Highlight
Cotton MYB108 interacts with CML11 and acts as a positive regulator in defense against V. dahliae infection.
Key words: Calcium, calmodulin, cotton, MYB, plant defense, Verticillium dahliae.
Abstract
Accumulating evidence indicates that plant MYB transcription factors participate in defense against pathogen attack, but their regulatory targets and related signaling processes remain largely unknown. Here, we identified a defense-related MYB gene (GhMYB108) from upland cotton (Gossypium hirsutum) and characterized its functional mechanism. Expression of GhMYB108 in cotton plants was induced by Verticillium dahliae infection and responded to the application of defense signaling molecules, including salicylic acid, jasmonic acid, and ethylene. Knockdown of GhMYB108 expression led to increased susceptibility of cotton plants to V. dahliae, while ecotopic overexpression of GhMYB108 in Arabidopsis thaliana conferred enhanced tolerance to the pathogen. Further analysis demonstrated that GhMYB108 interacted with the calmodulin-like protein GhCML11, and the two proteins form a positive feedback loop to enhance the transcription of GhCML11 in a calcium-dependent manner. Verticillium dahliae infection stimulated Ca2+ influx into the cytosol in cotton root cells, but this response was disrupted in both GhCML11-silenced plants and GhMYB108-silenced plants in which expression of several calcium signaling-related genes was down-regulated. Taken together, these results indicate that GhMYB108 acts as a positive regulator in defense against V. dahliae infection by interacting with GhCML11. Furthermore, the data also revealed the important roles and synergetic regulation of MYB transcription factor, Ca2+, and calmodulin in plant immune responses.
Introduction
Increasing numbers of transcription factors (TFs), including members of the WRKY, NAC, bHLH, bZIP, ERF/AP2, and MYB families, have been reported to play crucial roles in plant immunity (Singh et al., 2002; Buscaill and Rivas, 2014). In the defense against pathogen attack, TFs modulate the transcription of defense-related genes, and they often execute such functions through interaction with other regulators (Tsuda and Somssich, 2015). MYB proteins represent one of the largest TF families and have a characteristic MYB domain consisting of 52 amino acid repeats that form helix–turn–helix structures required for binding to cis-elements in the promoters of target genes (Dubos et al., 2010). Based on the numbers of adjacent repeats, MYB proteins are divided into four classes: R1-MYB, R2R3-MYB, 3R-MYB, and 4R-MYB (Dubos et al., 2010). MYB proteins play important roles in plant development and responses, as shown for various species such as Arabidopsis (Arabidopsis thaliana), tobacco (Nicotiana tabacum), rice (Oryza sativa), and cotton (Gossypium hirsutum), and the molecular mechanisms by which these MYBs fulfill their functions are very well established (Lippold et al., 2009; Liu et al., 2009; Zhang et al., 2010; Walford et al., 2011; Yang et al., 2012; Lee et al., 2015). Several MYBs have been reported to function in defense against pathogens, including AtMYB30, AtBOS1 (AtMYB108), and TaPIMP1 (Vailleau et al., 2002; Mengiste et al., 2003; Zhang et al., 2012), yet the regulatory mechanisms and signaling processes mediated by MYB proteins in defense responses remain largely unknown.
Ca2+ is an important second messenger for the transduction of signals regulating plant development and the response to environmental cues (Hepler, 2005; Sarwat et al., 2013). Influx of Ca2+ into the cytosol is an important early event in pathogen attack (Lecourieux et al., 2006). The major Ca2+ sensors include calmodulin (CaM) and CaM-like proteins, which localize in various cellular compartments such as the cytoplasm, apoplast, nucleus, and peroxisome (Yang and Poovaiah, 2003). CaMs regulate a number of downstream targets involved in diverse plant processes (Bouché et al., 2005). After pathogen challenge, expression of multiple CaM genes is induced or suppressed as part of the plant defense response (Heo et al., 1999; Chiasson et al., 2005). Several studies reported that CaMs regulate gene expression by interacting with TFs such as members of the WRKY and CAMTA families, in plant innate immunity responses (Park et al., 2005; Galon et al., 2008). These studies have begun to reveal the molecular mechanisms by which Ca2+/CaM and TFs co-operate to modulate defense-related transcriptional responses.
Cotton Verticillium wilt is a highly destructive vascular disease that is mainly caused by the soil-borne fungus Verticillium dahliae, and this disease leads to severe loss of cotton yields worldwide and threatens most cotton-producing areas (Fradin and Thomma, 2006). Although long-term efforts have been made to produce wilt-resistant cotton cultivars by traditional breeding, very few varieties of upland cotton are resistant to Verticillium wilt (Cai et al., 2009). During the past years, progress has been made in exploring the molecular mechanism of the disease tolerance against V. dahliae invasion in cotton, with the ultimate aim of generating Verticillium wilt-resistant cultivars by molecular breeding. Accumulating evidence indicates that sets of V. dahliae-responsive genes, such as GhNDR1, GhNaD1, GhSSN, GbWRKY1, and GhMLP28 (Gao et al., 2011; Gaspar et al., 2014; Li et al., 2014; Sun et al., 2014; Yang et al., 2015), are functionally related to defense responses against V. dahliae infection in cotton.
In this study, we identified the V. dahliae-responsive gene GhMYB108 from upland cotton. Functional characterization indicates that it participates in the defense response through interaction with the CaM-like protein GhCML11. Moreover, the two proteins form a positive feedback loop to regulate the transcription of GhCML11. Another interesting finding of this study is that GhCML11 proteins localize in the apoplast as well as in the nucleus and cytoplasm. Apoplastic GhCML11 may be required for Ca2+ influx in response to pathogen attack, and nuclear GhCML11 may act with GhMYB108 to activate the transcription of defense genes. Our results provide important insights into the significance of the synergetic interaction between a MYB transcription factor and Ca2+/CaM in plant immune responses.
Materials and methods
Plant materials and growth conditions
Gossypium hirsutum variety BD18, kindly provided by Professor Guiliang Jian (Institute of Plant Protection, CAAS), which is a Verticillium wilt-tolerant breeding line of upland cotton, was used in this study. Cotton plants were grown in pots at 28 °C under 16h/8h light/dark conditions.
Nicotiana benthamiana and A. thaliana (ecotype Columbia-1) plants were grown in the greenhouse under 16h/8h light/dark conditions at 23 °C and watered weekly with Murashige and Skoog nutrient solution.
Arabidopsis transformation
The ORF of GhMYB108 was cloned under control of the 35S promoter in the plant expression vector pBI121. The resulting plasmid pBI121-GhMYB108 was introduced into the Agrobacterium tumefaciens strain EHA105. Transformation of Arabidopsis plants was performed using the floral-dip method (Clough and Bent, 1998).
Pathogen cultivation and inoculation
The V. dahliae strain V991 originally isolated from an infected upland cotton, which is a strong pathogenic defoliating isolate (W.W. Zhang et al., 2012), was used as the pathogen. Fungal colonies were cultured on potato dextrose agar plates for 1 week at 26 °C. For V. dahliae infection, the roots of cotton seedlings grown under hydroponic conditions for 12 d were inoculated with a spore suspension (106 spores ml−1), and then harvested at the indicated time for RNA extraction. To infect VIGS (virus-induced gene silencing) cotton plants, the spore suspensions were stem-inoculated into cotton plants at a position 1cm under the cotyledons with a syringe needle (Bolek et al., 2005), at a dose of 3 μl per plant. For Arabidopsis infection, roots of 4-week-old plants were incubated in spore suspensions for 3min. Subsequently, plants were transplanted into fresh steam-sterilized vermiculite. The disease index was calculated according to the following formula: disease index=[(∑disease grades×number of infected plants)/(total checked plants×4)]×100. Seedlings were classified into five grades (grade 0, 1, 2, 3, and 4) based on the disease severity after V. dahliae infection, as described by Wang et al. (2004).
Pseudomonas syringae pv. tomato strain DC3000 was grown in King’s B medium at 28 °C. Overnight culture cells were resuspended in 10mM MgCl2. The cell density was adjusted to 2×105 colony-forming units (cfu) ml−1 for inoculation, and the bacterial growth was detected 3 d after inoculation. Botrytis cinerea strain BO5-10 was grown on potato dextrose agar at 23 °C for 10–14 d. Spores were harvested and adjusted to a concentration of 105 spores ml−1 with distilled water. A 6 μl aliquot of spore suspension was dropped on Arabidopsis leaves and the lesion size was measured at 3 d after inoculation.
Hormone, CaCl2, and LaCl3 treatments
Cotton roots were treated with 0.1mM salicylic acid, 0.15mM jasmonic acid, 1mM ethylene, and different concentration of CaCl2. Cotton roots were treated with 300 μM LaCl3 before and after V. dahliae infection. Roots treated with sterile water were used as mock control.
RNA extraction and qRT-PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. The quantitative real-time PCR (qRT-PCR) assay was conducted using the SYBR Green Real-Time PCR Master Mix (Toyobo, Japan) and the DNA Engine Opticon 2 Real-Time PCR Detection System (MJ Research). The cotton Histone3 gene or Arabidopsis EF-1α gene was used as the internal control. The expression levels of genes were calculated by using the 2–ΔΔCT or 2–ΔCT method, where CT is the cycle threshold. ΔCT=CTTarget–CTHistone3/EF-1α. ΔΔCT=ΔCTsample–ΔCTcontrol. The amplification efficiency of the samples was quantified and adjusted. All reactions were conducted in triplicate. The primers used in qRT-PCR are listed in Supplementary Table S1 at JXB online.
Electrophoretic mobility shift assay
The ORF of GhMYB108 was cloned into the pMAL-p2X vector to produce maltose-binding protein (MBP)–GhMYB108 fusion protein. The ORF of GhCML11 was fused to the pGEX6P-1 vector to produce glutathione S-transferase (GST)–GhCML11 fusion protein. Fusion proteins were expressed in Escherichia coli strain BL21 and purified. EMSA was performed using biotin-labeled probes and a Pierce LightShift Chemiluminescent EMSA kit (Thermo). The binding reaction was carried out in a 20 μl reaction mixture at room temperature for 30min and then separated on a native 6% polyacrylamide gel in 0.5× Tris-borate/EDTA buffer. To test the effect of GhCML11 on the DNA binding activity of GhMYB108, the reaction mixtures were supplemented with CaCl2 (10 μM) or EGTA (0.5mM), or purified GST–GhCML11 fusion proteins. The labeled probes were detected based on the manufacturer’s instructions.
Dual-luciferase reporter (DLR) assay
The TF activity of GhMYB108 was examined by DLR assay as described by Ohta et al. (2001). The reporter plasmid contains the Luc gene, controlled by the minimal TATA region of the 35S promoter with five GAL4-binding elements upstream. The Renilla luciferase gene driven by the 35S promoter was used as an internal control. To construct effector plasmids, the ORF of GhMYB108 was introduced into the pRT-BD vector to generate 35S-BD-GhMYB108. The Arabidopsis protoplasts were transfected with a mixture of 6 μg of effector plasmid, 6 μg of reporter plasmid, and 1 μg of internal control plasmid by polyethylene glycol (PEG) transformation. After 16h under dark conditions, the luciferase assay was conducted using the DLR assay system (Promega) and a GloMax 20-20 luminometer (Bio-rad).
Subcellular localization
The ORF of GhMYB108 was fused to GFP (green fluorescent protein) and the ORF of GhCML11 was fused to mCherry under the control of the 35S promoter in the expression vector pPZP111 (Hajdukiewicz et al., 1994) to generate pPZP-GhMYB108-GFP and pPZP-GhCML11-mCherry constructs, respectively. Agrobacterium cells (strain GV3101) containing recombinant plasmids were infiltrated into N. benthamiana leaves. The infiltrated plants were incubated for 40h at 23 °C under dark conditions. The cells expressing GFP proteins were stained with DAPI to indicate the nucleus. The signals were visualized with a confocal microscope (Leica TCS SP8, Germany).
For visualization of GhCML11 in onion epidermal cells, 2 μg of plasmid DNA was used to coat gold particles, and the plasmid harboring GFP alone was used as the control. The inner layer of the onion epidermis was bombarded by a gene gun (PDS-1000/He, Bio-Rad). For plasmolysis experiment, cells were treated with 20% sucrose. Signals were visualized by confocal microscopy.
Virus-induced gene silencing
pTRV1 and pTRV2 (Liu et al., 2002) vectors were used for VIGS experiments. The gene-specific fragment for GhMYB108 (Supplementary Fig. S1) or GhCML11 was inserted into pTRV2. Agrobacterium cultures (OD600=1.5) harboring pTRV1 and pTRV2-GhMYB108 or pTRV2-GhCML11 were mixed at a 1:1 ratio and agroinoculated into cotton plants by vacuum infiltration as described by Qu et al. (2012). Alternatively, cotyledons of seedlings were transfected with the mixture using a needle-less syringe as described by Gao et al. (2011). GhCLA1 was used as the positive control (Gao et al., 2011).
Yeast two-hybrid (Y2H) assay
Y2H assay was performed according to the instructions of the manufacturer of the Matchmaker Gold Yeast Two-Hybrid System (Clontech). The ORF of GhMYB108 was cloned into the BD vector pGBKT7 to construct BD-GhMYB108 as bait. The ORF of GhCML11 was inserted into the AD vector pGADT7 to produce the prey constructs. Yeast strain AH109 cells co-transformed with the bait and prey constructs were plated onto SD/–Leu/–Trp DO (DDO) medium. After growth at 30 °C for 72h, the independent colony with the same size was transferred to SD/–Leu/–Trp/–Ade/–His DO (QDO) medium supplemented with 5mM 3-aminotriazole (3-AT). The interactions were also visually detected using an X-gal filter assay.
Pull-down assay
MBP–GhMYB108 and GST–GhCML11 fusion proteins were expressed and purified as described above. The in vitro protein–protein interaction assay was carried out according to the ProFound Pull-Down GST Protein Kit. The eluted proteins were separated on an SDS–PAGE gel and detected by western blot using anti-GST or anti-MBP antibodies (1:4000; Sungene Biotechnology).
Firefly luciferase complementation imaging (LCI) assay
The ORF of GhMYB108 was inserted into the pCAMBIA-NLuc vector. The ORF of GhCML11 was cloned into the pCAMBIA-CLuc vector. Equal amounts of Agrobacterium cultures containing CLuc and NLuc constructs were mixed, and then co-infiltrated into N. benthamiana leaves. The infiltrated leaves were analyzed for relative Luc activity 48h after infiltration using a low-light cooled charge-coupled device camera (Night owl LB985, Germany). Quantitative analysis was performed using the IndiGo software (Berthold Technologies).
Transient expression assay
The transient expression assay was performed as described by Shang et al. (2010). The GhPR4, GhPR5, and GhPDF1.2 promoters were fused with the Luc reporter gene in the plant binary vector pGWB435 (Invitrogen) to generate the reporter constructs GhPR4 pro :Luc, GhPR5 pro :Luc, and GhPDF1.2 pro :Luc, respectively. The ORF of GhMYB108 was cloned into the pBI121 vector to generate the effector construct 35S pro :GhMYB108, and the ORF of GhCML11 was cloned into the pPZP111 vector to generate the effector construct 35S pro :GhCML11. The Agrobacterium strains containing different constructs were infiltrated into N. benthamiana leaves. The infiltrated plants were incubated for 48h at 23 °C. Image capture used the low-light cooled charge-coupled device camera, and calculation of the relative Luc activity was performed using the IndiGo software.
Ca2+-dependent mobility shift assay
The Ca2+-dependent mobility shift assay was conducted according to Garrigos et al. (1991). The GST-tag in GST–GhCML11 was cleaved using PreScission Protease (GE Healthcare), and GhCML11 proteins were electrophoresed on a 15% SDS–PAGE gel in the presence of either 5mM CaCl2 or 5mM EGTA, and then visualized by Coomassie brilliant blue staining.
[Ca2+]cyt staining
Staining of cytosolic Ca2+ was performed as described by Zhang et al. (1998). Cotton seedlings were grown under hydroponic conditions. Agrobacterium cultures harboring pTRV1 and pTRV2 (control), pTRV2-GhMYB108, or pTRV2-GhCML11 were mixed at a 1:1 ratio and agroinoculated into cotton plants by vacuum infiltration, and then the plants were transferred to steam-sterilized vermiculite. After 2 weeks, seedlings were gently uprooted and rinsed with sterile water, and then placed in sterile water for 24h to adapt to hydroponic conditions. The roots were infected by spore suspensions (106 spores ml−1). The cotton roots were then loaded with Ca2+-sensitive fluorescent dye Fluo-4/AM (Invitrogen) at 4 °C for 2h followed by 2h at 25 °C in the dark. The fluorescence of the cotton root cells was visualized with a confocal microscopy. The fluorescence intensity of root cells was determined using Leica LAS AF Lite software.
Transcriptome analysis
For transcriptome analysis, total RNAs were extracted from control (TRV:00) and GhMYB108-silenced (TRV:GhMYB108) plants. The library construction and Illumina sequencing were conducted by BGI (http://www.genomics.cn/en/index). After eliminating the adaptors and low-quality sequences, the sequence reads were used for further analysis. Genes with differentially expressed transcripts [fold change ≥2 and false discovery rate (FDR) <0.001] in GhMYB108-silenced plants compared with control plants were identified. The accession number of the raw transcriptomic data is SRP067059.
Accession numbers
Sequence data for the genes described in this study can be found in the GenBank/EMBL database under the following accession numbers: GhMYB108 (KT281917), GhCML11 (KT281918), AtPDF1.2 (AT5G44420), AtPR4 (AT3G04720), AtPR5 (AT1G75040), AtWRKY18 (AT4G31800), AtWRKY33 (AT2G38470), AtWRKY50 (AT5G26170), AtbHLH87 (AT3G21330), AtWAK2 (AT1G21270), AtFLS2 (AT5G46330), AtBAK1 (AT4G33430), AtLYK4 (AT2G23770), AtANP3 (AT3G06030), AtMKK4 (AT1G51660), AtMKK6 (AT5G 56580), AtAHK4 (AT2G01830), AtRLP12 (AT1G71400), AtCYP82G1 (AT3G25180), AtCYP707A1 (AT4G19230), AtRGA2 (AT1G14920), AtRPP13 (AT3G46530), AtH2A (AT5G54640), AtSOT17 (AT1G18590), and AtPUB23 (AT2G35930).
Results
Expression of GhMYB108 responds to V. dahliae infection
In our ongoing studies of the defense-related genes acting in the response against cotton Verticillium wilt, we frequently noticed the presence of MBS (MYB-binding site) cis-elements in the promoters of the defense-responsive genes. To investigate the role of cotton MYB genes in defense against V. dahliae infection, we first conducted a database search and randomly selected six candidate MYB genes from different subfamilies to compare the pathogen-responsive expression of the MYB genes in upland cotton. Among these MYB genes, one gene (GhMYB108) showed strong induction of transcription upon pathogen inoculation (Supplementary Fig. S2). Since two members of this subfamily of MYB genes were shown to participate in defense against fungus infection in Arabidopsis or wheat (Mengiste et al., 2003; Z. Zhang et al., 2012), we focused our study on the functional mechanism of the GhMYB108 gene in protection against V. dahliae infection in cotton.
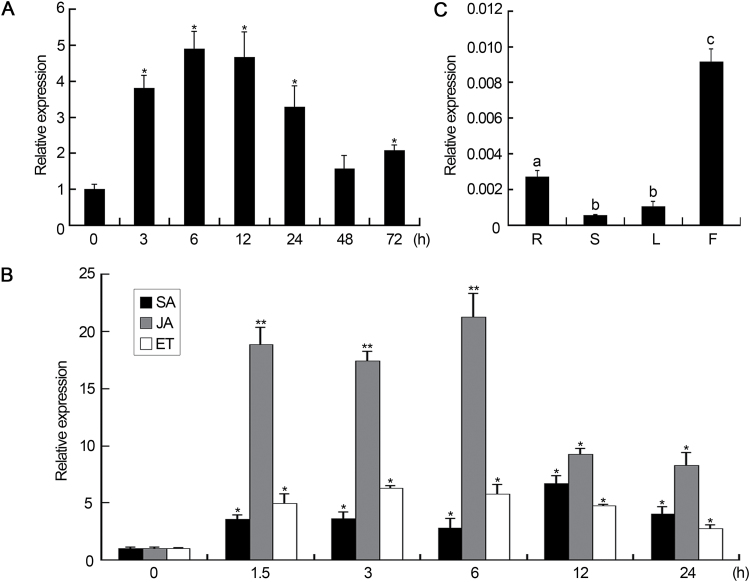
qRT-PCR analysis was performed to measure the time course of pathogen-responsive expression of GhMYB108. As shown in Fig. 1A, the expression of GhMYB108 increased in roots after V. dahliae infection and reached a maximal level at 6h post-inoculation. Next, GhMYB108 expression was analyzed after treatment with the defense-related signaling molecules salicylic acid, jasmonic acid, and ethylene. The results showed that these three signaling molecules enhanced the accumulation of GhMYB108 transcripts to different extents (Fig. 1B), supporting the idea that GhMYB108 could be involved in defense against V. dahliae invasion in cotton plants. Expression of GhMYB108 was also examined in various organs of the cotton plant. GhMYB108 transcripts accumulated to a higher level in the root, which is the site of the V. dahliae invasion, as compared with the stem and leaf (Fig. 1C). The expression of GhMYB108 was the highest in flowers, implying that GhMYB108 may also function in flower development.
Fig. 1.
Expression pattern of the GhMYB108 gene in cotton plants. (A) Accumulation of GhMYB108 transcripts in cotton roots in response to V. dahliae infection. Error bars represent the SD of three biological replicates. Asterisks indicate statistically significant differences, as determined by Student’s t-test (*P<0.05). (B) Expression of GhMYB108 after treatments with salicylic acid, jasmonic acid, and ethylene. Asterisks indicate statistically significant differences, as determined by Student’s t-test (*P<0.05, **P<0.01). (C) qRT-PCR analysis of GhMYB108 expression in root (R), stem (S), leaf (L), and flower (F) of cotton plants. Different letters indicate statistically significant differences at P<0.05 (Student’s t-test, three biological replicates).
GhMYB108 is a functional transcription activation factor
EMSA was used to test the DNA-binding activity of GhMYB108. The results showed that GhMYB108 proteins and labeled probe could form a complex, and addition of non-labeled probes dramatically reduced the observed DNA binding activity, indicating that GhMYB108 could bind specifically to the MBS cis-element (Fig. 2A).
Fig. 2.
Transcriptional activity of GhMYB108 and subcellular localization of GhMYB108–GFP fusion proteins. (A) EMSA analysis of the binding of GhMYB108 to the MBS cis-elements. GhMYB108 proteins were incubated with biotin-labeled probe (2× TAACGGAC) in the absence or presence of a 20-, 50-, or 100-fold excess of unlabeled competitor. (B) Transcriptional activation activity of GhMYB108 in Arabidopsis protoplasts. The empty vector pRT-BD and pRT-BD-VP16 were used as negative and positive control, respectively. Error bars represent the SD of three biological replicates. Asterisks indicate statistically significant differences, as determined by Student’s t-test (*P<0.05, **P<0.01). (C) Subcellular localization of intact and truncated fusion proteins. GFP, GhMYB108–GFP, GhMYB108ΔC–GFP, and GhMYB108ΔN–GFP fusion proteins were transiently expressed in N. benthamiana leaves. GFP fluorescence was visualized by confocal microscopy. Numbers represent amino acid residues. Scale bars=20 μm. (This figure is available in colour at JXB online.)
The TF activity of GhMYB108 was examined using the DLR assay in Arabidopsis protoplasts. Isolation and transformation of Arabidopsis protoplasts were carried out as described by He et al. (2007). Compared with the negative control, the protoplasts harboring GhMYB108 showed significantly higher luciferase activity (Fig. 2B), indicating that GhMYB108 can activate the transcription of the Luc reporter gene in vivo.
The region containing the R2R3 domain is required for the nuclear localization of GhMYB108
To examine the nuclear distribution of GhMYB108, Agrobacterium cells transformed with the GhMYB108-GFP fusion and GFP control constructs were infiltrated into N. benthamiana leaves. Transiently expressed GhMYB108–GFP proteins were mainly localized in the nucleus, whereas GFP control was diffusely localized throughout the cytoplasm and nucleus (Fig. 2C).
As no nuclear localization signal was found in the GhMYB108 protein sequence, we wished to know which region of the protein might be responsible for its nuclear distribution. To this end, plasmids harboring cDNA fragments encoding either C-terminus-deleted GhMYB108-GFP (GhMYB108ΔC-GFP) or N-terminus-deleted GhMYB108-GFP (GhMYB108ΔN-GFP) were constructed, and Agrobacterium cells transformed with these constructs were separately infiltrated into N. benthamiana leaves. GhMYB108ΔC–GFP proteins were localized in the nucleus, while GhMYB108ΔN–GFP proteins were distributed in the cytoplasm without entry into the nucleus (Fig. 2C). These results indicate that the region containing the R2R3 domain of GhMYB108 is required for the nuclear localization of GhMYB108.
Silencing of GhMYB108 in cotton plants impairs the tolerance to V. dahliae
The VIGS strategy was employed to study the role of GhMYB108 in response to V. dahliae. GhCLA1 (produces an albino phenotype when silenced) was used as the positive control. At 14 d after agroinfiltration with the GhCLA1 construct, the cotton leaves displayed the expected photobleaching phenotype (Supplementary Fig. S3), showing that the VIGS system worked well under our experimental conditions. Compared with the control, expression of GhMYB108 was obviously reduced in GhMYB108-silenced plants (Fig. 3A). To see if off-target silencing of other MYB genes was caused in the VIGS plants, we examined the transcription levels of six potential off-target genes, and the results showed that expression of these genes was not affected (Supplementary Figs S1, S4).
Fig. 3.
Increased susceptibility of GhMYB108-silenced cotton plants to V. dahliae. (A) Analysis of GhMYB108 expression levels. Total RNAs were extracted from leaves of cotton plants at 14 d post-agroinfiltration, and the expression level of GhMYB108 in VIGS plants was compared with that of the control plant (TRV:00). Asterisks indicate statistically significant differences, as determined by Student’s t-test (**P<0.01). (B) Disease symptoms of control (TRV:00) and GhMYB108-silenced (TRV:GhMYB108) plants infected by V. dahliae. (C) Rate of diseased plants and disease index of the control and GhMYB108-silenced plants. Error bars represent the SD of three biological replicates (n≥30). Asterisks indicate statistically significant differences, as determined by Student’s t-test (*P<0.05). (D) Comparison of a longitudinal section of stem between control and GhMYB108-silenced cotton plants 20 d after V. dahliae infection. Arrows indicate the vascular part of the stem. (E) Fungal recovery assay. The stem sections from cotton plants 20 d after V. dahliae infection were plated on potato dextrose agar medium. Photos were taken at 6 d after plating. The number of stem sections on which the fungus grew showed the extent of fungal colonization. (This figure is available in colour at JXB online.)
GhMYB108-silenced and control plants were challenged with V. dahliae. Before inoculation of the pathogen, no significant phenotype change was observed in VIGS plants as compared with the control plants (Fig. 3B, left panel). At 20 d after infection, GhMYB108-silenced plants began to show increased susceptibility to V. dahliae, with more severe wilting and yellowing symptoms than control plants. After a further 10 d, the disease symptoms of GhMYB108-silenced plants became more evident (Fig. 3B, right panel). The rate of diseased plants and the disease index were higher for the GhMYB108-silenced plants than for the control plants (Fig. 3C). In addition, the vascular tissue of GhMYB108-silenced cotton plants turned brown 20 d after infection with V. dahliae, but that of the control plants was not chlorotic when tested at the same time (Fig. 3D). These observations indicated that silencing of GhMYB108 enhanced the susceptibility to V. dahliae. The fungal recovery assay from stem sections of inoculated cotton plants also confirmed the role of GhMYB108 in cotton defense against V. dahliae (Fig. 3E).
Overexpression of GhMYB108 enhances tolerance to V. dahliae in transgenic Arabidopsis plants
A gain-of-function approach was also employed to study the function of GhMYB108 in the defense response. Due to technical difficulties and long duration of cotton transformation, we used the model plant Arabidopsis, which is also a host for V. dahliae. Three lines (7-4, 35-3, and 39-2) of transgenic plants with different expression levels of GhMYB108 were selected for further study (Fig. 4A). Arabidopsis plants at ~4 weeks old were inoculated with V. dahliae. Fifteen days after inoculation, the leaves of Arabidopsis began to show wilting and yellowing symptoms, and the plants grew stunted and short. Compared with the wild type, the transgenic plants showed much weaker symptoms at 22 d post-inoculation (Fig. 4B). The rate of diseased plants and disease index of the transgenic plants were significantly lower than those of the wild-type plants (Fig. 4C, D), showing that ectopic overexpression of GhMYB108 conferred increased disease tolerance to V. dahliae in Arabidopsis plants. To verify the observed phenotype further, the fungal biomass was measured by real-time PCR. Less fungal DNA was measured in transgenic plants than in wild-type plants (Fig. 4E), supporting the conclusion that GhMYB108-transgenic plants were more tolerant to V. dahliae infection.
Fig. 4.
Enhanced disease tolerance of Arabidopsis plants overexpressing GhMYB108. (A) Expression levels of GhMYB108 in WT (wild-type) and transgenic Arabidopsis lines (7-4, 35-3, and 39-2). (B) Symptoms of WT and GhMYB108 transgenic plants inoculated with V. dahliae for 22 d. (C and D) Rate of diseased plants and disease index of WT and transgenic plants. Error bars indicate the SD of three biological replicates with 36 plants per repeat. (E) Quantification of fungal biomass. Real-time PCR analysis was conducted to compare the transcript levels between the ITS gene (as a measure for fungal biomass) of V. dahliae and the Rubisco gene of Arabidopsis (for equilibration) at 22 d post-inoculation. Relative amounts of fungal DNA were set to 100% for the WT. Asterisks indicate statistically significant differences, as determined by Student’s t-test (*P<0.05, **P<0.01). (This figure is available in colour at JXB online.)
In addition to V. dahliae, we also inoculated the GhMYB108-overexpressing Arabidopsis plants with two other pathogens, the bacterium Pst DC3000 and the fungus B. cinerea. The results showed that these plants were less susceptible to B. cinerea as compared with the wild type, but similar disease symptoms were found between the wild-type and transgenic plants infected with Pst DC3000, indicating that GhMYB108 overexpression rendered the transgenic Arabidopsis plants specifically more tolerant to the fungal pathogen (Supplementary Fig. S5).
GhMYB108 interacts with GhCML11
The Y2H system was employed to identify protein(s) that may interact with GhMYB108. Screening the cDNA library of cotton roots infected by V. dahliae identified a cDNA that encodes a CaM-like protein (designated GhCML11). Direct Y2H assays confirmed the interaction between the two proteins (Fig. 5A). A pull-down assay was performed to verify further the interaction of the two proteins (Fig. 5B). Equal amounts of lysates containing GST–GhCML11 were incubated with immobilized MBP or MBP–GhMYB108 proteins. As expected, GhCML11 bound to GhMYB108, but not to the control MBP proteins. Subsequently, lysates containing MBP–GhMYB108 were incubated with immobilized GST or GST–GhCML11 proteins. GhMYB108 bound to GhCML11, but not to the control GST proteins. These results confirmed that GhMYB108 and GhCML11 could interact.
Fig. 5.
Interaction of GhMYB108 and GhCML11 proteins. (A) Yeast two-hybrid assay to detect interaction between GhMYB108 and GhCML11. The yeast strain containing the indicated plasmids was grown on SD/–Leu/–Trp DO (DDO) plates and SD/–Leu/–Trp/–Ade/–His DO (QDO) plates (containing 5mM 3-AT) for 3 d. Interaction of GhMYB108 with the AD domain in the pGADT7 empty vector was used as a negative control. (B) Pull-down assay. GST–GhCML11 fusion protein was used as bait, and MBP–GhMYB108 fusion protein was used as prey. Alternatively, MBP–GhMYB108 fusion protein was used as bait, and GST–GhCML11 fusion protein was used as prey. The anti-MBP and anti-GST antibodies were used to detect bait and prey proteins. MBP and GST proteins were used as negative controls. (C) LCI analysis of the interaction between GhMYB108 and GhCML11. Agrobacterium strains containing the indicated pairs were co-expressed in N. benthamiana. The luminescent signal was collected at 48h after infiltration. (D) Quantification of relevant Luc activities in (C). Error bars represent the SD of three biological replicates. Asterisks indicate statistically significant differences, as determined by Student’s t-test (**P<0.01). (This figure is available in colour at JXB online.)
To verify the interaction of the two proteins in planta, an LCI assay (Chen et al., 2008) was conducted. As shown in Fig. 5C and D, strong Luc activity was detected in N. benthamiana leaves, but no significant Luc activity was detected in the negative controls.
Since GhCML11 interacts with GhMYB108, we investigated whether the subcellular localization of GhCML11 was similar with GhMYB108. Agrobacterium cells containing GhMYB108-GFP and GhCML11-mCherry were co-infiltrated into N. benthamiana leaves. Indeed, GhCML11 co-localized with GhMYB108 in the nucleus (Fig. 6A). In addition to the nucleus, we also noticed GhCML11 in the periphery of the N. benthamiana pavement cells (Fig. 6A). To see this subcellular localization of GhCML11 more clearly, we bombarded the GhCML11-GFP construct into onion epidermal cells and used plasmolysis to examine the plasma membrane and apoplast. GhCML11–GFP fluorescence was observed in both the nucleus and cytoplasm (Fig. 6B). Interestingly, we found that some GhCML11 proteins remained in the apoplast after plasmolysis. However, no free GFP signal was detected in the extracellular region after plasmolysis in the cells transformed with GFP alone. Thus, as reported for some CaMs in other plants (Cui et al., 2005; Wang et al., 2013), GhCML11 is probably also an apoplastic protein. As a protein that lacks a signal peptide but can be secreted from the cell independent of the endoplasmic reticulum/Golgi system can be defined as a non-classically secreted protein (Nickel and Rabouille, 2009; Drakakaki and Dandekar, 2013), GhCML11 belongs to such a protein group based on its sequence and localization. Indeed, GhCML11 is predicted to be a non-classically secreted protein by the online software http://www.cbs.dtu.dk/services/SecretomeP-1.0/.
Fig. 6.
Subcellular localization of GhCML11 proteins. (A) Co-localization of GhMYB108 and GhCML11 in the nucleus. Agrobacterium strains containing the indicated pair of GhMYB108-GFP and GhCML11-mCherry were co-expressed in N. benthamiana. The signal was visualized with confocal microscopy. Scale bars=20 μm. (B) Localization of GhCML11 transiently expressed in onion epidermal cells. The two left-hand panels show the cells containing the empty vector before and after plasmolysis. The two right-hand panels show the cells harboring the GhCML11–GFP construct before and after plasmolysis. Arrows indicate the cell wall region after plasmolysis. Scale bars=20 μm. (This figure is available in colour at JXB online.)
GhCML11 promotes the transcriptional function of GhMYB108
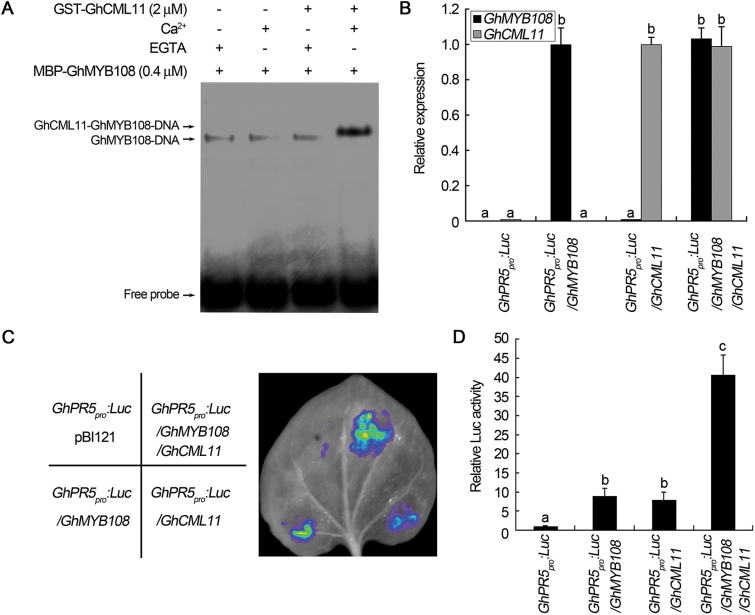
Since GhMYB108 acts as a TF, the interaction between GhCML11 and GhMYB108 may have an effect on its activity. To test this possibility, EMSA was performed in the presence of GhCML11. As shown in Fig. 7A, GhMYB108 bound to the MBS cis-elements and formed a band representing the DNA–protein complex; when GhCML11 and Ca2+ were present in the reaction simultaneously, a supershifted band with markedly enhanced intensity appeared. When GhCML11 was included in the reaction without addition of Ca2+, no effect was observed on the DNA binding activity of GhMYB108 either. The result indicated that the DNA binding activity of GhMYB108 was enhanced by its interaction with GhCML11 in a Ca2+-dependent manner in vitro. The EMSA was conducted to determine the Ca2+ binding property of GhCML11. It is known that CaMs undergo conformational changes and exhibit an increase in their electrophoretic migration rates after binding Ca2+ (Garrigos et al., 1991; Wang et al., 2015). As shown in Supplementary Fig. S6, the mobility of GhCML11 was increased in the presence of Ca2+, demonstrating that GhCML11 is a functional Ca2+-binding protein.
Fig. 7.
GhCML11 promotes transcriptional activity of GhMYB108. (A) Effect of GhCML11 on the DNA binding activity of GhMYB108. (B) qRT-PCR analysis of GhMYB108 and GhCML11 expression in the infiltrated N. benthamiana leaves transformed with the indicated constructs in (C). Different letters indicate statistically significant differences at P<0.01 (Student’s t-test, n≥15, three biological repeats). (C) Effect of GhCML11 on the transcription factor activity of GhMYB108. Luminescence imaging was performed 48h after co-infiltration. (D) Quantitative analysis of luminescence intensity in (C). Different letters indicate statistically significant differences at P<0.05 (Student’s t-test, n=30, three biological repeats).
We next conducted an in vivo test to see if the effect of GhCML11 on GhMYB108 DNA binding activity reflects its role in the TF activity of GhMYB108. As it was reported that a plant MYB could bind to the promoter sequence of PR5 (thaumatin-like protein) and regulate its transcription (Kenton et al., 2000; Z. Zhang et al., 2012), we performed a transient expression assay by using the promoter sequence of a cotton PR5 gene to drive the expression of the reporter gene with or without the presence of GhCML11 (Fig. 7B–D). First, the binding of GhMYB108 to the GhPR5 promoter was tested by EMSA. As shown in Supplementary Fig. S7C, GhMYB108 bound to the GhPR5 promoter efficiently. The GhPR5 promoter was then fused to the Luc reporter gene (GhPR5 pro :Luc) and infiltrated into N. benthamiana leaves. Two days later, the expression of GhMYB108 and GhCML11 was confirmed by qRT-PCR (Fig. 7B) and Luc expression was examined. The results showed that the GhPR5 promoter drove Luc expression weakly on its own, but co-expression of GhPR5 Pro :Luc with GhMYB108 created an obvious increase in Luc activity, indicating that GhMYB108 activated the expression of Luc driven by the PR5 promoter. Luc activity was also enhanced when 35S:GhCML11 was co-transformed with GhPR5 Pro :Luc, probably caused by endogenous GhMYB108 homolog(s) in N. benthamiana, which might act co-operatively with GhCML11 and promote the GhPR5 promoter activity. Co-expression of the GhPR5 Pro :Luc reporter with GhMYB108 and GhCML11 led to much stronger Luc intensity than in the cells injected with the GhPR5 Pro :Luc reporter and GhMYB108 (Fig. 7C, D), demonstrating that GhCML11 could promote the transcriptional activation activity of GhMYB108 in plant cells.
GhMYB108 regulates the transcription of GhCML11
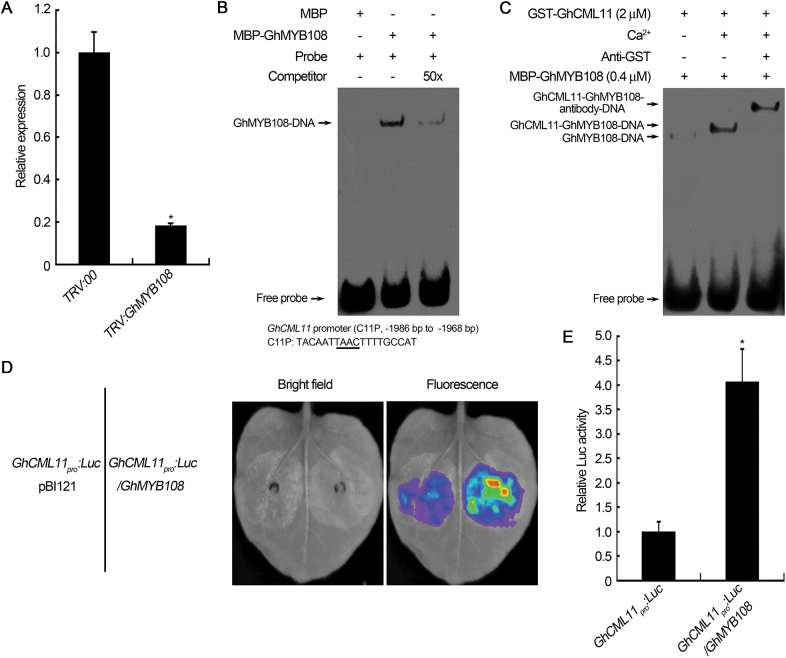
In GhMYB108-silenced cotton plants, the expression of GhCML11 was also suppressed (Fig. 8A). This raised the possibility that GhMYB108 may affect the transcription of GhCML11. To test this, the promoter sequence of GhCML11 was isolated and subjected to EMSA analysis. As shown in Fig. 8B, GhMYB108 protein could form a complex with the promoter sequence of GhCML11, and non-labeled probes considerably decreased the binding of GhMYB108 proteins to the labeled probes, indicating that GhMYB108 could specifically bind to the tested sequence of the GhCML11 promoter. As it was observed that binding of GhMYB108 to MBS cis-elements could be increased by GhCML11, we also tested if GhCML11 could enhance the binding of GhMYB108 to its own promoter. As shown in Fig. 8C, a supershifted signal with enhanced intensity appeared when GhCML11 proteins and Ca2+ were added in the reaction; when an anti-GST antibody was added to the binding mixture, the band was shifted even further, indicating that GhCML11 was present in the supershifted band.
Fig. 8.
GhMYB108 regulates the transcription of GhCML11. (A) Expression analysis of GhCML11 in control (TRV:00) and GhMYB108-silenced (TRV:GhMYB108) plants. Asterisks indicate statistically significant differences, as determined by Student’s t-test (*P<0.05). (B) EMSA of the binding of GhMYB108 to the promoter of GhCML11. The underlined sequence indicates the core motif of the MYB-binding site. (C) Analysis of the effect of GhCML11 proteins on the binding activity of GhMYB108 to the GhCML11 promoter. Anti-GST antibody against GST-tagged GhCML11 was added in the reaction to detect the presence of GhCML11 in the GhMYB108–DNA complexes. (D) Activation of GhCML11 transcription by GhMYB108. Luminescence imaging was performed 48h after co-infiltration of N. benthamiana leaves with equal amounts of Agrobacterium cells containing the indicated constructs on the left panel. (E) Quantitative analysis of luminescence intensity in (D). Error bars represent the SD (n=30) of three biological replicates. Asterisks indicate statistically significant differences, as determined by Student’s t-test (*P<0.05). (This figure is available in colour at JXB online.)
To investigate further whether GhMYB108 could activate the transcription of GhCML11 in plant cells, the promoter of GhCML11 was inserted into the vector pGWB435 with the Luc reporter gene. Co-expression of the GhCML11 promoter fused to the Luc reporter gene with 35S:GhMYB108 showed an obvious increase in Luc activity as compared with the control (Fig. 8D, E), indicating that GhMYB108 activated the expression of Luc driven by the GhCML11 promoter.
Calcium signaling is active in response to V. dahliae infection in cotton
Ca2+ plays an important role in plant immune responses (Lecourieux et al., 2006). To gain more insight into the involvement of Ca2+-mediated signaling in the cotton defense response against V. dahliae, we performed a time course experiment to assess the change of [Ca2+]cyt in response to V. dahliae infection. The cotton root cells were loaded with the Ca2+ indicator Fluo-4/AM. The fluorescence intensity in the root cells of control plants increased significantly after inoculation with the pathogen, reaching a peak at 4min and then decreasing quickly (Fig. 9). The result indicated that Ca2+ influx into the cytosol occurred in response to V. dahliae infection.
Fig. 9.
Ca2+ levels in cytosol of root cells in control, GhMYB108-silenced, and GhCML11-silenced cotton plants. (A) Change in fluorescent intensity of control, GhMYB108-, and GhCML11-silenced cotton root cells treated with Fluo-4/AM at the indicated time points after inoculation with V. dahliae. Error bars represent the SD (n≥10) of three biological replicates. Asterisks indicate statistically significant differences, as determined by Student’s t-test (*P<0.05). (B) Fluorescence images of cotton root cells at 0, 4, and 60min post-inoculation with V. dahliae. The fluorescence signals were visualized by confocal microscopy. Scale bars=20 μm.
The fluorescence intensity in the root cells of GhMYB108-silenced and GhCML11-silenced plants was compared with that of the control plants. Before V. dahliae infection, the fluorescence intensity in GhMYB108- and GhCML11-silenced root cells was similar to that of control root cells, but it increased relatively less upon pathogen inoculation, indicating that the influx of [Ca2+]cyt upon V. dahliae infection was influenced in these cells (Fig. 9). These results show that Ca2+ influx into the cytosol occurs in response to V. dahliae invasion and the expression levels of GhCML11 and GhMYB108 had an impact on this process.
Transcriptomic analysis of genes affected in GhMYB108-silenced cotton plants
Comparative transcriptome analysis was employed to identify genes possibly regulated by GhMYB108. A total of 391 differentially expressed genes (fold change ≥2 and FDR <0.001) were identified, of which 181 genes were up-regulated and 210 genes were down-regulated (Supplementary Table S2). Among the differentially expressed genes, a large number were involved in the biological processes of transcriptional regulation, signal transduction, developmental process, biosynthesis, and metabolism (Fig. 10A). In accordance with the above results on the relationship between GhMYB108 and Ca2+/GhCML11, several calcium signaling genes were down-regulated in GhMYB108-silenced cotton plants (Fig. 10B).
Fig. 10.
Transcript profiling analysis of differentially expressed genes in the GhMYB108-silenced cotton plants. (A) Functional classification of genes up- or down-regulated in GhMYB108-silenced cotton plants. The percentage of each category of up-regulated or down-regulated genes indicates the number of genes in that category relative to the 181 annotated up-regulated or 210 annotated down-regulated genes. (B) The expression levels of calcium signaling genes between control (TRV:00) and GhMYB108-silenced (TRV:GhMYB108) plants. These genes included Ca2+-binding protein genes GhEHD2 (EPS15 homology domain protein), GhPBP1 (PINOID-binding protein), GhNRT1.2 (Nitrate transporter1.2), GhRBOHF (Respiratory burst oxidase homolog protein), calmodulin-binding protein genes GhIQD1, GhIQD14, and GhIQD31 (IQ-domain protein), and the CBL-binding protein gene GhCIPK6. Error bars represent the SD of three biological replicates. Asterisks indicate statistically significant differences, as determined by Student’s t-test (*P<0.05).
Among the identified differentially expressed genes, 23 defense-related genes were inhibited in GhMYB108-silenced plants (Supplementary Table S3). The expression of these genes in GhMYB108-silenced cotton plants was then evaluated by qRT-PCR, which verified the down-regulation of these genes (Supplementary Fig. S8). We also analyzed the expression of these genes in GhMYB108-overexpressing Arabidopsis (Supplementary Figs S7B, S9), and observed that 14 out of 23 genes were up-regulated. These results indicate that there is a correlation between expression of GhMYB108 and these defense-related genes. As a previous study suggested that MYB may bind to the promoter sequences of some defense-related genes, we analyzed the promoter sequences of these 14 genes for the presence of MBS cis-elements, and found that several of these genes harbored the MBS motifs in their promoters. Of these, we chose PDF1.2 (defensin-like gene), PR4, and PR5 (see above), which showed altered expression in both GhMYB108-overexpressing and GhMYB108-silenced plants (Supplementary Fig. S7A, B), and tested the binding of GhMYB108 to their promoter sequences by EMSA (Supplementary Fig. S7C, D). GhMYB108 could bind to the promoter fragments of these three genes. In addition, GhMYB108 activated expression of Luc driven by the PDF1.2, PR4, and PR5 promoters (Supplementary Fig. S10). These results suggest that GhMYB108 may also be able to bind to the promoters of these genes and activate their transcription.
Discussion
GhMYB108 and GhCML11 form a positive feedback loop to regulate GhCML11 transcription
MYB proteins are one of the largest families of TFs and have been implicated to function in plant defense by regulating defense-related transcriptional responses (Buscaill and Rivas, 2014). In this study, we show that GhMYB108 is a pathogen-responsive gene and that inhibition of its expression by VIGS resulted in increased disease susceptibility of cotton plants, indicating that GhMYB108 is involved in the defense response against V. dahalie in cotton. By Y2H assay, we found that GhMYB108 interacted with GhCML11 and the two proteins had a synergetic relationship. On the one hand, GhMYB108 functions as a TF to activate GhCML11 expression. On the other hand, GhCaM11 acts as a transcriptional activator to enhance the activity of GhMYB108. Thus, the two proteins form a positive feedback loop to enhance GhCML11 transcription. Previous work reported that the Arabidopsis MYB2 interacted with Glycine max CaMs for abiotic stress tolerance in Arabidopsis (Yoo et al., 2005). Here, we provide a novel line of evidence showing the interaction and co-operative function of MYB and CaM, which contributed to the biotic stress tolerance in cotton.
The V. dahliae-induced redistribution of Ca2+ may depend on the apoplastic GhCML11
Ca2+ plays a critical role in plant innate immunity, and Ca2+ influx is an early event in plant defense response against pathogen attack (Ma, 2011). In our study, we observed a clear Ca2+ influx into the cytosol of cotton root cells upon V. dahliae infection. Through comparative transcriptome analysis, we found altered expression of a number of genes encoding calcium-binding proteins. These results show that Ca2+ influx is tightly associated with the defense against V. dahliae infection in cotton.
The apoplast is a large Ca2+ pool in plants, and some cytosolic CaMs can be secreted into the apoplast. These apoplastic CaMs promote the entry of Ca2+ from the apoplast into the cytosol (Wang et al., 2009; Jiang et al., 2014). It was demonstrated that Ca2+ redistribution across the plasma membrane is required for pollen tube growth (Wang et al., 2013). Using onion epidermis as an experimental system, we found that a portion of GhCML11 proteins is distributed in the apoplast. It will be interesting to investigate whether the apoplastic localization is involved in modulating the Ca2+ influx, which contributes to subsequent defense responses in cotton cells. In support of this notion, we found that the pathogen-induced Ca2+ influx was disturbed in root cells in GhCML11-silenced cotton plants, which was coupled with the increased disease susceptibility. It is likely that when expression of GhCML11 was reduced, less GhCML11 protein was secreted into the apoplasts, resulting in reduced influx of Ca2+ into the cytosol and, as a consequence, disturbed defense responses. This result provides novel hints on the function of apoplastic CaMs in the plant immune response. Further study is required to assess the links between dynamic redistribution of Ca2+ and GhCML11 in defense response.
In GhMYB108-silenced cotton root cells, Ca2+ influx was also altered upon pathogen attack (Fig. 9). This could be due to reduced expression of GhCML11, which was caused by silencing of GhMYB108. In this regard, GhMYB108 is also functionally linked to the Ca2+ redistribution during responses to pathogen infection.
GhMYB108, calcium, and GhCML11 function interdependently to mediate defense responses
A mechanism by which TFs, CaM, and Ca2+ function co-operatively to de-repress the expression of the immune system has been proposed based on studies on the Arabidopsis TF CAMTA3 (Zhang et al., 2014). According to this model, plant TFs such as CAMTA3 bind to CaM and repress target gene expression prior to pathogen attack (Du et al., 2009; Nie et al., 2012). Upon pathogen infection, with the elevation of nuclear Ca2+ that binds to the CaM–TF complex, the TF is dissociated from CaM and degraded by ubiquitin-mediated destruction and, as a consequence, expression of the immune system is de-repressed (Zhang et al., 2014; Fromm and Finkler, 2015). Here, we found that GhMYB108 is a transcriptional activator and GhCML11 enhances its activity in the presence of Ca2+. The expression of defense genes upon pathogen attack is by a mechanism of activation in this case, thus different from the mechanism involving CAMTA3.
EMSA analysis showed that GhCML11 interacted with GhMYB108 and enhanced the transcriptional activity of GhMYB108 in a Ca2+-dependent manner, indicating that Ca2+ is critical for GhMYB108–GhCML11–DNA binding efficiency. Accordingly, transcription of GhMYB108 or GhCML11 in cotton roots was induced by CaCl2, and when cotton roots were treated with LaCl3 (a Ca2+ influx blocker), the induction of GhMYB108 and GhCML11 expression was inhibited upon pathogen attack (Supplementary Fig. S11). On the other hand, we found that the pathogen-responsive Ca2+ influx was interrupted upon silencing of GhCML11 or GhMYB108, indicating that these proteins in turn had an effect on Ca2+ uptake which may affect the calcium-mediated signaling in the defense response. Taken together, our results demonstrate that GhMYB108, Ca2+, and GhCML11 can form a functional unit to regulate gene expression in cotton’s response to V. dahliae invasion.
GhMYB108 and GhCML11 contribute to protection against V. dahliae invasion in cotton
Verticillium wilt is the most serious disease affecting cotton production. In our study we observed that the expression of GhMYB108 is induced by pathogen attack and by defense-related signaling molecules; knock down of GhMYB108 expression by VIGS impaired the disease tolerance to V. dahliae in cotton plants, and ectopic overexpression of GhMYB108 enhanced disease tolerance to V. dahliae in transgenic Arabidopsis plants. Also, expression of a number of defense-related genes was inhibited when GhMYB108 was silenced, and this may be the cause of the impaired disease tolerance to V. dahliae invasion, by either a direct or an indirect mechanism. Based on our results, we speculate that GhMYB108 is a positive regulator of the cotton defense response to V. dahliae.
Some CaM genes have been reported to be involved in defense responses (Yamakawa et al., 2001; Park et al., 2004). For example, overexpression of GmCaM-4/-5 in wild-type Arabidopsis enhances disease resistance and induces PR gene expression (Park et al., 2004). In this study, we found that the expression of GhCML11 was highest in the root compared with the stem and leaves, and its expression was also induced by V. dahliae invasion (Supplementary Fig. S12). Cotton plants with reduced expression of GhCML11 showed decreased disease tolerance compared with control plants (Supplementary Fig. S13). These results indicate that GhCML11 is also an important contributor in defense against Verticillium wilt in cotton. It should be mentioned that in addition to the nucleus and apoplast, GhCML11 proteins are also present in the cytoplasm. It is known that CaM in the cytosol acts as a calcium sensor and transmits the Ca2+ signal by interacting with target proteins (Yang and Poovaiah, 2003). Thus, apart from its roles in the nucleus and apoplast, GhCML11 may also participate in calcium signaling in the cytosol as do other CaMs.
Due to the difficulty in generating Verticillium-resistant cotton cultivars by traditional breeding, it is desirable to make breakthroughs in this field through genetic manipulation. Based on our data, we suggest that GhMYB108 and GhCML11 may be suitable candidate genes for molecular breeding of upland cotton cultivars with high tolerance to Verticillium wilt.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Multiple sequence alignments of GhMYB108 and potential off-target MYB genes.
Figure S2. Expression pattern of GhMYB genes upon V. dahliae infection in cotton plant.
Figure S3. Photobleaching phenotype in GhCLA1-silenced cotton plants.
Fig.ure S 4. qRT-PCR analysis of expression levels of six potential off-target MYB genes in control and GhMYB108-silenced plants.
Figure S5. Disease symptoms of GhMYB108-overexpressing Arabidopsis plants inoculated with B. cinerea or Pst DC3000.
Figure S6. The Ca2+-dependent mobility shift assay of GhCML11.
Figure S 7. Expression of PDF1.2, PR4, and PR5 genes in GhMYB108-silenced and GhMYB108-overexpressing plants, and binding of GhMYB108 to their promoter sequences.
Figure S8. Verification of transcriptomic data in GhMYB108-silenced cotton plants.
Figure S9. Expression levels of defense-related genes in GhMYB108 transgenic Arabidopsis plants.
Figure S10. Transient expression analysis of GhCML11-enhanced transcriptional activation activity of GhMYB108.
Figure S11. Effects of Ca2+ on the expression of GhMYB108 and GhCML11.
Figure S12. Expression pattern of GhCML11 in cotton plants.
Figure S13. Increased susceptibility of GhCML11-silenced cotton plants to V. dahliae.
Table S1. Primers used in this study.
Table S2. Genes differentially expressed in GhMYB108-silenced plants determined by comparative transcript profiling analysis.
Table S3. Defense-related genes down-regulated in GhMYB108-silenced cotton plants.
Acknowledgements
We are grateful to Lei Su and Yao Wu (Institute of Microbiology, Chinese Academy of Sciences) for technical assistance with confocal microscopy analysis. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB11040600) and the National Science Foundation of China (grant no. 31401033).
References
- Bolek Y, El-Zik KM, Pepper AE, Bell AA, Magill CW, Thaxton PM, Reddy OUK. 2005. Mapping of verticillium wilt resistance genes in cotton. Plant Science 168, 1581–1590. [Google Scholar]
- Bouché N, Yellin A, Snedden WA, Fromm H. 2005. Plant-specific calmodulin-binding proteins. Annual Review of Plant Biology 56, 435–466. [DOI] [PubMed] [Google Scholar]
- Buscaill P, Rivas S. 2014. Transcriptional control of plant defence responses. Current Opinion in Plant Biology 20, 35–46. [DOI] [PubMed] [Google Scholar]
- Cai Y, He X, Mo J, Sun Q, Yang J, Liu J. 2009. Molecular research and genetic engineering of resistance to Verticillium wilt in cotton. African Journal of Biotechnology 8, 7363–7372. [Google Scholar]
- Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X, Zhou JM. 2008. Firefly luciferase complementation imaging assay for protein–protein interactions in plants. Plant Physiology 146, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson D, Ekengren SK, Martin GB, Dobney SL, Snedden WA. 2005. Calmodulin-like proteins from Arabidopsis and tomato are involved in host defense against Pseudomonas syringae pv. tomato . Plant Molecular Biology 58, 887–897. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Cui S, Guo X, Chang F, Cui Y, Ma L, Sun Y, Sun D. 2005. Apoplastic calmodulin receptor-like binding proteins in suspension-cultured cells of Arabidopsis thaliana . Journal of Biological Chemistry 280, 31420–31427. [DOI] [PubMed] [Google Scholar]
- Drakakaki G, Dandekar A. 2013. Protein secretion: how many secretory routes does a plant cell have? Plant Science 203–204, 74–78. [DOI] [PubMed] [Google Scholar]
- Du L, Ali GS, Simons KA, Hou J, Yang T, Reddy AS, Poovaiah BW. 2009. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 457, 1154–1158. [DOI] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis . Trends in Plant Science 15, 573–581. [DOI] [PubMed] [Google Scholar]
- Fradin EF, Thomma BP. 2006. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum . Molecular Plant Pathology 7, 71–86. [DOI] [PubMed] [Google Scholar]
- Fromm H, Finkler A. 2015. Repression and de-repression of gene expression in the plant immune response: the complexity of modulation by Ca2+ and calmodulin. Molecular Plant 8, 671–673. [DOI] [PubMed] [Google Scholar]
- Galon Y, Nave R, Boyce JM, Nachmias D, Knight MR, Fromm H. 2008. Calmodulin-binding transcription activator (CAMTA) 3 mediates biotic defense responses in Arabidopsis . FEBS Letters 582, 943–948. [DOI] [PubMed] [Google Scholar]
- Gao X, Wheeler T, Li Z, Kenerley CM, He P, Shan L. 2011. Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt. The Plant Journal 66, 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigos M, Deschamps S, Viel A, Lund S, Champeil P, Moller JV, le Maire M. 1991. Detection of Ca2+-binding proteins by electrophoretic migration in the presence of Ca2+ combined with 45Ca2+ overlay of protein blots. Analytical Biochemistry 194, 82–88. [DOI] [PubMed] [Google Scholar]
- Gaspar YM, McKenna JA, McGinness BS, Hinch J, Poon S, Connelly AA, Anderson MA, Heath RL. 2014. Field resistance to Fusarium oxysporum and Verticillium dahliae in transgenic cotton expressing the plant defensin NaD1 . Journal of Experimental Botany 65, 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P. 1994. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Molecular Biology 25, 989–994. [DOI] [PubMed] [Google Scholar]
- He P, Shan L, Sheen J. 2007. The use of protoplasts to study innate immune responses. Methods in Molecular Biology 354, 1–9. [DOI] [PubMed] [Google Scholar]
- Heo WD, Lee SH, Kim MC, et al. 1999. Involvement of specific calmodulin isoforms in salicylic acid-independent activation of plant disease resistance responses. Proceedings of the National Academy of Sciences, USA 96, 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK. 2005. Calcium: a central regulator of plant growth and development. The Plant Cell 17, 2142–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Gao Y, Zhou H, Chen J, Wu J, Zhang S. 2014. Apoplastic calmodulin promotes self-incompatibility pollen tube growth by enhancing calcium influx and reactive oxygen species concentration in Pyrus pyrifolia . Plant Cell Reports 33, 255–263. [DOI] [PubMed] [Google Scholar]
- Kenton P, Darby RM, Shelley G, Draper J. 2000. A PR-5 gene promoter from Asparagus officinalis (AoPRT-L) is not induced by abiotic stress, but is activated around sites of pathogen challenge and by salicylate in transgenic tobacco. Molecular Plant Pathology 1, 367–378. [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Ranjeva R, Pugin A. 2006. Calcium in plant defence-signalling pathways. New Phytologist 171, 249–269. [DOI] [PubMed] [Google Scholar]
- Lee K, Lee HG, Yoon S, Kim HU, Seo PJ. 2015. The Arabidopsis MYB96 transcription factor is a positive regulator of ABSCISIC ACID-INSENSITIVE4 in the control of seed germination. Plant Physiology 168, 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, He X, Luo X, Xu L, Liu L, Min L, Jin L, Zhu L, Zhang X. 2014. Cotton WRKY1 mediates the plant defense-to-development transition during infection of cotton by Verticillium dahliae by activating JASMONATE ZIM-DOMAIN1 expression. Plant Physiology 166, 2179–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippold F, Sanchez DH, Musialak M, Schlereth A, Scheible WR, Hincha DK, Udvardi MK. 2009. AtMyb41 regulates transcriptional and metabolic responses to osmotic stress in Arabidopsis . Plant Physiology 149, 1761–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Ren G, Guirgis A, Thornburg RW. 2009. The MYB305 transcription factor regulates expression of nectarin genes in the ornamental tobacco floral nectary. The Plant Cell 21, 2672–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP. 2002. Virus-induced gene silencing in tomato. The Plant Journal 31, 777–786. [DOI] [PubMed] [Google Scholar]
- Ma W. 2011. Roles of Ca2+ and cyclic nucleotide gated channel in plant innate immunity. Plant Science 181, 342–346. [DOI] [PubMed] [Google Scholar]
- Mengiste T, Chen X, Salmeron J, Dietrich R. 2003. The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis . The Plant Cell 15, 2551–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Rabouille C. 2009. Mechanisms of regulated unconventional protein secretion. Nature Reviews Molecular Cell Biology 10, 148–155. [DOI] [PubMed] [Google Scholar]
- Nie H, Zhao C, Wu G, Wu Y, Chen Y, Tang D. 2012. SR1, a calmodulin-binding transcription factor, modulates plant defense and ethylene-induced senescence by directly regulating NDR1 and EIN3 . Plant Physiology 158, 1847–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M. 2001. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. The Plant Cell 13, 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Heo WD, Yoo JH, et al. 2004. Pathogenesis-related gene expression by specific calmodulin isoforms is dependent on NIM1, a key regulator of systemic acquired resistance. Molecules and Cells 18, 207–213. [PubMed] [Google Scholar]
- Park CY, Lee JH, Yoo JH, et al. 2005. WRKY group IId transcription factors interact with calmodulin. FEBS Letters 579, 1545–1550. [DOI] [PubMed] [Google Scholar]
- Qu J, Ye J, Geng YF, Sun YW, Gao SQ, Zhang BP, Chen W, Chua NH. 2012. Dissecting functions of KATANIN and WRINKLED1 in cotton fiber development by virus-induced gene silencing. Plant Physiology 160, 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwat M, Ahmad P, Nabi G, Hu X. 2013. Ca2+ signals: the versatile decoders of environmental cues. Critical Reviews in Biotechnology 33, 97–109. [DOI] [PubMed] [Google Scholar]
- Shang Y, Yan L, Liu ZQ, et al. 2010. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of WRKY transcription repressors to relieve ABA-responsive genes of inhibition. The Plant Cell 22, 1909–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Foley RC, Oñate-Sánchez L. 2002. Transcription factors in plant defense and stress responses. Current Opinion in Plant Biology 5, 430–436. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhu L, Xu L, Yuan D, Min L, Zhang X. 2014. Cotton cytochrome P450 CYP82D regulates systemic cell death by modulating the octadecanoid pathway. Nature Communications 5, 5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Somssich IE. 2015. Transcriptional networks in plant immunity. New Phytologist 206, 932–947. [DOI] [PubMed] [Google Scholar]
- Vailleau F, Daniel X, Tronchet M, Montillet JL, Triantaphylidès C, Roby D. 2002. A R2R3-MYB gene, AtMYB30, acts as a positive regulator of the hypersensitive cell death program in plants in response to pathogen attack. Proceedings of the National Academy of Sciences, USA 99, 10179–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford SA, Wu Y, Llewellyn DJ, Dennis ES. 2011. GhMYB25-like: a key factor in early cotton fibre development. The Plant Journal 65, 785–797. [DOI] [PubMed] [Google Scholar]
- Wang L, Lv X, Li H, Zhang M, Wang H, Jin B, Chen T. 2013. Inhibition of apoplastic calmodulin impairs calcium homeostasis and cell wall modeling during Cedrus deodara pollen tube growth. PLoS One 8, e55411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Chen B, Liu P, et al. 2009. Calmodulin binds to extracellular sites on the plasma membrane of plant cells and elicits a rise in intracellular calcium concentration. Journal of Biological Chemistry 284, 12000–12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Diao WZ, Yang X, Qiao Z, Wang M, Acharya BR, Zhang W. 2015. Arabidopsis thaliana CML25 mediates the Ca2+ regulation of K+ transmembrane trafficking during pollen germination and tube elongation. Plant, Cell and Environment 38, 2372–2386. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Chen DJ, Wang DM, Huang QS, Yao ZP, Liu FJ, Wei XW, Li RJ, Zhang ZN, Sun YR. 2004. Over-expression of Gastrodia anti-fungal protein enhances Verticillium wilt resistance in coloured cotton. Plant Breeding 123, 454–459. [Google Scholar]
- Yamakawa H, Mitsuhara I, Ito N, Seo S, Kamada H, Ohashi Y. 2001. Transcriptionally and post-transcriptionally regulated response of 13 calmodulin genes to tobacco mosaic virus-induced cell death and wounding in tobacco plant. European Journal of Biochemistry 268, 3916–3929. [DOI] [PubMed] [Google Scholar]
- Yang A, Dai X, Zhang WH. 2012. A R2R3-type MYB gene, OsMYB2, is involved in salt, cold, and dehydration tolerance in rice. Journal of Experimental Botany 63, 2541–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CL, Liang S, Wang HY, Han LB, Wang FX, Cheng HQ, Wu XM, Qu ZL, Wu JH, Xia GX. 2015. Cotton major latex protein 28 functions as a positive regulator of the ethylene responsive factor 6 in defense against Verticillium dahliae . Molecular Plant 8, 399–411. [DOI] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW. 2003. Calcium/calmodulin-mediated signal network in plants. Trends in Plant Science 8, 505–512. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Park CY, Kim JC, et al. 2005. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis . Journal of Biological Chemistry 280, 3697–3706. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liang W, Yang X, Luo X, Jiang N, Ma H, Zhang D. 2010. Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. The Plant Cell 22, 672–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Du L, Shen C, Yang Y, Poovaiah BW. 2014. Regulation of plant immunity through ubiquitin-mediated modulation of Ca2+–calmodulin–AtSR1/CAMTA3 signaling. The Plant Journal 78, 269–281. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Rengel Z, Kuo J. 1998. Determination of intracellular Ca2+ in cells of intact wheat roots: loading of acetoxymethyl ester of Fluo-3 under low temperature. The Plant Journal 15, 147–151. [Google Scholar]
- Zhang WW, Jian GL, Jiang TF, Wang SZ, Qi FJ, Xu SC. 2012. Cotton gene expression profiles in resistant Gossypium hirsutum cv. Zhongzhimian KV1 responding to Verticillium dahliae strain V991 infection. Molecular Biology Reports 39, 9765–9774. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Wang X, Zhou M, Zhou X, Ye X, Wei X. 2012. An R2R3 MYB transcription factor in wheat, TaPIMP1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense- and stress-related genes. New Phytologist 196, 1155–1170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.