Highlight
Arabidopsis plants lacking type-f thioredoxins show an impairment of photosynthetic performance indicating specific functions for these thioredoxins not compensated for by other redox systems.These functions only affect growth under short-day conditions.
Key words: Carbon assimilation; chloroplast; fructose 1,6 bisphosphatase; photosynthesis; redox regulation; thioredoxin.
Abstract
Redox regulation plays a central role in the adaptation of chloroplast metabolism to light. Extensive biochemical analyses in vitro have identified f-type thioredoxins (Trxs) as the most important catalysts for light-dependent reduction and activation of the enzymes of the Calvin–Benson cycle. However, the precise function of type f Trxs in vivo and their impact on plant growth are still poorly known. To address this issue we have generated an Arabidopsis thaliana double knock-out mutant, termed trxf1f2, devoid of both f1 and f2 Trxs. Despite the essential function previously proposed for f-type Trxs, the visible phenotype of the trxf1f2 double mutant was virtually indistinguishable from the wild type when grown under a long-day photoperiod. However, the Trx f-deficient plants showed growth inhibition under a short-day photoperiod which was not rescued at high light intensity. The absence of f-type Trxs led to significantly lower photosynthetic electron transport rates and higher levels of non-photochemical energy quenching. Notably, the Trx f null mutant suffered from a shortage of photosystem I electron acceptors and delayed activation of carbon dioxide fixation following a dark–light transition. Two redox-regulated Calvin–Benson cycle enzymes, fructose 1,6-bisphosphatase (FBPase) and Rubisco activase, showed retarded and incomplete reduction in the double mutant upon illumination, compared with wild-type plants. These results show that the function of f-type Trxs in the rapid activation of carbon metabolism in response to light is not entirely compensated for by additional plastid redox systems, and suggest that these Trxs have an important role in the light adjustment of photosynthetic metabolism.
Introduction
Chloroplasts are essential for plant life because these organelles perform photosynthesis, the process that allows the conversion of light energy into biomass with the concomitant production of molecular oxygen. In addition, chloroplasts act as sensors of environmental conditions, particularly light quantity and quality, thus playing an important role in harmonizing the growth of plant photosynthetic and non-photosynthetic tissues as well as the adaptation of plants to the environment (Jarvis and López-Juez, 2013). To meet these requirements, chloroplast metabolism needs to respond rapidly to external and internal signals, redox regulation being an important aspect of this adaptability. Redox regulation is a post-translational modification consisting of the dithiol-disulphide interchange of selected and well-conserved cysteine residues of proteins. It is thus a reversible mechanism that allows the rapid regulation of metabolic pathways (Buchanan et al., 2012). Protein disulphide reductases such as thioredoxins (Trxs), small polypeptides of 12–14kDa with a highly conserved active site (WCGPC) and a characteristic structure, the so-called Trx-fold, play a central role in redox regulation. Trxs catalyse the reduction of disulphide bridges in target proteins and, as a consequence, the two cysteine residues at the Trx active site become oxidized as a disulphide bridge. Therefore, for a new catalytic cycle this disulphide needs to be reduced, reducing power being provided by NADPH in a reaction catalysed by NADPH-dependent Trx reductase (NTR). This two-component redox system formed by NTR and Trx is universally distributed in all kinds of organisms from bacteria to fungi, animals, and plants (Jacquot et al., 2009).
In contrast to heterotrophic organisms and non-photosynthetic plant tissues, redox regulation in chloroplasts has unique features. These organelles harbour a complex set of Trxs and Trx-like proteins (Lemaire et al., 2007; Chibani et al., 2009; Meyer et al., 2012; Balsera et al., 2014 ), which are not reduced by NADPH, but by ferredoxin (Fdx) reduced by photosynthetic electron transport in a process catalysed by a Fdx-dependent Trx reductase (FTR) found exclusively in plastids and cyanobacteria (Dai et al., 2007). Therefore, chloroplast redox regulation is mediated by the Fdx/FTR/Trx system and, thus, is dependent on light. Initial biochemical analyses in vitro led to the identification of two types of Trxs in chloroplasts, termed f and m, based on their ability to reduce and activate fructose-1,6-bisphophate phosphatase (FBPase) and NADP-malic dehydrogenase (NADP-MDH), respectively (Wolosiuk et al., 1979). The availability of genome sequences from different plants has uncovered the complex set of chloroplastic Trxs, which in Arabidopsis thaliana include two isoforms of f-type, four isoforms of m-type, two isoforms of the y-type, and an x-type Trx (Lemaire et al., 2007; Meyer et al., 2012; Balsera et al., 2014). Trxs f and m were proposed to play a predominant role in the redox regulation of the central biosynthetic pathways, such as the Calvin–Benson cycle, whereas Trxs x and y show a capacity to reduce peroxiredoxins (Prxs) and so were considered to have an antioxidant function (Collin et al., 2003, 2004). In addition, a novel Trx, type-z, was identified in Arabidopsis, which is involved in the redox regulation of plastid transcription (Arsova et al., 2010; Schröter et al., 2010; Steiner et al., 2011; Wimmelbacher and Börnke, 2014). Beside these canonical Trxs, several atypical Trxs have been described in the chloroplast. This is the case of HCF164, which is localized in the thylakoid membrane facing the lumen (Motahashi and Hisabori, 2006), and the small family of atypical Trxs identified in Arabidopsis termed AtACHTs (for atypical Cys His-rich Trxs) (Dangoor et al., 2009). Finally, different Trx-like proteins were identified in chloroplasts, among which the so-called CDSP32 is the best characterized (Broin et al., 2002).
In addition, chloroplasts harbour an NADPH-dependent redox system based on the activity of a bimodular enzyme consisting of an NTR with a joint Trx domain at the C-terminus, termed NTRC (Serrato et al., 2004; Kirchsteiger et al., 2012). NTRC is able to conjugate both NTR and Trx activities for the efficient reduction of 2-Cys Prxs (Pérez-Ruiz et al., 2006; Moon et al., 2006; Alkhalfioui et al., 2007), thus suggesting an antioxidant function for this enzyme (Puerto-Galán et al., 2013). Additional reports indicate a function of NTRC in the redox regulation of starch synthesis (Michalska et al., 2009; Lepistö et al., 2013) and in different reactions of the biosynthesis of tetrapyrroles (Richter et al., 2013; Pérez-Ruiz et al., 2014). The high affinity of NTRC for NADPH introduces the notion that redox regulation in chloroplasts relies not only on photosynthetically reduced Fdx but also on NADPH which can be produced during the night from sugars by the oxidative pentose phosphate pathway (Spínola et al., 2008; Cejudo et al., 2012).
In parallel with the knowledge of the increasing complexity of the plastidial Trxs, the advance in proteomics has allowed the identification of a large number of putative targets of Trxs (Balmer et al., 2003; Buchanan and Balmer, 2005; Montrichard et al., 2009), which indicates that redox regulation is important for virtually any process taking place in the chloroplast. However, the question of the level of specificity or redundancy of the different Trxs in redox regulation in this organelle is still poorly understood. An Arabidopsis knockout mutant deficient in Trx z showed a severe phenotype indicating that the function of this Trx in chloroplast transcription is not redundant with other plastidial Trxs (Arsova et al., 2010). By contrast, an Arabidopsis knockout mutant lacking Trx x shows almost a wild-type phenotype, indicating that this deficiency is compensated for by other plastidial redox systems (Pulido et al., 2010). More uncertain is the specificity of Trxs with several isoforms, such as those of type m or f, which are considered to play a relevant role in the redox regulation of photosynthetic metabolism. Trx m4 is involved in alternative photosynthetic electron transport (Courteille et al., 2013), and the simultaneous deficiency of Trxs m1, m2, and m4 resulted in impaired photosystem II biogenesis (Wang et al., 2013).
Proteomic and biochemical studies in vitro have shown the relevant function of type-f Trxs in the redox regulation of most of the enzymes of the Calvin–Benson cycle (Michelet et al., 2013). In addition, an Arabidopsis mutant lacking Trx f1 shows impaired light-dependent reductive activation of ADP-glucose pyrophosphorylase (AGPase) and starch turnover (Thormählen et al., 2013). Surprisingly, despite the key role proposed for type-f Trxs in the redox regulation of chloroplast metabolism, the Trx f1-deficient mutant shows a wild-type phenotype (Thormählen et al., 2013). Moreover, a double mutant devoid of both Trx f1 and Trx f2 showed a visible phenotype indistinguishable from wild-type plants (Yoshida et al., 2015), suggesting that other plastidial redox systems are able to compensate for the deficiency of f-type Trxs. Therefore, with the aim of establishing the role of type f Trxs in chloroplast performance and plant growth, we generated a double knockout mutant of Arabidopsis devoid of Trx f1 and Trx f2, and have performed the analysis of its phenotype under different growth conditions. The double mutant shows no visible phenotype, compared with wild-type plants, when grown under long-day conditions, however, it shows retarded growth under short-day conditions which is not rescued by high light intensity. Analysis of photosynthetic parameters and changes in the redox status of FBPase and Rubisco activase in response to light showed that type-f Trxs are required for the rapid reduction of the Calvin–Benson cycle enzymes in response to light, a function not compensated for by other plastidial redox systems.
Materials and methods
Growth conditions and plant material
Arabidopsis thaliana wild-type (ecotype Columbia) and mutant plants were grown in soil in growth chambers under long-day (16/8h light/dark) or short-day (8/16h light/dark) conditions at 22 °C during the light and 20 °C during the dark periods and a light intensity of 125 μE m−2 s−1. For experiments addressing the effects of irradiance, plants were grown under short-day conditions at 125, 350, and 950 μE m−2 s−1 light intensity. A homozygous line, GK-020E05-013161, with a T-DNA insertion in the TRX f2 gene (see Supplementary Fig. S1 at JXB online) from Arabidopsis, termed the trxf2 mutant, was selected by PCR analysis with the oligonucleotides described in Supplementary Table S1. This mutant was manually crossed with the trxf1 mutant, which was previously reported by Pérez-Ruiz et al. (2014). Seeds resulting from this cross were tested for heterozygosity of the T-DNA insertions in the TRX f1 and TRX f2 genes. Plants were then self-crossed and double homozygous lines were identified in the progeny by PCR analysis of genomic DNA using the oligonucleotides described in Supplementary Table S1.
RNA extraction and qRT-PCR analysis
Total RNA was extracted using the TRIsure RNA extraction reagent (BIOLINE) and cDNA synthesis was performed with 1 μg of total RNA using the Maxima first strand cDNA synthesis kit (Thermo Scientific) according to the manufacturer’s instructions. The content of Trx f1 and Trx f2 transcripts was determined by real time quantitative PCR (qRT-PCR) with RNA samples extracted from seedlings. qRT-PCR was performed with oligonucleotides shown in Supplementary Table S2 in an IQ5 real-time PCR detection system (Bio-Rad) following a standard thermal profile (95 °C, 3min, 40 cycles of 95 °C for 10s, and 60 °C for 30s). The relative level of each transcript was referred to the level of the UBIQUITIN transcript.
Protein extraction, alkylation assays, and Western blot analysis
For protein extraction, leaves were ground in liquid nitrogen and 10% (v/v) trichloroacetic acid (TCA) was immediately added to quench thiol oxidation. Samples were incubated on ice for 20min and then centrifuged at 16 200 g at 4 °C for 10min. The pellets were washed with acetone, resuspended in alkylation buffer (2% SDS, 50mM TRIS–HCl pH 7.8, 2.5% glycerol, and 4M urea) with 10mM methyl-maleimide polyethylene glycol (MM-PEG24) and incubated for protein thiol alkylation for 20min at room temperature. Samples were subjected to SDS-PAGE (9.5% polyacrylamide), transferred on to nitrocellulose membranes, and probed with an anti-FBPase antibody which was kindly provided by Dr M Sahrawy, Estación Experimental del Zaidín, Granada, Spain, or with an anti-Rubisco activase antibody which was kindly provided by Dr AR Portis, USDA, Urbana, USA. The anti-Trx f antibody was raised by rabbit immunization with purified recombinant Trx f1 from Arabidopsis at the Servicio de Producción Animal, University of Seville, Spain. This antibody also detected Trx f2 although with somewhat lower efficiency (see Supplementary Fig. S2).
Measurements of chlorophyll a fluorescence and P700 absorbance
Room temperature chlorophyll fluorescence was measured using a pulse-amplitude modulation fluorimeter (DUAL-PAM-100, Walz, Effeltrich, Germany). The maximum PSII quantum yield, determined as variable fluorescence (F v) to maximal fluoresence (F m), F v/F m, was measured after the dark adaptation of the plants for 30min and a single saturating pulse of red (635nm) light at 10 000 μE m−2 s−1 was applied. Induction-recovery curves were performed using red (635nm) actinic light at 75 μE m−2 s−1 for 8min. Saturating pulses of red light at 10 000 μE m−2 s−1 intensity and 0.6s duration were applied every 60s and recovery in darkness was recorded for up to 10min. The parameters Y(II) and Y(NPQ) corresponding to the respective quantum yields of PSII photochemistry and non-photochemical quenching (NPQ) were calculated by the DUAL-PAM-100 software according to the equations in Kramer et al. (2004). Relative linear electron transport rates were measured in leaves of pre-illuminated plants by applying stepwise increasing actinic light intensities up to 2 000 μE m−2 s−1. The redox state of photosystem I P700 was monitored by following the changes in absorbance at 830nm versus 875nm using the DUAL-PAM-100. Plants were kept in the dark for 30min and then, to probe the maximum extent of P700 oxidation, leaves were illuminated with far red (730nm) light superimposed on the actinic light. Thereafter, absorbance traces were recorded during a 5min illumination with 126 μE m−2 s−1 red (635nm) actinic light followed by 5min darkness. Saturating pulses of red light at 10 000 μE m−2 s−1 were applied every 20s. The quantum yields of PSI photochemistry Y(I), donor side limitations Y(ND), and acceptor side limitations Y(NA) were based on saturating pulse analysis and calculated by the DUAL-PAM-100 software.
Determination of the rate of carbon assimilation A N
Net CO2 assimilation rate (A N) was measured using an open gas exchange system Li-6400 equipped with the chamber head (Li-6400–40). All measurements were conducted in dark-adapted leaves of short-day grown plants (50 d-old) at 500 μmol mol−1 of CO2, a constant leaf temperature of 20 °C, and a vapour pressure deficit between leaf and air of below 1 kPa. Before the light, at an intensity of 70 μE m−2 s−1, was turned on in the leaf chamber, A N was recorded for 30min every 5s, then recording continued until equilibrium was reached. Six leaves were measured per line.
Determination of chlorophylls
Leaf discs were weighed and incubated in 1ml methanol overnight at 4 °C. After extraction, chlorophyll levels were measured spectrophotometrically, as described in Porra et al. (1989), and normalized to fresh weight or leaf area. The values were compared with a Tukey Test (Anova) using a confidence interval of 99%.
Determination of starch content
Starch content was determined in leaves of wild-type and the Trx f-deficient mutant grown under long-day conditions harvested before flowering (22–26-d-old), essentially as described in Lin et al. (1988). For starch extraction, leaves (100–200mg fresh weight) harvested at the end of the day were ground in liquid nitrogen and washed with 80% (v/v) ethanol in 10mM HEPES pH 7.6, for 2h at 80 °C before being washed with the same solution at room temperature until the tissue was free of pigments. Dry pellets, after centrifugation, were resuspended in 1ml of 0.2N KOH and heated at 100 °C for 30min. After cooling, samples were centrifuged at 17 000 g for 10min and the supernatant was adjusted to pH 5.0 with 1N acetic acid. An aliquot of 200 μl of this solution was used to determine the amount of starch using the enzymatic method described by Lin et al. (1988). The values were compared with a Tukey Test (Anova) using a confidence interval of 99%.
Results
Type-f Trxs are dispensable for plant growth
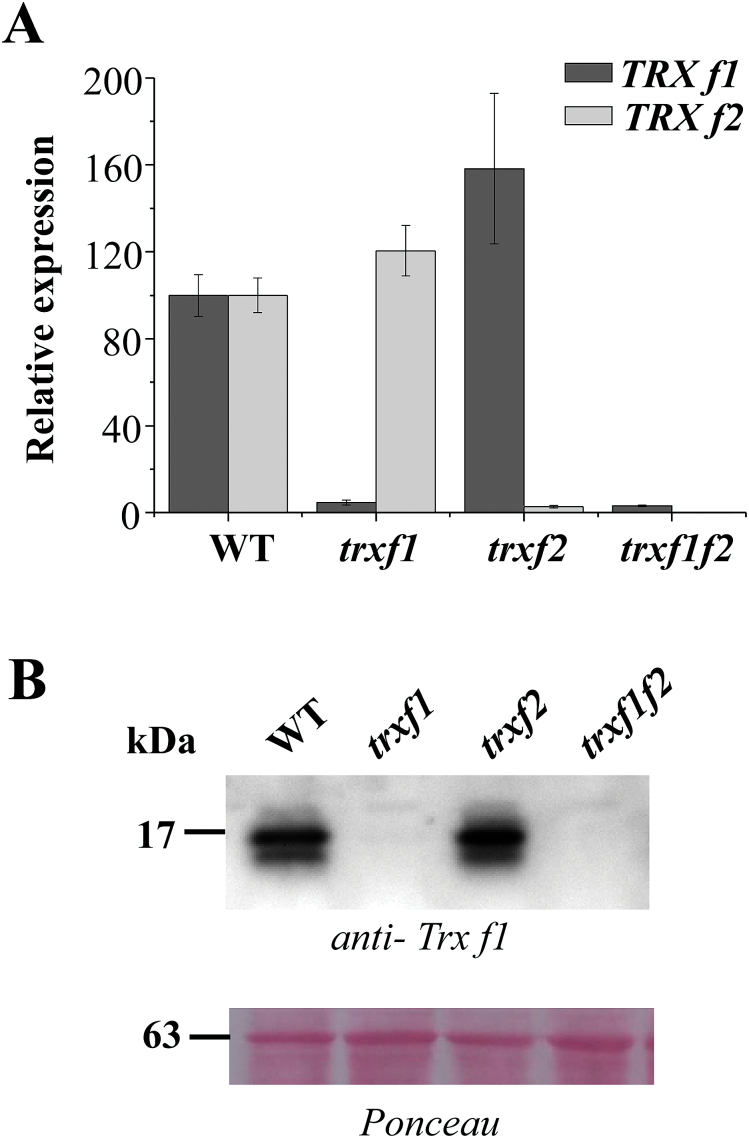
The Arabidopsis genome encodes two isoforms of f-type Trxs, f1 and f2. It was recently reported that Arabidopsis knockout mutants lacking either Trx f1 (Thormählen et al., 2013) or both Trx f1 and Trx f2 (Yoshida et al., 2015) show a wild-type phenotype with respect to growth and pigmentation. These results suggest that the loss of f-type Trxs might be compensated for by other plastidial redox systems. To address this issue in more detail, we have generated a double knockout mutant of Arabidopsis devoid of both Trx f1 and Trx f2 and have analysed its growth under different conditions. To that end, the Arabidopsis line (GK-020E05-013161) with a T-DNA insertion at the AT5G16400.1 locus encoding Trx f2 was isolated (Supplementary Fig. S1). This line was manually crossed with the Trx f1 knockout mutant (SALK_128365.45.75.x) previously reported by our group (Pérez-Ruiz et al., 2014), which was also characterized by Thormählen et al. (2013). Plants homozygous for both T-DNAs were selected by PCR analysis of genomic DNA (Supplementary Fig. S1). The double mutant, here termed trxf1f2, was effectively devoid of both Trx f1 and Trx f2 as shown by the lack of transcripts of the two genes, based on qRT-PCR (Fig. 1A), and the corresponding polypeptides, as shown by Western blots probed with an antibody raised against Trx f1 (Fig. 1B), which also cross-reacted with Trx f2 (Supplementary Fig. S2). These results confirmed that Trx f1 is much more abundant than Trx f2 in wild-type plants. In addition, we tested whether the absence of type-f Trxs had any effect on the expression of other plastidial redox systems, but only minor differences in the levels of the transcripts of genes encoding NTRC, type-m and x Trxs were detected (see Supplementary Fig. S3 ).
Fig. 1.
The level of Trx f1 and Trx f2 transcripts and proteins in wild-type and mutant plants. (A) The content of Trx f1 and f2 transcripts was determined by qRT-PCR from total RNA, which was extracted from wild-type, trxf1, trxf2, and trxf1f2 seedlings. The pairs of oligonucleotides used for cDNA amplification are indicated in Supplementary Table S2. Transcript levels were normalized to UBIQUITIN amplification and referred to the level of TRX f1 and TRX f2 in wild-type plants. Determinations were performed three times and mean values ±SD are represented. (B) Western blot analysis of the content of type-f Trxs in wild-type and mutant plants. Proteins were extracted from leaves (100mg fresh weight) of wild-type, trxf1, trxf2, and trxf1f2 plants grown under short-day conditions and subjected to SDS-PAGE (13% polyacrylamide) under reducing conditions, transferred to a nitrocellulose membrane, and probed with an anti-Trx f1 antibody. Ponceau staining was used as a loading control. Molecular weight markers in kDa are indicated on the left.
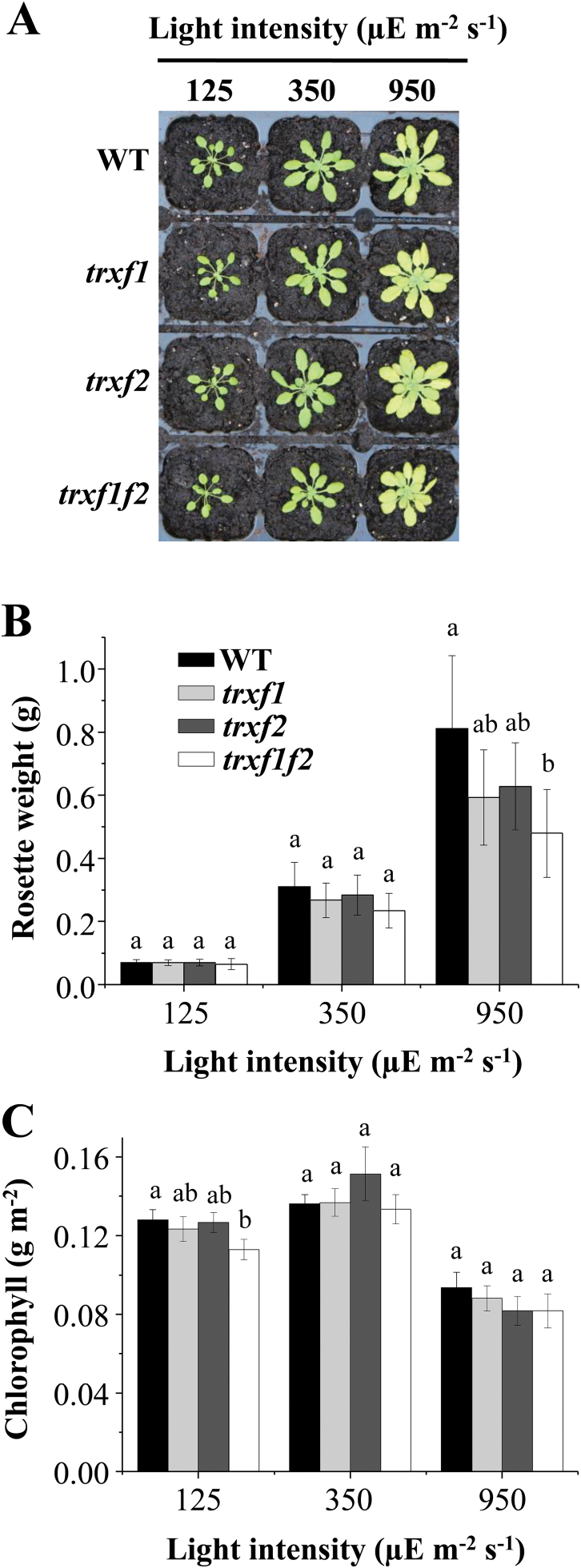
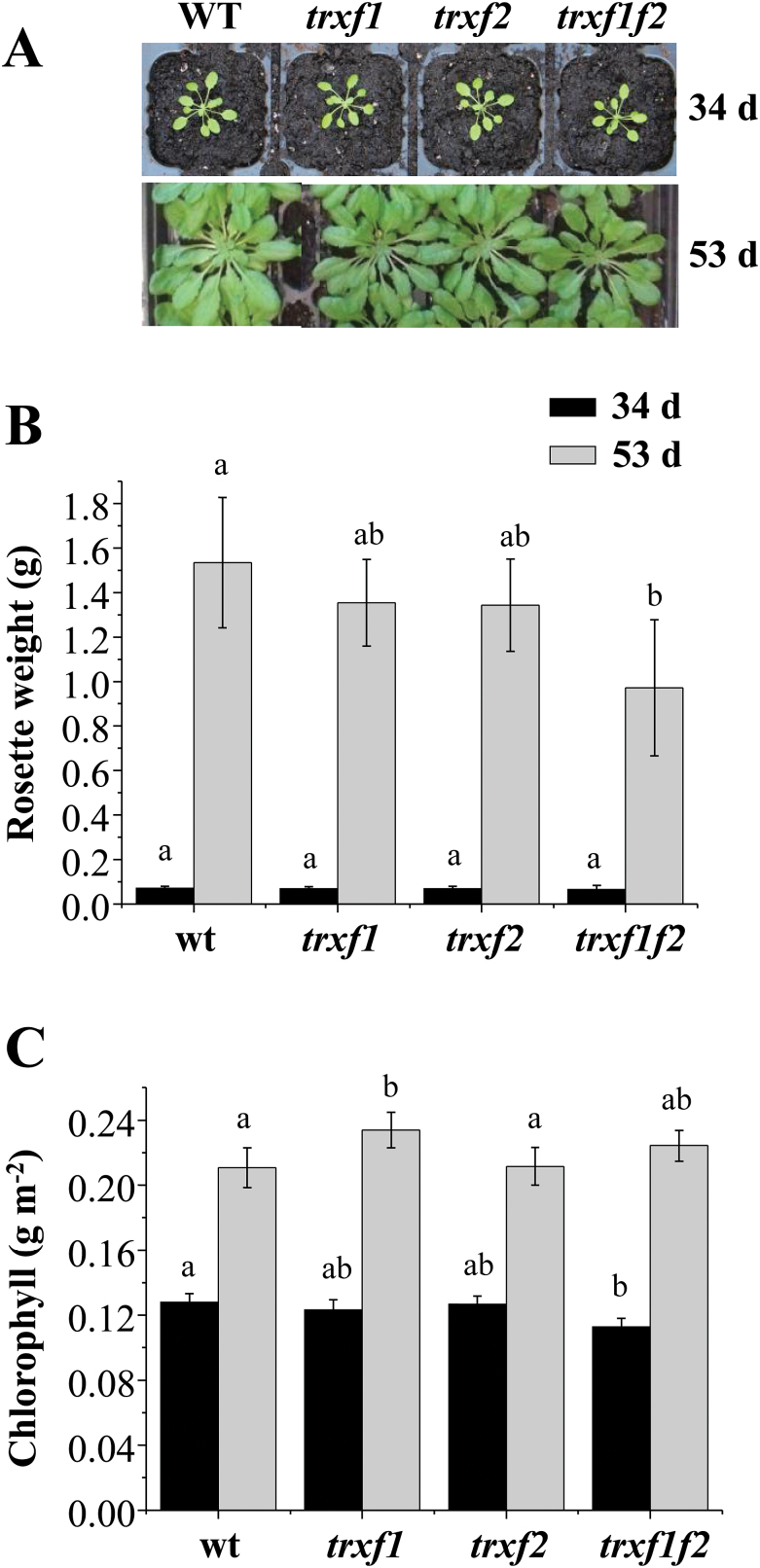
The trxf1f2 double mutant, like the single mutants trxf1 and trxf2, showed the wild-type phenotype when grown under the long-day photoperiod (Fig. 2A), as confirmed by the weight of the rosette leaves (Fig. 2B) and leaf chlorophyll content (Fig. 2C) of mature plants immediately before bolting. To analyse in more detail the effect of Trx f deficiency on plant growth, the different lines were grown under short-day conditions. Wild-type and Trx f-deficient mutants showed an indistinguishable growth rate up to the stage of young rosette leaves (34 d of growth), as determined by fresh weight (Fig. 3A, B). However, after 53 d of growth, the double mutant displayed a significant growth inhibition, as shown by the lower weight of rosette leaves (Fig. 3B), although the chlorophyll content was unaffected (Fig. 3C). Data of the long growth period, 53 d, are only shown for short-day (Fig. 3) and not for long-day conditions (Fig. 2), because the plant developmental stages, adult plants before bolting and advanced leaf senescence, respectively, are not comparable. We then analysed the effect of different light intensities on these mutants grown under short-day conditions. While at 125 and 350 μE m−2 s−1 light intensity no difference was observed between the wild type and the mutant lines after 34 d of growth, Trx f-deficient mutants grown at 950 μE m−2 s−1 light intensity displayed lower rosette weights than the wild-type plants (Fig. 4A, B). At high light intensity the leaf chlorophyll content was decreased, but no significant differences were observed between wild-type and mutant plants (Fig. 4C). Therefore, despite the central function previously attributed to type f Trxs in the redox regulation of chloroplast metabolism, these Trxs are dispensable for plant growth under the long-day photoperiod. Nevertheless, the retarded growth of the trxf1f2 double mutant under the short-day photoperiod at adult plant stages, even under high light conditions, confirms the light-dependent participation of f-type Trxs in chloroplast photosynthetic metabolism.
Fig. 2.
Growth of Arabidopsis lines lacking type-f Trxs under a long-day photoperiod. (A) Plants of the wild type and mutant lines grown under long-day conditions (16/8h light/dark, light intensity 125 μE m−2 s−1) for 22 d. (B) The weight of the rosette leaves was determined from nine plants, except for the trxf1f2 double mutant which was determined from 14 plants. (C) Chlorophyll content was determined from leaf discs (n=6) and average values ±SD are represented. Letters indicate significant differences with the Tukey Test and a confidence interval of 99%.
Fig. 3.
Growth of Arabidopsis lines lacking type-f Trxs under a short-day photoperiod. (A) Representative plants of the wild type and mutant lines grown under short-day conditions (8/16h light/dark, light intensity 125 μE m−2 s−1) for 34 d and 53 d. (B) The weight of the rosette leaves was determined from 12 plants (34 d of growth) or seven plants (53 d of growth), except for the trxf1f2 double mutant which was determined from 14 plants in both cases. (C) Chlorophyll content was determined from leaf discs (n=6). Average values ±SD are represented. Letters indicate significant differences with the Tukey Test and a confidence interval of 99%.
Fig. 4.
Effect of light intensity on growth of Arabidopsis lines lacking type-f Trxs under a short-day photoperiod. (A) Representative plants of the wild type and mutant lines grown under short-day conditions (8/16h light/dark) for 34 d at increased light intensities, as indicated. (B) The weight of the rosette leaves from all lines was determined from 12 plants. (C) Chlorophyll content was determined from leaf discs (n=6). Average values ±SD are represented. Letters indicate significant differences with the Tukey Test and a confidence interval of 99%.
Activation of photosynthesis upon a dark–light transition is retarded in plants lacking type f Trxs
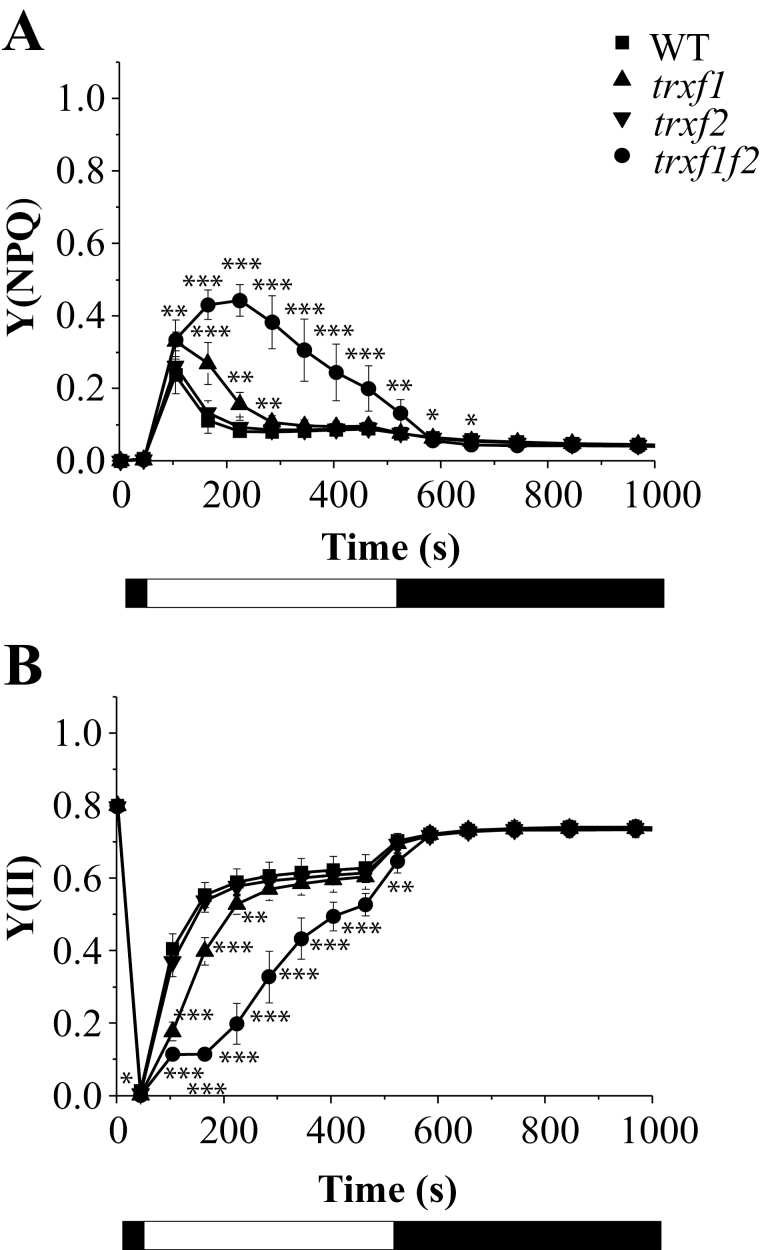
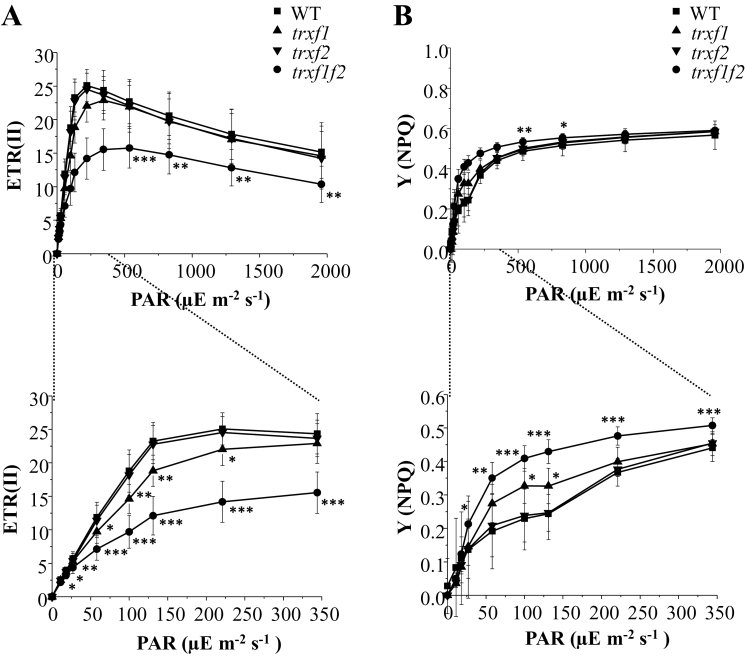
To explore the function of f-type Trxs in chloroplast metabolism, different photosynthetic parameters of plants lacking either or both of the Trx f enzymes were first examined using chlorophyll fluorescence. The integrity of PSII, determined as the ratio of variable to maximal fluorescence in dark-adapted leaves, was not affected in the single or double trxf1f2 mutants (Table 1). Non-photochemical quenching (NPQ) is a loss of chlorophyll fluorescence in the light which is not due to photochemistry and reflects adaptation mechanisms regulating the fraction of absorbed light that reaches the PSII reaction centre (Szabó et al., 2005; Baker, 2008). The energy-dependent quenching, qE, which involves thermal dissipation of the absorbed light energy, is the main component of NPQ in plants under moderate light intensities and depends on the proton gradient across the thylakoid membrane (Szabó et al., 2005; Baker, 2008; Ruban et al., 2012). Following the onset of actinic light at low or moderate intensity, a brief peak of NPQ is normally observed in wild-type plants due to transient acidification of the thylakoid lumen before activation of photosynthesis (Kalituho et al., 2007). Such an initial peak of NPQ was also found in the trxf2 mutant and a somewhat broader peak in the trxf1 mutant (Fig. 5A; see Supplementary Fig. S4). By contrast, the double mutant displayed an extensive NPQ which did not relax completely even after 8min of illumination at 75 μE m−2 s−1 (Fig. 5A). As a result, the PSII effective quantum yield, Y(II), increased more slowly in the light and remained lower in the trxf1f2 double mutant (Fig. 5B). In agreement with these results, the relative linear photosynthetic electron transport rates were considerably lower in the double mutant at all light intensities examined (Fig. 6A). By contrast, only a small but significant reduction in photosynthetic electron transport rates was observed in the trxf1 mutant, whereas the trxf2 mutant displayed wild-type rates (Fig. 6A). Notably, the yields of NPQ were higher in trxf1f2 plants, particularly at low light intensities (Fig. 6B). These measurements were performed using plants grown under short-day conditions and very similar results were obtained when these photosynthetic parameters were analysed in plants grown under the long-day photoperiod (see Supplementary Figs S5 and S6).
Table 1.
F v/F m and kinetics of response of net CO2 assimilation rate to light
| Genotype | WT | trxf1 | trxf2 | trxf1f2 |
|---|---|---|---|---|
| F v/F m | 0.81±0.01 | 0.82±0.01 | 0.81±0.01 | 0.82±0.01 |
| t 1/2 (s) | 106.0±2.3 a | 187.9±7.5 b | 134.3±9.5 a | 237.2±10.1 c |
The maximum PSII quantum yield was determined as variable fluorescence (F v) to maximal fluorescence (F m), F v/F m, in dark-adapted leaves of plants grown under short-day conditions. The F v/F m values (±SD) are the average of 12 measurements. t 1/2 represents the time to achieve 50% of the final rate of CO2 assimilation, as shown in the data of Supplementary Fig. S5. Data presented are the means (±SE; n=6). The differences between mutants and the wild type, when significant, are indicated by different letters (P <0.01; Anova, Tukey test).
Fig. 5.
Photosystem II activity in wild type and Trx f-deficient mutants. Chlorophyll fluorescence of photosystem II (P680) was measured using a pulse-amplitude modulation fluorimeter. After incubation of the plants for 30min in darkness, an induction–recovery curve was performed to determine the quantum yields of non-photochemical quenching, Y(NPQ) (A) and photosystem II, Y(II) (B). During the 8min induction period, a red (653nm) actinic light at 75 μE m−2 s−1 intensity was applied. Thereafter, the actinic light was switched off and measurements were continued for another 10min in the dark. Saturation pulses (10 000 μE m−2 s−1, 0.6s) every 60s were applied. Values are the mean ±SD of 6 plants grown for 53 d under short-day conditions. Light and dark periods are indicated by the white and black bars. Statistical significance (*P <0.05; **P <0.01; ***P <0.001) was determined with Student’s t test comparing values for each of the mutant lines with the wild type.
Fig. 6.
Light-dependent linear photosynthetic electron transport and NPQ in the wild type and Trx f-deficient mutants. (A) Relative linear electron transport rates of photosystem II, ETR (II) was measured in pre-illuminated attached leaves of plants grown at 125 μE m−2 s−1 under short-day conditions. Chlorophyll fluorescence of photosystem II was determined using a pulse-amplitude modulation fluorimeter. Each data point is the mean ±SD of the ETR (II) from eight plants under short-day conditions. PAR (photosynthetically active radiation). (B) Quantum yields of NPQ from the corresponding light-titration curves in (A). Statistical significance (*P <0.05; **P <0.01; ***P <0.001) was determined with Student’s t test comparing values for each of the mutant lines with the wild type.
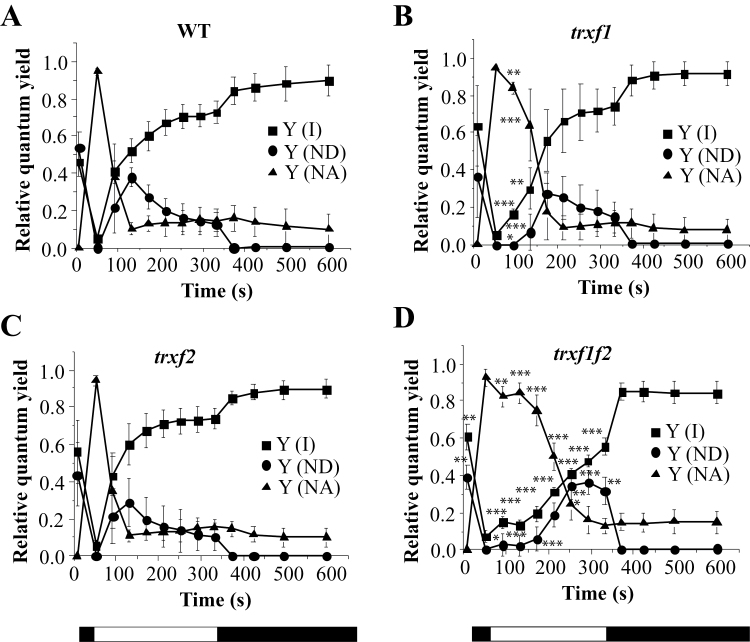
The effect of the type f Trxs on the control of energy quenching and photosynthetic yield might be through direct interaction with the photosynthetic apparatus or an indirect consequence of limited biosynthesis and demand for ATP, leading to lower luminal pH. The latter case should be revealed by a lower rate of consumption of electrons from photosynthetic electron transport. Therefore, we measured the PSI quantum yield, Y(I), based on P700 absorbance changes, in mutant and wild-type plants (Fig. 7; see Supplementary Fig. S7). Indeed, the double mutant had a lower PSI activity due to prolonged limitations on the acceptor side, Y(NA), after turning on the light (Fig. 7D). This indicates retardation of the activation of the biosynthetic processes which consume reducing equivalents derived from photosynthetic electron transport.
Fig. 7.
Comparative analysis of photosystem I activity in the wild type and Trx f-deficient mutants. The absorbance of oxidized phtotosystem I (P700) at 830nm was measured using a pulse-amplitude modulation fluorimeter. The redox state of photosystem I (P700) was inferred from changes in this absorbance and monitored for 10min. After 30min dark adaptation (maximum P700 reduced), leaves were illuminated with far-red light superimposed on actinic red light (126 μE m−2 s−1, 635nm) to achieve the maximum oxidation of P700 and, subsequently, the actinic light was kept on for 5min followed by another 5min of darkness. White light saturation pulses (10 000 μE m−2 s−1, 0.6s) were applied every 20s. The quantum yields of PSI Y(I), donor side limitations Y(ND), and acceptor side limitations Y(NA) are based on saturating pulse analysis. Data were collected from six plants grown under short-day conditions and mean ±SD are represented. Statistical significance (*P <0.05; **P <0.01; ***P <0.001) was determined with Student’s t test comparing values for each of the mutant lines (B, C, D) with the wild type (A).
The response of the rate of CO2 fixation (A N) to illumination was more retarded in the trxf1 than in the trxf2 single mutant while the trxf1f2 double mutant displayed even slower kinetics (Table 1; see Supplementary Fig. S8). The half-time for reaching the maximal CO2 fixation rate after turning on the light was more than twice as long in the double mutant compared with wild-type plants (Table 1). These results demonstrate the function of f-type Trxs in the rapid response of the chloroplast assimilatory metabolism to light.
Light-dependent reduction of FBPase and Rubisco activase is impaired in the trxf1f2 double mutant
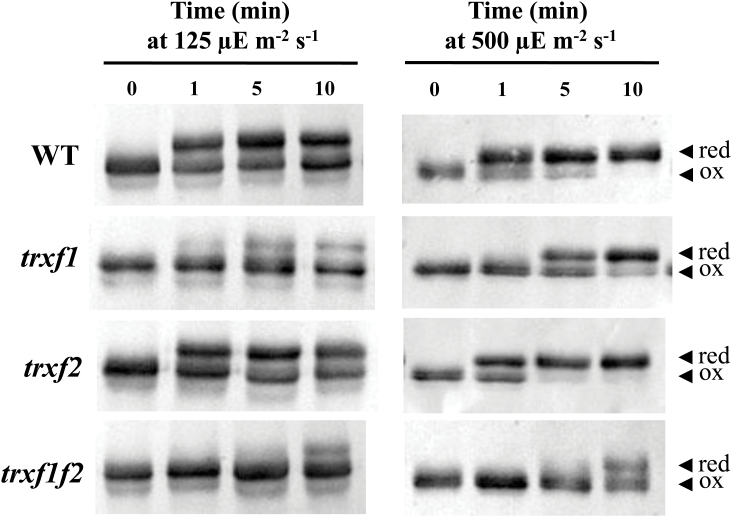
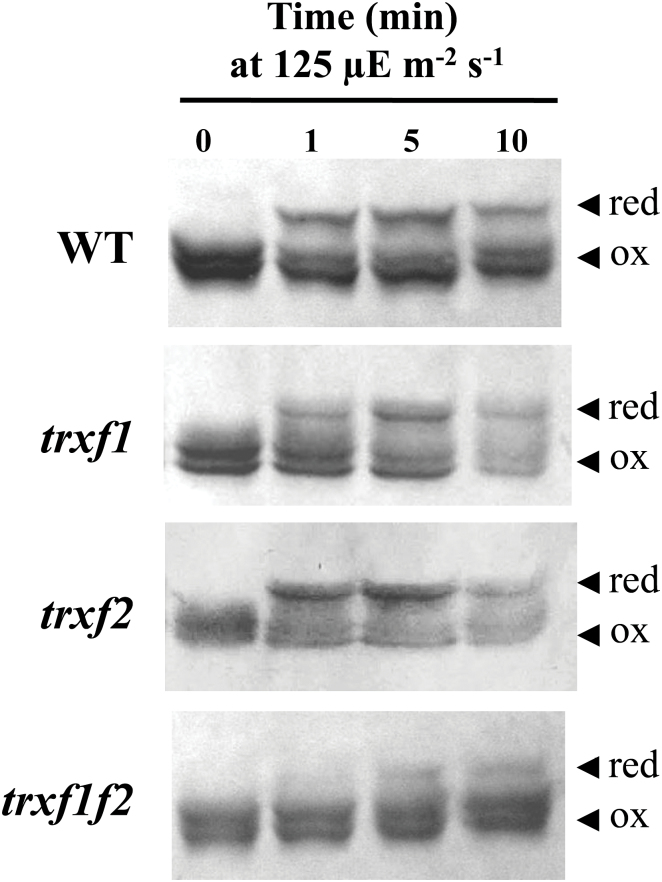
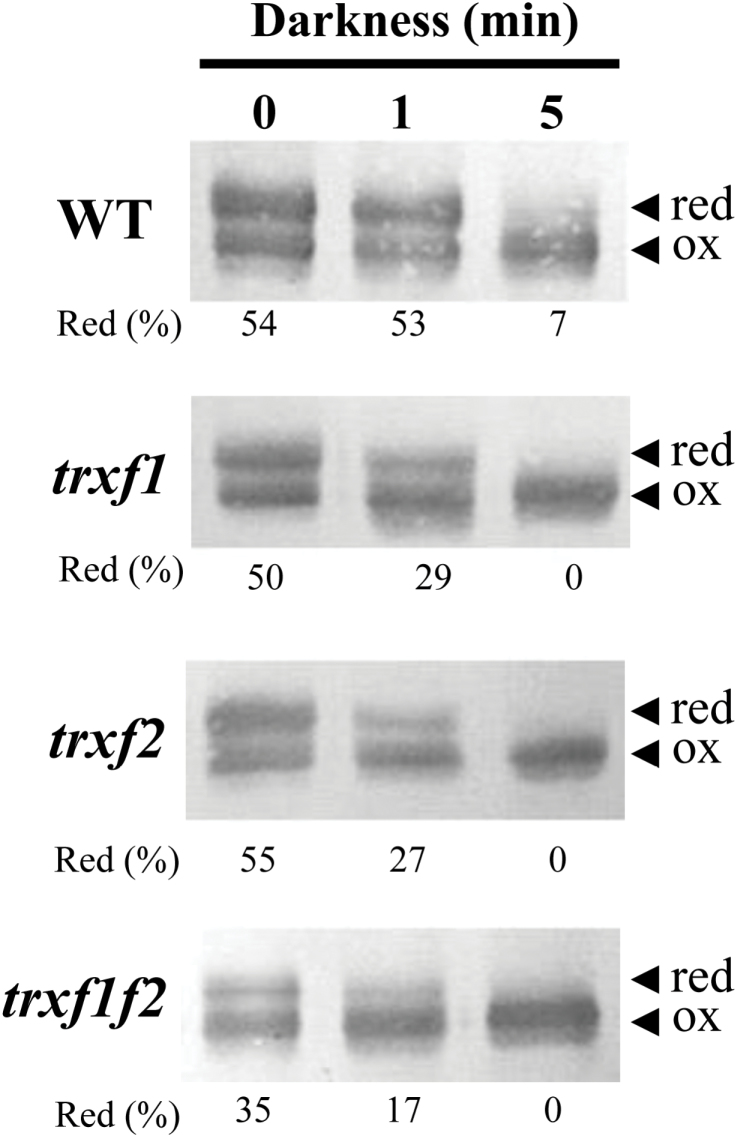
There is extensive evidence in vitro supporting the relevant function of f-type Trxs in light-dependent reductive activation of different chloroplast enzymes including those of the Calvin–Benson cycle (recently reviewed by Michelet et al., 2013). To analyse further the function of f-type Trxs in vivo, we examined the change of the redox status of two well-known in vitro targets of type f Trxs, FBPase and Rubisco activase, in response to light in the mutant lines. To this end, samples were taken at the end of the night period from plants that had been grown at a light intensity of 125 μE m−2 s−1 and then subjected to illumination with the same or higher (500 μE m−2 s−1) light intensity. Short-term changes of the redox status of these enzymes were analysed with the aid of the thiol-alkylating agent methyl maleimide-polyethylene glycol24 (MM-PEG24), which adds 1.24kDa per thiol group, thus producing a shift in the electrophoretic mobility of the labelled proteins that reflects their redox status. In dark-adapted plants FBPase was detected as a single band indicating that, as expected, the enzyme was fully oxidized under these conditions (Fig. 8). In wild-type plants, FBPase becomes rapidly reduced in response to light within seconds after illumination (Fig. 8). The level of reduction of FBPase proved to be dependent on light intensity since the enzyme became fully reduced after 10min at 500 μE m−2 s−1, whereas the growth light intensity of 125 μE m−2 s−1 only promoted a partial reduction, approximately 50%, of the enzyme (Fig. 8). While the deficiency of Trx f2 exerted almost no effect on FBPase reduction in response to light, the deficiency of Trx f1 significantly impaired the level of reduction of the enzyme at both light intensities (Fig. 8). The trxf1f2 double mutant showed not only a pronounced delay of FBPase reduction in response to light but also a lower level of reduction of the enzyme after 10min of illumination, even at the higher light intensity tested here (Fig. 8). A similar pattern of reduction in response to light was observed for another prominent redox-regulated enzyme of the Calvin–Benson cycle, Rubisco activase (Fig. 9). In dark-adapted samples Rubisco activase was detected as a double band corresponding to isoforms of 46 and 43kDa, respectively, due to alternative splicing. The long isoform has a C-terminal extension that includes two cysteines which may form an intramolecular disulphide that renders the enzyme inactive (Zhang and Portis, 1999).
Fig. 8.
In vivo redox status of FBPase in response to light in the wild type and Trx f mutant lines. The redox status of FBPase from the different lines under analysis, as indicated on the left, was determined in leaf extracts from plants grown under short-day conditions and harvested at the end of the dark period (time 0), and after 1, 5, and 10min of illumination at the indicated light intensities. Total leaf proteins were extracted in the presence of 10% TCA and protein thiols were alkylated with 10mM MM-PEG24. Proteins were resolved in SDS-PAGE (9.5% polyacrylamide) under non-reducing conditions, transferred to nitrocellulose filters, and probed with an anti-FBPase antibody; red, reduced; ox, oxidized.
Fig. 9.
In vivo redox status of Rubisco activase in response to light in the wild type and Trx f mutant lines. The redox status of Rubisco activase from the different lines under analysis, as indicated on the left, was determined in leaf extracts from plants grown under short-day conditions and harvested at the end of the dark period (time 0), and after 1, 5, and 10min of illumination. Total leaf proteins were extracted in the presence of 10% TCA and protein thiols were alkylated with 10mM MM-PEG24. Samples were fractionated in SDS-PAGE (9.5% polyacrylamide) under non-reducing conditions, transferred to nitrocellulose filters, and probed with an anti-Rubisco activase antibody; red, reduced; ox, oxidized.
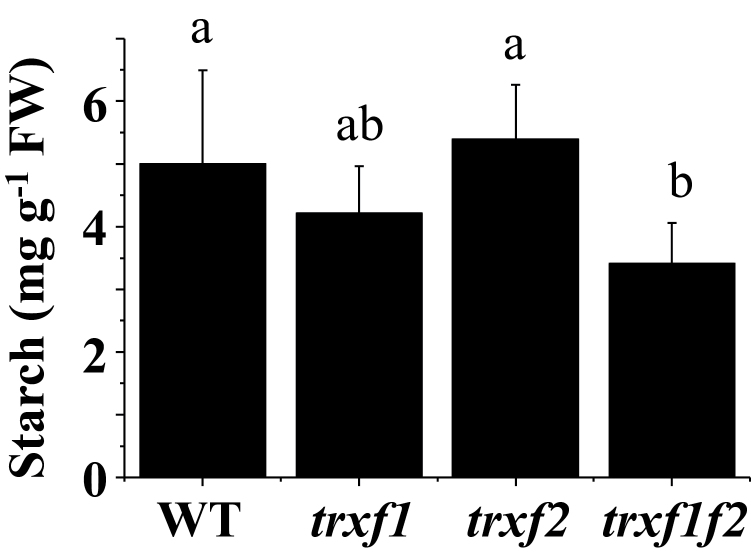
Quantification of the reduction of FBPase and Rubisco activase from a series of experiments (such as those presented in Figs 8 and 9), revealed that the levels of reduction of both enzymes remained at 50% of the wild-type level in the trxf1f2 double mutant after 10min illumination at the growth light intensity (see Supplementary Fig. S9A, B). Furthermore, we analysed the change of the redox status of FBPase following a light–dark transition. Re-oxidation of FBPase in the dark was faster in all three Trx f deficient mutants than in wild-type plants (Fig. 10). However, after 5min darkness, FBPase was also nearly completely oxidized in the wild type (Fig. 10). Finally, we compared the starch content in leaves which was significantly lower in the trxf1f2 double mutant than in the wild type and the single mutants (Fig. 11).
Fig. 10.
Re-oxidation of FBPase in response to darkness. The redox status of FBPase from the different lines under analysis, as indicated on the left, was determined in leaf extracts from plants grown under short-day conditions and harvested at the end of the light period (time 0), and after 1min and 5min of darkness. Total leaf proteins were extracted in the presence of 10% TCA and protein thiols were alkylated with 10mM MM(PEG)24. Proteins were resolved in SDS-PAGE (9.5% polyacrylamide) under non-reducing conditions, transferred to nitrocellulose filters, and probed with an anti-FBPase antibody; red, reduced; ox, oxidized. Band intensities were quantified and the percentage of reduced FBPase is indicated.
Discussion
Following the demonstration of the function of Trxs in the light-dependent regulation of chloroplast photosynthetic metabolism (Wolosiuk and Buchanan, 1976), extensive biochemical and proteomic work has shown the central role of f-type Trxs in the reductive activation of most of the Calvin–Benson cycle enzymes. In this regard, the findings that overexpression of Trx f, but not Trx m, enhanced starch accumulation and increased the content of sugars in tobacco leaves (Sanz-Barrio et al., 2013) lend further support to the idea that, among the complex set of plastidial Trxs, those of type f play a key role in the redox regulation of carbon metabolism. However, Arabidopsis knockout mutants for Trx f1, which accounts for up to 95% of the total f-type Trxs in this plant, showed impairment of AGPase redox regulation and leaf diurnal starch turnover alterations, yet the visible phenotype of these mutants was indistinguishable from the wild type (Thormählen et al., 2013). Moreover, double mutants lacking both Trx f1 and f2 were also indistinguishable from wild type plants (Yoshida et al., 2015). Thus, the lack of f-type Trxs seems not to affect plant growth despite the relevant function proposed for these Trxs in the redox regulation of chloroplast metabolism based on biochemical analyses. These results suggest that additional redox systems may compensate for the function of f-type Trxs being sufficient to support the redox regulation that allows plant growth.
To address this issue, in the present work we have generated a double mutant of Arabidopsis, here termed trxf1f2, lacking both Trx f1 and Trx f2. Both qRT-PCR and Western blot analyses (Fig. 1A, B) confirmed that the trxf1f2 double mutant is a null mutant devoid of f-type Trxs. Western blot analysis of leaf extracts from wild-type plants detected a double band, which might correspond to f1 and f2 Trxs. However, this possibility was ruled out because the double band was detected in the trxf2 mutant but not in the trxf1 mutant. Thus, the double band is most probably indicative of a post-translational modification, the nature of which is still unknown. Under long-day conditions the growth of the single mutants, trxf1 and trxf2, was indistinguishable from that of wild-type plants, consistent with previous studies on Trx f1-deficient mutants (Thormählen et al., 2013). Similarly, the double mutant displayed the wild-type phenotype when grown under long-day conditions, as shown for type-f null mutants recently reported (Yoshida et al., 2015). However, under short-day conditions, single mutants trxf1 and trxf2 showed slightly retarded growth (Fig. 3B), in agreement with the behaviour of the Trx f1-deficient mutant, which shows retarded growth under short-days, but not under the long-day photoperiod (Thormälen et al., 2015). Adult plants of the trxf1f2 double mutant showed retarded growth (Fig. 3) which was observed only at later stages of development, indicating that the effect of the deficiency of f-type Trxs reported here (lower efficiency of photosynthetic parameters, impaired reduction of Calvin–Benson enzymes, lower starch content) requires time to affect plant growth. Most likely, the delayed growth of the trx f-deficient mutant under short-day conditions is not attributable to short-term kinetics of activation, but rather to the lower final level of reduction of enzymes, such as FBPase and Rubisco activase, which can be seen after 10min of illumination (Figs 8, 9) and also at the end of the day (Fig. 10). Moreover, high light intensity (950 μE m−2 s−1), which caused the decrease in chlorophyll content of both the wild type and mutant lines (Fig. 4C), resulted in retarded growth not only of the double trxf1f2 mutant but also of the single mutants after 34 d of growth (Fig. 4B). Therefore, deficiency of f-type Trxs affects the plant’s response to high light suggesting the participation of these Trxs in plant adaptation to light intensities that might produce oxidative stress. Therefore, despite the central function attributed to type f Trxs in chloroplast redox regulation based on biochemical in vitro analyses, the in vivo approach reported here shows that these Trxs are dispensable for plant growth at least under the standard long-day conditions performed in this study. It should be noted, however, that conditions that may cause light limitation, such as growth under a short-day photoperiod, have a negative effect on the growth rate of Trx f-deficient mutants. Moreover, higher light intensity failed to stimulate the growth of mutant plants to the extent that it was stimulated in wild-type plants (Fig. 4), indicating the relevant function of type-f Trxs is for plant adaptation to varying light conditions.
The genes encoding Trx f1 and Trx f2 isoforms are subject to different regulation in Arabidopsis. The expression of the TRX f1 gene responds to light, whereas the TRX f2 gene is under circadian control (Barajas-López et al., 2011). The differential pattern of expression of these genes might indicate different functions for the respective Trx f isoforms. However, all the photosynthetic parameters analysed in this study, such as photosynthetic electron transport and response to illumination of the rate of CO2 fixation (A N), were more affected in the trxf1 than in the trxf2 mutant, while the double mutant showed an additive effect. Most probably, these results reflect the higher content of Trx f1 in wild-type plants (Fig. 1B) and support a redundant function for both f-type Trxs in Arabidopsis.
A relevant question concerning chloroplast redox regulation is the level of redundancy or specificity among the many different types of Trx in this organelle. This issue is currently being addressed with the aid of mutants or transgenic plants with altered levels of the different plastidial Trxs. In this regard, the redox status in vivo of the Mg chelatase CHLI subunit from pea plants was affected by the simultaneous silencing of the TRX f and TRX m genes, but not by the silencing of the TRX f gene alone (Luo et al., 2012), showing the compensatory effect of Trx m on the regulation of this enzyme of the chlorophyll biosynthesis pathway. Therefore, one might assume that m-type Trxs could substitute for f-type Trxs in the light-dependent redox regulation of the Calvin–Benson cycle enzymes which is in line with the relevant role proposed for m-type Trxs in the redox regulation of these enzymes (Okegawa and Motohashi, 2015). However, comparative analyses in vitro of a large number of the Arabidopsis plastid Trxs (f1, f2, m1, m2, m3, m4, x, y1, and y2) confirmed that only the f-type Trxs are capable of activating FBPase using recombinant enzymes (Collin et al., 2003, 2004). The relevant function of f-type Trxs in the redox regulation of chloroplast enzymes was further confirmed by in vivo studies showing the incomplete reduction of FBPase in mutant plants devoid of Trx f1 (Thormählen et al., 2015) and the double mutant devoid of both Trx f1 and Trx f2 (Yoshida et al., 2015). In addition, purified Rubisco activase has been shown to be readily reduced and activated by spinach Trx f, whereas spinach Trx m is unable to reduce this enzyme (Zhang and Portis, 1999). In line with the proposed central function of f-type Trxs in light-dependent redox regulation of the Calvin–Benson cycle enzymes, our studies in vivo show that reduction of both FBPase and Rubisco activase upon illumination is impaired in the trxf1f2 knockout mutant (Figs 8, 9;http://jxb.oxfordjournals.org/lookup/suppl/doi:10.1093/jxb/erw017/-/DC1 Supplementary Fig. S9). Nevertheless, despite the complete absence of Trx f, both enzymes become partially reduced during illumination. These results suggest that the in vitro studies showing the predominant function of f-type Trxs in the redox regulation of the Calvin–Benson cycle enzymes cannot be extrapolated to the physiological situation and show that the double mutant relies on alternative system(s) capable of limited reductive activation of these enzymes in response to light. In this regard, it is worth mentioning that mutant plants devoid of NTRC, an alternative chloroplast redox system, show an incomplete level of FBPase reduction, this effect being even more dramatic in the ntrc-trxf1 double mutant (Thormählen et al., 2015). Therefore, light-dependent redox regulation of Calvin–Benson cycle enzymes, such as FBPase, seems to be the result of the action of different redox systems including type-f Trxs (Fig. 8, this work; Thormählen et al., 2015; Yoshida et al., 2015), type m Trxs (Okegawa and Motohashi, 2015), and NTRC (Thormählen et al., 2015). The finding that NTRC and Trx f1 act in concert (Thormählen et al., 2015) indicates the existence of cross-talk among these redox systems.
The light-dependent reduction of FBPase and Rubisco activase show very similar patterns in wild-type plants on the one hand and in Trx f-deficient plants on the other (Figs 8, 9; Supplementary Fig. S9), suggesting common regulatory mechanisms for the two enzymes. This may be significant since both enzymes are involved in the pathway of CO2 fixation in the chloroplast. It should be noted that plant chloroplasts contain two types of FBPase, termed FBPase I and II, but only one of them, FBPase I, is redox-regulated (Serrato et al., 2009). Our results show that, upon illumination with a higher light intensity, 500 μE m−2s−1, FBPase becomes fully reduced (Fig. 8) indicating that the redox-insensitive form, FBPase II, is present in minor amounts in chloroplasts, in agreement with previous results (Rojas-González et al., 2015).
An interesting observation regarding the redox state of FBPase and Rubisco activase is that these enzymes do not become fully reduced under the growth light intensity even in wild-type plants. This is in agreement with recent reports (Yoshida et al. 2014, 2015) showing that Arabidopsis plants display only partial reduction of the FBPase when illuminated at low light intensity. However, we observed the complete reduction of FBPase in wild-type plants after 10min illumination at 500 μE m−2 s−1 light intensity, while the trxf1f2 double mutant showed a partial reduction of the enzyme (Fig. 8). A remarkable implication of these results is that plants that have been adapted to low light conditions have a significant pool of inactive, not fully reduced, enzymes. A possible advantage of this seemingly wasteful synthesis of excess Calvin–Benson cycle enzymes is that, after a sudden increase in photon flux leading to higher rates of photosynthetic electron transport, the ATP and NADPH generated would immediately be utilized for carbon dioxide fixation and, thus, would not be a problem of light stress. Obviously, a prerequisite for this to occur is that there are sufficiently high amounts of f-type Trxs to catalyse the reductive activation of these enzymes. In addition, we have analysed the changes of redox status of FBPase in light–dark transitions. Re-oxidation of FBPase in response to darkness is again a very rapid process which is essentially completed in 5min in wild-type plants (Fig. 10). The fact that re-oxidation is faster in the mutants could indicate that, until exhausted, a pool of reduced f-type Trxs continues to catalyse reduction in the dark.
Light-harvesting efficiency and photosynthetic electron transport are highly sensitive to changes in carbon assimilation. For example, sub-atmospheric levels of CO2, which result in limited regeneration of ADP and NADP+, lead to elevated NPQ and decreased effective PSII quantum yield at low or moderate light intensities (Kramer et al., 2004). Similarly, inhibition of the Calvin–Benson cycle enzymes in vivo by iodoacetamide leads to higher NPQ and slower linear photosynthetic electron transport (Joliot and Alric, 2013). Notably, the qE component of NPQ ensures safe dissipation of the light energy absorbed and prevents excess excitation of PSII (Szabó et al., 2005; Baker, 2008; Ruban et al., 2012). Such a negative feedback control that arises from hampered CO2 fixation is the most likely explanation for the results concerning the activities of PSII and PSI obtained from the trxf1f2 mutant plants using chlorophyll fluorescence and P700 absorbance. Hence, despite normal maximum PSII quantum efficiency, F v/F m, the linear photosynthetic electron transport rate is significantly decreased in the double trxf1f2 knockout mutant compared with the wild type, particularly at lower light intensities. At 100–150 μE m−2 s−1, which corresponds to growth light intensity, the electron transport in the double mutant was only half that in wild-type plants (Fig. 6A) and the yield of NPQ was twice as high (Fig. 6B).
Although in plants devoid of f-type Trxs the response of the rate of carbon assimilation (A N) to light was delayed (Table 1) these plants still reached carbon assimilation rates similar to those of the wild type (Supplementary Fig. S8). However, the content of starch was diminished in the trxf1f2 mutant (Fig. 11) which is in line with the impaired activation of the AGPase of the trxf1 single mutant (Thormählen et al., 2013). Nevertheless, the leaf starch accumulated during the day under a long-day light regime seems sufficient for the correspondingly short night and to support wild-type growth rates (Fig. 2).
Fig. 11.
The content of starch in leaves of the wild type and Trx f mutant lines. Starch content was determined at the end of the day in leaves from plants grown under long-day conditions for 22–26 d. Data are the mean ±SD of five replicates from two different experiments (with a total of three starch extractions). Letters indicate significant differences with the Tukey Test and a confidence interval of 99%.
Conclusion
In summary, the in vivo approach undertaken in this work identifies the impact of type f Trxs on photosynthetic performance in Arabidopsis, such as the kinetics of activation of carbon assimilation, the redox status of Calvin–Benson cycle enzymes upon a dark–light transition, and the control of photosynthetic electron transport. The fact that these parameters were impaired in plants devoid of type f Trxs indicates that the functions of these Trxs are specific and are not compensated for by other Trxs or chloroplast redox systems. On the other hand, FBPase and Rubisco activase showed a significant level of reduction upon dark–light transition in the trxf1f2 double mutant indicating that additional chloroplast redox systems participate in light-dependent reduction of these Calvin–Benson cycle enzymes. This stands out against the well-established notion, based on biochemical in vitro analyses, of the almost exclusive role attributed to type f Trxs in the redox regulation of these enzymes. Surprisingly, the growth of plants devoid of type f Trxs is indistinguishable from wild-type plants when grown under standard conditions with a long-day photoperiod, indicating that the function of type f Trxs are dispensable for growth.
Supplementary data
Supplementary data can be found at JXB online.
Table S1. Sequence of oligonucleotides used for genotype analyses.
Table S2. Sequence of oligonucleotides used for gene expression analyses.
Figure S1. Genotype of Trx f-deficient mutants.
Figure S2. Cross-reaction of the anti Trx f1 antibody with Trx f1 and Trx f2.
Figure S3. Level of NTRC, m- and x-type TRX gene transcripts in Trx f-deficient mutants.
Figure S4. Chlorophyll a fluorescence of PSII.
Figure S5. Photosystem II activity in wild type and Trx f deficient mutants grown under long-day conditions.
Figure S6. Light-dependent linear photosynthetic electron transport and NPQ in wild type and Trx f deficient mutants grown under long-day conditions.
Figure S7. Absorbance of the oxidized form of PSI.
Figure S8. Net CO2 assimilation rate (A N) in the wild type and Trx f deficient mutants.
Figure S9. Light-dependent reduction FBPase and Rubisco activase.
Acknowledgements
This work was supported by grant number BIO2013-43556-P from the Ministerio de Economía y Competitividad, Spain. The anti-FBPase and anti-Rubisco activase antibodies were provided by Dr Sahrawy, Estación Experimental del Zaidín, Granada, Spain and by Dr Portis, USDA, Urbana, USA, respectively. BN was the recipient of an FPI pre-doctoral fellowship from the Ministerio de Economía y Competitividad, Spain.
References
- Alkhalfioui F, Renard M, Montrichard F. 2007. Unique properties of NADP-thioredoxin reductase C in legumes. Journal of Experimental Botany 58, 969–978. [DOI] [PubMed] [Google Scholar]
- Arsova B, Hoja U, Wimmelbacher M, Greiner E, Ustün S, Melzer M, Petersen K, Lein W, Börnke F. 2010. Plastidial thioredoxin z interacts with two fructokinase-like proteins in a thiol-dependent manner: evidence for an essential role in chloroplast development in Arabidopsis and Nicotiana benthamiana . The Plant Cell 22, 1498–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology 59, 89–113. [DOI] [PubMed] [Google Scholar]
- Balmer Y, Koller A, del Val G, Manieri W, Schürmann P, Buchanan BB. 2003. Proteomics gives insight into the regulatory function of chloroplast thioredoxins. Proceedings of the National Academy of Sciences, USA 100, 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsera M, Uberegui E, Schürmann P, Buchanan BB. 2014. Evolutionary development of redox regulation in chloroplasts. Antioxidants and Redox Signaling 20, 1327–1355. [DOI] [PubMed] [Google Scholar]
- Barajas-López J, Serrato AJ, Cazalis R, Meyer Y, Chueca A, Reichheld JP, Sahrawy M. 2011. Circadian regulation of chloroplastic f and m thioredoxins through control of the CCA1 transcription factor. Journal of Experimental Botany 62, 2039–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broin M, Cuiné S, Eymery F, Rey P. 2002. The plastidic 2-cysteine peroxiredoxin is a target for a thioredoxin involved in the protection of the photosynthetic apparatus against oxidative damage. The Plant Cell 14, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan BB, Balmer Y. 2005. Redox regulation: a broadening horizon. Annual Review of Plant Biology 56, 187–220. [DOI] [PubMed] [Google Scholar]
- Buchanan BB, Holmgren A, Jacquot JP, Scheibe R. 2012. Fifty years in the thioredoxin field and a bountiful harvest. Biochimica et Biophysica Acta 1820, 1822–1829. [DOI] [PubMed] [Google Scholar]
- Cejudo FJ, Ferrández J, Cano B, Puerto-Galán L, Guinea M. 2012. The function of the NADPH thioredoxin reductase C-2-Cys peroxiredoxin system in plastid redox regulation and signalling. FEBS Letters 586, 2974–2980. [DOI] [PubMed] [Google Scholar]
- Chibani K, Wingsle G, Jacquot JP, Gelhaye E, Rouhier N. 2009. Comparative genomic study of the thioredoxin family in photosynthetic organisms with emphasis on Populus trichocarpa . Molecular Plant 2, 308–322. [DOI] [PubMed] [Google Scholar]
- Collin V, Issakidis-Bourguet E, Marchand C, Hirasawa M, Lancelin J-M, Knaff DB, Miginiac-Maslow M. 2003. The Arabidopsis plastidial thioredoxins. New functions and new insights into specificity. Journal of Biological Chemistry 278, 23747–23752. [DOI] [PubMed] [Google Scholar]
- Collin V, Lamkemeyer P, Miginiac-Maslow M, Hirasawa M, Knaff DB, Dietz KJ, Issakidis-Bourguet E. 2004. Characterization of plastidial thioredoxins from Arabidopsis belonging to the new y-type. Plant Physiology 136, 4088–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courteille A, Vesa S, Sanz-Barrio R, Cazalé AC, Becuwe-Linka N, Farran I, Havaux M, Rey P, Rumeau D. 2013. Thioredoxin m4 controls photosynthetic alternative electron pathways in Arabidopsis. Plant Physiology 161, 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Friemann R, Glauser DA, Bourquin F, Manieri W, Schürmann P, Eklund H. 2007. Structural snapshots along the reaction pathway of ferredoxin-thioredoxin reductase. Nature 448, 92–96. [DOI] [PubMed] [Google Scholar]
- Dangoor I, Peled-Zehavi H, Levitan A, Pasand O, Danon A. 2009. A small family of chloroplast atypical thioredoxins. Plant Physiology 149, 1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot JP, Eklund H, Rouhier N, Schürmann P. 2009. Structural and evolutionary aspects of thioredoxin reductases in photosynthetic organisms. Trends in Plant Science 14, 336–343. [DOI] [PubMed] [Google Scholar]
- Jarvis P, López-Juez E. 2013. Biogenesis and homeostasis of chloroplasts and other plastids. Nature Reviews Molecular and Cellular Biology 14, 787–802. [DOI] [PubMed] [Google Scholar]
- Joliot P, Alric J. 2013. Inhibition of CO2 fixation by iodoacetamide stimulates cyclic electron flow and non-photochemical quenching upon far-red illumination. Photosynthesis Research 115, 55–63. [DOI] [PubMed] [Google Scholar]
- Kalituho L, Beran KC, Jahns P. 2007. The transiently generated nonphotochemical quenching of excitation energy in Arabidopsis leaves is modulated by zeaxanthin. Plant Physiology 143, 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchsteiger K, Ferrández J, Pascual MB, González M, Cejudo FJ. 2012. NADPH thioredoxin reductase C is localized in plastids of photosynthetic and nonphotosynthetic tissues and is involved in lateral root formation in Arabidopsis . The Plant Cell 24, 1534–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DM, Johnson G, Kiirats O, Edwards GE. 2004. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynthesis Research 79, 209–218. [DOI] [PubMed] [Google Scholar]
- Lemaire SD, Michelet L, Zaffagnini M, Massot V, Issakidis-Bourguet E. 2007. Thioredoxins in chloroplasts. Current Genetics 51, 343–365. [DOI] [PubMed] [Google Scholar]
- Lepistö A, Pakula E, Toivola J, Krieger-Liszkay A, Vignols F, Rintamäki E. 2013. Deletion of chloroplast NADPH-dependent thioredoxin reductase results in inability to regulate starch synthesis and causes stunted growth under short-day photoperiods. Journal of Experimental Botany 64, 3843–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TP, Caspar T, Somerville C, Preiss J. 1988. Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiology 86, 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Fan T, Liu Y, Rothbart M, Yu J, Zhou S, Grimm B, Luo M. 2012. Thioredoxin redox regulates ATPase activity of magnesium chelatase CHLI subunit and modulates redox-mediated signaling in tetrapyrrole biosynthesis and homeostasis of reactive oxygen species in pea plants. Plant Physiology 159, 118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y, Belin C, Delorme-Hinoux V, Reichheld JP, Riondet C. 2012. Thioredoxin and glutaredoxin systems in plants: molecular mechanisms, crosstalks, and functional significance. Antioxidants and Redox Signaling 17, 1124–1160. [DOI] [PubMed] [Google Scholar]
- Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P. 2009. NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proceedings of the National Academy of Sciences, USA 106, 9908–9913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet L, Zaffagnini M, Morisse S, et al. 2013. Redox regulation of the Calvin–Benson cycle: something old, something new. Frontiers in Plant Science 4, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrichard F, Alkhalfioui F, Yano H, Vensel WH, Hurkman WJ, Buchanan BB. 2009. Thioredoxin targets in plants: the first 30 years. Journal of Proteomics 72, 452–474. [DOI] [PubMed] [Google Scholar]
- Moon JC, Jang HH, Chae HB, et al. 2006. The C-type Arabidopsis thioredoxin reductase ANTR-C acts as an electron donor to 2-Cys peroxiredoxins in chloroplasts. Biochemical and Biophysical Research Communications 348, 478–484. [DOI] [PubMed] [Google Scholar]
- Motohashi K, Hisabori T. 2006. HCF164 receives reducing equivalents from stromal thioredoxin across the thylakoid membrane and mediates reduction of target proteins in the thylakoid lumen. Journal of Biological Chemistry 281, 35039–35047. [DOI] [PubMed] [Google Scholar]
- Okegawa Y, Motohashi K. 2015. Chloroplastic thioredoxin m functions as major regulator of Calvin cycle enzymes during photosynthesis in vivo . The Plant Journal doi: 10.1111/tpj.13049. [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz JM, Guinea M, Puerto-Galán L, Cejudo FJ. 2014. NADPH thioredoxin reductase C is involved in redox regulation of the Mg-chelatase I subunit in Arabidopsis thaliana chloroplasts. Molecular Plant 7, 1252–1255. [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz JM, Spínola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ. 2006. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. The Plant Cell 18, 2356–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta – Bioenergetics 975, 384–394. [Google Scholar]
- Puerto-Galán L, Pérez-Ruiz JM, Ferrández J, Cano B, Naranjo B, Nájera VA, González M, Lindahl AM, Cejudo FJ. 2013. Overoxidation of chloroplast 2-Cys peroxiredoxins: balancing toxic and signaling activities of hydrogen peroxide. Frontiers in Plant Science 4, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido P, Spínola MC, Kirchsteiger K, Guinea M, Pascual MB, Sahrawy M, Sandalio LM, Dietz K-J, González M, Cejudo FJ. 2010. Functional analysis of the pathways for 2-Cys peroxiredoxin reduction in Arabidopsis thaliana chloroplasts. Journal of Experimental Botany 61, 4043–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter AS, Peter E, Rothbart M, Schlicke H, Toivola J, Rintamäki E, Grimm B. 2013. Posttranslational influence of NADPH-dependent thioredoxin reductase C on enzymes in tetrapyrrole synthesis. Plant Physiology 162, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-González JA, Soto-Súarez M, García-Díaz Á, Romero-Puertas MC, Sandalio LM, Mérida Á, Thormählen I, Geigenberger P, Serrato AJ, Sahrawy M. 2015. Disruption of both chloroplastic and cytosolic FBPase genes results in a dwarf phenotype and important starch and metabolite changes in Arabidopsis thaliana . Journal of Experimental Botany 66, 2673–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Johnson MP, Duffy CDP. 2012. The photoprotective molecular switch in the photosystem II antenna. Biochimica et Biophysica Acta 1817, 167–181. [DOI] [PubMed] [Google Scholar]
- Sanz-Barrio R, Corral-Martinez P, Ancin M, Segui-Simarro JM, Farran I. 2013. Overexpression of plastidial thioredoxin f leads to enhanced starch accumulation in tobacco leaves. Plant Biotechnology Journal 11, 618–627. [DOI] [PubMed] [Google Scholar]
- Schröter Y, Steiner S, Matthäi K, Pfannschmidt T. 2010. Analysis of oligomeric protein complexes in the chloroplast sub-proteome of nucleic acid-binding proteins from mustard reveals potential redox regulators of plastid gene expression. Proteomics 10, 2191–2204. [DOI] [PubMed] [Google Scholar]
- Serrato A, Pérez-Ruiz JM, Spínola MC, Cejudo FJ. 2004. A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana . Journal of Biological Chemistry 279, 43821–43827. [DOI] [PubMed] [Google Scholar]
- Serrato AJ, Yubero-Serrano EM, Sandalio LM, Muñoz-Blanco J, Chueca A, Caballero JL, Sahrawy M. 2009. cpFBPaseII, a novel redox-independent chloroplastic isoform of fructose-1,6-bisphosphatase. Plant, Cell and Environment 32, 811–827. [DOI] [PubMed] [Google Scholar]
- Spinola MC, Pérez-Ruiz JM, Pulido P, Kirchsteiger K, Guinea M, Gonzalez M, Cejudo FJ. 2008. NTRC new ways of using NADPH in the chloroplast. Physiologia Plantarum 133, 516–524. [DOI] [PubMed] [Google Scholar]
- Steiner S, Schröter Y, Pfalz J, Pfannschmidt T. 2011. Identification of essential subunits in the plastid-encoded RNA polymerase complex reveals building blocks for proper plastid development. Plant Physiology 157, 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó I, Bergantino E, Giacometti GM. 2005. Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation. EMBO Reports 6, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormählen I, Meitzel T, Groysman J, Öchsner AB, von Roepenack-Lahaye E, Naranjo B, Cejudo FJ, Geigenberger P. 2015. Thioredoxin f1 and NADPH-dependent thioredoxin reductase C have overlapping functions in regulating photosynthetic metabolism and plant growth in response to varying light conditions. Plant Physiology 169, 1766–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thormählen I, Ruber J, von Roepenack-Lahaye E, Ehrlich SM, Massot V, Hümmer C, Tezycka J, Issakidis-Bourguet E, Geigenberger P. 2013. Inactivation of thioredoxin f1 leads to decreased light activation of ADP-glucose pyrophosphorylase and altered diurnal starch turnover in leaves of Arabidopsis plants. Plant, Cell and Environment 36, 16–29. [DOI] [PubMed] [Google Scholar]
- Wang P, Liu J, Liu B, et al. 2013. Evidence for a role of chloroplastic m-type thioredoxins in the biogenesis of photosystem II in Arabidopsis. Plant Physiology 163, 1710–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmelbacher M, Börnke F. 2014. Redox activity of thioredoxin z and fructokinase-like protein 1 is dispensable for autotrophic growth of Arabidopsis thaliana . Journal of Experimental Botany 65, 2405–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosiuk RA, Buchanan BB. 1976. Studies on the regulation of chloroplast NADP-linked glyceraldehyde-3-phosphate dehydrogenase. Journal of Biological Chemistry 251, 6456–6461. [PubMed] [Google Scholar]
- Wolosiuk RA, Crawford NA, Yee BC, Buchanan BB. 1979. Isolation of three thioredoxins from spinach leaves. Journal of Biological Chemistry 254, 1627–1632. [PubMed] [Google Scholar]
- Yoshida K, Hara S, Hisabori T. 2015. Thioredoxin selectivity for thiol-based redox regulation of target proteins in chloroplasts. Journal of Biological Chemistry 290, 14278–14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Matsuoka Y, Hara S, Konno H, Hisabori T. 2014. Distinct redox behaviors of chloroplast thiol enzymes and their relationships with photosynthetic electron transport in Arabidopsis thaliana . Plant and Cell Physiology 55, 1415–1425. [DOI] [PubMed] [Google Scholar]
- Zhang N, Portis AR. 1999. Mechanism of light regulation of Rubisco: a specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-f. Procedings of the National Academy of Sciences, USA 96, 9438–9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.