Summary
The vaccination efficacy in the elderly is significantly reduced compared to younger populations due to thymic involution and age‐related intrinsic changes affecting their naïve T‐cell compartment. Interleukin (IL)‐21 was recently shown to display thymostimulatory properties. Therefore, we hypothesized that its administration to ageing hosts may improve T‐cell output and thus restore a competent peripheral T‐cell compartment. Indeed, an increase in the production of recent thymic emigrants (RTEs) attributable to intrathymic expansion of early thymic progenitors (ETPs), double‐negative (DN), and double‐positive (DP) thymocytes as well as thymic epithelial cell (TEC) was observed in recombinant (r)IL‐21‐treated aged mice. In sharp contrast, no alterations in the frequency of bone marrow (BM)‐derived progenitors were detected following rIL‐21 administration. Enhanced production of naïve T cells improved the T‐cell receptor (TCR) repertoire diversity and re‐established a pool of T cells exhibiting higher levels of miR‐181a and diminished amounts of the TCR‐inhibiting phosphatases SHP‐2 and DUSP5/6. As a result, stimulation of T cells derived from rIL‐21‐treated aged mice displayed enhanced activation of Lck, ZAP‐70, and ERK, which ultimately boosted their IL‐2 production, CD25 expression, and proliferation capabilities in comparison with T cells derived from control aged mice. Consequently, aged rIL‐21‐treated mice vaccinated using a tyrosinase‐related protein 2 (Trp2)‐derived peptide exhibited a substantial delay in B16 tumor growth and improved survival. The results of this study highlight the immunorestorative function of rIL‐21 paving its use as a strategy for the re‐establishment of effective immunity in the elderly.
Keywords: interleukin‐21, miR‐181a, T cells, thymic involution, tumor vaccine, signaling
Introduction
In addition for being the key site of T lymphopoiesis in jawed vertebrates, the thymus maintains a competent peripheral T‐cell pool with a broad spectrum of TCR specificities (Lynch et al., 2009). It is, however, well established that immunity declines with ageing owing to two key factors impeding thymic function: a defect in the survival/proliferation ability of the prethymic hematopoietic progenitor pool coupled to the precocious loss of TECs (Boehm & Swann, 2013). These age‐related changes, collectively known as thymic involution, represent major driving forces for homeostatic expansion of preexisting peripheral T cells (Lynch et al., 2009). The net outcome culminates in TCR repertoire skewing with a noticeable increase in the number of effector/memory T cells (Zanni et al., 2003). Notably, a growing body of literature established that while the size of the peripheral T‐cell compartment remains unchanged throughout ageing, an increase in post‐thymic lifespan of T cells takes place consequently leading to the emergence of T‐cell intrinsic defects (Haynes & Swain, 2006; Maue et al., 2009; Tsukamoto et al., 2009). For instance, naïve CD4+ T cells derived from aged mice display defects in TCR threshold calibration, do not readily form immunological synapses, and have a marked reduction in the recruitment of TCR‐associated signaling molecules when compared to younger mice (Garcia & Miller, 1997, 2001, 2002; Tamir et al., 2000). Furthermore, increased expression of inhibitory receptors such as PD1, LAG3, 2B4, and CD160 were observed on the surface of ageing CD8+ T cells (Decman et al., 2012), while both IL‐2 secretion and proliferation potential are limited in naïve CD4+ and CD8+ T cells derived from aged mice (Eaton et al., 2004). Thus, stifled thymopoiesis combined to global qualitative changes affecting the ageing peripheral T‐cell pool limits the host's ability to mount effective responses against new antigenic challenges and accounts for the eroded immunity commonly observed in the elderly.
Primarily produced by activated CD4+ T cells, IL‐21 is a prominent member of the common γ‐chain family of cytokines (Spolski & Leonard, 2014). Besides its wide‐ranging effects on immune cells, IL‐21 overexpression in vivo triggers expansion of BM‐derived progenitors (Ozaki et al., 2006). Furthermore, we recently reported a novel mitogenic function for IL‐21 on peptide‐mediated TCR‐engaged DP thymocytes using a newly developed in vitro coculture system designed for T‐cell differentiation (Rafei et al., 2013b). Likewise, rIL‐21 administration to mice with glucocorticoid‐induced thymic atrophy dramatically accelerates thymic function recovery by stimulating the proliferation of ETPs, DN, and positively selected DP thymocytes (Rafei et al., 2013a). Such unprecedented thymopoiesis‐supporting function suggests that rIL‐21 is indeed a promising therapeutic tool endowed with the capacity of improving T‐cell output in aged hosts owing to the expression of the IL‐21 receptor (IL‐21R) on both BM and thymic progenitors (Ozaki et al., 2006; Rafei et al., 2013a,b). We wished therefore to scrutinize whether rIL‐21 administration to aged mice can rejuvenate their T‐cell immunity by targeting de novo thymopoiesis as a mean to enhance their antitumoral response following vaccination.
Results
Administration of rIL‐21 enhances thymopoiesis in aged mice
To ensure maximal thymopoiesis‐stimulating effects in vivo, we first conducted a dose–response study in young (2 months—2M) versus old (15 months—15M) RAG2p‐GFP mice by intraperitoneally (IP) administering rIL‐21 (Fig. S1A). The use of the RAG2p‐GFP model allows to easily assess de novo thymopoiesis as expression of the GFP transgene, marking newly developed T cells, is controlled by the Rag2 promoter activity (Monroe et al., 1999; Yu et al., 1999; Rafei et al., 2011). Thymic analysis 1 week following the last injection revealed a progressive increase in total thymocyte count in 15M but not 2M‐old rIL‐21‐treated mice with an optimal response rate achieved at a dose of 50 μg kg−1 (Fig. S1B). Similarly, only aged mice receiving rIL‐21 exhibited an increase in the counts of all thymic subsets (DN, DP and single positive) (Fig. 1A) including ETPs (Fig. 1B,C). Even though the percentage of GFP+ thymocytes remained unchanged in all studied groups (Fig. S1C), the increased thymic count observed in the rIL‐21‐treated aged mice was sustained over a 3‐week period postcytokine administration (Fig. S1D). To determine whether rIL‐21‐enhanced thymopoiesis involves the expansion of BM progenitors, which could have increased their migration rate to the thymus, we next monitored the frequency of LSK cells (Lin− Sca1+ c‐Kit+) and its subpopulations including the long‐term (LT; Lin− Sca1+ c‐Kit+ CD34− CD135−) and short‐term (ST; Lin− Sca1+ c‐Kit+ CD34+ CD135−) hematopoietic stem cells (HSCs), as well as multipotent progenitors (MPPs; Lin− Sca1+ c‐Kit+ CD34+ CD135+) following rIL‐21 treatment. Despite IL‐21R expression on the surface of wild‐type (WT) LT, ST‐HSCs, and MPPs (Fig. S2A), the overall proportion of LSK cells (Fig. S2B) or its subpopulations (Fig. S2C) was not affected by rIL‐21 administration. Likewise, no increase in the number of LSK subpopulations (Fig. S2D) nor in the more differentiated common lymphoid progenitor (CLP; Lin− IL‐7R+ Sca1+ c‐Kit+) population (Fig. S2E) was observed. Furthermore, only in vitro cultured young LSK cells proliferated when cultured with rIL‐21 (Fig. S2F) clearly suggesting a defective response in ageing BM.
Figure 1.
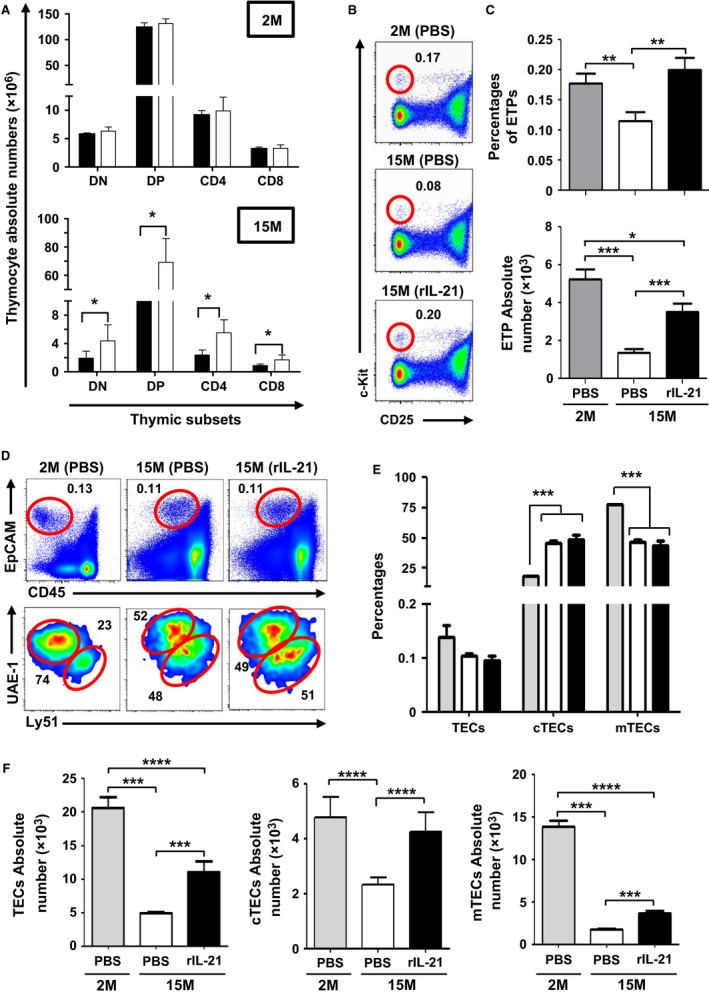
Administration of rIL‐21 promotes de novo thymopoiesis in aged but not young mice. (A) Counts of thymocyte subpopulations. Groups are displayed as PBS (■) and IL‐21 (□). (B) Representative flow cytometry analysis of ETPs. (C) Absolute count of ETPs. (D, E) Percentages of total, cTECs, and mTECs. (F) Absolute counts of total, cTECs, and mTECs in comparison with PBS‐treated aged mice. For panels C, E, and F, groups are displayed as: 2M (PBS  ), 15M (PBS □), or 15M (rIL‐21 ■). All data are representative of three independent experiments (n = 5/group with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
), 15M (PBS □), or 15M (rIL‐21 ■). All data are representative of three independent experiments (n = 5/group with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
We recently reported that TECs are devoid of IL‐21R (Rafei et al., 2013a). Therefore, we presumed that the thymic effects observed following rIL‐21 infusion strictly affect hematopoietic cells. Indeed, when given to aged mice, rIL‐21 does not fluctuate the frequency of total TECs (EpCAM+ CD45−), nor it did affect the ratio of cortical (c)TEC (EpCAM+ CD45− UAE‐1− Ly51+) relative to medullary (m)TEC (EpCAM+ CD45− UAE‐1+ Ly51−/lo) populations (Fig. 1D,E). Conversely, absolute counts analysis showed marked improvements in the stromal compartment as total, cTEC, and mTEC populations were significantly higher in rIL‐21‐treated aged mice compared to the PBS group (Fig. 1F). Higher production of IL‐7/thymus production was also noticed in the rIL‐21‐treated aged mice (Fig. S1E). These data suggest that rIL‐21 administration is beneficial to aged mice by directly targeting thymopoiesis in situ without triggering the expansion of BM‐derived LSK cells.
Physiological levels of RTE are restored in aged mice following rIL‐21 treatment
To interrogate the functional relevance of rIL‐21‐enhanced thymopoiesis on the peripheral T‐cell pool of aged RAG2p‐GFP mice, we next assessed the percentage of circulating RTEs mirrored by the level of peripheral GFP+ CD3ε+ T cells. In contrast to 2M‐old animals, where RTEs represent roughly 2.3% of total circulating lymphocytes, lower percentages (~0.5%) are detected in the peripheral blood of 15M PBS‐treated aged mice (Fig. 2A). Following rIL‐21 treatment, the percentage of GFP+ CD3ε+ T cells reached a range of 1.3–1.7% over a period of 3‐week postcytokine treatment (Fig. 2A,B) with absolute numbers attaining physiological levels according to RTE counts calculated using blood derived from young mice (Fig. 2C).
Figure 2.
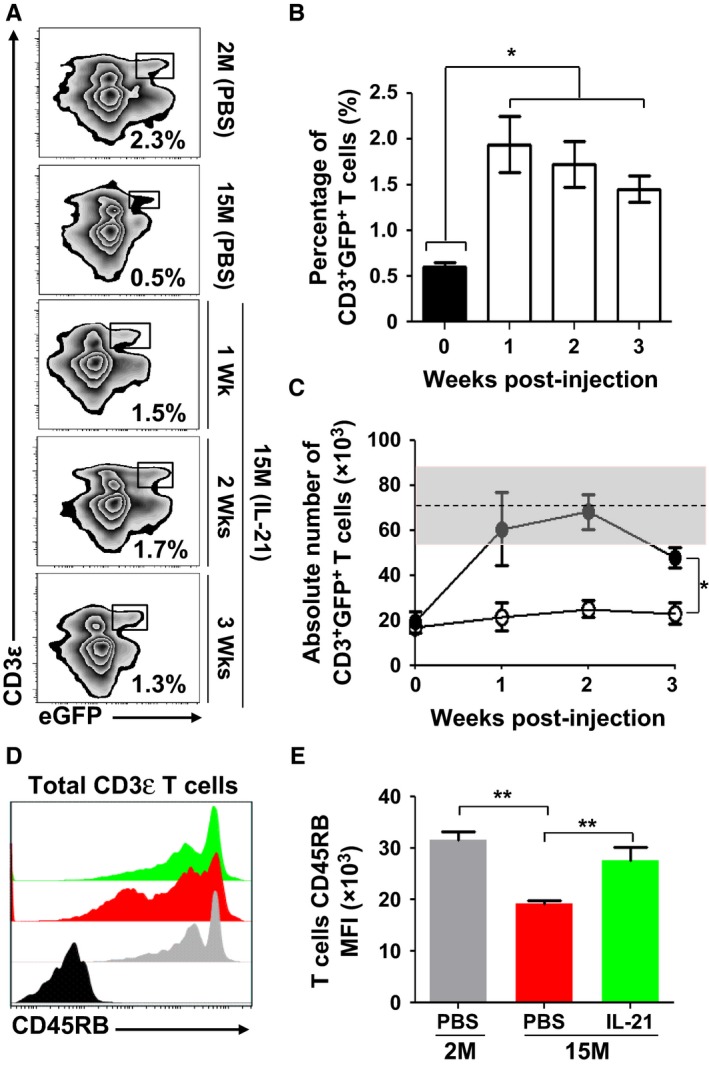
Increased levels of circulating RTEs in rIL‐21‐treated aged mice. (A) Representative flow cytometry analysis of RTEs in peripheral blood of young (2M) or aged (15M) mice 1, 2, or 3 weeks post‐rIL‐21 administration. Young mice treated with PBS were used as comparative positive controls. (B, C) Analysis of overall percentages (B) and counts (C) of RTEs in the weeks following rIL‐21 administration to aged mice. The black histogram represents the 2M PBS‐treated mice. The gray zone in (C) represents the RTE level calculated using 2M young mice (n = 10) and displayed as the average RTE number ± two SD. Black circles in (C) represent rIL‐21‐treated mice. (D) Representative flow cytometry analysis of CD45RB on the surface of all CD3+ T cells derived from 2M (PBS
 ), 15M (PBS
), 15M (PBS
 ), and 15M (rIL‐21
), and 15M (rIL‐21 ). CD45RB isotype is displayed in black. (E) Compiled MFIs for CD45RB expression in treated mice. All data are representative of three independent experiments (n = 5/group with *P < 0.05 and **P < 0.01).
). CD45RB isotype is displayed in black. (E) Compiled MFIs for CD45RB expression in treated mice. All data are representative of three independent experiments (n = 5/group with *P < 0.05 and **P < 0.01).
With increased encountered antigens and declined RTE levels, qualitative changes in the phenotype of peripheral T‐cell composition occur with ageing (Boursalian et al., 2004). More specifically, the expression of various cell surface makers including the glycoprotein CD45RB is downregulated as T cells become activated and progress from a naïve to a memory phenotype (Tough & Sprent, 1994). We therefore hypothesized that enhancing RTE generation in aged mice would increase the overall expression pattern of CD45RB on peripheral T‐cell pool and found that it was indeed the case in aged mice treated with rIL‐21 as depicted by histogram overlaps (Fig. 2D) and compiled mean fluorescent intensities (MFIs) (Fig. 2E). We therefore conclude that rIL‐21 treatment enhances the de novo generation of RTEs, which incorporate the peripheral T‐cell pool of aged mice.
The nature of ageing T‐cell pool is greatly affected by rIL‐21 administration
Following thymic egress, RTEs continue their maturation in the periphery to eventually become fully competent mature naïve T cells (Boursalian et al., 2004). To do so, they require access to secondary lymphoid organs (SLO) as a mean to encounter other cell types and cytokines required for their maturation (Houston et al., 2008). Although the percentage of GFP+ T cells increased significantly in the spleen of rIL‐21‐treated aged mice in the 3 weeks following cytokine treatment (Fig. 3A), the overall number of splenocytes remained steady (Fig. 3B). Further in‐depth analysis revealed a progressive time‐dependent increase in the absolute counts of GFP+ CD4+ and GFP+ CD8+ T cells in the spleens of rIL‐21‐treated aged mice (Fig. 3C) suggesting that SLO‐resident aged T cells were displaced by newly migrating RTEs. We next examined by flow cytometry the differentiation stages of spleen‐derived CD4+ and CD8+ T cells and detected increased abundance of T cells with a naïve phenotype (CD44loCD62Lhi) in IL‐21‐treated aged mice (Fig. 3D,E) bolstering the notion of rIL‐21‐mediated enhanced T‐cell output.
Figure 3.
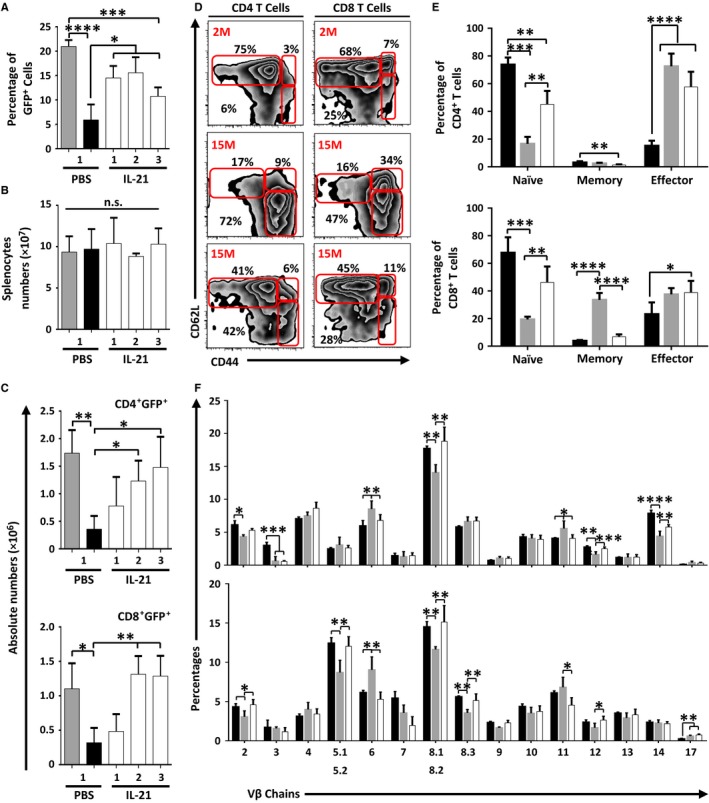
Aged mice treated with rIL‐21 display increased proportion of naïve T cells with enhanced TCR diversity. (A) Percentages of GFP
+ events in the spleen of treated mice. (B) Splenocyte counts in all experimental groups. (C) Absolute counts of CD4+ GFP
+ and CD8+ GFP
+ T cells in treated mice. (D) A representative flow cytometry analysis of naïve (CD62Lhi
CD44lo), memory (CD62Lhi
CD44hi), and effector (CD62Llo
CD44hi) T cells in all experimental groups. (E) Compiled percentages of all three subpopulations in CD4+ (top panel) and CD8+ (lower panel) T cells. (F) Flow cytometry analysis of 15 TCRVβ chains using peripheral CD4+ (top panel) or CD8+ (lower panel) T cells. For panels A–C, groups are displayed as 2M (PBS
 ), 15M (PBS ■), and 15M (rIL‐21 □). For panels E and F, groups are displayed as 2M (PBS ■), 15M (PBS
), 15M (PBS ■), and 15M (rIL‐21 □). For panels E and F, groups are displayed as 2M (PBS ■), 15M (PBS
 ), and 15M (rIL‐21 □). All data are representative of three independent experiments (n = 5/group with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
), and 15M (rIL‐21 □). All data are representative of three independent experiments (n = 5/group with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
Given that thymic involution compromises the TCR repertoire (Yager et al., 2008; Ahmed et al., 2009), we continued our analysis by investigating the effect of rIL‐21 administration on TCR diversity. To address this question, the expression profile of 15 TCRVβ‐chains was analyzed on the surface of spleen‐derived CD4+ and CD8+ T cells collected from treated mice. Although no changes occurred in the TCRVβ‐chains of young mice treated with PBS or rIL‐21 (Fig. S3), significant improvements were observed in the proportion of Vβ2‐, 6‐, 8.1/8.2‐, 11‐, 12‐, and 14‐expressing CD4+ T cells as well as Vβ2‐, 5.1/5.2‐, 6‐, 8.1/8.2‐, 8.3‐, 11‐, and 12‐expressing CD8+ T cells following rIL‐21 administration to ageing mice (Fig. 3F). These results indicate that rIL‐21‐mediated de novo RTE generation is associated with major qualitative changes in the peripheral T‐cell pool of aged mice including improved TCR diversity.
Characterizing the biochemical responses of T cells
Thymic involution cannot solely account for impaired immune responses as additional T‐cell intrinsic defects appear in ageing naïve T cells due to prolonged post‐thymic lifespan (Haynes & Swain, 2006; Maue et al., 2009; Tsukamoto et al., 2009). More specifically, TCR activation is blunted with ageing due to increased cytoplasmic concentration of phosphatases known for inhibiting TCR signaling (Li et al., 2012). Given such fluctuation in TCR threshold calibration, we next explored whether the previously observed changes in the nature of the peripheral T‐cell pool induced by rIL‐21 affect the expression levels of the TCR‐targeting phosphatases SHP‐2, PTPN‐22, and DUSP5/6. Delineation of phosphatase levels by Western blotting (Fig. 4A) and densitometry‐based quantification (Fig. 4B) revealed diminished SHP‐2 and DUSP5/6 levels in T cells derived from rIL‐21‐treated aged mice at all tested time points. Only PTPN‐22 remained unchanged in all groups throughout treatments (Fig. 4A,B). Previous studies conducted in mice demonstrated that SHP‐2 and DUSP5/6 were among several phosphatases controlled by miR‐181a, a ~22nt microRNA molecule capable of repressing the translation of over 40 phosphatases (Li et al., 2007). Consistently, the progressive decline in miR‐181a levels observed in ageing human naïve CD4+ T cells dovetails the decreased T‐cell responsiveness following TCR stimulation (Li et al., 2012). To examine the possibility that rIL‐21 could have reversed such defect through enhanced generation of RTEs expressing normal levels of miR‐181a, quantitative (q)PCR studies were conducted using T cells sorted from spleens of treated mice. Our analysis revealed a two‐ to threefold increase in miR‐181a levels in T cells derived from rIL‐21‐treated aged mice (Fig. 4C), which is consistent with the diminished SHP‐2 and DUSP5/6 levels observed earlier (Fig. 4A,B). We next tested the responses of T cells derived from treated mice by probing early signaling events triggered by TCR stimulation. Although not efficient as younger lymphocytes, CD4+ or CD8+ T cells derived from rIL‐21‐treated aged mice displayed higher Lck, ZAP‐70, and ERK phosphorylation compared to control PBS aged mice as shown by phospho‐flow analysis (Fig. 4D). Taken collectively, these data indicate that enhanced TCR responses in the pool of T cells derived from rIL‐21‐treated aged mice are attributable to the increase in the proportion of naïve T cells displaying lower SHP‐2 and DUSP5/6 phosphatase levels owing to improved miR‐181a expression.
Figure 4.
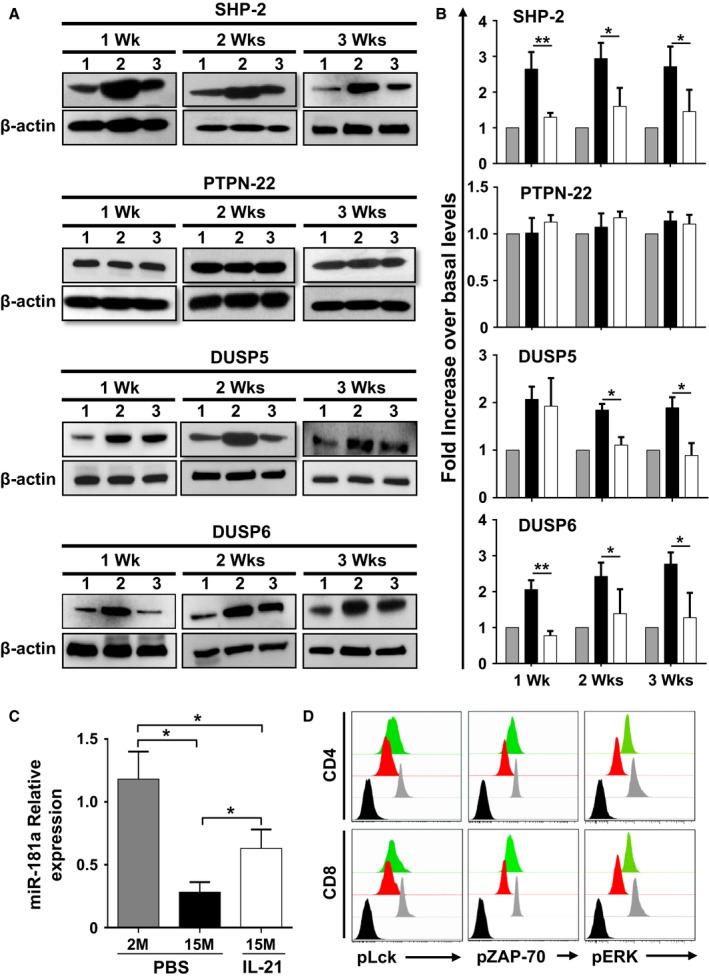
The peripheral pool of T cells in rIL‐21‐treated aged mice displays improved TCR signaling responses. (A) Representative Western blot analyses of phosphatases at 1, 2, or 3 weeks post‐treatments of 2M PBS (1), 15M PBS (2), and 15M rIL‐21 (3) aged mice. β‐actin was used as internal loading control. (B) Compiled densitometry analysis of phosphatase expression levels. The displayed groups are as follows: 2M (PBS
 ), 15M (PBS ■), and 15M (rIL‐21 □) aged mice. C) qPCR analysis of miR‐181a in freshly isolated T cells from 2M (PBS
), 15M (PBS ■), and 15M (rIL‐21 □) aged mice. C) qPCR analysis of miR‐181a in freshly isolated T cells from 2M (PBS
 ), 15M (PBS ■), and 15M (rIL‐21 □) treated mice. D) Representative intracellular flow cytometry staining of pLck, pZAP‐70, and pERK in 2M (PBS
), 15M (PBS ■), and 15M (rIL‐21 □) treated mice. D) Representative intracellular flow cytometry staining of pLck, pZAP‐70, and pERK in 2M (PBS
 ), 15M (PBS
), 15M (PBS
 ), and 15M (rIL‐21
), and 15M (rIL‐21  ) aged mice. Nonstimulated T cells derived from young 2M mice are displayed by black histograms. All data are representative of three independent experiments (n = 5/group with *P < 0.05, and **P < 0.01).
) aged mice. Nonstimulated T cells derived from young 2M mice are displayed by black histograms. All data are representative of three independent experiments (n = 5/group with *P < 0.05, and **P < 0.01).
rIL‐21 administration improves the biological responses of T cells derived from aged mice
The dramatic reduction in naïve T‐cell output coupled to the relative increase in the proportion of effector and central memory T cells in aged mice negatively impact T‐cell responses to neo‐antigens (Yager et al., 2008). Repeated observations revealed that beside scarcity of T‐cell precursors in the pre‐immune repertoire, IL‐2 production, cell surface expression of CD25, and T‐cell proliferation are all greatly reduced in ageing hosts (Haynes & Swain, 2006; Maue et al., 2009; Tsukamoto et al., 2009). Elevated secretion levels of pro‐inflammatory cytokines such as interferon (IFN)γ caused by the accumulation of CD44hi T cells were also reported to play a role in accelerating age‐related immunosenescence (Zhang et al., 2002; Decman et al., 2012). The observations made so far led us to speculate that the beneficial effect of rIL‐21 on thymopoiesis in aged mice should improve the effector function of their rejuvenated T‐cell pool. To test this hypothesis, spleen‐derived CD3ε+ T cells were isolated 3 weeks following the last cytokine treatment (to ensure that they had completed their necessary post‐thymic maturation) and stimulated using CD3‐CD28 beads (Berkley et al., 2013). Not only were IFNγ and IL‐2 secretion profiles by TCR‐stimulated T cells derived from rIL‐21‐treated mice comparable to younger animals, but a striking decrease in IL‐17 production was observed as well (Fig. 5A). In addition, the intensity of CD25 cell surface expression on spleen‐purified TCR‐stimulated CD4+ or CD8+ T cells and their proliferation rates were comparable between young and rIL‐21‐treated aged mice as determined by flow cytometry (Fig. 5B,D) and compiled MFIs data (Fig. 5C,E). To gain further insights, we quantified by qPCR the expression level of several genes known to influence the differentiation and/or effector function of T cells. Although no difference was observed in Nfat, Ap‐1, and Gata3 expression in all tested groups, a decrease in T‐bet and RORC (encoding for RORγt) transcript levels was detected in T cells derived from rIL‐21‐treated aged mice compared to aged PBS control mice (Fig. S4). Foxp3 expression level, on the other hand, was equivalent between PBS‐ and rIL‐21‐treated aged mice but significantly higher than younger mice (Fig. S4). These qPCR results are consistent with the observed decrease in IFNγ (T‐bet) and IL‐17 (RORC) secretion levels and are particularly interesting as several investigations have associated IL‐21 to autoimmune diseases (Korn et al., 2007; Peluso et al., 2007; Attridge et al., 2012). Further analyses conducted on rIL‐21‐treated aged mice showed no unusual signs of inflammation or autoimmunity (Fig. S5). More specifically, we found (i) that the spleen size and weight of PBS‐ or rIL‐21‐treated aged mice were similar to younger mice (Fig. S5A), (ii) that the total serum IgG levels were lower in young mice but comparable in both PBS‐ and rIL‐21‐treated animals indicating an age‐related factor at play independent of rIL‐21 treatment (Fig. S5B), and (iii) no immune infiltrates in lungs, liver, or kidneys of aged mice receiving rIL‐21 (Fig. S5C). In agreement with previous studies reporting accumulation of CD25+ CD4+ Tregs in the periphery and SLO of ageing mice and humans (Lages et al., 2008), we detected a significantly higher percentage and absolute number of spleen‐derived Tregs in both PBS‐ and rIL‐21‐treated aged mice when compared to their younger counterparts (Fig. S5D,E). Globally, these findings indicate that rIL‐21‐mediated boosting of T‐cell output in aged mice corrects the T‐cell dysfunctionalities commonly seen with ageing without inducing signs of autoimmunity.
Figure 5.
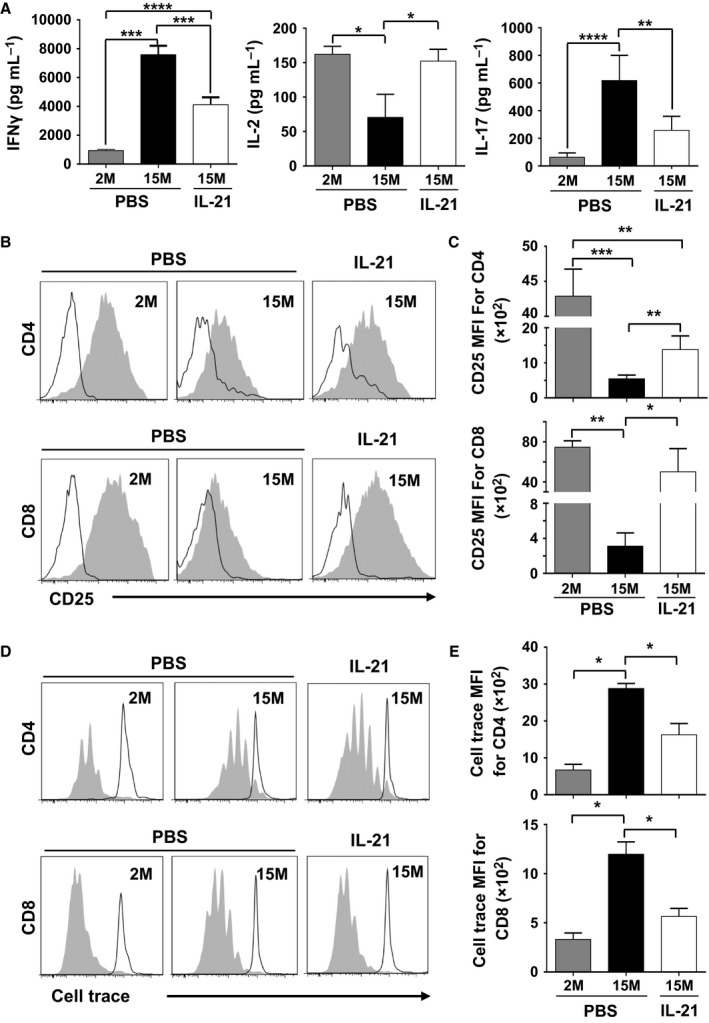
Enhanced biological responses of peripheral T‐cell pool derived from rIL‐21‐treated aged mice. (A) Cytokine quantification from stimulated CD3+ T cells isolated from the spleen of treated mice. (B) Representative flow cytometry analysis of CD25 cell surface expression on CD4+ (top panels) and CD8+ (lower panels) T cells. (C) Compiled MFIs for CD25 expression on CD4+ and CD8+ T cells. (D) Representative flow cytometry analysis of cell trace dilution following TCR stimulation of CD4+ (top panels) and CD8+ (lower panels) T cells derived from treated animals. (E) Compiled MFIs for cell trace dilution of CD4+ and CD8+ T cells following stimulation. All data are representative of three independent experiments (n = 5/group with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
Enhanced vaccine‐elicited antitumoral response in rIL‐21‐treated aged mice
To evaluate the effectiveness of our T‐cell rejuvenation therapy, a proof‐of‐concept vaccination study was conducted using an experimental melanoma antigen (Parkhurst et al., 1998). Briefly, aged mice were first treated with PBS or rIL‐21 according to our established protocol, then left for 3 weeks to allow for RTE maturation (Fig. 6A). All mice were then vaccinated weekly (for a total of three injections) using in vitro generated BM‐derived mature DCs (Fig. S6) pulsed with SIINFEKL (a control epitope derived from chicken ovalbumin) or SVYDFFVWL peptides (the experimental epitope derived from the melanoma differentiation antigen Trp2). Two weeks following the last DC boost, mice were challenged subcutaneously (SC) with syngeneic B16 tumor cells and monitored thereafter for tumor growth and survival. Although all SIINFEKL‐vaccinated mice died within the same time frame (day 20–24) with no statistically significant difference between young, PBS‐, or rIL‐21‐treated mice, a slower tumor growth (Fig. 6B) and a significant delay in survival (Fig. 6C) were observed in the Trp2‐vaccinated rIL‐21‐treated aged mice in comparison with the Trp2‐vaccinated PBS control group (survived up to 40 versus 22 days, respectively). We then retrieved the spleens of mice from each group and analyzed their IFNγ production level and proliferation following Trp2 restimulation in vitro. All Trp2‐vaccinated animals responded to peptide restimulation with a significant increase in IFNγ response achieved in rIL‐21‐treated aged mice compared to control PBS aged mice (Fig. 6D). Similarly, enhanced proliferation responses were observed in the rIL‐21‐treated aged mice as evaluated by cell trace dilution overtime by flow cytometry (Fig. 6E) and compiled MFIs data (Fig. 6F). In sum, these data clearly show that rIL‐21 preconditioning of aged mice leads to marked improvements in the control of B16 tumor growth following Trp2 vaccination.
Figure 6.
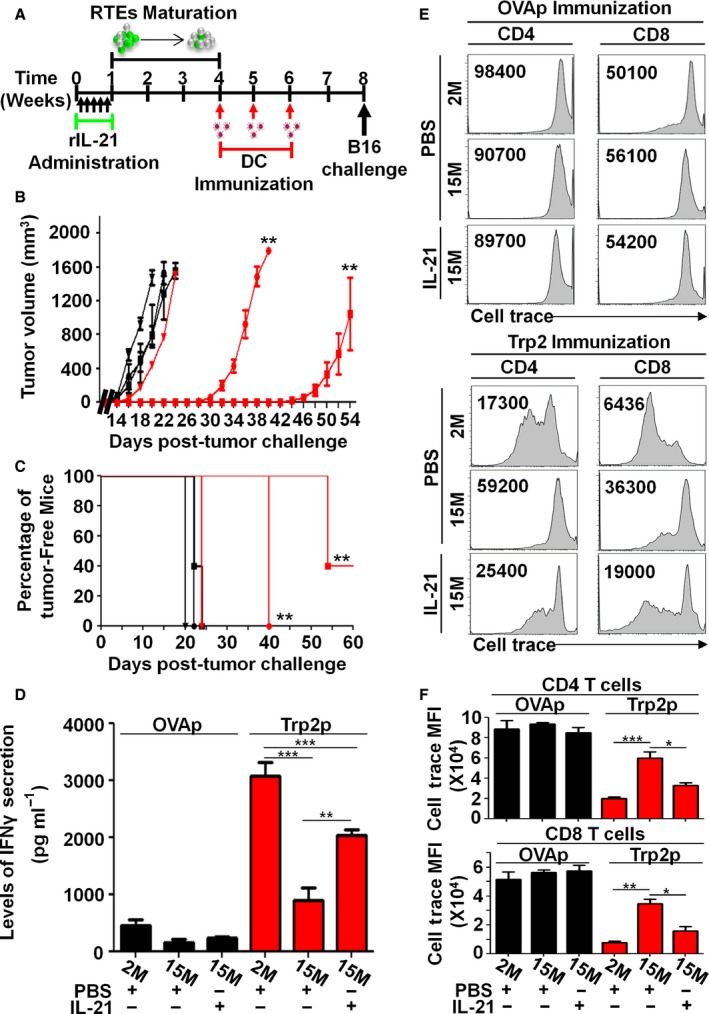
T‐cell rejuvenation of aged mice enhances their antitumoral immunity. (A) Timeline used for vaccination. (B, C) Tumor volumes and percentage of tumor‐free mice post‐B16 tumor cell challenge in OVAp‐DC‐vaccinated 2M (PBS ■), 15M (PBS ▼), and 15M (rIL‐21 ●); or Trp2p‐DC‐vaccinated 2M (PBS
 ), 15M (PBS
), 15M (PBS
 ) and 15M (rIL‐21
) and 15M (rIL‐21  ). (D) IFNγ quantification following in vitro Trp2p stimulation of T cells derived from vaccinated animals. (E) Representative flow cytometry analysis of cell trace dilution in CD4+ and CD8+ T cells derived from vaccinated animals following in vitro Trp2 stimulation. (F) Compiled MFIs for cell trace dilutions in CD4+ (top panel) and CD8+ (lower panel) T cells following in vitro Trp2 stimulation. OVAp (SIINFEKL) and Trp2p (SVYDFFVWL). All data are representative of three independent experiments (n = 5/group with *P < 0.05, **P < 0.01, and ***P < 0.001).
). (D) IFNγ quantification following in vitro Trp2p stimulation of T cells derived from vaccinated animals. (E) Representative flow cytometry analysis of cell trace dilution in CD4+ and CD8+ T cells derived from vaccinated animals following in vitro Trp2 stimulation. (F) Compiled MFIs for cell trace dilutions in CD4+ (top panel) and CD8+ (lower panel) T cells following in vitro Trp2 stimulation. OVAp (SIINFEKL) and Trp2p (SVYDFFVWL). All data are representative of three independent experiments (n = 5/group with *P < 0.05, **P < 0.01, and ***P < 0.001).
Discussion
Thymic involution deprives aged hosts from a competent immune system capable of effectively responding to vaccination and invading pathogens (Boehm & Swann, 2013). Although some aspects of age‐related decline in T‐cell responses reflect systemic changes, others are due to cell intrinsic defects (Haynes & Swain, 2006; Maue et al., 2009; Tsukamoto et al., 2009). We show in this report that administration of rIL‐21 enhances thymopoiesis in aged mice through expansion of both the stromal and responsive thymocytes compartments without the induction of any apparent pathology in peripheral organs. Consequently, a competent peripheral lymphoid pool containing larger proportion of naïve CD4+ and CD8+ T cells (CD62LhiCD44lo) displaying potent effector functions in response to TCR stimulation was restored. This increase in the availability and potency of naïve T cells augmented the responsiveness of aged mice to Trp2 vaccination and accounts for their improved survival in response to B16 tumor challenge (Fig. S7).
T lymphopoiesis remains functional at older age albeit to a limited extent (Hale et al., 2006). As the thymus lacks self‐renewing progenitors, it heavily relies on sustained seeding with BM‐derived CLPs and/or ETPs (Min et al., 2004). Unfortunately, however, BM‐derived progenitor numbers decline markedly with age due to increased apoptosis rates as well as reduced proliferative capacities (Min et al., 2004). This in turn negatively impacts the delicate thymic stromal compartment, which is dependent on cross talk interactions with thymic progenitors for its sustained survival and morphogenesis (Shores et al., 1991; Hollander et al., 1995; van Ewijk et al., 2000; Dudakov et al., 2012). In this regard, two major points can be concluded based on observations made in rIL‐21‐treated aged mice. First, a report by Ozaki et al. previously demonstrated that IL‐21 overexpression in WT young mice expands LSK cells in vivo (Ozaki et al., 2006). Such effect is certainly beneficial as it can increase the pool of BM progenitor cells available for thymic migration. Although we did not directly assess the level of thymus‐migrating BM progenitors, we confirmed that all LSK subpopulations in ageing mice express IL‐21R but fail to proliferate in response to rIL‐21 administration (Fig. S2). Such apparent discrepancy between our observations and those reported by Ozaki and colleagues may be explained by the lower dose or bioavailability of rIL‐21 in comparison with in vivo overexpression. However, age‐related changes in the functional properties of LSK cells should also be taken into account. While the frequency of LT‐HSCs increases drastically in the BM of ageing mice, a decrease in the ST‐HSC and the transiently reconstituting MPP populations occurs in parallel (confirmed in Fig. S2) (Rossi et al., 2005). Such alterations are elemental to thymopoiesis as the ST‐HSC and MPP fractions both lie upstream of CLP and contribute to lymphoid reconstitution (Rossi et al., 2005). These observations are consistent with microarray analyses revealing the existence of systematic downregulation of LT‐HSC‐specific genes mediating lymphoid specification and function with ageing (Rossi et al., 2005). This implies that rIL‐21 can compensate for deficiencies affecting BM‐derived progenitors by stimulating the expansion of progenitor cells such as ETPs that have already seeded the thymic compartment. Second, the increase in the absolute number of cTEC and mTEC subpopulations in rIL‐21‐treated aged mice cannot be mediated by a direct action of rIL‐21 due to the absence of IL‐21R on TEC surface (Rafei et al., 2013a). The simplest interpretation of these results is that increased thymocyte numbers enhanced lympho‐stromal interactions consequently promoting TECs expansion (Hollander et al., 1995; van Ewijk et al., 2000). Indeed, BM transplantation studies using RAGnull mice as recipients revealed a central role played by developing thymocytes in the functional organization of thymic microenvironments (van Ewijk et al., 2000). By first providing signals to cTECs, DN thymocytes initiate the creation of a functional 3D organized cortical microenvironment. Such restructuring facilitates the differentiation of DN thymocytes to DP and SP stages, which ends up improving the survival/expansion of the mTEC compartment (van Ewijk et al., 2000). This is certainly a plausible explanation as the adult thymus contains nonsenescent progenitor stromal cells believed to be involved in TEC maintenance (Dumont‐Lagace et al., 2014). Thus, an involuted stroma possibly retains the capacity to regenerate in response to increased progenitor numbers. Under such context, the lack of rIL‐21‐induced thymopoiesis in younger mice may not be surprising as both thymic size and function are optimal at that age.
The concept that miR‐181a acts as a rheostat for TCR signaling is not novel (Li et al., 2007). Although expression of signaling molecules involved in TCR signaling is not influenced by age (Tamura et al., 2000), cumulative homeostatic proliferation is believed to promote progressive loss of miR‐181a in ageing naïve T cells overtime (Li et al., 2012). Only a continuous stream of newly developed T cells capable of replenishing the peripheral T‐cell compartment can compensate for such intrinsic defect. In accordance with these studies, we detected lower expression of miR‐181a in T cells of aged mice compared to their younger counterparts, which was further reflected on their poor TCR responsiveness (Fig. 4). However, in vivo provision of exogenous rIL‐21 reversed the miR‐181a decrease through enhanced de novo generation of RTEs that have incorporated the peripheral compartment. RTE maturation thereafter led to an increase in mature naïve T cells, which were competent enough to effectively respond to Trp2 vaccination and trigger a substantial delay in B16 tumor growth (Figs 3, 5, and 6). This, however, does not preclude the possibility that rIL‐21 administration to aged mice affects other preexisting immune cells in periphery. Additional studies involving thymectomized mice or adoptive transfer into athymic nude are required to confirm this hypothesis. In addition, there are data indicating that RTEs generated in aged hosts retain some intrinsic defects following TCR cross‐linking (Clise‐Dwyer et al., 2007). If so, how can we interpret the improved effector functions observed in the rejuvenated T‐cell pool of rIL‐21‐treated mice? Clise‐Dwyer et al. demonstrated that RTEs generated either following transplantation of younger hosts with aged BM or after antibody‐mediated depletion of peripheral T‐cell in ageing hosts exhibit normal effector function in comparison with RTEs developed in untreated aged animals (Clise‐Dwyer et al., 2007). The authors explained this conundrum by suggesting that under lymphopenic conditions, levels of IL‐7 are either elevated or competition for IL‐7 is reduced resulting in rescued T‐cell development. As stromal cells are the main producers of IL‐7 in the thymus, an ageing thymic microenvironment with increased TECs turnover would indeed compromise RTEs number, quality, and function due to limited IL‐7 availability. This highlights one of the distinctive thymopoiesis‐stimulating properties of rIL‐21 as it can raise the level of intrathymic IL‐7 production (Fig. 1SE) most likely by indirectly mediating the expansion of the stromal compartment through enhanced thymocyte proliferation therefore resulting in the generation of defects‐free RTEs.
Besides physiological ageing, contraction of the TCR repertoire is commonly observed in patients suffering from infections, cancers, or following BM transplantation (van den Brink et al., 2004). There are currently no effective therapies capable of exerting a positive impact on broadening the spectrum of TCR. Studies involving IL‐21R−/− mice clearly showed that IL‐21 is dispensable for immune cell development as normal proportions of lymphocytes, monocytes, and granulocytes have been reported (Spolski & Leonard, 2014). Our data nevertheless suggest that rIL‐21 administration to ageing hosts could have potent clinical uses related to its ability to promote the expansion of thymic progenitor cells, which can be further enhanced if combined with other thymostimulatory compounds.
Experimental procedures
Cell line and mice
The B16F0 mouse melanoma cell line was kindly provided by Dr. J. Galipeau (Atlanta, GA, USA). The RAG2p‐GFP transgenic mice were kindly provided by Dr. M. Nussenzweig (Rockefeller University, New York, NY, USA). Female WT C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). To generate IL‐21R−/− C57BL/6 mice, commercially available sperm was purchased from the MMRRC repository and used to fertilize female WT C57BL/6 mice. All mice were housed at the Institute for Research in Immunology and Cancer (IRIC) animal facility under specific pathogen‐free conditions. All animal protocols were approved by the Animal Care Committee of Université de Montréal.
Antibodies, cytokines and reagents
Mouse rIL‐21 and granulocyte–macrophage colony‐stimulating factor (rGM‐CSF) were purchased from Peprotech (Rocky Hill, NJ, USA). All flow cytometry antibodies and Cytofix/Cytoperm Kits were purchased from BD Pharmingen (San Diego, CA, USA). The anti‐SHP‐2, PTPN‐22, DUSP5/6, and β‐actin antibodies used in Western blotting were purchased from Abcam (Cambridge, MA, USA). Quantikines were purchased from R&D System (Minneapolis, MN, USA). The CD3‐CD28 beads, cell trace, and Trizol were purchased from Invitrogen (Burlington, ON, Canada). The cell Lytic M buffer and lipopolysaccharide (LPS) were purchased from Sigma (St‐Louis, MO, USA). T‐cell enrichment kits were purchased from StemCell Technologies (Vancouver, BC, Canada). Peptides were synthesized by GenScript (Piscataway, NJ, USA).
Administration of rIL‐21 and T‐cell analyses
For IL‐21 dosage establishment, WT young (2M), or aged (15M) female mice were IP‐injected with rIL‐21 in 200 μL PBS on a daily interval (total of five injections) in the first week. Control mice received equal volumes of PBS. All subsequent in vivo experiments were performed using rIL‐21 at 50 μg kg−1. Peripheral T‐cell absolute numbers were obtained by combining the analysis of blood samples collected from treated RAG2p‐GFP mice using the Scil‐Vet ABC+ hematological analyzer (to obtain absolute counts of lymphocytes) and flow cytometry (to obtain percentages of specific subpopulations).
Enrichment of primary TECs
Primary TECs were enriched from thymic lobes of WT C57Bl/6 mice as previously described (Gray et al., 2006).
Western blots
Splenocytes were first isolated to sort total CD3+ T cells. Whole T‐cell extracts were obtained through using the Cell Lytic M reagent (Sigma). Extracts were separated by electrophoresis, transferred onto Hybond‐ECL membrane, and probed using primary antibodies. Densitometry analysis was conducted by first imaging the chemiluminescent blots with the ImageQuant LAS 4000 imager (GE Healthcare Life Sciences, Mississauga, ON, Canada) followed by analysis using the ImageQuant TL software (GE Healthcare Life Sciences).
Expression analysis of miR‐181a
T cells were first sorted directly in 900 μL Trizol (106 cells per tube) and lysed followed by RNA extraction in Trizol reagent (Invitrogen). RNA purification was further carried out using the RNA extraction kit (QIAgen, Toronto, On, Canada). Reverse transcription was performed using the High Capacity cDNA reverse transcription kit and qPCR was performed with a 7900HT Fast Real‐Time PCR system at IRIC's genomic core facility. Target gene values were normalized to endogenous control Gapdh.
Intracellular staining
For analysis of phosphorylated (p)Lck, ZAP‐70, and ERK, isolated splenocytes were first stimulated in vitro using CD3‐CD28 Dynabeads (Invitrogen) for 5 min, then washed and surface stained for CD4 and CD8 prior to intracellular staining according to manufacturer's instructions.
Histological analyses
Chosen organs were harvested from treated mice, fixed in 10% formalin before mounting in paraffin. Sections were then stained with hematoxylin and eosin, then scanned using the NanoZoomer Digital Pathology system and NPD.scan 2.3.4 software (Hamamatsu, Hamamatsu city, Japan).
DC vaccination and tumor challenge
To generate DCs for vaccination, BM cells were extracted from tibia/femur bones of 8‐week‐old male mice and plated in 10‐cm nontissue culture‐treated Petri dishes containing 10 mL of complete RPMI 1640 medium (0.048 mmol L−1 β‐mercaptoethanol, 2 mmol L−1 l‐glutamine, 10% fetal bovine serum, 2 mmol L−1 penicillin–streptavidin) supplemented with 10 ng mL−1 rGM‐CSF (Peprotech). The media were changed at days 3 and 6 of culture. To induce DC maturation, 1 μg mL−1 LPS (Sigma) was added at day 8 for 24 h. Following confirmation of a mature phenotype (>80% of adherent cells were CD80+ CD86+ MHCI+ MHCII+), DCs were pulsed with OVA‐ or Trp2‐derived peptides (GenScript) for 4 h, then harvested for intravenous vaccination. Two weeks following the last boost, mice were SC‐challenged with 5 × 105 B16 tumor cells. Tumor volume was measured as [(length × width2)/2], and mice were sacrificed when the tumor volume reached 2000 mm3.
Proliferation and cytokine measurements
For all assays conducted using nonvaccinated mice, total or fractionated T cells were first isolated by negative selection, then plated at 105 cells well−1 in 96‐well plates. To trigger proliferation/activation, T cells were stimulated with CD3‐CD28 Dynabeads in a 1:1 ratio. For in vitro recall responses following vaccination, splenocytes were pulsed with 1 μg mL−1 Trp2 peptide. Cells or supernatants were collected 48 h poststimulation for further analyses.
Statistical analyses
P‐values were calculated using the ANOVA and log‐rank statistical test where applicable. Log‐rank testing was performed using software available at the Walter and Eliza Hall Institute Web site (http://bioinf.wehi.edu.au/software/russell/logrank/).
Author contributions
EAC designed most of the study, carried out experiments, analyzed the data, prepared the figures, and wrote the first draft of the manuscript. AT, SP, RK, and SZ performed some experiments and contributed to data analysis and manuscript preparation. MR designed the study, discussed the results with all authors, and wrote the manuscript. The authors declare that they have no conflict of interest.
Supporting information
Fig. S1 Analysis of thymic GFP+ content, absolute counts and IL‐7 production following rIL‐21 administration.
Fig. S2 Administration of rIL‐21 to aged mice has no beneficial effect on the BM compartment.
Fig. S3 Assessment of TCR repertoire diversity following rIL‐21 administration to young mice.
Fig. S4 Transcription factors quantification in T cells derived from treated mice.
Fig. S5 Assessment of autoimmune signs following rIL‐21 administration.
Fig. S6 Generation and characterization of BM‐derived DC for vaccination.
Fig. S7 rIL‐21 administration to aged mice increases thymic output leading to improved immune functions.
Acknowledgments
We are grateful to Danièle Gagné and Serge Sénéchal (for sorting and flow cytometry assistance), Raphaëlle Lambert (for qPCR studies), and to the staff of the IRIC histology and animal care facility for their assistance. This work was supported by a Cancer Research Society (CRS) operating grant. Moutih Rafei holds a Fonds de la Recherche en Santé du Québec Junior 1 award.
References
- Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA (2009) Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J. Immunol. 182, 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attridge K, Wang CJ, Wardzinski L, Kenefeck R, Chamberlain JL, Manzotti C, Kopf M, Walker LS (2012) IL‐21 inhibits T cell IL‐2 production and impairs Treg homeostasis. Blood 119, 4656–4664. [DOI] [PubMed] [Google Scholar]
- Berkley AM, Hendricks DW, Simmons KB, Fink PJ (2013) Recent thymic emigrants and mature naive T cells exhibit differential DNA methylation at key cytokine loci. J. Immunol. 190, 6180–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T, Swann JB (2013) Thymus involution and regeneration: two sides of the same coin? Nature reviews . Immunology 13, 831–838. [DOI] [PubMed] [Google Scholar]
- Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ (2004) Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 5, 418–425. [DOI] [PubMed] [Google Scholar]
- van den Brink MR, Alpdogan O, Boyd RL (2004) Strategies to enhance T‐cell reconstitution in immunocompromised patients. Nat. Rev. Immunol. 4, 856–867. [DOI] [PubMed] [Google Scholar]
- Clise‐Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL (2007) Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. J. Immunol. 178, 1321–1331. [DOI] [PubMed] [Google Scholar]
- Decman V, Laidlaw BJ, Doering TA, Leng J, Ertl HC, Goldstein DR, Wherry EJ (2012) Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus‐specific precursors. J. Immunol. 188, 1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, Boyd RL, van den Brink MR (2012) Interleukin‐22 drives endogenous thymic regeneration in mice. Science 336, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont‐Lagace M, Brochu S, St‐Pierre C, Perreault C (2014) Adult thymic epithelium contains nonsenescent label‐retaining cells. J. Immunol. 192, 2219–2226. [DOI] [PubMed] [Google Scholar]
- Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L (2004) Age‐related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J. Exp. Med. 200, 1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk W, Hollander G, Terhorst C, Wang B (2000) Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development 127, 1583–1591. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA (1997) Differential tyrosine phosphorylation of zeta chain dimers in mouse CD4 T lymphocytes: effect of age. Cell. Immunol. 175, 51–57. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA (2001) Single‐cell analyses reveal two defects in peptide‐specific activation of naive T cells from aged mice. J. Immunol. 166, 3151–3157. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA (2002) Age‐dependent defects in TCR‐triggered cytoskeletal rearrangement in CD4 + T cells. J. Immunol. 169, 5021–5027. [DOI] [PubMed] [Google Scholar]
- Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL (2006) Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 108, 3777–3785. [DOI] [PubMed] [Google Scholar]
- Hale JS, Boursalian TE, Turk GL, Fink PJ (2006) Thymic output in aged mice. Proc. Natl Acad. Sci. USA 103, 8447–8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Swain SL (2006) Why aging T cells fail: implications for vaccination. Immunity 24, 663–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander GA, Wang B, Nichogiannopoulou A, Platenburg PP, van Ewijk W, Burakoff SJ, Gutierrez‐Ramos JC, Terhorst C (1995) Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature 373, 350–353. [DOI] [PubMed] [Google Scholar]
- Houston EG Jr, Nechanitzky R, Fink PJ (2008) Cutting edge: contact with secondary lymphoid organs drives postthymic T cell maturation. J. Immunol. 181, 5213–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK (2007) IL‐21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448, 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C (2008) Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J. Immunol. 181, 1835–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ (2007) miR‐181a is an intrinsic modulator of T cell sensitivity and selection. Cell 129, 147–161. [DOI] [PubMed] [Google Scholar]
- Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ (2012) Decline in miR‐181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 18, 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD (2009) Thymic involution and immune reconstitution. Trends Immunol. 30, 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, Haynes L (2009) T‐cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 30, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H, Montecino‐Rodriguez E, Dorshkind K (2004) Reduction in the developmental potential of intrathymic T cell progenitors with age. J. Immunol. 173, 245–250. [DOI] [PubMed] [Google Scholar]
- Monroe RJ, Seidl KJ, Gaertner F, Han S, Chen F, Sekiguchi J, Wang J, Ferrini R, Davidson L, Kelsoe G, Alt FW (1999) RAG2:GFP knockin mice reveal novel aspects of RAG2 expression in primary and peripheral lymphoid tissues. Immunity 11, 201–212. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Hishiya A, Hatanaka K, Nakajima H, Wang G, Hwu P, Kitamura T, Ozawa K, Leonard WJ, Nosaka T (2006) Overexpression of interleukin 21 induces expansion of hematopoietic progenitor cells. Int. J. Hematol. 84, 224–230. [DOI] [PubMed] [Google Scholar]
- Parkhurst MR, Fitzgerald EB, Southwood S, Sette A, Rosenberg SA, Kawakami Y (1998) Identification of a shared HLA‐A*0201‐restricted T‐cell epitope from the melanoma antigen tyrosinase‐related protein 2 (TRP2). Cancer Res. 58, 4895–4901. [PubMed] [Google Scholar]
- Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, Pallone F, Monteleone G (2007) IL‐21 counteracts the regulatory T cell‐mediated suppression of human CD4 + T lymphocytes. J. Immunol. 178, 732–739. [DOI] [PubMed] [Google Scholar]
- Rafei M, Hardy MP, Williams P, Vanegas JR, Forner KA, Dulude G, Labrecque N, Galipeau J, Perreault C (2011) Development and function of innate polyclonal TCRalphabeta+ CD8 + thymocytes. J. Immunol. 187, 3133–3144. [DOI] [PubMed] [Google Scholar]
- Rafei M, Dumont‐Lagace M, Rouette A, Perreault C (2013a) Interleukin‐21 accelerates thymic recovery from glucocorticoid‐induced atrophy. PLoS ONE 8, e72801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafei M, Rouette A, Brochu S, Vanegas JR, Perreault C (2013b) Differential effects of gammac cytokines on postselection differentiation of CD8 thymocytes. Blood 121, 107–117. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL (2005) Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA 102, 9194–9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores EW, Van Ewijk W, Singer A (1991) Disorganization and restoration of thymic medullary epithelial cells in T cell receptor‐negative scid mice: evidence that receptor‐bearing lymphocytes influence maturation of the thymic microenvironment. Eur. J. Immunol. 21, 1657–1661. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ (2014) Interleukin‐21: a double‐edged sword with therapeutic potential. Nat. Rev. Drug Discovery 13, 379–395. [DOI] [PubMed] [Google Scholar]
- Tamir A, Eisenbraun MD, Garcia GG, Miller RA (2000) Age‐dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J. Immunol. 165, 1243–1251. [DOI] [PubMed] [Google Scholar]
- Tamura T, Kunimatsu T, Yee ST, Igarashi O, Utsuyama M, Tanaka S, Miyazaki S, Hirokawa K, Nariuchi H (2000) Molecular mechanism of the impairment in activation signal transduction in CD4(+) T cells from old mice. Int. Immunol. 12, 1205–1215. [DOI] [PubMed] [Google Scholar]
- Tough DF, Sprent J (1994) Turnover of naive‐ and memory‐phenotype T cells. J. Exp. Med. 179, 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Clise‐Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, Haynes L, Swain SL (2009) Age‐associated increase in lifespan of naive CD4 T cells contributes to T‐cell homeostasis but facilitates development of functional defects. Proc. Natl Acad. Sci. USA 106, 18333–18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA (2008) Age‐associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 205, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, Melchers F, Meffre E, Nussenzweig MC (1999) Continued RAG expression in late stages of B cell development and no apparent re‐induction after immunization. Nature 400, 682–687. [DOI] [PubMed] [Google Scholar]
- Zanni F, Vescovini R, Biasini C, Fagnoni F, Zanlari L, Telera A, Di Pede P, Passeri G, Pedrazzoni M, Passeri M, Franceschi C, Sansoni P (2003) Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp. Gerontol. 38, 981–987. [DOI] [PubMed] [Google Scholar]
- Zhang X, Fujii H, Kishimoto H, LeRoy E, Surh CD, Sprent J (2002) Aging leads to disturbed homeostasis of memory phenotype CD8(+) cells. J. Exp. Med. 195, 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Analysis of thymic GFP+ content, absolute counts and IL‐7 production following rIL‐21 administration.
Fig. S2 Administration of rIL‐21 to aged mice has no beneficial effect on the BM compartment.
Fig. S3 Assessment of TCR repertoire diversity following rIL‐21 administration to young mice.
Fig. S4 Transcription factors quantification in T cells derived from treated mice.
Fig. S5 Assessment of autoimmune signs following rIL‐21 administration.
Fig. S6 Generation and characterization of BM‐derived DC for vaccination.
Fig. S7 rIL‐21 administration to aged mice increases thymic output leading to improved immune functions.


