Highlight
The molecular mechanisms underlying the elevated inducibility of stilbene in pathogen-resistant Vitis sylvestris can be explained by the increased inducibility of the MYB14 promoter.
Key words: flg22, grapevine (V. sylvestris), MYB14, Plasmopara viticola, stilbene synthase, UV.
Abstract
Stilbene synthase is a key enzyme for the production of the phytoalexin resveratrol. Some clones of Vitis sylvestris, a wild European grapevine species which is almost extinct, have been shown to accumulate more resveratrol in response to different forms of stress. In the current study, we asked whether the induction of stilbene synthase transcripts in Hoe29, one of the V. sylvestris clones with elevated stilbene inducibility, might result from the elevated induction of the transcription factor MYB14. The MYB14 promoter of Hoe29 and of Ke83 (a second stilbene-inducible genotype) harboured distinct regions and were applied to a promoter–reporter system. We show that stilbene synthase inducibility correlates with differences in the induction of MYB14 transcripts for these two genotypes. Both alleles were induced by UV in a promoter–reporter assay, but only the MYB14 promoter from Hoe29 was induced by flg22, consistent with the stilbene synthase expression of the donor genotypes, where both respond to UV but only Hoe29 is responsive to Plasmopara viticola during defence. We mapped upstream signals and found that a RboH-dependent oxidative burst, calcium influx, a MAPK cascade, and jasmonate activated the MYB14 promoter, whereas salicylic acid was ineffective. Our data suggest that the Hoe29 allele of the MYB14 promoter has potential as a candidate target for resistance breeding.
Introduction
Grapevine is an economically important crop, which is highly susceptible to various biotic diseases, and, therefore, requiring extensive plant protection measures. Viticulture accounts for 70% of the European fungicide consumption. In order to get the ‘pure wine’ with fewer chemicals, more environmentally friendly approaches are warranted. An important element for sustainable viticulture is resistance breeding using resistance factors originating from North American grapes (Gessler et al., 2011).
These resistance factors have to be understood in context with the complex evolution of plant immunity: The plant innate immune system is composed of two distinct layers (Jones and Dangl, 2006). The first layer exploits ubiquitous molecules in pathogenic microorganisms, termed pathogen-associated molecular patterns (PAMPs), to recognize pathogen attack by receptors at the plasma membrane and to activate a basal, so-called PAMP triggered immunity, PTI (Jones and Dangl, 2006; Boller and He, 2009). Most of these PAMPs are essential for the life cycle of the invader, such that the PAMPs are preserved. Pathogens that have undergone coevolution with their hosts, have usually developed an alternative strategy: They can quell PTI by injecting chemical signals, so-called effectors, into the host cell and thus reinstate pathogenicity, which is termed effector-triggered immunity, ETI (Takken and Tameling, 2009). This ETI has evolved during a long battle between the pathogen and the host plant. The molecular mechanisms underlying these two layers of plant immunity differ, but can also overlap (Thomma et al., 2011), which contributes to the complexity of the plant–pathogen interaction.
The economically important grapevine pathogen Downy Mildew (Plasmopara viticola) has co-evolved over millions of years together with wild American grapes, such that these wild grapes had enough time to evolve ETI and, therefore, can cope with these pathogens. The recent discovery of Plasmopara viticola strains that can infect specific grapevine genotypes (Rouxel et al., 2013; Gómez-Zeledón et al., 2013) support the hypothesis that the resistance of these American grapes is based upon a canonical ETI. However, these wild grapes are not suited for winery, due to their unpleasant ‘foxy taste’ (off-flavour). As a strategy to separate the desired immunity from the undesired flavour, those wild grapes have been crossed with cultivated grape varieties. This strategy has been successful and has culminated in economically important new varieties with good resistance against downy mildew (P. viticola). However, the rising success of these new varieties is expected to initiate the next round of the evolutionary warfare. In fact, the resistance conferred by the genetic factor ‘Resistance to P. viticola 3 (Rpv3)’ which forms the base of most current disease-resistant grapevine varieties, has already been observed to become eroded by new strains of Plasmopara (Peressotti et al., 2010; Gessler et al., 2011; Gómez-Zeledón et al., 2013).
As a strategy to render the success of resistance breeding more sustainable, new sources of resistance are required. In this context, it might also be rewarding to exploit factors stimulating the basal immunity (PTI). The ancestor of cultivated grapevine, V. vinifera L. ssp. vinifera, the European Wild Grape (V. vinifera L. ssp. sylvestris Hegi) lacks a history of coevolution with these pathogens and, therefore, should not harbour any ETI-like defence against downy mildew. Nevertheless, many genotypes of the European Wild Grape show good tolerance against several grapevine diseases (Tisch et al., 2014), such as downy mildew (P. viticola), powdery mildew (Erysiphe necator), and black rot (Guignardia bidwelli), which means that some genotypes of V. sylvestris might command a more efficient basal immunity. In fact, during previous work, we have identified several genotypes of V. sylvestris with a strong induction of antimicrobial stilbenes in response to either a short pulse of UV irradiation or to infection with P. viticola (Duan et al., 2015) indicating that early signals shared between these two stress factors must be involved. These genotypes were also endowed with a partial resistance to downy mildew and, therefore, have potential as new breeding resources to be exploited for sustainable viticulture in the future.
Stilbenes, as important phytoalexins, are a central factor for the basal immunity of grapevine. Transcription of the key enzyme stilbene synthase (StSy) can be activated by the PAMP flg22 (Chang and Nick, 2012) as part of PTI, but also by the bacterial trigger Harpin (mimicking an ETI-like pattern of defence). Although both immunity responses culminate in an accumulation of stilbene synthase transcripts and share a part of early signalling events, they differ in perception, the role of oxidative burst, and integration into a qualitatively different output of stilbene metabolites. The transcriptional regulation of the stilbene biosynthetic pathway is mediated by two R2R3-MYB-type transcription factors, MYB14 and MYB15 that were shown to activate the stilbene synthase promoter (Höll et al., 2013). In our previous work (Duan et al., 2015), the V. sylvestris genotypes Hoe29 and Ke83 were found to be endowed with an elevated stilbene inducibility in response to UV light, which was correlated with a strong induction of stilbene synthase transcripts. Interestingly, in response to inoculation with P. viticola, stilbene synthase transcripts were elevated for Hoe29, but not for Ke83.
In the current study, we test the idea whether the strong inducibility of stilbene synthase transcripts in Hoe29 might result from the elevated induction of MYB14 and MYB15. Specific differences were discovered by Illumina-based next-generation sequencing and confirmed by cloning for the MYB14 promoter of Hoe29, whereas MYB15 did not reveal obvious changes. Both sylvestris genotypes shared certain MYB14 promoter domains (whereas they differ in others), which were absent from the promoter of the cultivated variety Augster Weiss (that is a weak stilbene producer). Using a promoter–reporter assay in grapevine suspension cells (Höll et al., 2013), we show that differences in the inducibility of the MYB14 promoter can account for the stress-specific differences in the expression of stilbene synthase observed between the three genotypes (Hoe29, Ke83, and Augster Weiss). Although both sylvestris genotypes show good UV inducibility of MYB14, only the Hoe29 allele of this promoter is competent for induction by PTI (triggered by flg22). We also mapped known signalling events for PTI, such as dependence on NADPH oxidase (RboH) or induction by jasmonic acid, and calcium influx. Our data suggest that a particular region in the promoter of a specific sylvestris allele of MYB14 harbours potential as a candidate target for resistance breeding.
Materials and methods
Plant materials
The Vitis vinifera ssp. sylvestris genotypes ‘Ke83’ and ‘Hoe29’, as well as the V. vinifera cultivar Augster Weiss used in this study, have already been described in Ledesma-Krist et al. (2014) and Duan et al. (2015). All accessions are maintained as living specimens in the grapevine collection of the Botanical Garden of the Karlsruhe Institute of Technology (Olmo, 1976). For DNA and RNA extraction, the leaves were harvested from greenhouse-grown plants cultivated at a day/night temperature of 22/18 °C and a 14/10h light/dark photoperiod.
Preparation of leaf samples
The fully expanded leaves were excised and subjected to UV-C stress as described in Duan et al. (2015). The leaves of the different genotypes were harvested at different time points after the treatment: C (control fresh leaf, without UV-C treatment), 0.5h, and 6h, respectively, immediately frozen in liquid nitrogen, and stored at −80 °C until RNA extraction.
Downy mildew (P. viticola) infection was carried out as described previously (Genet et al., 1997; Kortekamp, 2006; Duan et al., 2015). Three experimental situations were used: fresh leaf without inoculation (C), control leaf incubated in the absence of P. viticola but kept under the same conditions (120 h-C), and controlled inoculation with a suspension of P. viticola and incubation for 120h (120 h-S), respectively. The leaf material was immediately frozen in liquid nitrogen and stored at −80 °C until RNA extraction.
cDNA synthesis and quantitative Real-Time RT-PCR
Total RNA was isolated using the SpectrumTM Plant Total RNA Kit (Sigma, Deisenhofen) according to the protocol of the manufacturer. The extracted RNA was treated with a DNA-free DNase (Qiagen, Hilden, Germany) to remove any potential contamination by genomic DNA. The mRNA was transcribed into cDNA using the M-MuLV cDNA Synthesis Kit (New England Biolabs; Frankfurt am Main, Germany) according to the instructions of the manufacturer. The RNase inhibitor (New England Biolabs; Frankfurt am Main, Germany) was used to protect the RNA from degradation. The amount of RNA template was 1 μg.
Quantitative real-time RT-PCR was performed on an Opticon 2 system (Bio-Rad, München) as described by Svyatyna et al. (2014). To compare the mRNA expression levels between different samples, the C t values from each sample were normalized to the value for the EF-1α internal standard obtained from the same sample. For each triplicate, these normalized C t values were averaged. The difference between the C t values of the target gene X and those for the EF-1α reference R were calculated as follows: △Ct(X)=Ct(X)–Ct(R). The final result was expressed as 2–△Ct(X). Each experiment was repeated with three biological replicates.
Illumina next generation sequencing and data analysis
Genomic DNA was extracted from young leaves of Hoe29 using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the instructions of the manufacturer. For Illumina Next Generation Sequencing, 1.5 μg genomic DNA were sheared using Covaris (Woburn, Massachusetts), and the subsequent library preparation was carried out with the TruSeq DNA LT Sample Prep Kit (Illumina Inc., San Diego, CA). The resulting library was sequenced in a 100bp paired-end run on an Illumina HiSeq2000 generating approximately 200 million reads (IMSB, Mainz, Germany). The subsequent analysis of NGS data was conducted with the CLC bio Genomics Workbench (Aarhus, Denmark). Raw reads were filtered for quality (P=0.01, no ambiguity nucleotides) and adapter trimmed. De novo assembly of remaining trimmed reads (approximately 187 million reads) was conducted using standard parameters. The resulting contigs served as the database for BLASTn searches using the MYB14 sequence of PN40024 (NW_003724037.1) as a query. Trimmed reads were mapped to cloned promoter sequences of Hoe29, Ke83, and Augster Weiss with varying lengths and similarity fractions: 1.0/1.0; 0.98/0.98; 0.96/0.96.
Cloning of the MYB14 promoter
In order to conduct the transient expression assay, the MYB14 fragments of genomic DNA – Hoe29, Ke83, and Augster Weiss were amplified with the Phusion DNA polymerase (NEB, Frankfort, Germany), including the promoter sequences of Hoe29 (1351bp), Ke83 (1245bp), and Augster Weiss (1347bp). The primers MYB14-F (5′-CTACTGACGTGCACTAGCCT-3′) and MYB14-R (5′-GCAG AGTGAAAGTGCAACACG-3′) were designed according to the reference sequence in GenBank (NW_003724037.1). The isolated sequences were compared with the database sequence using the multiple sequence alignment program ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). After the alignments, specific primers for GATEWAY cloning (see Supplementary Table S1 at JXB online) were designed to amplify the chosen promoter sequence of Hoe29, Ke83, and Augster Weiss using the GATEWAY®-Cloning technology (Invitrogen Corporation, Paisley, UK). The promoter regions of these three genotypes were ligated into the luciferase vector pLuc (Horstmann et al., 2004) and verified by DNA sequencing (GATC Biotech, Cologne, Germany).
Transient transfection and dual-luciferase assay
A transient assay using a cell suspension culture of a ‘Chardonnay’ petiole callus maintained on Grape Cormier (GC) medium (Bao Do and Cormier 1991), was performed as described previously (Czemmel et al., 2009; Merz et al., 2014) with minor modifications as follows: after a transfection (48h), the cells were harvested after a treatment with different stresses at specified time points, and then lysed by grinding on ice in 200 μl of 2× passive lysis buffer (PLB, Promega, Madison, Wl) for 1.5min using a pestle and mortar. After centrifugation of the lysates for 1min at 1 000 g, luciferase activities were measured with the dual-luciferase reporter assay system (PJK, Kleinblittersdorf, Germany), by the sequential addition of 50 μl Beetle Juice and Renilla Glow Juice to 20 μl of the individual lysate supernatants. Light emission was measured with a lumat LB9507 Luminometer (Berthold Technologies, Bad Wildbad, Germany). The specific promoter linked to a firefly luciferase gene was co-bombarded with the Renilla luciferase plasmid pRluc as an internal standard and the relative luciferase activity was calculated as the ratio between the firefly and Renilla (control) luciferase activity. All transfection experiments were performed in triplicate with similar relative ratios to the respective control.
Treatment of the cells for transient promoter assays
For the UV-C experiment, the cells were treated for 2min at a distance of 12.5cm by UV-C (15W, OSRAMHNS, OFR) and then harvested at 3h (Duan et al., 2015). The bacterial peptide flg22 QRLSTGSRINSAKDDAAGLQIA (Felix et al., 1999), a 22-amino-acid peptide, was purchased from a commercial producer (Antikörper online, Aachen, Germany) and diluted in sterile H2O as a stock solution of 1mM. Diphenyleneiodonium chloride (DPI) (Sigma-Aldrich, Germany) was dissolved in dimethyl sulphoxide (DMSO) as a stock solution of 10mM. The calcium ionophore A23187 (Sigma-Aldrich, Germany) was diluted in DMSO as a stock solution of 50mM. Gadolinium chloride (GdCl3) (Sigma-Aldrich, Germany) was used as an inhibitor of mechanosensitive calcium channels and diluted with DMSO to a 20mM stock solution. PD98059, a chemical inhibitor for the mitogen-activated protein kinase (MAPK) cascade (Sigma-Aldrich, Deisenhofen, Germany), was dissolved and sterilized into a 100mM stock solution in DMSO. Salicylic acid (SA) (Sigma-Aldrich, Germany) and (±)-jasmonic acid (JA) (Sigma-Aldrich, Germany) were dissolved in ethanol (EtOH) to obtain stock solutions of 50mM and 500mM, respectively. The inhibitor 1-phenylpyrazolidinone (phenidone) (Sigma-Aldrich, Germany) was dissolved in DMSO to obtain a 2M stock solution. Polyoxyethylen-20-sorbitan monolaurate (Tween® 20), required in a low concentration (1 ‰) to keep phenidone soluble, was obtained from Carl Roth in Germany. All treatments were accompanied by appropriate solvent controls, and the maximal concentration of solvent used in the test samples did not exceed 1‰.
Results
Specific alleles of the MYB14 promoters in sylvestris
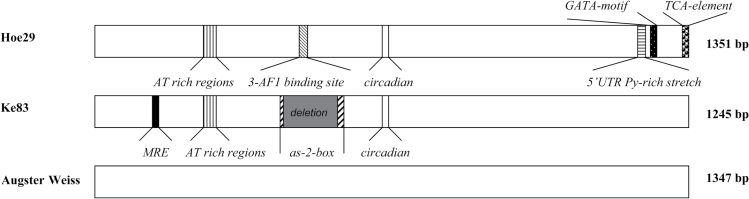
During the comparison of the sylvestris genotype Hoe29, which has high stilbene-inducibility (Duan et al., 2015), significant differences in the region of the MYB14 promoter were discovered with respect to the reference genome (established for the vinifera variety ‘Pinot Noir’) by next-generation sequencing. This region was, therefore, cloned from the two sylvestris genotypes Hoe29, and Ke83 (a second sylvestris genotype with high stilbene-inducibility), together with the ancient vinifera variety ‘Augster Weiss’, which is male-sterile and, therefore, often used for breeding. The alignment of these promoters showed significant differences that were then analysed with respect to predicted promoter motives using the PlantCARE algorithm (Lescot et al., 2002).
In Fig. 1, the significant differential cis-elements are summarized for the MYB14 promoters of Hoe29 and Ke83, compared with Augster Weiss (the full alignment with the details on these cis-elements are given in Supplementary Fig. S1 at JXB online). The Hoe29 promoter harboured a specific 5′-UTR Py-rich stretch, which was absent in the alleles from Ke83 and Augster Weiss due to a deletion of 10bp. This cis-acting element has been reported to confer high transcriptional levels (Daraselia et al., 1996; Wang et al., 2013). Furthermore, a 19-bp-long AT-rich insertion was found in Hoe29 and Ke83 (ATTTATTAAATTTATTTTT) which has been found to act as an enhancer (Bustos et al., 1989; Sandhu et al., 1998). In addition, several cis-elements linked to light responsiveness have also been found, such as a 3-AF1 binding site and a GATA-motif specific for Hoe29; and a MRE and an as-2-box in Ke83. Most notably, there was a big deletion of 107bp in Ke83 removing two putative CAAT boxes present in Hoe29. These putative enhancer elements were also absent in Augster Weiss (although this genotype did not carry the 107bp deletion). In addition, the promoter in Hoe29 displayed a TCA element shown to confer salicylic-acid responsiveness (Goldsborough et al., 1993).
Fig. 1.
Comparison of MYB14 promoters in the two sylvestris genotypes Hoe29 and Ke83, along with the cultivated variety Augster Weiss.  3-AF1 binding site (11bp): a light-responsive element.
3-AF1 binding site (11bp): a light-responsive element.  circadian (10bp): cis-acting regulatory element involved in circadian control.
circadian (10bp): cis-acting regulatory element involved in circadian control.  5′UTR Py-rich stretch (10bp): cis-acting element conferring high transcriptional levels.
5′UTR Py-rich stretch (10bp): cis-acting element conferring high transcriptional levels.  GATA-motif (7bp): part of a light-responsive element.
GATA-motif (7bp): part of a light-responsive element.  TCA-element (10bp): cis-acting element involved in salicyclic acid responsiveness.
TCA-element (10bp): cis-acting element involved in salicyclic acid responsiveness.  MRE (7bp): MYB binding site involved in light responsiveness.
MRE (7bp): MYB binding site involved in light responsiveness. Deletion (107bp) in Ke83 compared with Hoe29 and Augster Weiss.
Deletion (107bp) in Ke83 compared with Hoe29 and Augster Weiss.  as-2-box (10bp): involved in shoot-specific expression and light responsiveness.
as-2-box (10bp): involved in shoot-specific expression and light responsiveness.  AT rich regions (19bp) in Hoe29 and Ke83.
AT rich regions (19bp) in Hoe29 and Ke83.
Responses of MYB14 to UV-C and P. viticola
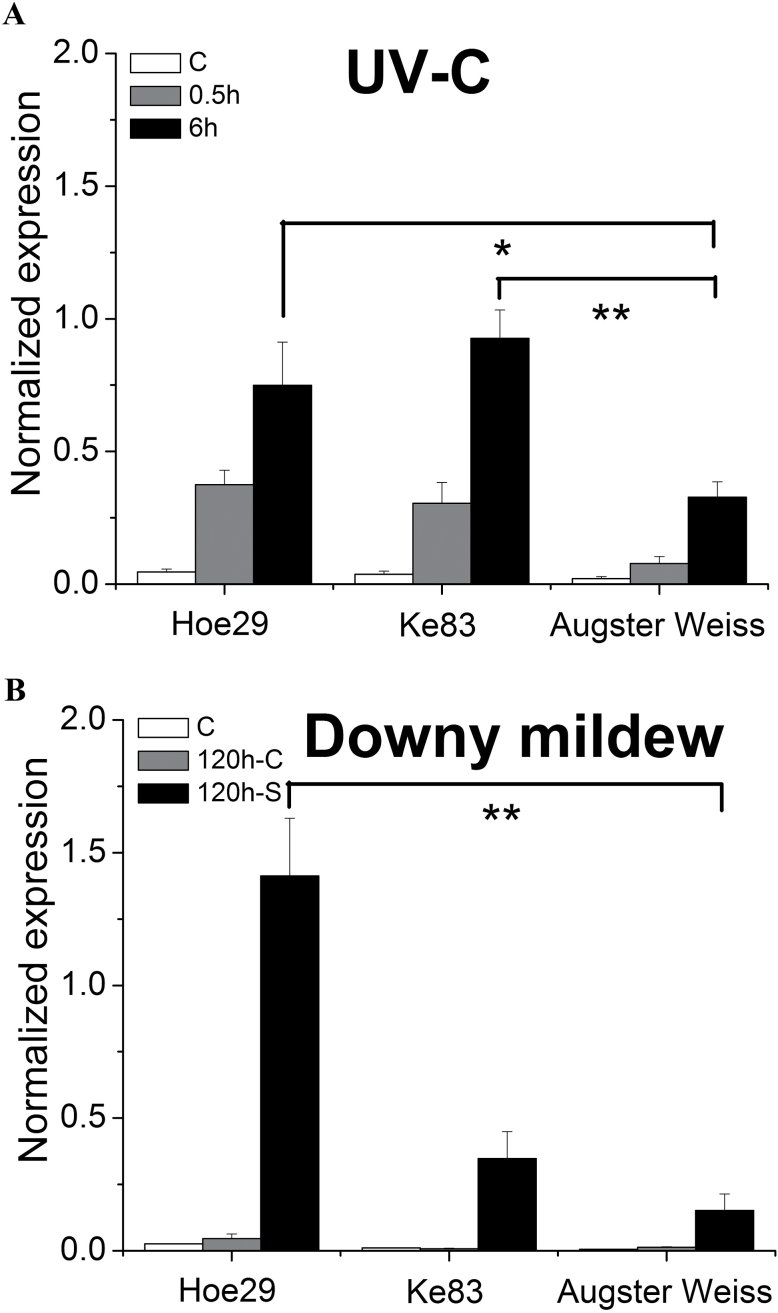
To verify whether the transcription factor MYB14 is indu ced under conditions where the stilbene branch of the phenylpropanoid pathway is activated, we followed the transcript levels of MYB14 in Hoe29, Ke83, and Augster Weiss in response to UV-C and P. viticola by quantitative real-time PCR.
As shown in Fig. 2A, for all the genotypes tested, no significant transcript levels can be detected in the controls. However, as early as 0.5h after a UV pulse, these transcripts had been clearly induced, with the response of Hoe29 and Ke83 being much stronger than that of Augster Weiss. This difference was magnified at 6h, when the induction in Hoe29 was 16-fold compared with the control and more than two times compared with Augster Weiss; also in Ke83, this induction was nearly three times higher compared with Augster Weiss.
Fig. 2.
Expression of MYB14 in response to UV-C and downy mildew. (A) UV-C irradiation for 10min. (B) Downy mildew infection for 120h. Quantification of transcripts by quantitative real-time PCR normalized to the expression of elongation factor EF1-α. * indicate differences that are statistically significant on the P <0.05 level and ** indicate P <0.01 level. Data represent mean values from three independent experimental series, error bars represent standard errors.
For infection with downy mildew, the expression of MYB14 in Hoe29 was strongly induced by 30-fold compared with the control (Fig. 2B), which was more than nine times higher compared with Augster Weiss. The induction in Ke83, although also higher than in Augster Weiss, was not comparable with that in Hoe29.
Differential activation of MYB14 promoters from different genotypes
Using a promoter–reporter assay in grapevine suspension cells, we mapped known early signalling events such as the dependence on NADPH oxidase (RboH), or induction by jasmonates and calcium influx to investigate whether the differences in the inducibility of the MYB14 promoter can account for the stress-specific differences in the expression of stilbene synthase observed between the three genotypes (Hoe29, Ke83, and Augster Weiss).
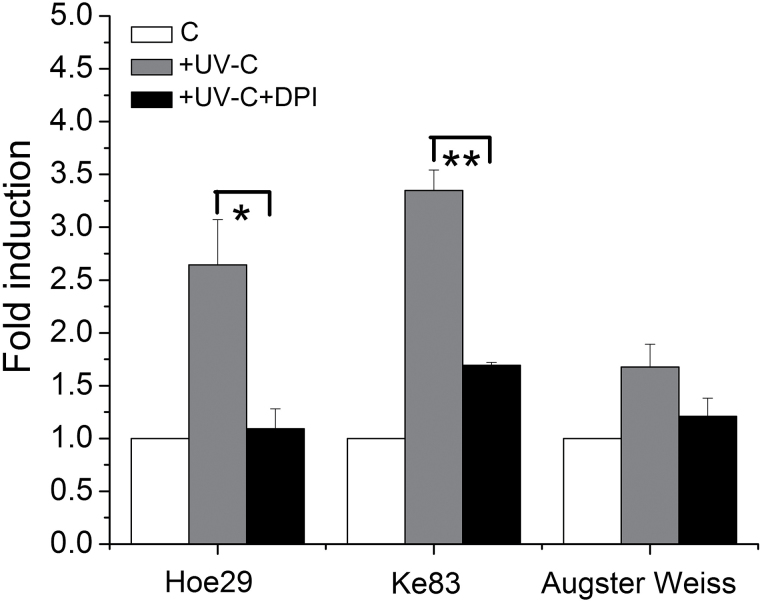
Activation of MYB14 by UV-C requires an oxidative burst
The rapid generation of reactive oxygen species (ROS), termed an oxidative burst, is an early inducible plant response during pathogen invasion or after treatment with elicitors (Wojtaszek, 1997). To test whether the induction of MYB14 by UV-C requires an oxidative burst, the NADPH oxidase inhibitor DPI was used to quell the increase in ROS abundance following challenge with UV irradiation.
As shown in Fig. 3, the promoters were activated in all genotypes after UV irradiation, but were stronger in Hoe29 and Ke83 (around 3-fold) compared with Augster Weiss (only around 1.5-fold). Pretreatment with DPI nearly abolished the induction by UV; in Hoe29, the induction was even completely eliminated.
Fig. 3.
Effect of DPI on promoter activity of MYB14 in response to UV-C. Values give fold induction levels of promoter activity in the presence of UV-C and UV-C with NADPH oxidase inhibitor (DPI) relative to the respective control (C) (promoter activity without any treatments). +UV-C: fold induction of promoter activity for 3h after the UV-C irradiation for 2min; +UV-C+DPI: fold induction of promoter activity for 3h after the addition of DPI (10 μM) and UV-C for 2min. Values were calibrated against the co-bombarded Renilla luciferase plasmid pRluc as an internal standard. * indicate differences that are statistically significant on the P <0.05 level and ** indicate P <0.01 level. Mean values and standard errors from three independent experimental series.
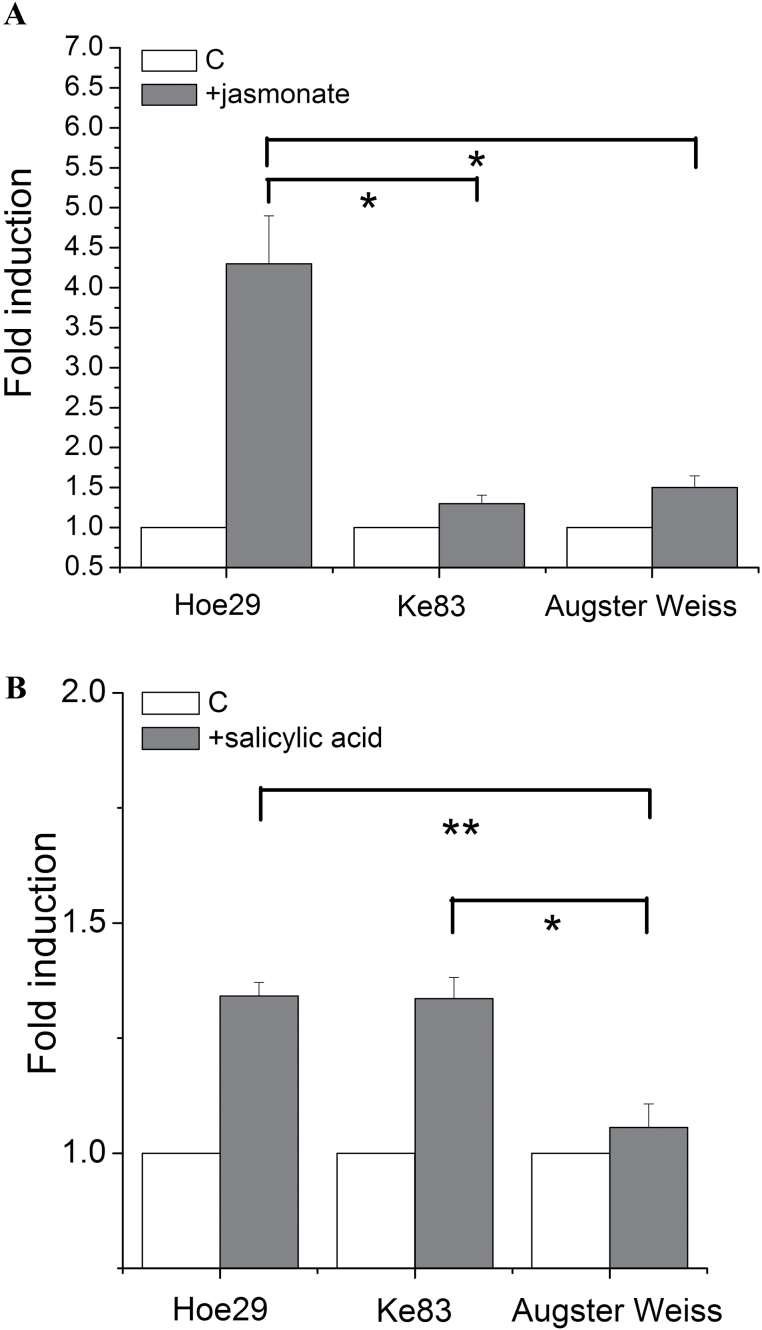
Impact of JA and SA on the activation of MYB14 promoters
Since jasmonic acid (JA) signalling is activated in response to herbivores, necrotrophic pathogens, and to wounding, we tested whether JA signalling was involved in the activation of the MYB14 promoters at the early signalling stage, by applying exogenous jasmonates. As shown in Fig. 4A, this induced the promoter activity by around 4-fold in Hoe29, whereas there was hardly any induction in Ke83 and Augster Weiss.
Fig. 4.
Activities of MYB14 promoters in response to (±)-jasmonic acid (JA) and salicylic acid (SA). Values show promoter activities relative to the untreated control after the treatments of 50 μM (±)-jasmonic acid (JA) for 4h (A) and 50 μM salicylic acid (SA) for 4h (B), respectively. * indicate differences that are statistically significant on the P <0.05 level and ** indicate P <0.01 level. Mean values and standard errors from three independent experimental series.
The salicylic acid (SA) pathway acts antagonistically to JA signalling, and is triggered during both PTI and ETI. Since a putative SA-response element had been found in the promoter from Hoe29 (Fig. 1), we therefore tested the effect of exogenous SA. As shown in Fig. 4B, the induction in Hoe29 and Ke83, although significantly higher than in Augster Weiss, was very weak (around 30%) and thus not comparable with the JA induction in Hoe29 (Fig. 4A). This means that while jasmonate can effectively activate the Hoe29 allele of the MYB14 promoter, SA seems to be fairly marginal.
The MYB14 promoters are activated in response to a calcium ionophore
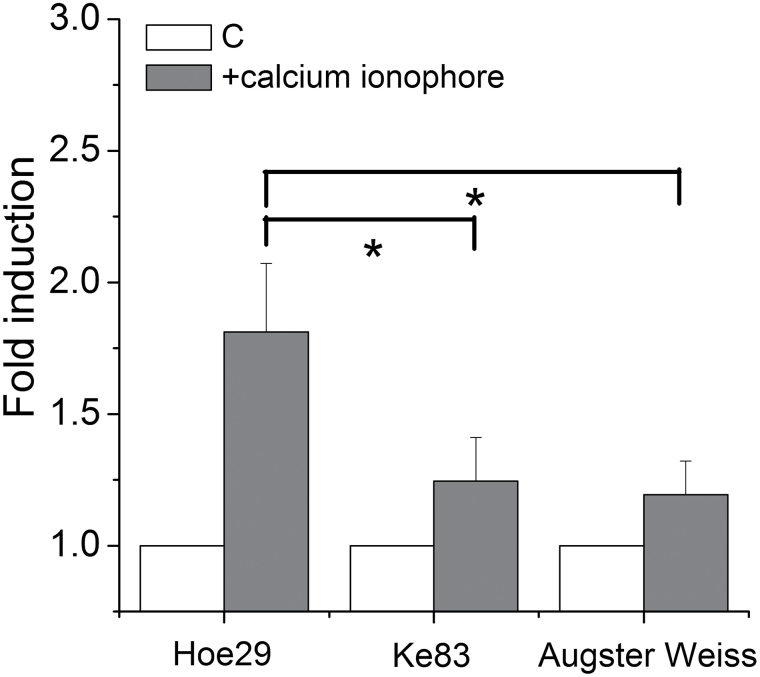
The influx of Ca2+ is considered to be the earliest signalling event in basal immunity and we therefore used the antibiotic ionophore A23187 as a probe which can permeate through the cell membrane and release Ca2+ in the cytoplasm. This allows calcium to be triggered in the absence of an external stimulus which provides an important tool for functional analysis. To test whether the influx of Ca2+ is sufficient for the activation of the MYB14 promoters, the calcium ionophore was applied. As shown in Fig. 5, this treatment induced promoter activity by around 80% for Hoe29, whereas the induction (although significant) was much weaker (around 20%) in Ke83 and Augster Weiss.
Fig. 5.
Activities of MYB14 promoters in response to the calcium ionophore A23187. Values show promoter activities relative to the untreated control after treatment with 50 μM of A23187 for 4h. * indicate differences that are statistically significant on the P <0.05 level. Mean values and standard errors from three independent experimental series.
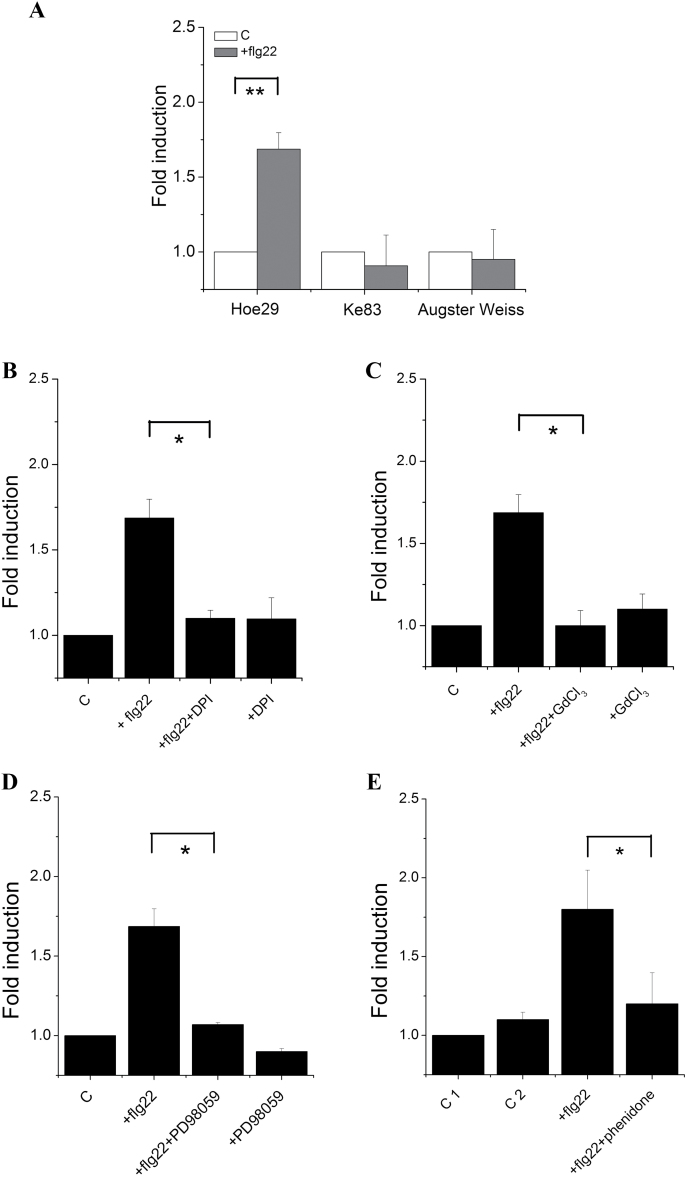
The PAMP flg22 can induce the MYB14 promoter in Hoe29 but not in Ke83
In order to test whether the stronger induction of MYB14 by the calcium ionophore in Hoe29 was linked with a higher sensitivity of this allele to basal immunity, the responses to the PAMP flg22 were monitored (Fig. 6A). Treatment with this PAMP induced the MYB14 promoter activity in Hoe29 by a similar factor (+70%) as treatment with the calcium ionophore. Again, Ke83 and Augster Weiss did not show this induction. Since this pattern suggested that the Hoe29 allele of the MYB14 promoter was activated by PTI, whereas the Ke83 allele was not, we decided to map known upstream events involved in flg22-triggered basal immunity for Hoe29.
Fig. 6.
Activities of the MYB14 promoter from Hoe29 in response to flg22 and the effect of inhibitors on this activity. Values show promoter activities relative to the untreated control after treatment with 1 μM flg22 for 4h (A), the modulation of this activity by pretreatment with: 10 μM of the NADPH oxidase inhibitor DPI (B), 20 μM of the calcium-channel blocker GdCl3 (C), 100 μM of the MAPK cascade inhibitor PD98059 (D), and 2mM of the lipoxygenase inhibitor phenidone (E). Activities were measured 4h after the addition of flg22; pretreatments were for30min. In the phenidone experiment (E), 2mM phenidone containing 0.1% Tween® 20 was used, control 1 was pretreated with 0.1% Tween® 20 for 30min alone and then kept for 4h without flg22, control 2 was pretreated with 0.1% Tween® 20 for 30min and then incubated with 1 μM flg22 for 4h. * indicate differences that are statistically significant on the P <0.05 level. ** indicate differences that are statistically significant on the P <0.01 level. Mean values and standard errors from three independent experimental series.
RboH-dependent oxidative bursts are necessary. In order to test whether a RboH-dependent oxidative burst was involved in the activation of the Hoe29 allele of the MYB14 promoter, DPI was used which almost eliminated the activation in response to flg22 (Fig. 6B). A negative control showed that application of DPI alone did not cause a significant change of activity.
Calcium influx is necessary. Since the activity of a calcium influx channel is essential for the activation of early defence, inhibition of this influx by GdCl3, an inhibitor of mechanosensitive calcium channels should therefore suppress the activation of PTI by flg22 (Chang and Nick, 2012). Since a calcium ionophore was able specifically to activate the Hoe29 allele of the MYB14 promoter of Hoe29 (Fig. 5), we tested whether the specific activation of the Hoe29 allele in response to flg22 required a calcium influx. As shown in Fig. 6C, this was the case. This finding suggests the Ca2+ influx is essential for the flg22-induced activation of the MYB14 promoter.
MAPK signalling is necessary. A mitogen-activated protein kinase (MAPK) cascade is implied in the activation of defence gene expression which can be inhibited in grapevine cell cultures by the specific inhibitor PD98059 (Chang and Nick, 2012). As shown in Fig. 6D, this inhibitor can efficiently suppress the flg22-induced activation of the MYB14 promoter, demonstrating that MAPK signalling is necessary for this activation.
JA synthesis is necessary. Since exogenous JA could efficiently activate the Hoe29 allele of the MYB14 promoter (Fig. 4A), we tested whether endogenous jasmonate was necessary for the activation of MYB14 promoter in response to flg22. Since jasmonate synthesis initiates from a peroxidation of lipids, phenidone, an inhibitor of lipoxygenases, can block the synthesis of JA. As shown in Fig. 6E, a pretreatment with phenidone can efficiently inhibit the flg22-triggered induction of the Hoe29 allele of the MYB14 promoter. Thus, JA synthesis is necessary for activation.
Discussion
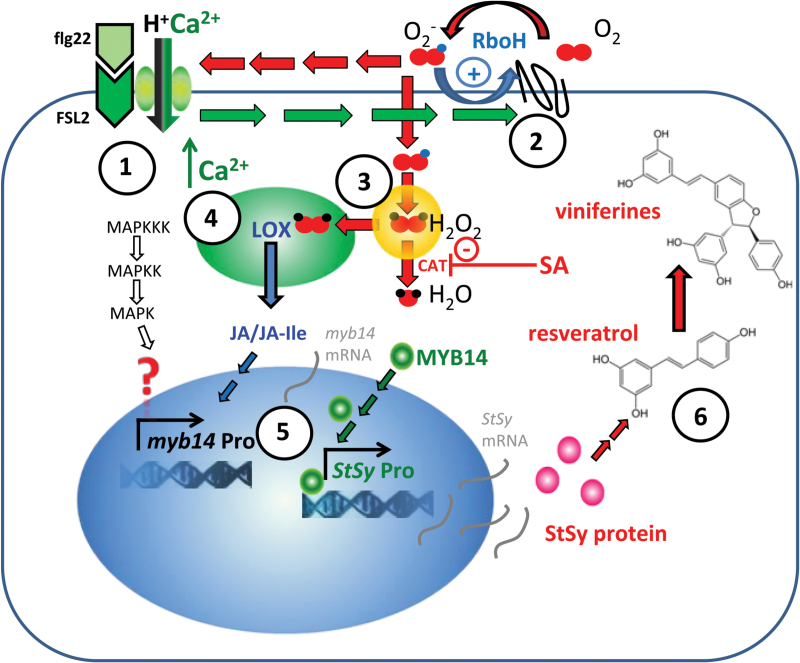
In the current study, to understand the functional relevance of these specific promoters and to map the upstream signalling, we employed a promoter–reporter assay (Höll et al., 2013). We show that both sylvestris promoters (but not the promoter from the weak stilbene producer Augster Weiss) are induced by UV light in cell culture and that this induction requires the activity of a NADPH oxidase. However, only the MYB14 promoter from Hoe29 was also induced by jasmonic acid and flg22 (again dependent on the activity of a NADPH oxidase) indicating that this allele of MYB14 was, in addition, a target of the signalling driving basal immunity (PTI). By contrast, the MYB14 promoter from Ke83, although inducible by UV light, was not induced in the context of PTI. This is consistent with our previous findings (Duan et al., 2015), where StSy transcripts were strongly induced by UV light in both genotypes whereas, for infection by P. viticola, induction was only observed in Hoe29, but not in Ke83. We will present a signature model of immunity signalling that can explain most, if not all of our observations (Fig. 7).
Fig. 7.
The signature model of immunity signalling. Details are explained in the discussion.  Binding of flg22 to the receptor will therefore result in a substantial increase of cytosolic calcium within a few minutes.
Binding of flg22 to the receptor will therefore result in a substantial increase of cytosolic calcium within a few minutes.  The stimulation of the membrane-bound NADPH oxidase RboH through specific calcium-dependent protein kinases, leading to an apoplastic oxidative burst generating superoxide anions.
The stimulation of the membrane-bound NADPH oxidase RboH through specific calcium-dependent protein kinases, leading to an apoplastic oxidative burst generating superoxide anions.  Hydrogen peroxide is generated in the peroxisomes from superoxide.
Hydrogen peroxide is generated in the peroxisomes from superoxide.  Calcium reaching the plastid will activate specific lipoxygenases there as the first committed step of the oxylipin pathway generating jasmonates.
Calcium reaching the plastid will activate specific lipoxygenases there as the first committed step of the oxylipin pathway generating jasmonates.  MYB14 transcription factor.
MYB14 transcription factor.  Stilbenes.
Stilbenes.
The earliest known cellular response of basal immunity is the activation of a rapid influx of Ca2+ and H+ (Nürnberger, 1999). In the case of the PAMP flg22, perception is brought about by the receptor FLS2 (for reviews see Boller and Felix, 2009; Robatzek and Wirthmueller, 2013), for which a grapevine homologue has also been described (Di Gaspero and Cipriani, 2003). An immediate target of activated FLS2 signalling is cyclic-nucleotide gated calcium influx channels (reviewed in Ma and Berkowitz, 2011). Binding of flg22 to the receptor will therefore result within a few minutes in a substantial increase of cytosolic calcium (Fig. 7,  ).
).
Calcium, as an important second messenger, plays an important role as an activator of various signal chains (Harper and Harmon, 2005). One of these secondary signalling events is the stimulation of the membrane-bound NADPH oxidase RboH through specific calcium-dependent protein kinases (Kobayashi et al., 2007), leading to an apoplastic oxidative burst generating superoxide anions (Fig. 7,  ). Superoxide anions represent the second central input for plant stress signalling (for a review see Marino et al., 2011), and can also be formed in response to UV light (Hideg et al., 2012). The observation that both sylvestris alleles of the MYB14 promoter show elevated activation by UV that can be blocked by DPI (Fig. 3) suggests that this induction is triggered by RboH. By contrast, only the Hoe29 allele, but not the Ke83 allele, was activated by flg22 (Fig. 6A). This activation was also dependent on the activity of RboH, because it can also be blocked by DPI (Fig. 6B), suggesting that RboH acts downstream of flg22 or complements signalling triggered by flg22. Since activation of RboH by UV light can also activate the Ke83 allele of the MYB14 promoter, it would be expected, at first sight, that flg22 can also activate this promoter allele because the upstream signalling is provided by the identical recipient (suspension cells of ‘Chardonnay’) in the promoter–reporter system. This means that the differential activation of the two sylvestris promoters must be caused by differential activation with other branches of signalling that are independent of RboH. From our model we would predict that these RboH signalling events are activated by calcium influx directly. To test this prediction, we used a calcium ionophore and observed that this significantly activated the Hoe29 allele, whereas the Ke83 and Augster Weiss alleles did not produce significant activation (Fig. 5). When the flg22-triggered influx of calcium is blocked by Gd3+ ions, a specific inhibitor of mechanosensitive calcium channels (Ding and Pickard, 1993), this will also block the activation of the Hoe29 allele of the MYB14 promoter (Fig. 6C). Calcium influx is therefore necessary and sufficient for this activation.
). Superoxide anions represent the second central input for plant stress signalling (for a review see Marino et al., 2011), and can also be formed in response to UV light (Hideg et al., 2012). The observation that both sylvestris alleles of the MYB14 promoter show elevated activation by UV that can be blocked by DPI (Fig. 3) suggests that this induction is triggered by RboH. By contrast, only the Hoe29 allele, but not the Ke83 allele, was activated by flg22 (Fig. 6A). This activation was also dependent on the activity of RboH, because it can also be blocked by DPI (Fig. 6B), suggesting that RboH acts downstream of flg22 or complements signalling triggered by flg22. Since activation of RboH by UV light can also activate the Ke83 allele of the MYB14 promoter, it would be expected, at first sight, that flg22 can also activate this promoter allele because the upstream signalling is provided by the identical recipient (suspension cells of ‘Chardonnay’) in the promoter–reporter system. This means that the differential activation of the two sylvestris promoters must be caused by differential activation with other branches of signalling that are independent of RboH. From our model we would predict that these RboH signalling events are activated by calcium influx directly. To test this prediction, we used a calcium ionophore and observed that this significantly activated the Hoe29 allele, whereas the Ke83 and Augster Weiss alleles did not produce significant activation (Fig. 5). When the flg22-triggered influx of calcium is blocked by Gd3+ ions, a specific inhibitor of mechanosensitive calcium channels (Ding and Pickard, 1993), this will also block the activation of the Hoe29 allele of the MYB14 promoter (Fig. 6C). Calcium influx is therefore necessary and sufficient for this activation.
In addition to the activation of RboH through a calcium-dependent protein kinase, cytosolic calcium triggers two additional signalling chains: One of the targets is a MAPK cascade that conveys the signal from the membrane to the nucleus. This MAPK cascade pathway is highly conserved in eukaryotes and is composed of three hierarchical layers whereby MAPK kinase kinases phosphorylate MAPK kinases which, in turn, activate MAP kinases that will then activate different downstream targets. This pathway is central for basal immunity (reviewed in Nürnberger et al., 2004) and also mediates the activation of grapevine stilbene synthase in response to flg22, as concluded from experiments with the specific MAPK inhibitor PD98059 (Chang and Nick, 2012). We therefore tested, whether PD98059 can block the induction of the Hoe29 allele of MYB14 by flg22. This was what was observed (Fig. 6D), thus showing that the MAPK cascade is necessary.
There is an alternative calcium-dependent branch of defence signalling, though (Fig. 7,  ): Calcium reaching the plastid will activate specific lipoxygenases there (reviewed in Wasternack and Hause, 2013) as the first committed step of the oxylipin pathway generating jasmonates. In Arabidopsis, mutants affected in vacuolar calcium channels (Bonaventure et al., 2007) fail to activate AtLOX2, the lipoxygenase, which is the central trigger for the oxylipin pathway. The molecular mechanism is not clear but might be linked with the binding of lipoxygenase to the membrane due to a conserved calcium binding loop interspersed between two β-sheets (Tatulian et al., 1998). This will alter the specificity of lipoxygenase – whereas the free enzyme converts linoleic acid to conjugated dienes, however, upon binding to the membrane, it preferentially forms a conjugated ketodiene. In consequence, within a few minutes, cis-(+)-12-oxophytodienoic acid (OPDA) is exported from the plastid and converted to jasmonic acid (JA) and its potent conjugate JA-Ile.
): Calcium reaching the plastid will activate specific lipoxygenases there (reviewed in Wasternack and Hause, 2013) as the first committed step of the oxylipin pathway generating jasmonates. In Arabidopsis, mutants affected in vacuolar calcium channels (Bonaventure et al., 2007) fail to activate AtLOX2, the lipoxygenase, which is the central trigger for the oxylipin pathway. The molecular mechanism is not clear but might be linked with the binding of lipoxygenase to the membrane due to a conserved calcium binding loop interspersed between two β-sheets (Tatulian et al., 1998). This will alter the specificity of lipoxygenase – whereas the free enzyme converts linoleic acid to conjugated dienes, however, upon binding to the membrane, it preferentially forms a conjugated ketodiene. In consequence, within a few minutes, cis-(+)-12-oxophytodienoic acid (OPDA) is exported from the plastid and converted to jasmonic acid (JA) and its potent conjugate JA-Ile.
We therefore tested, whether exogenous jasmonic acid could activate MYB14 in the absence of flg22. This is, in fact, what we can observe (Fig. 4A), whereby this activation only works with the Hoe29 allele, whereas the alleles from Ke83, and Augster Weiss are not responsive to jasmonic acid. To test, whether induction of (endogenous) jasmonic acid is necessary for this activation, we treated the cells with phenidone, an inhibitor of jasmonate synthesis targeted to lipoxygenases (Ismail et al., 2012), and we found that phenidone can block the flg22-induced activation of MYB14. Thus, jasmonate is necessary and sufficient to convey the activation of flg22 to the Hoe29 allele.
This points to a scenario where flg22 activates the MAPK cascade, as well as jasmonate signalling, that converge on a target on the sylvestris MYB14 promoter that is present in Hoe29, but not in Ke83. Nevertheless, RboH seems to be necessary as well and this effect of RboH seems to be different from that in the UV-activation of MYB14 (which was similar in both sylvestris alleles of this promoter). This apparent discrepancy can be resolved when RboH dependent signalling converges with jasmonate synthesis. This point of convergence might again be the lipoxygenase that is not only activated by calcium, but requires hydrogen peroxide (Fig. 7,  ). Hydrogen peroxide is generated in the peroxisomes from superoxide and is then further converted to water by catalase (Fig. 7,
). Hydrogen peroxide is generated in the peroxisomes from superoxide and is then further converted to water by catalase (Fig. 7,  ). It has been known for a long time that the activity of catalase can be inhibited by the important stress factor salicylic acid, leading to elevated levels of hydrogen peroxide (Durner and Klessig, 1996).
). It has been known for a long time that the activity of catalase can be inhibited by the important stress factor salicylic acid, leading to elevated levels of hydrogen peroxide (Durner and Klessig, 1996).
Our model would therefore predict that salicylic acid, by blocking the reduction of hydrogen peroxide, should promote the activation of lipoxygenase and, therefore, the activation of the Hoe29 allele of MYB14 by flg22 should also depend on RboH. This prediction was tested experimentally and it was found that DPI can block the activation (Fig. 6B), consistent with the prediction. We have further found that salicylic acid can activate the Hoe29 allele of MYB14 (Fig. 4B). However, the activation was observed for both sylvestris alleles pointing to additional targets of salicylic acids (that are different from jasmonate). But, since this activation was also very weak (although significant), the impact of salicylic acid alone (i.e. without synergy with flg22) seems to be fairly marginal.
Open questions and outlook
The current work proposes a mechanism to explain the observed phenotype (Duan et al., 2015) of a specific sylvestris genotype, Hoe29, and draws a link between specific regions in the promoter of the transcription factor MYB14, elevated inducibility of this promoter by the signalling activated during basal immunity, and the observed strong accumulation of resveratrol and viniferins correlated with the improved tolerance of these genotypes against downy mildew. Although we can reproduce in the promoter–reporter system the response patterns observed in the plant, for instance the differential activation of stilbene synthase transcription in the Hoe29 versus the Ke83 genotypes, the general activation of the promoter is lower than the observed accumulation of transcripts in planta. This indicates that differentiated grapevine cells harbour enhancing factors that are not present in non-differentiated suspension cells. A similar phenomenon with similar ratios is observed for stilbene synthase when the induction of transcripts in planta is compared with the inductions observed in the promoter–reporter system (Höll et al., 2013). Whether these factors are of epigenetic nature or are simply additional signalling factors remains to be elucidated. In addition, the role of the MYB15 factor should be addressed as well as the MYB14-independent direct signalling to the stilbene synthase promoter. These aspects are currently being analysed and are expected to complement and refine the proposed working model. As proof of the concept for the approach, the Hoe29 allele of MYB14 will also be transformed into a vinifera host with poor stilbene performance to see whether up-regulation of StSy transcription is sufficient to produce high levels of bioactive stilbenes. The target of this research is to define targets for molecular breeding of grapevine varieties with elevated basal immunity due to enhanced MYB14 inducibility.
Supplementary data
Supplementary data can be found at JXB online.
Figure S1. The alignment and the significant differential cis-elements for the MYB14 promoters of Hoe29 and Ke83, compared with Augster Weiss and the reference genome (from the vinifera cultivar Pinot Noir).
Table S1. Sequence of oligonucleotide primers for GATEWAY® cloning of the MYB14 constructs for the promoter-reporter assay.
Acknowledgements
This work was supported by the BACCHUS Interreg IV Upper Rhine project co-financed by the European Union/European Regional Development Fund (ERDF), the German Federal Agency for Agriculture (Programme for Sustainable Agriculture, BÖLN), and by a fellowship from the Chinese Scholarship Council to Dong Duan. We gratefully acknowledge Joachim Daumann and Kerstin Huber (Karlsruhe Institute of Technology) for taking care of plants in the Botanical garden, and Claudia Vogel (Dienstleistungszentrum Laendlicher Raum Rheinpfalz, Neustadt) for kind assistance in the preparation of the promoter–reporter experiments.
References
- Bao Do C, Cormier F. 1991. Effects of low nitrate and high sugar concentrations on anthocyanin content and composition of grape (Vitis viniferin L.) cell suspension. Plant Cell Reports 9, 500–504. [DOI] [PubMed] [Google Scholar]
- Boller T, He SY. 2009. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324, 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. 2009. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annual Review of Plant Biology 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Bonaventure G, Gfeller A, Rodríguez VM, Armand F, Farmer EE. 2007. The fou2 gain-of-function allele and the wild-type allele of Two Pore Channel 1 contribute to different extents or by different mechanisms to defense gene expression in Arabidopsis. Plant and Cell Physiology 48, 1775–1789. [DOI] [PubMed] [Google Scholar]
- Bustos MM, Guiltinan MJ, Jordano J, Bequm D, Kalkan FA, Hall TC. 1989. Regulation of β-glucuronidase expression in transgenic tobacco plants by an A/T-rich, cis-acting sequence found upstream of a French bean β-phaseolin gene. The Plant Cell 1, 839–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Nick P. 2012. Defence signaling triggered by flg22 and harpin is integrated into a different stilbene output in Vitis cells. PLoS One 7, e40446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czemmel S, Stracke R, Weisshaar B, Cordon N, Harris NN, Walker AR, Robinson SP, Bogs J. 2009. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berriers. Plant Physiology 151, 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daraselia ND, Tarchevskaya S, Narita JO. 1996. The promoter for tomato 3-hydroxy-3-methylglutaryl coenzyme A reductase gene 2 has unusual regulatory elements that direct high-level expression. Plant Physiology 112, 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di G, aspero G, Cipriani G. 2003. Nucleotide biding site/leucine-rich repeats, Pto-like, receptor-like kinases related to disease resistance in grapevine. Molecular Genetics and Genomics 269, 612–623. [DOI] [PubMed] [Google Scholar]
- Ding JP, Pickard BG. 1993. Mechanosensory calcium-selective cation channels in epidermal cells. The Plant Journal 3, 83–110. [DOI] [PubMed] [Google Scholar]
- Durner J, Klessig DF. 1996. Salicylic acid is a modulator of tobacco and mammalian catalases. Journal of Biological Chemistry 271, 28492–28501. [DOI] [PubMed] [Google Scholar]
- Duan D, Halter D, Baltenweck R, Tisch C, Tröster V, Kortekamp A, Hugueney P, Nick P. 2015. Genetic diversity of stilbene metabolism in V. sylvestris . Journal of Experimental Botany 66, 3243–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. 1999. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. The Plant Journal 18, 265–276. [DOI] [PubMed] [Google Scholar]
- Genet JL, Steva H, Vincent O, Cazenave C. 1997. A method for measuring the level of sensitivity of Plasmopara viticola populations to cymoxanil. Bulletin OEPP/EPPO Bulletin 27, 217–225. [Google Scholar]
- Gessler C, Pertot I, Perazzolli M. 2011. Plasmopara viticola: a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathologia Mediterranea 50, 3–44. [Google Scholar]
- Goldsborough AP, Albrecht H, Stratford R. 1993. Salicylic acid-inducible binding of a tobacco nuclear protein to a 10bp sequence which is highly conserved amongst stress-inducible genes. The Plant Journal 3, 563–571. [DOI] [PubMed] [Google Scholar]
- Gómez-Zeledón J, Zipper R, Spring O. 2013. Assessment of phenotypic diversity of Plasmopara viticola on Vitis genotypes with different resistance. Crop Protection 54, 221–228. [Google Scholar]
- Harper JF, Harmon A. 2005. Plants, symbiosis and parasites: a calcium signaling connection. Nature Reviews Molecular Cell Biology 6, 555–566. [DOI] [PubMed] [Google Scholar]
- Hideg H, Jansen MAK, Strid Å. 2012. UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends in Plant Science 18, 107–115. [DOI] [PubMed] [Google Scholar]
- Höll J, Vannozzi A, Czemmel S, D’Onofrio C, Walker AR, Rausch T, Lucchin M, Boss PK, Dry IB, Bogs J. 2013. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera . The Plant Cell 25, 4135–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann V, Huether CM, Jost W, Reski R, Decker EL. 2004. Quantitative promoter analysis in Physcomitrella patens: A set of plant vectors activating gene expression within three orders of magnitude. BMC Biotechnology 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A, Riemann M, Nick P. 2012. The jasmonate pathway mediates salt tolerance in grapevines. Journal of Experimental Botany 63, 2127–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. 2006. The plant immune system. Nature 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H. 2007. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. The Plant Cell 19, 1065–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekamp A. 2006. Expression analysis of defence-related genes in grapevine leaves after inoculation with a host and a non-host pathogen. Plant Physiology and Biochemistry 44, 58–67. [DOI] [PubMed] [Google Scholar]
- Ledesma-Krist GM, Nick P, Daumann J, Maul E, Dister E. 2014. Überlebenssicherung der Wildrebe Vitis vinifera L. ssp. sylvestris (C.C. Gmel.) Hegi in den Rheinauen durch gezieltes in situ-Management. Bundesanstalt für Landwirtschaft und Ernährung . http://download.ble.de/06BM001/06BM001.pdf
- Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. 2002. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research 30, 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Berkowitz GA. 2011. Ca2+ conduction by plant cyclic nucleotide gated channels and associated signaling components in pathogen defense signal transduction cascades. New Phytologist 190, 566–572. [DOI] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N. 2011. A burst of plant NADPH oxidases. Trends in Plant Science 17, 9–15. [DOI] [PubMed] [Google Scholar]
- Merz PR, Moser T, Höll J, Kortekamp A, Buchholz G, Zyprian E, Bogs J. 2014. The transcription factor VvWRKY33 is involoved in the regulation of grapevine (Vitis vinifera) defense against the oomycete pathogen Plasmopara viticola . Physiologia Plantarum 153, 365–380. [DOI] [PubMed] [Google Scholar]
- Nürnberger T. 1999. Signal perception in plant pathogen defence. Cellular and Molecular Life Sciences 55, 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger T, Brunner F, Kemmerling B, Piater L. 2004. Innate immunity in plants and animals: striking similarities and obvious differences. Immunological Reviews 198, 249–266. [DOI] [PubMed] [Google Scholar]
- Olmo HP. 1976. Grapes: Vitis, Muscadinia (Vitaceae). In: Simmonds NW. ed. Evolution of crop plants . Longman: London and New York, 294–298. [Google Scholar]
- Peressotti E, Wiedemann-Merdinoglu S, Delmotte F, Bellin D, Di G, aspero G, Testolin R, Merdinoglu D, Mestre P. 2010. Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biology 10, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Wirthmueller L. 2013. Mapping FLS2 function to structure: LRRs, kinase and its working bits. Protoplasma 250, 671–681. [DOI] [PubMed] [Google Scholar]
- Rouxel M, Mestre P, Comont G, Lehman BL, Schilder A, Delmotte F. 2013. Phylogenetic and experimental evidence for host-specialized cryptic species in a biotrophic oomycete. New Phytologist 197, 251–263. [DOI] [PubMed] [Google Scholar]
- Sandhu JS, Webster CI, Gray JC. 1998. A/T-rich sequences act as quantitative enhancers of gene expression in transgenic tobacco and potato plants. Plant Molecular Biology 37, 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svyatyna K, Jikumaru Y, Brendel R, Reichelt M, Mithöfer A, Takano M, Kamiya Y, Nick P, Riemann M. 2014. Light induces jasmonate-isoleucine conjugation via OsJAR1-dependent and -independent pathways in rice. Plant, Cell and Environment 37, 827–839. [DOI] [PubMed] [Google Scholar]
- Takken FL, Tameling WI. 2009. To nibble at plant resistance proteins. Science 324, 744–746. [DOI] [PubMed] [Google Scholar]
- Tatulian SA, Steczko J, Minor W. 1998. Uncovering a calcium-regulated membrane-binding mechanism for soybean lipoxygenase-1. Biochemistry 37, 15481–15490. [DOI] [PubMed] [Google Scholar]
- Thomma BP, Nürnberger T, Joosten MH. 2011. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. The Plant Cell 23, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch C, Nick P, Kortekamp A. 2014. Rescue to be rescued: European wild grape as genetic resources of resistance towards fungal diseases. Proceedings of the 7th International Workshop on Gapevine Downy and Powdery Mildew : 61–62. ISBN: 978-84-7821-827-1. [Google Scholar]
- Wang K, Zhang X, Zhao Y, Chen F, Xia G. 2013. Structure, variation and expression analysis of glutenin gene promoters from Triticum aestivum cultivar Chinese Spring shows the distal region of promoter 1Bx7 is key regulatory sequence. Gene 527, 484–490. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Hause B. 2013. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany 111, 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszek P. 1997. Oxidative burst: an early plant response to pathogen infection. Biochemical Journal 332, 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.