphe or the nuclear core of the locus coeruleus in the rat. Brain Research. 2013;1490:72–82. doi: 10.1016/j.brainres.2012.08.022. [DOI] [PubMed] [Google Scholar]