Highlight
Species, storage products, and moisture have large effects on the nature and quantity of volatile emission from dry seeds, but storage time and seed viability do not.
Key words: Fermentation, gas chromatography, methanol, pentane, peroxidation, seed aging, seed storage, seed quality markers, volatile organic compounds, water content.
Abstract
The nature and kinetics of reactions in dry seeds determines how long the seeds survive. We used gas chromatography to assay volatile organic compounds (VOCs) emitted from seeds of three unrelated species as a means to non-invasively probe chemical changes during very dry, dry, and humid storage (seeds were dried to 5.5, 33, and 75% relative humidity at room temperature). VOCs emitted from seeds stored in humid conditions reflected fermentation-type reactions, with methanol and ethanol being predominant in Lactuca sativa and Carum carvi, and acetaldehyde and acetone being predominant in Eruca vesicaria. Dried C. carvi seeds continued to emit fermentation-type products, although at slower rates than the seeds stored in humid conditions. In contrast, drying caused a switch in VOC emission in L. sativa and E. vesicaria seeds towards higher emission of pentane and hexanal, molecules considered to be byproducts from the peroxidation of polyunsaturated fatty acids. Longevity correlated best with the rate of fermentation-type reactions and appeared unrelated to the rate of lipid peroxidation. Emission of VOCs decreased when seed species were mixed together, indicating that seeds adsorbed VOCs. Adsorption of VOCs did not appear to damage seeds, as longevity was not affected in seed mixtures. Collectively, the study shows similarity among species in the types of reactions that occur in dry seeds, but high diversity in the substrates, and hence the byproducts, of the reactions. Moreover, the study suggests that the most abundant VOCs arise from degradation of storage reserves within seed cells, and that these reactions and their byproducts are not, in themselves, damaging.
Introduction
Seeds are a major class of desiccation-tolerant organisms, which can survive in the absence of water. Metabolism changes dramatically in these organisms as water is removed, and there are few measurable reactions in cells containing less than 15% water (Walters et al., 2005). Despite this highly restricted chemical activity, it is clear that some reactions occur in dry organisms, because they deteriorate with time and inevitably die. The effects of this deterioration, or aging, are usually identified as lost growth potential in rehydrated organisms, and the initial stage is extremely hard to detect. Eventually, a threshold is reached and very rapid deterioration ensues. In order to understand aging and predict when the threshold marking high morbidity will occur, we need a better understanding of the nature and kinetics of chemical activity in dry organisms.
Currently, several classes of chemical reactions are considered to occur under dry conditions, including fermentation, glycation (e.g. Maillard reaction), oxidation, and peroxidation, the latter reactions being initiated or stimulated by activated oxygen molecules or free radicals (Bailly, 2004; Colville et al. 2012; Job et al., 2005; Kranner et al., 2006, 2010; Labuza, 1980; Mira et al., 2010; Walters et al., 2010). Substrates for these reactions can be diverse, leading to a wide range of products that are classified into two main groups: (i) small molecular weight carbonyl compounds that escape into the airspace as volatile molecules and (ii) advanced glycation end-products, in which molecules become cross-linked (Chan, 1987; Frankel, 1983; Grosch, 1987; Halliwell and Gutteridge, 1999; Knutson et al., 2000). These reactions usually occur at low rates in the dry state, and it remains unclear whether enzyme catalysis occurs. We speculate that the involvement of enzymes would narrow the types of substrates and products produced.
Because volatile organic compounds (VOCs) are a major byproduct of catabolic reactions, assessment of these molecules provides an accessible method to evaluate chemical reactions occurring in dry seeds. A growing number of studies have measured VOCs using gas chromatography (GC) or gas chromatography/mass spectrometry (GCMS) to monitor metabolism non-invasively. For example, volatile alka(e)nes and aldehydes are major byproducts of lipid peroxidation (Aldini et al., 2010; Grotto et al, 2009; Rodríguez et al., 1989). Pentane and ethane are commonly derived from oxidation of polyunsaturated fatty acids such as linoleic and linolenic acid, and have been used as biomarkers of aging in plant tissue culture (Rodríguez et al., 1989), human breath (Mendis et al., 1994), and human or animal tissues (Grotto et al. 2009; Halliwell and Chirico, 1993; Hartmut et al., 1980). Saturated aldehydes, such as hexanal, are a potential biomarker of lipid peroxidation during storage of seeds (Colville et al., 2012; Mira et al., 2010; Zhang et al., 1995a), animal tissues (Orhan et al., 2006; Pignoli et al., 2009), and human milk (Elisia and Kitts 2011). Lipid peroxidation reactions also yield unsaturated aldehydes such as hexenal and hydroxyalkenals (4-hydroxynonenal), which are common biomarkers of lipid peroxidation and oxidative stress (Aldini et al., 2010; Grotto et al., 2009).
Many VOCs are reactive and might be toxic, perpetuating reactions that lead to deterioration and accelerating the rate at which seeds lose viability, as has been confirmed for high concentrations of ethanol and acetaldehyde (Akimoto et al., 2004; Zhang et al., 1995a). Being potentially both a cause and an effect of deterioration, VOCs might serve as a biomarker for seed quality loss (Fielding and Goldsworthy, 1982; Hailstones and Smith, 1989; Smith and Adamson, 1989; Wilson and McDonald, 1986). Ethanol and methanol are reported to be major constituents of the airspace above seeds stored at a relative humidity (RH) of 65–90% (Akimoto et al., 2004; Colville et al., 2012; Lee et al., 2000; Mira et al., 2010; Obendorf et al., 1990; Schwember and Bradford, 2005; Trawatha et al., 1995; Zhang et al., 1993, 1995a). In a previous study, we suggested that products reflecting glycolysis/fermentation-like reactions did not indicate specific damaging reactions, but rather indicated the fluidity of the glassy matrix in which aging reactions occurred (Mira et al., 2010). In that work, we also showed a low correlation between the presence of lipid oxidation and peroxidation products and the rate of aging in lettuce seeds.
The present study continues to explore how measurement of VOCs might reveal details of chemical activity occurring in dry seeds. Our first objective was to explore ethanol and methanol emission in diverse species, to test whether these were ubiquitously produced and whether emission was regulated by water content during storage. We reasoned that a higher rate of VOC emission implies a higher reaction rate, which, in turn, would lead to faster deterioration. Hence, our second objective was to test the hypothesis that seeds with a poor shelf life emit more VOCs. We also wanted to test the hypothesis that physiologically relevant concentrations of volatile compounds were toxic and would promote faster aging. Without knowing the specific molecule to test, we opted to mix seeds, with the logic that a shorter-lived seed might emit compounds that would increase the rate of aging in a longer-lived seed. We used three species to make these comparisons, namely, Lactuca sativa (Asteraceae), Eruca vesicaria (Brassicaceae) and Carum carvi (Apiaceae).
Materials and methods
Seed material and measurement of lipid and water content
Lettuce seeds (Lactuca sativa L., cv. ‘Black-seeded Simpson’) were purchased from Gurney Seed Company, Greendale, IN, USA, in 2004 and 2009. Arugula seeds (Eruca vesicaria L. Cav.) were purchased from Richters in 2007. Caraway seeds (Carum carvi L.) were purchased from Hazzard’s Seeds in 2007. Seeds were stored at 5 °C and 30% RH until used for these experiments, which were initiated in 2007 for all the treatments (2800 days of storage). The experiment for L. sativa seeds (unmixed with other species) was repeated in 2009 using a 2009-harvested seed lot (2100 days of storage). All seed samples had high initial quality, with normal germination greater than 97%.
Total lipid content was measured using a protocol modified from Bligh and Dyer (1959). Pre-weighed samples of ground seeds (0.5–2.0g) were mixed in a chloroform:methanol (2:1) solution for 10min. The solvent was collected and the pellet rewashed in chloroform:methanol solution two more times. After separation, the bottom layer (i.e. the solvent and dissolved lipids) was retained and washed twice with 1:1 methanol:0.9% NaCl solution. The solvent fraction was then evaporated to remove chloroform. The remaining lipids were weighed and the amount of lipid was calculated per gram of seed dry weight (dw). Values are expressed as the average of two replicate extractions. Fatty acids were esterified using the protocol of Metcalfe and Schmitz (1961) and then characterized using GC. The fatty acid derivatives were separated on a gas chromatograph with a flame ionization detector (FID; model 8500, Perkin-Elmer, Waltham, MA, USA) using a Supelco Nukol 30 m, 0.25mm internal diameter fused silica capillary column (Sigma-Aldrich, St. Louis, MO, USA). Helium was used as the carrier gas at flow rates set to 0.14MPa (20 psi). Injector and detector temperatures were set to 220 °C and oven temperature was 100 °C increasing to 190 °C at 10 °C min−1.
Seed storage
Experiments consisted of adjusting the water content of seeds, hermetically sealing seeds in vials, storing vials at 35 °C, and sampling intermittently for airspace analysis, seed viability, and seed water content. Seed water content was adjusted by maintaining seeds at different RH at 25 °C. RH was controlled in desiccators containing saturated solutions of ZnCl2 (very dry: 5.5% RH), MgCl2 (dry: 33% RH) or NaCl (humid: 75% RH) (Vertucci and Roos, 1993). Water content was determined from a comparison of sample mass (sample size ranged from 0.01–0.09g) before and after drying at 95 °C for 72h using a microbalance (Orion Cahn C-33, Thermo Fisher Scientific, Inc., Beverly, MA, USA) and is expressed as g H2O g−1 dw. Water content at the beginning of the experiment was measured on two replicated samples prior to sealing vials and then periodically during the storage experiment as a test that vials were properly sealed. The three RH treatments gave water content ranges of 0.030–0.039, 0.042–0.063, and 0.089–0.131g H2O g−1 dw.
Approximately 30 aliquots of 50 seeds each (0.15g for L. sativa and 0.6g for E. vesicaria or C. carvi) were sealed into individual 2.7ml crimp-top vials (National Scientific, Rockwood, TN, USA). Seeds of L. sativa (0.15g) were also mixed with seeds of E. vesicaria or C. carvi (0.6g) and sealed into crimp-top vials. All vials were placed at 35 °C and samples were removed for testing periodically over a 7.5-year (2800 days) period.
Germination tests and seed longevity
Deterioration of seeds during storage was detected by changes in percentage germination. Seeds from a stored vial were sown on 14cm Petri plates containing 1% agar and incubated under a 16h light/8h dark cycle at 20 °C for 7 days for L. sativa and E. vesicaria seeds, and at 25 °C for 14 days for C. carvi seeds. Petri dishes were photographed after the incubation period and germination was measured using ImageJ software (Rasband, 1997–2008). Each germination assay consisted of two replicates of 25 seeds. In the first series of experiments in which longevity among species was compared, the 2009-harvested L. sativa seed lot was used, and this gave us comparisons of seeds tested within 6 months of harvest. The second series of experiments was initiated in 2007 and the 2004-harvested L. sativa seed lot was mixed with 2007-harvested E. vesicaria or C. carvi seeds. Germination time-course data for the unmixed 2004-harvested cohort of L. sativa seeds were presented previously (Mira et al., 2010), with sampling times for the dry treatment added.
The response to storage time in terms of percentage germination was modeled using the glm function with a binomial distribution available in the statistical package R (R Core Team, 2015). Time for seed viability to decline (i.e. longevity) was determined for each species, humidity treatment, and replicate. Time for germination percentage to decrease to 75% or 50% of maximum (P75 and P50, respectively) was calculated using the dose.p function available in R (R Core Team, 2015). We used the value of P75 to indicate the duration of the initial asymptomatic phase of seed deterioration.
Characterization of volatile production from seeds
Volatile compounds emitted by seeds during storage were characterized using GC. Periodically, a crimp-top vial containing seeds was removed from storage at 35 °C and allowed to cool to room temperature for 1–2 hours before a 1ml sample of air was taken from the headspace of the vial using a gas-tight syringe (Hamilton, Reno, NV, USA). The air sample was injected directly into the gas chromatograph (Perkin Elmer, Autosystem XL, Norwalk, CT, USA). Injector and FID detector temperatures were set at 200 °C; helium was used as the carrier gas and set at a flow rate of 0.8ml min−1. Volatile compounds were separated using a 30 m, 0.32mm internal diameter DB624 capillary column (Agilent Technologies, Santa Clara, CA, USA) that was held at 35 °C for 5 minutes, increased at 8 °C min−1 to 200 °C, and then held at 200 °C for 10min. Volatile compounds were identified by retention times (RTs) of known standards. We measured RT for 58 standards, 33 of which were also included in the manufacturer’s list of RTs for 216 non-halogenated compounds (Agilent Technical Overview 5991-5017EN, 2014). RTs for all analytes were time-corrected by pentane and/or acetone (RT = 6.05 and 7.26, respectively), which were reliably detected in most samples, obviating the need for an internal standard.
To confirm compound identity, a subset of eight samples was also analyzed using GCMS by an outside analytical laboratory (Edison Analytical Laboratories Inc., Schenectady, NY, USA). Vials representing different species and humidities that had been stored for 2–3 years were shipped to the laboratory in an insulated box to prevent major temperature changes. Volatile compounds were collected on to a solid-phase microextraction fiber (50/30 μm DVB/Carboxen/PDMS; Supelco, Bellefonte, PA, USA) that was inserted through the septum of the sample vial and held in place with a support for 40min at room temperature. The fiber was then desorbed for 2min in the injection port set at 260 °C of a HP5890 GC connected to a HP5972 mass selective detector (Hewlett Packard/Agilent, Santa Clara, CA, USA). Separation by GC was conducted using a DB624 capillary column (Agilent Technologies) held at 35 °C for 5min, increased at 8 °C min–1 to 200 °C, and then held at 200 °C for 10min. Library searches of analytes used the Wiley/NIST library of mass spectra (2008). A total of 56 compounds were identified by GCMS, of which 32 were included as VOC standards as described above.
A library of 266 compounds and associated RTs specific to our GC protocols was constructed using the 58 standards we tested and translating the 184 RTs for compounds unique to the manufacturer and 24 compounds unique to the GCMS analyses with correlation models of the ~30 standards we had in common with the manufacturer’s list and GCMS data. In addition, we developed relationships between RTs and carbon length for different carbonyl groups (alcohols, alkanes, aldehydes, acids, esters, diols, diones, and di-oxy and -ene groups) and used these relationships to infer RTs of compounds not included in any list.
Peaks with area greater than 225 µVs were analyzed. This high sensitivity allowed us to detect compounds emitted in very low quantities. Previously published data for L. sativa seeds containing 0.089g g−1 water content (Mira et al., 2010) were reanalyzed using this more sensitive threshold. VOC levels were calculated from peak size and relationships established between peak size and carbon chain length for different carbonyl groups (Mira et al., 2010). Kinetic models of volatile production were developed by linear regression of moles VOC emitted and storage time.
Results
Lipid composition varied among seed species. Seeds of L. sativa had the highest total lipid content (32%) and seeds of C. carvi the lowest (10%) (Table 1). Linoleic and oleic acids were prominent in seeds of all three species. Seeds of E. vesicaria also had high levels of erucic acid (Table 1). At any specific RH, water content was higher in seed species that had a lower lipid content (Table 2).
Table 1.
Percentage of fatty acid in total lipids of seeds of three species
| Fatty acids (%±SE) |
Chain length | L. sativa (2004) | L. sativa (2009) | E. vesicaria | C. carvi |
|---|---|---|---|---|---|
| Lauric | C12 | 0 | 0 | 0 | 2.9±0.4 |
| Myristic | C14 | 0.2±0.1 | 0 | 2.3±1.0 | 0 |
| Palmitic | C16 | 9.9±0.6 | 8.8 | 7.3±0.9 | 6.1±0.7 |
| Palmitoleic | C16:1 | 0 | 0 | 0 | 0 |
| Stearic | C18 | 3.2±0.1 | 3.2 | 1.3±0.1 | 1.2±0 |
| Oleic | C18:1 | 25.5±0.2 | 26.7 | 18.1±1.1 | 52.5±4.3 |
| Linoleic | C18:2 | 58.6±0.5 | 60.3 | 12.2±1.4 | 32.7±1.0 |
| Linolenic | C18:3 | 0.2±0.1 | 0 | 13.2±0.1 | 0.3±0.1 |
| Arachidate | C20 | 0.8±0.1 | 0.4 | 0.7±0.0 | 4.2±4.1 |
| Eicosenoate | C20:2 | 0 | 0 | 9.3±2.6 | 0 |
| Behenic | C22 | 1.6±0.9 | 0.6 | 1.3±1.3 | 0 |
| Erucic | C22:1 | 0 | 0 | 34.4±3.2 | 0 |
| Lipid content | 32±6 | 33±0.3 | 19±0 | 10±0 | |
Table 2.
Kinetics of deterioration in seeds stored at 35 °C and different water contents. Deterioration is expressed as the time for seed quality to decrease to 75 and 50% of initial germination (P75 and P50, respectively)
| Treatment | Species | Water content (g g-1 dw±SE) |
Seed longevity (days±SE) |
|
|---|---|---|---|---|
| P75 | P50 | |||
| Humid | L. sativa | 0.099±0.003 | 46±1 | 58±1 |
| E. vesicaria | 0.100±0.001 | 45±1 | 58±1 | |
| C. carvi | 0.131±0.001 | 0.4±0.1 | 0.8±0.1 | |
| Dry | L. sativa | 0.042±0.001 | 1 486±73 | 2 261±113 |
| E. vesicaria | 0.048±0.001 | 1 244±77 | 1 952±80 | |
| C. carvi | 0.063±0.001 | 1±8 | 81±4 | |
| Very dry | L. sativa | 0.030±0.001 | 2 537±3.805 | 3 882±518 |
| E. vesicaria | 0.034±0.001 | 2 334±125 | 3 379±195 | |
| C. carvi | 0.039±0.001 | 35±17 | 222±14 | |
Changes in germination with storage time followed the characteristic reverse-sigmoidal time course, which starts with an initial asymptomatic stage and concludes with rapid loss in seed viability (Fig. 1). Water content affected the duration of the asymptomatic phase. At 35 °C, seeds stored under humid conditions lost capacity to germinate within 3–100 days, depending on species (Fig. 1A). In contrast, seeds stored under dry and very dry conditions maintained high germination percentages for ~2000 (L. sativa and E. vesicaria) and 50 (C. carvi) days (Fig. 1B, C).
Fig. 1.
Changes in percentage germination of L. sativa (solid curve), E. vesicaria (long dash), and C. carvi (short dash) seeds during storage at 35 °C and three moisture treatments: (A) Humid (0.099–0.131g g–1), (B) Dry (0.042–0.063g g–1), and (C) Very Dry (0.030–0.039g g–1). Each point represents a germination assay in which the percentage of normal seedlings was measured for a particular treatment, storage time, and replicate. Data were fitted to a logistic regression model and values for 75 and 50% seed germination (P75 and P50) were calculated (Table 2).
The initial asymptomatic stage of seed deterioration was considered to be the time before significant loss in viability and characterized as the time for germination to decrease to 75% of maximum germination (P75). Duration of P75 ranged from 1 to 2545 days depending on water content during storage and species (Table 2). P75 increased with decreasing water content for all three seed species (P≤0.0005). Within each water content treatment, C. carvi aged significantly faster than L. sativa and E. vesicaria (P≤0.0005). The latter two species had similar longevities under all storage conditions (Fig. 1; Table 2).
Different compounds, varying in carbon chain length and carbonyl group, were detected in the airspace above stored seeds and identified as 1–10 C acid, alcohols, aldehydes, alkanes, furans, ketones, and terpenes. To facilitate comparisons at similar stages of deterioration, VOC composition was evaluated by averaging samples with germination between 100 and 75% (P75) and samples with germination between 75 and 50% (P50) (Fig. 2). Depending on species and water content, between seven and 23 compounds were detected in the vial airspace. L. sativa and C. carvi emitted a similar number of compounds for each water content treatment (~15–20), while the number of compounds emitted from E. vesicaria varied from 4 to 24 depending on the humidity and extent of deterioration (Fig. 2A–C).
Fig. 2.
Volatile emissions by L. sativa, E. vesicaria, and C. carvi seeds during storage at 35 °C and three moisture treatments: (A, D) Humid (0.099–0.131g g–1), (B, E) Dry (0.042–0.063g g–1), and (C, F) Very Dry (0.030–0.039g g–1). The number and quantity of VOCs are given as seed germination decreased to 75 and 50% (P75 and P50). The number of molecular species (A–C) and total molar quantity emitted (D–F) were classified by the carbonyl group. Data were not available for the treatment indicated by asterisks because samples were depleted before sufficient deterioration was detected. misc, Miscellaneous.
The total amount of emitted VOCs ranged among species and water contents (Fig. 2D–F, also summarized by the numbers shown above the bars in Fig. 3). The VOC concentration at P75 and P50 for humid storage was greatest for C. carvi (~27 nmol g−1 seed) and least for E. vesicaria (~1 nmol g−1 seed). For drier storage, VOC concentration at P75 and P50 was lower for C. carvi, comparable to L. sativa (~15 nmol g−1 seed), and higher for E. vesicaria seeds (~5 nmol g−1 seed) compared with counterparts stored under humid conditions. Data for VOC emission at P50 for L. sativa seeds stored under very dry conditions are not available because all samples were used before P50 was reached (i.e. >2100 days). Volatile composition in the humid-stored seeds at P25 and when all seeds had died appeared similar to our observations at P75 and P50 (data not shown), confirming our previous report (Mira et al., 2010). The error bars in Fig. 2 represent variation among vials sampled before P75, and between P75 and P50. While the number of VOCs detected appeared consistent among samples within a given time period (Fig. 2A–C), the quantity of VOCs varied greatly among samples, especially for the dry and very dry treatments (Fig. 2E, F), which were necessarily longer storage times.
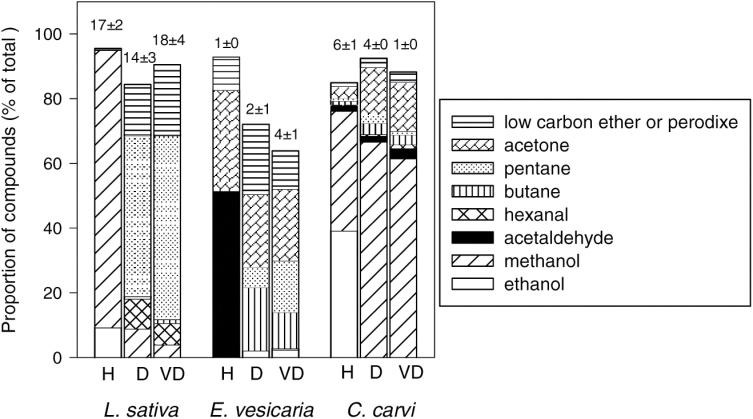
Fig. 3.
Volatile emissions by L. sativa, E. vesicaria, and C. carvi seeds during storage at 35 °C and three moisture treatments: Humid (H, 0.099–0.131g g–1), Dry (D, 0.042–0.063g g–1), and Very Dry (VD, 0.030–0.039g g–1). Bars represent average quantities (in percentage over total quantity) among storage times (<1200 days or when monitoring stopped) of major components: ethanol, methanol, acetaldehyde, hexanal, butane, pentane, acetone and a low-carbon ether or peroxide. Values above the bars are the average of total quantity of VOCs emitted (nmol g−1). Total VOCs included all detected compounds except terpenes; bars do not sum to 100% because amounts of individual minor compounds are not shown.
In order to confirm the consistent prominence of certain analytes, despite variation in the total amount (Fig. 2E, F), we analyzed GC profiles by the quantity of compound (nmol g–1) relative to the total quantity of compounds in the sample. For this analysis, we pooled data from samples that had been stored for ≤1200 days (L sativa and E. vesicaria) or upon completion of the germination time course (C. carvi), a treatment that we felt was justified because there were no perceived time-trends in VOC composition during this period (data not shown). The types of compounds emitted from seeds varied among species and moisture treatments. A few compounds were added to the list already published for lettuce (Mira et al., 2010) as a result of a higher sensitivity threshold. Alcohols were the major constituent in the airspace above humid-stored L. sativa seeds (Fig. 2D, water content 0.099g g−1), with ethanol and methanol comprising ~90% of detected molecules (Fig. 3). The VOC profile of dry-stored L. sativa seeds was markedly different from that of those exposed to the humid treatment, with low alcohol content, high aldehyde and alkane content, and several minor molecules (water content ≤0.042g g−1, Fig. 2E, F; see also Fig. 3). Pentane, hexanal, and an as yet unidentified molecule with low RT, believed to be a short-chain ether or peroxide, were consistently detected in dry L. sativa samples. The headspace composition of vials containing E. vesicaria seeds differed substantially from profiles from L. sativa seeds (Fig. 3), with acetaldehyde and acetone comprising over 83% of detected VOCs in the humid treatment. Acetaldehyde was replaced by butane and pentane in VOCs above dry E. vesicaria (Fig. 3), and compounds such as butanal or butanone appeared and were more pronounced than was observed from L. sativa. Most of the compounds emitted by C. carvi were terpenes and terpenoids (e.g. carvone, limonene, α-pinene, β-pinene) (Fig. 2D–F), which are interpreted as flavor compounds rather than degradation products per se (Tammela et al., 2003). To facilitate observations of molecules believed to be associated with changes in seed quality, the concentrations of terpenes were subtracted from the total VOCs measured and are omitted from further analyses. Once this correction was made, it was clear that, as for L. sativa seeds, methanol and ethanol were prevalent compounds in the headspace above humid-stored C. carvi seeds, and, unlike L. sativa seeds, methanol comprised a large proportion of VOC emission in dry-stored C. carvi seeds (Fig. 3). During dry storage, this species also emitted butane, pentane, acetone, and the unidentified short-chain ether or peroxide that was also observed for E. vesicaria and dry L. sativa seeds. For all three species, ~10% of total VOCs from dry-stored seeds was composed of several minor compounds.
Initial accumulation of total VOCs (except terpenes) was characterized for each seed species and moisture environment (Fig. 4). Under humid conditions, VOC levels increased linearly with storage time, and the slope for C. carvi seeds was greater than those for the other two species (slopes were 1.42, 0.42, and 0.02 nmol g−1 day−1 for C. carvi, L. sativa, and E. vesicaria seeds, respectively) (Fig. 4A). VOC emission continued unabated even after all seeds had died. For the dry treatment, VOC emission increased with storage time for L. sativa and E. vesicaria seeds throughout the storage period, although data are fairly scattered (Fig. 4B; data for L. sativa and E. vesicaria seeds at storage time >500 days are not shown). VOC emission appeared to level off in vials containing C. carvi seeds between 100 and 320 days, when monitoring ended (Fig. 4B). Calculations of slopes suggested comparable emission rates for C. carvi and L. sativa (0.02 and 0.03 nmol g−1 day−1, respectively) and considerably slower emission for E. vesicaria seeds (0.002 nmol g−1 day−1; Fig. 4B). For the very dry treatment, VOC emission increased with storage time (Fig. 4C; data for all species not shown at storage time >1000 days). The emission rate for very dry C. carvi seeds was much reduced compared with emission rates under other moisture treatments (slope was 0.003 nmol g−1 day−1) and was comparable to emission rates for E. vesicaria seeds at this moisture level (slope 0.003 nmol g−1 day−1). VOC data for very dry L. sativa were highly scattered, but suggested faster emission in very dry compared with dry storage (slope was 0.06 nmol g−1 day−1), contrasting with observations made for very dry C. carvi or E. vesicaria seeds.
Fig. 4.
Time course for total production of volatile compounds (except terpenes) emitted by L. sativa, E. vesicaria, and C. carvi seeds stored at 35 °C and three moisture treatments: (A) Humid (0.099–0.131g g–1), (B) Dry (0.042–0.063g g–1), and (C) Very Dry (0.030–0.039g g–1). Values correspond to the slope for each species and treatment.
To test the hypothesis that volatile production from seeds affected longevity, we compared viability within seed mixtures of L. sativa and either E. vesicaria or C. carvi. The longevity of seeds was not significantly affected by the presence of another species in most cases (Fig. 5; also compare P75 values in Table 3 and those for E. vesicaria and C. carvi in Table 2). Results for L. sativa seeds in mixtures with E. vesicaria were inconsistent, showing slightly beneficial effects of mixing under humid conditions (P≤0.0005), slightly detrimental effects under dry conditions, and beneficial effects under very dry conditions (Fig. 5, Table 3). There is high uncertainty in the longevity values for L. sativa alone (very dry treatment, Fig. 5C) and in combination with C. carvi (dry treatment, Fig. 5B), because these were extrapolated from sampling times <1000 days.
Fig. 5.
Changes in percentage germination of L. sativa when stored alone (solid curve) and in the presence of E. vesicaria (long dash) or C. carvi (short dash) seeds at 35 °C and three moisture treatments: (A) Humid (0.090–0.086g g–1); (B) Dry (0.048–0.042g g–1), and (C) Very Dry (0.033–0.029g g–1). Data were fitted to a logistic regression model and values for P75 and P50 were calculated (Table 3). The dotted line in (C) corresponds to a different extrapolation of longevity for the same dataset. Water contents are for L. sativa seeds only and there is slight variation between seeds alone and in mixtures.
Table 3.
Kinetics of deterioration in seed samples of pure and mixed mixtures stored at 35 °C and different water contents. Deterioration is expressed as the time for seed quality to decrease to 75 and 50% of initial germination (P75 and P50, respectively). Time course data for L. sativa seeds (2004 cohort) is provided in Fig. 5. Data for unmixed L. sativa seeds are from Mira et al. (2010) with additional sample times for the dry treatment added
| Treatment | Species/mixture | Water content (g g–1 dw±SE) | Seed longevity (days±SE) | |
|---|---|---|---|---|
| P75 | P50 | |||
| L. sativa | 0.089 | 16±4 | 27±4 | |
| L. sativa (+E. vesicaria) | 0.086 | 25±1 | 35±1 | |
| L. sativa (+C. carvi) | 0.090 | 19±1 | 25±1 | |
| E. vesicaria (+L. sativa) | 0.102 | 42±1 | 55±1 | |
| C. carvi (+L. sativa) | 0.125 | 0.3±0.1 | 0.9±0.1 | |
| Dry | L. sativa | 0.046 | 550±67 | 1 056±109 |
| L. sativa (+E. vesicaria) | 0.046 | 445±29 | 675±34 | |
| L. sativa (+C. carvi) | 0.048 | 643±175 | 1 108±331 | |
| E. vesicaria (+L. sativa) | 0.048 | 1 178±66 | 1 703±73 | |
| C. carvi (+L. sativa) | 0.064 | 7±6 | 76±4 | |
| Very dry | L. sativa | 0.030 | 567±70 | 882±18a |
| L. sativa (+E. vesicaria) | 0.029 | 3 021±753 | 4 470±1 185 | |
| L. sativa (+C. carvi) | 0.033 | 2 341±475 | 3 407±723 | |
| E. vesicaria (+L. sativa) | 0.036 | 2 281±132 | 3 379±203 | |
| C. carvi (+L. sativa) | 0.043 | 61±17 | 225±17 | |
a This value was obtained by extrapolating a logistic model beyond the last sampling time of 550 days (Mira et al., 2010).
Although longevity in seed mixtures appeared mostly unaffected, the composition of compounds in the headspace was different in vials containing a combination of seed species compared with vials containing a single species. The total amount of compounds detected in the headspace when L. sativa seeds were mixed was not additive as we expected, but rather was characteristic of E. vesicaria or C. carvi seeds alone (most treatments) or intermediate between L. sativa and C. carvi (humid treatment; compare numbers above bars in Fig. 3 and Fig. 6). VOC composition reflected a mixture of prevalent molecules from both species averaged in the airspace of the mixture (Fig. 6). Two exceptions to this observation are noted: (i) a complete loss of the methanol signal (from L. sativa) in the humid and dry treatments of L. sativa mixed with E. vesicaria seeds, and (ii) an increase in minor compounds in the very dry L. sativa+C. carvi mixture (Fig. 6). Another notable difference in VOC composition in L. sativa+C. carvi mixtures was the diminished presence of terpenes, which were highly abundant in C. carvi-only samples (data not shown).
Fig. 6.
Volatile emissions from seed mixtures containing L. sativa+E. vesicaria and L. sativa+C. carvi seeds stored together at 35 °C and three moisture treatments as described in Fig. 5. Bars represent the average quantities of major components of the volatile profile: ethanol, methanol, acetaldehyde, hexanal, butane, pentane, acetone and a low-carbon ether or peroxide. Values above the bars are total VOC levels (nmol g−1) averaged for storage times less than 1200 days. Total VOCs included all detected compounds except terpenes; bars do not sum to 100% because amounts of individual minor compounds are not shown.
Discussion
In this paper, we address the problem of characterizing the nature and kinetics of reactions that occur in dry biological systems. The problem is difficult because reactions tend to be slow (detection time is days to years) and because subtle chemical changes are difficult to discern through small changes in substrates. Further, dry biological systems are amorphous solids in which the reaction rate is typically regulated by both the concentration of substrate and structural relaxation or localized organization of substrates or reaction sites. This interaction confounds direct correlations between kinetics and substrate levels or the presence of catalysts (Walters et al., 2010). Characterizing the nature and kinetics of reactions in dry systems is important in order to understand slow changes in physiology in organisms that are typically regarded as inert. The practical goal of this research is to gain better insight into the mechanisms by which seeds deteriorate and eventually die during dry storage.
To focus on reaction products, rather than substrates, and to exploit the rare opportunity of using water as an experimental variable rather than a component of the analysis, we explored the production of VOCs from seeds. Analysis of VOCs in breathalyzer tests for animals is increasingly being used to identify reactions associated with aging or pathology (Aldini et al., 2010; Grotto et al., 2009; Hartmut et al., 1980). VOC analysis accommodates small sample size and does not require destructive sampling of tissues. In a previous study using L. sativa seeds, we established prominent VOCs produced by these seeds under humid and dry conditions and demonstrated that drying to ~30% RH induces a switch away from fermentation-type reactions and towards peroxidation-type reactions (Mira et al., 2010). We also suggested that lower rates of VOC production in seeds dried to 30% RH reflected the loss of molecular mobility in solidifying cellular matrices. Questions arising from that study include the apparent increase in peroxidation products as seeds are progressively dried below 30% RH and the poor correlation between peroxidation products and rate of aging, despite current wisdom that aging is a consequence of oxidation (Bailly, 2004; Groot et al., 2015; Job et al., 2005; Kranner et al., 2006; McDonald, 1999; Walters, 1998; Walters et al., 2010). In addition, other laboratories studying VOCs emitted from seeds (Colville et al., 2012; Zhang et al., 1993, 1995a) have indicated a broader array of molecules than we previously detected. Our goal in the present study was to address some of these questions by using additional species and greater sensitivity in our GC methods.
The VOC analysis technique is fraught with problems of sensitivity and interpretation. Dynamic interactions among compounds emitted into the headspace influence the interpretation of the nature and kinetics of reactions occurring in the solid matrix below (Aldini et al., 2010; Grotto et al., 2009; Halliwell and Chirico, 1993). For example, ethanol and acetaldehyde may interconvert through alcohol dehydrogenase, and pentane might convert to pentanol. Molecules may be highly unstable and change rapidly, leading to multiple or undetected signals. Some byproducts of lipid peroxidation, such as malondialdehyde and 4-hydroxynonenal, are so unstable that derivatization to a more stable compound is required for detection (Aldini et al., 2010; Grotto et al., 2009; Halliwell and Chirico, 1993; Zhang et al., 1993). In addition, it is difficult to give unambiguous assignments of isomers with similar RTs, which occurs more frequently as RT and molecular mass increase (Mendis et al., 1994). Very small molecules (e.g. alkanes and ethers with three or fewer carbon atoms) were not detectable using our protocols because they elute in the void volume.
Quantification of VOCs produced by chemical reactions in seeds is confounded by adsorption–desorption relationships between VOCs and molecular interfaces. Cells naturally contain compounds and structures analogous to solid-phase microextraction fibers that trap VOCs, and we should expect these interactions to influence VOC quantification. Early work has considered the kinetics of VOC production and sorption/desorption in animal (Harmut et al., 1980) and seed (Zhang et al., 1995a) cells, but more work is needed to accurately model the partitioning of VOC products between solids and airspaces of storage containers. In this study, we demonstrate that some seeds, such as E. vesicaria or C. carvi, can adsorb VOCs produced by L. sativa seeds and effectively lower the VOC concentrations in the airspace (compare the total emission values in Fig. 6 with those in Fig. 3). From this experiment, we conclude that accurate quantification of VOC production in seeds must include considerations of sorption–desorption dynamics. The seminal literature in this regard comes from studies of seed fumigation (Lubatti and Smith, 1948). In general, our study supports work showing a relationship between storage atmosphere and changes in physiology (Gonzalez-Benito et al., 2011; Groot et al., 2015; Halliwell and Chirico, 1993), but there is a need for more in-depth analyses to establish the bases for these relationships.
VOC analyses reflect the most prominent types of reactions, but these may not be the most physiologically relevant. For example, the high production of pentane in L. sativa seeds (Fig. 3) indicates peroxidation of linoleic acid (Frankel, 1983; Knutson et al., 2000). High rates of this reaction probably reflect the high level of available substrate in the form of storage reserves in the seed (Table 1) rather than a reaction with pathological consequences. That said, pentane production might reflect the fluidity of lipid bodies and, if so, suggests an interesting probe of the non-aqueous environment within seeds and water interactions. Future experiments will monitor the kinetics of alkane production in seeds with varying lipid content and liposome structure and in response to temperature changes, with the goal of describing reaction kinetics as non-aqueous cellular components solidify. It may be that the minor constituents of the GC profile more directly describe aging or pathological reactions in seeds. Degradation of substrates in lower abundance than storage reserves would appear as minor compounds. These are more difficult to characterize because they are barely detectable and appear inconsistently among chromatograms. Previously, we overlooked many of these molecules as noise in the chromatogram baseline. Indeed, baselines become increasingly ‘messy’ as storage time progresses (data not shown).
Previously, we reported high production of ethanol and methanol in L. sativa seeds, diminishing emission rates as seed moisture decreased from 0.09 to 0.05g H2O g−1 dw, and a strong correlation between emission rate and aging rate within this range of moisture content (Mira et al., 2010). We postulated that these molecules arose from fermentation-type reactions and that emission kinetics reflected decreasing molecular mobility within drying seeds. In the present study, we investigated whether these fermentation products are ubiquitously produced in seeds in the humid to dry moisture range and whether rate of emission corresponds with aging rate. Methanol and ethanol production was also observed in C. carvi seeds (Fig. 3) and has previously been reported in other species (Bicanic et al., 2003; Buckley and Buckley, 2009; Colville et al., 2012; Kodde et al., 2012; Lee et al., 2000; Min, 2012; Mira et al., 2010; Rutzke et al., 2008; Taylor et al., 1999; Zhang et al., 1993, 1994, 1995b; Zhang and Roos, 1997). In contrast, seeds of E. vesicaria produced acetaldehyde and acetone under humid conditions (Fig. 3), molecules that have been linked to fermentation of pyruvate in single-cell organisms, especially in low-oxygen and high-metal contexts (Jardine et al., 2010; Jensen, 1976; Lehninger et al., 1993). Differences in fermentation products (if these reactions are, indeed, related to fermentation) among species suggest different substrates or modes of catalysis; both possibilities require further investigation to gain insights about the nature of reactions in drying seeds. Emission of the supposed fermentation products was fastest for C. carvi under humid conditions (Fig. 4A), and this species was also the fastest to age among our study samples (Fig. 1A); however, L. sativa and E. vesicaria aged at similar rates and had considerably different emission rates.
The presence of ethanol and methanol in the headspace above stored seeds has been implicated as a marker of seed quality for some species (Buckley and Buckley, 2009; Kodde et al., 2012; Rutzke et al., 2008; Taylor et al., 1999; Zhang et al., 1993, 1994, 1995b; Zhang and Roos, 1997). However, we do not believe these molecules are a direct cause of poor quality. E. vesicaria adsorbed VOCs produced by L. sativa, and there was no detrimental effect on longevity (Tables 2 and 3). The slight improvement in longevity of L. sativa seeds when mixed with E. vesicaria seeds (Fig. 5A) may have arisen from E. vesicaria scavenging these molecules or from the slight drying that was also indicated.
The distinct change in the VOC profile when L. sativa and E. vesicaria seeds were dried to <0.065g g−1 water content (the dry and very dry treatments; Figs 2 and 3) suggest a switch in the types of reactions that occur. We suggest this arises from reactions in aqueous domains of seed cells becoming increasingly restricted and reactions in lipid domains becoming increasingly facilitated as seeds dry. The continued emission of methanol from dry and very dry C. carvi seeds suggests a different response to drying, and this might contribute to the overall poor longevity of seeds of this species.
We present longevity data for two cohorts of L. sativa (cv. ‘Black Seeded Simpson’) seeds that differ substantially (Tables 2 and 3) even though storage conditions were similar. We suggest that the primary reasons for these differences are that (i) the 2004 cohort was stored for 3 years before it was used, while the 2009 cohort was used within 6 months of harvest, and (ii) there is uncertainty in longevity predictions when storage time is considerably less than response time. Extending storage time data for the dry treatment (Table 3) increased P75 and P50 estimates compared with those reported by Mira et al. (2010), and we would expect to observe a similar effect on the longevity estimates of the very dry treatment if data for extended times were available. Other minor effects include variation in storage condition, maternal effects, post-harvest handling differences, and intrinsic variation in longevity values as longevity increases.
Conclusions
Emission of volatile compounds is a dynamic process that involves chemical reactions, mobility of molecules within drying cells, and sorption/desorption processes. Observed differences in VOC emission among species can be attributed to substrate levels in seeds and varying responses to drying. Different suites of molecules were detected in seeds stored under humid and dry conditions and appear to reflect decreasing propensity for fermentation-type reactions and increasing propensity for triacylglycerol degradation in aqueous and non-aqueous domains, respectively, as cells dry. Our study suggests an indirect association of fermentation-type reactions, but no association of triacylglycerol oxidation, with seed longevity. Longevity was mostly unaffected in mixtures of seeds emitting different VOCs, suggesting that the molecules we detected were not damaging. Our study shows differences among seeds in the adsorption of VOCs on to molecular matrices, mechanisms of fermentation-type reactions under water-stress conditions, and possible effects of moisture on lipid body behavior. Understanding these differences may provide greater insight into the mechanisms by which dry seeds succumb with time.
Acknowledgements
This work was supported by the project CGL2006-10536 (Ministerio de Educación, Spain). SM was supported by the FPU program (Ministerio de Educación, Spain), a grant from the Consejo Social (Universidad Politécnica de Madrid), and by the Jose Castillejo program (Ministerio de Educación, Spain).
References
- Aldini G, Yeum KJ, Niki E, Russell RM. 2010. Biomarkers for antioxidant defense and oxidative damage: principles and practical applications . Ames: Wiley-Blackwell. [Google Scholar]
- Akimoto T, Cho SY, Yoshida H, Furuta H, Esashi Y. 2004. Involvement of acetaldehyde in seed deterioration of some recalcitrant woody species through the acceleration of aerobic respiration. Plant and Cell Physiology 45, 201–210. [DOI] [PubMed] [Google Scholar]
- Bailly C. 2004. Active oxygen species and antioxidants in seed biology. Seed Science Research 14, 93–107. [Google Scholar]
- Bicanic D, Persijn S, Taylor AG, Cozijnsen J, van Veldhuyzen B, Lenssen G, Wegh H. 2003. Detection of ethanol and acetaldehyde released from cabbage seeds of different quality: Laser photoacoustic spectroscopy versus FTIR and headspace gas chromatography. Review of Scientific Instruments 74, 689–693. [Google Scholar]
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Buckley WT, Buckley KE. 2009. Low-molecular-weight volatile indicators of canola seed deterioration. Seed Science and Technology 37, 676–690. [Google Scholar]
- HWS Chan. (ed). 1987. Autoxidation of unsaturated lipids . London: Academic Press. [Google Scholar]
- Colville L, Bradley EL, Lloyd AS, Pritchard HW, Castle L, Kranner I. 2012. Volatile fingerprints of seeds of four species indicate the involvement of alcoholic fermentation, lipid peroxidation, and Maillard reactions in seed deterioration during ageing and desiccation stress. Journal of Experimental Botany 63, 6519–6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisia I, Kitts DD. 2011. Quantification of hexanal as an index of lipid oxidation in human milk and association with antioxidant components. Journal of Clinical Biochemistry and Nutrition 49, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding JL, Goldsworthy A. 1982. The evolution of volatiles in relation to ageing in dry wheat seed. Seed Science and Technology 10, 277–282. [Google Scholar]
- Frankel EN. 1983. Volatile lipid oxidation-products. Progress in Lipid Research 22, 1–33. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Benito ME, Perez-Garcia F, Tejeda G, Gomez-Campo C. 2011. Effect of the gaseous environment and water content on seed viability of four Brassicaceae species after 36 years storage. Seed Science and Technology 39, 443–451. [Google Scholar]
- Groot SPC, de Groot L, Kodde J, van Treuren R. 2015. Prolonging the longevity of ex situ conserved seeds by storage under anoxia. Plant Genetic Resources 13, 18–26. [Google Scholar]
- Grosch W. 1987. Reactions of hydroperoxides – products of low molecular weight. In: Chan HWS, ed. Autoxidation of unsaturated lipids . London: Academic Press, 95–139. [Google Scholar]
- Grotto D, Santa Maria L, Valentini J, Paniz C, Schmitt G, Garcia SC, Juarez Pomblum V, Rocha JBT, Farina M. 2009. Importance of the lipid peroxidation biomarkers and methodological aspects FOR malondialdehyde quantification. Química Nova 32, 169–174. [Google Scholar]
- Hailstones MD, Smith MT. 1989. Thermally-derived volatile aldehydes in relation to seed viability in soybean seeds. Seed Science and Technology 17, 649–658. [Google Scholar]
- Halliwell B, Chirico S. 1993. Lipid peroxidation: its mechanism, measurement, and significance. American Journal of Clinical Nutrition 57, 715–724. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. 1999. Free radicals in biology and medicine . Oxford: Oxford University Press. [Google Scholar]
- Hartmut F, Hintze T, Bimboes D, Remmer H. 1980. Monitoring lipid peroxidation by breath analysis: endogenous hydrocarbons and their metabolic elimination. Toxicology and Applied Pharmacology 56, 337–344. [DOI] [PubMed] [Google Scholar]
- Jardine KJ, Sommer ED, Saleska SR, Huxman TE, Harley PC, Abrell L. 2010. Gas phase measurements of pyruvic acid and its volatile metabolites. Environmental Science and Technology 44, 2454–2460. [DOI] [PubMed] [Google Scholar]
- Jensen RA. 1976. Enzyme recruitment in evolution of new function. Annual Review of Microbiology 30, 409–425. [DOI] [PubMed] [Google Scholar]
- Job C, Rajjou L, Lovigny Y, Belghazi M, Job D. 2005. Patterns of protein oxidation in Arabidopsis seeds during germination. Plant Physiology 138, 790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodde J, Buckley WT, de Groot CC, Retiere M, Zamora AMV, Groot SPC. 2012. A fast ethanol assay to detect seed deterioration. Seed Science Research 22, 55–62. [Google Scholar]
- Knutson MD, Handelman GJ, Viteri FE. 2000. Methods for measuring ethane and pentane in expired air from rats and humans. Free Radical Biology and Medicine 28, 514–519. [DOI] [PubMed] [Google Scholar]
- Kranner I, Birtic S, Anderson KM, Pritchard HW. 2006. Glutathione half-cell reduction potential: a universal stress marker and modulator of programmed cell death? Free Radical Biology and Medicine 40, 2155–2165. [DOI] [PubMed] [Google Scholar]
- Kranner I, Minibayeva FV, Beckett RP, Seal CE. 2010. What is stress? Concepts, definitions and applications in seed science. New Phytologist 188, 655–673. [DOI] [PubMed] [Google Scholar]
- Labuza TP. 1980. The effect of water activity on reaction kinetics of food deterioration. Food Technology 34, 36–41. [Google Scholar]
- Lee P-C, Taylor AG, Zhang M, Esashi Y. 2000. Volatile compounds and accumulation of acetaldehyde-protein adducts in relation to seed quality and storage conditions. Journal of New Seeds 2, 59–76. [Google Scholar]
- Lehninger AL, Nelson DL, Cox MM. 1993. Principles of biochemistry . New York: Worth Publishers. [Google Scholar]
- Lubatti OP, Smith B. 1948. Determination of fumigants. XIX. Sorption of methyl bromide by onion seed. Journal of the Society of Chemical Industry 67, 297–309. [Google Scholar]
- McDonald MB. 1999. Seed deterioration: physiology, repair and assessment. Seed Science and Technology 27, 177–237. [Google Scholar]
- Mendis S, Sobotka PA, Euler DE.1994. Pentane and isoprene in expired air from humans: gas-chromatographic analysis of single breath. Clinical Chemistry 40, 1485–1488. [PubMed] [Google Scholar]
- Metcalfe LD, Schmitz AA. 1961. The rapid preparation of fatty acid esters for gas chromatographic analysis. Analytical Chemistry 33, 363–364. [Google Scholar]
- Min TG. 2012. Detection of ethanol released from aged radish (Raphanus sativus L.) seeds using resazurin. Horticulture Environment and Biotechnology 53, 66–71. [Google Scholar]
- Mira S, González-Benito ME, Hill LM, Walters C. 2010. Characterization of volatile production during storage of lettuce (Lactuca sativa) seeds. Journal of Experimental Botany 61, 3915–3924. [DOI] [PubMed] [Google Scholar]
- Obendorf RL, Koch JL, Gorecki RJ, Amable RA, Aveni MT. 1990. Methanol accumulation in maturing seeds. Journal of Experimental Botany 41, 489–495. [Google Scholar]
- Orhan H, Gurer-Orhan H, Vriese E, Vermeulen NP, Meerman JH. 2006. Application of lipid peroxidation and protein oxidation biomarkers for oxidative damage in mammalian cells. A comparison with two fluorescent probes. Toxicology in Vitro 20, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Pignoli G, Bou R, Rodriguez-Estrada MT, Decker EA. 2009. Suitability of saturated aldehydes as lipid oxidation markers in washed turkey meat. Meat Science 83, 412–416. [DOI] [PubMed] [Google Scholar]
- Rasband WS. 1997. –2008. ImageJ . Bethesda: US National Institutes of Health; http://rsb.info.nih.gov/ij/. [Google Scholar]
- R Core Team 2015. R: a language and environment for statistical computing . Vienna: R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- Rodríguez R, Sánchez Tamés R, Durzan DJ. 1989. Plant aging: basic and applied approaches . Ribadesella: NATO Advanced Study Institute on Molecular Basic of Plant Aging. [Google Scholar]
- Rutzke CFJ, Taylor AG, Obendorf RL. 2008. Influence of aging, oxygen, and moisture on ethanol production from cabbage seeds. Journal of the American Society for Horticultural Science 133, 158–164. [Google Scholar]
- Schwember AR, Bradford KJ. 2005. Drying rates following priming affect temperature sensitivity of germination and longevity of lettuce seeds. Hortscience 40, 778–781. [Google Scholar]
- Smith MT, Adamson IH. 1989. Volatile lipid peroxidation breakdown products and viability in lettuce (Lactuca sativa L.). South African Journal of Science 85, 63–64. [Google Scholar]
- Tammela P, Nygren M, Laakso I, Hopia A, Vuorela H, Hiltunen R. 2003. Volatile compound analysis of ageing Pinus sylvestris L. (Scots pine) seeds. Flavour and Fragrance Journal 18, 290–295. [Google Scholar]
- Taylor AG, Lee PC, Zhang M. 1999. Volatile compounds as indicators of seed quality and their influence on seed aging. Seed Technology 21, 57–65. [Google Scholar]
- Trawatha SE, TeKrony DM, Hildebrand DF. 1995. Relationship of soybean seed quality to fatty acid and C6-aldehyde levels during storage. Crop Science 35, 1415–1422. [Google Scholar]
- Vertucci CW, Roos EE. 1993. Theoretical basis of protocols for seed storage II. The influence of temperature on optimal moisture levels. Seed Science Research 3, 201–213. [Google Scholar]
- Walters C. 1998. Understanding the mechanisms and kinetics of seed aging. Seed Science Research 8, 223–244. [Google Scholar]
- Walters C, Ballesteros D, Vertucci VA. 2010. Structural mechanics of seed deterioration: standing the test of time. Plant Science 179, 565–573. [Google Scholar]
- Walters C, Hill LM, Wheeler LJ. 2005. Dying while dry: kinetics and mechanisms of deterioration in desiccated organisms. Integrative and Comparative Biology 45, 751–758. [DOI] [PubMed] [Google Scholar]
- Wiley/NIST 2008. Wiley registry of mass spectral data, with NIST 2008 , 8th edition Hoboken: John Wiley & Sons. [Google Scholar]
- Wilson DO, McDonald MB. 1986. A convenient volatile aldehyde assay for measuring soybean seed vigor. Seed Science and Technology 14, 259–268. [Google Scholar]
- Zhang M, Liu Y, Torii I, Sasaki H, Esashi Y. 1993. Evolution of volatile compounds by seeds during storage periods. Seed Science and Technology 21, 359–373. [Google Scholar]
- Zhang M, Maeda Y, Furihata Y, Nakamaru Y, Esashi Y. 1994. A mechanism of seed deterioration in relation to the volatile compounds evolved by dry seeds themselves. Seed Science Research 4, 49–56. [Google Scholar]
- Zhang M, Nakamaru Y, Tsuda S, Nagashima T, Esashi Y. 1995. b. Enzymatic conversion of volatile metabolites in dry seeds during storage. Plant and Cell Physiology 36, 157–164. [Google Scholar]
- Zhang M, Roos EE. 1997. Using seed volatile as a possible indicator for seed deterioration during storage. Hortscience 32, 526. [Google Scholar]
- Zhang M, Yajima H, Umezawa Y, Nakagawa Y, Esashi Y. 1995. a. GC-MS identification of volatile compounds evolved by dry seeds in relation to storage-conditions. Seed Science and Technology 23, 59–68. [Google Scholar]