Highlight
The relationship between an adenine isopentenyltransferase transgene, reactive oxygen species (ROS) content and the ROS-scavenging system, mechanistically contributing to improved root growth under drought stress in creeping bentgrass, is discussed.
Key words: Antioxidant, drought stress, qRT-PCR, root respiration, ROS scavenging system, turfgrass.
Abstract
Drought stress limits root growth and inhibits cytokinin (CK) production. Increases in CK production through overexpression of isopentenyltransferase (ipt) alleviate drought damages to promote root growth. The objective of this study was to investigate whether CK-regulated root growth was involved in the alteration of reactive oxygen species (ROS) production and ROS scavenging capacity under drought stress. Wild-type (WT) creeping bentgrass (Agrostis stolonifera L. ‘Penncross’) and a transgenic line (S41) overexpressing ipt ligated to a senescence-activated promoter (SAG12) were exposed to drought stress for 21 d in growth chambers. SAG12-ipt transgenic S41 developed a more extensive root system under drought stress compared to the WT. Root physiological analysis (electrolyte leakage and lipid peroxidation) showed that S41 roots exhibited less cellular damage compared to the WT under drought stress. Roots of SAG12-ipt transgenic S41 had significantly higher endogenous CK content than the WT roots under drought stress. ROS (hydrogen peroxide and superoxide) content was significantly lower and content of total and free ascorbate was significantly higher in S41 roots compared to the WT roots under drought stress. Enzymatic assays and transcript abundance analysis showed that superoxide dismutase, catalase, peroxidase, and dehydroascorbate reductase were significantly higher in S41 roots compared to the WT roots under drought stress. S41 roots also maintained significantly higher alternative respiration rates compared to the WT under drought stress. The improved root growth of transgenic creeping bentgrass may be facilitated by CK-enhanced ROS scavenging through antioxidant accumulation and activation of antioxidant enzymes, as well as higher alternative respiration rates when soil water is limited.
Introduction
Drought stress is a primary limiting factor of plant growth and productivity in semi-arid and arid climatic areas. Drought inhibition of shoot and root growth has been associated with the induction of oxidative damage due to over-production of reactive oxygen species (ROS), such as superoxide (O2 -) and hydrogen peroxide (H2O2) (Halliwell, 2006). Excessive ROS can lead to lipid peroxidation, protein degradation, and nucleotide damage further inhibiting a wide range of plant cellular processes (Smirnoff, 1993; Kültz, 2005, Halliwell, 2006; Anjum et al., 2011). Therefore, suppressing ROS production or enhancing the capacity for ROS scavenging by antioxidant enzymes [i.e. superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), monohydroascorbate reductase (MR), dehydroascorbate reductase (DR), and glutathione reductase (GR)] and antioxidant compounds [i.e. ascorbate (ASA) and glutathione (GSH)] can suppress drought-induced oxidative damage (Asada 1992; Bowler et al., 1992; Willekens et al., 1997; Noctor and Foyer, 1998; Mittler 2002). Despite extensive knowledge of ROS scavenging regulating shoot responses to drought stress, limited information is available describing mechanisms of ROS detoxification for maintaining root growth during drought stress. ROS are mainly produced in root mitochondria during respiration; the cytochrome respiration pathway involves electron transfer from the ubisemiquinone radical to oxygen, prompting superoxide formation (Halliwell and Gutteridge, 1999). Activating both enzymatic and non-enzymatic antioxidant defense systems could suppress drought-induced ROS formation in roots. In addition, alternative respiration pathways, which divert the high-energy electrons to alternative oxidase can also avoid ROS over-production in roots (Purvis and Shewfelt, 1993; Wagner and Krab, 1995). However, it is uncertain how to maintain active ROS defense systems in roots that are typically weakened by prolonged drought stress (Jain et al., 2006).
Cytokinins (CK) are a class of plant hormones synthesized primarily in actively growing root tips, which play critical roles in regulating plant growth and responses to abiotic stresses (Li et al., 1992; Peres and Kerbauy, 1999; Takei et al., 2001). Drought stress inhibits CK synthesis and accelerates CK degradation, reducing CK levels in roots and shoots (Kudoyarova et al., 2007; Nishiyama et al., 2011). Increasing endogenous CK content through exogenous application of CK (Zhang and Ervin, 2004; Zavaleta-Mancera et al., 2007; Baque et al., 2010) or genetic modification to overexpress isopentenyltransferase (ipt) controlling cytokinin synthesis has positive effects on improving plant drought tolerance, which has been attributed to the promotion of photosynthesis, water use efficiency, and antioxidant metabolism of shoots in various plant species (Zhang and Ervin, 2004; Rivero et al., 2007, 2009; Merewitz et al., 2010, 2011a; Ghanem et al., 2011). CK have been shown to modulate leaf enzymatic antioxidant activities (i.e. POD, SOD, and CAT), activating leaf defenses to abiotic stresses (Chaloupková and Smart 1994; Kurepa et al. 1997; Petit-Paly et al. 1999; Synkova et al., 2006; Zavaleta-Mancera et al., 2007). In addition, Zhang and Ervin (2008) also reported antioxidant properties of CK, protecting leaves from stress-induced oxidation. Most of previous work including the aforementioned literature on CK regulation of drought tolerance has focused on enhancing shoot growth and suppressing leaf senescence.
Few studies have examined CK-regulation of root responses to drought stress involving oxidative stress. CK have been widely reported to play negative roles in primary root elongation and lateral root formation of dicot species, such as Arabidopsis, under non-stress conditions (Werner et al., 2003; Kuderová et al., 2008). However, increasing CK content promoted growth of fibrous root systems in monocot species, such as barley (Hordeum vulgare) through RNAi silencing of HvCKX1 encoding cytokinin oxidase catalyzing CK cleavage (Zalewski et al., 2010) and creeping bentgrass through overexpressing ipt gene under drought stress (Merewitz et al., 2010, 2011a, b). The PSARK::IPT transgenic tobacco plants with increased CK content also exhibited greater root biomass than the WT under drought stress (Rivero et al., 2007). The mechanisms of CK-promotion of root growth under drought stress, with particular focus on whether CK mediates antioxidant defense systems protecting roots from drought-induced oxidative damages, are not well understood.
Since drought-inhibition of root growth is associated with the production of ROS, and CK may enhance root growth under drought stress, it is reasonable to postulate that CK may contribute towards maintaining or enhancing root growth under drought stress or alleviate drought damages in roots by suppressing ROS accumulation, increasing production of non-enzymatic antioxidant compounds, and/or activating enzymatic ROS scavenging systems. Therefore, the objective of this study was to investigate whether CK-regulated root growth under drought stress was involved in the alteration of reactive oxygen species (ROS) production and ROS scavenging capacity using a perennial grass species, creeping bentgrass, overexpressing ipt ligated to a senescence-associated promoter (SAG12). Previous work in our lab revealed improvement in drought tolerance and associated proteins and metabolites involved in the CK-regulation of shoot growth by increasing endogenous CK content in the SAG12-ipt creeping bentgrass lines (Merewitz et al., 2010, 2011a, b, 2012). The present study focuses on examining root tolerance to drought stress and antioxidant metabolism of roots in SAG12-ipt creeping bentgrass, which may enable transgenic plants to maintain active root growth under prolonged periods of drought stress.
Materials and methods
Plant materials and growth conditions
The plants tested in this experiment were A. stolonifera cv. Penncross wild-type line WT, which was transformed with the empty vector used for SAG12-ipt transgene, and SAG12-ipt transgenic line S41. S41 was examined in this study as previous studies have shown that this transgenic line exhibited superior drought tolerance, as manifested by greater overall turf quality, improved root viability, and increased root iPA and total CK content, as well as other physiological factors compared to the WT (Merewitz et al., 2010, 2011a). Four individual plants, each of which contained 30 uniform-size tillers of either WT or S41, were transferred to a plastic container (57×44×30cm; 12 drainage holes in base) filled with fritted clay medium (Profile Products, Deerfield, IL). Both WT and S41 were planted in eight containers (of which four were for well-watered conditions and four for were for drought stress conditions). Plants were established for 35 d in a greenhouse maintained at an average day/night temperature of 23/20°C and 780 µmol m−2 s−1 photosynthetically active radiation (PAR) at the canopy level from natural sunlight and supplemental lighting. Plants were irrigated daily, fertilized twice per week with half-strength Hoagland’s nutrient solution (Hoagland and Arnon, 1950), and trimmed to 2cm once per week during establishment. Plants were not trimmed during the final week of establishment to allow for sufficient foliar regrowth prior to stress imposition. After 35 d of plant establishment, plants were transferred to four controlled-environment growth chambers (Environmental Growth Chamber, Chagrin Falls, Ohio) and acclimated in the chambers for a week before drought stress was imposed. The growth chambers were set to maintain 22/18°C (day/night), 60% relative humidity, 12-h photoperiod, and 650 µmol m−2 s−1 PAR at the canopy level.
Drought treatments and experimental design
Non-stressed control plants were continually supplied with adequate water and nutrients as previously described, while other plants were subjected to drought stress by withholding irrigation for 21 d. Soil volumetric water content was measured using the time domain reflectometry method (Trase TDR, Soil Moisture Equipment, Santa Cruz, CA) By 21 d of drought stress, soil water content declined to 7.8% while soil water content was maintained at 28.1% under well-watered conditions for both the WT and transgenic plants.
The experiment was arranged as a completely randomized design with each plant line (WT or S41) and each watering treatment (well-watered or drought stress) replicated four times (four containers per treatment). Plants were randomly placed in four growth chambers.
iPa extraction, purification, and analysis
Isopentenyladenine (iPA) was extracted from roots and purified using the procedure as described by Zhang et al. (2013). Briefly, root tissues were ground with a mortar and a pestle in liquid nitrogen and a sample (50mg) was mixed with 1.8ml Na-phosphate buffer (50mM, pH 7.0) containing 0.02% sodium diethyldithiocarbamate as an antioxidant and iPA was extracted by continuous shaking for 1h at 4ºC. The pH for each sample was adjusted to ~2.6, and then the sample was slurried with Amberlite XAD-7 (150mg) (Sigma, St Louis, MO) for 30min. After removal of the buffer, the XAD-7 was washed twice with 1ml 1% acetic acid before being slurried two times with 1ml dichloromethane for 30min. The combined dichloromethane fractions were reduced to dryness with nitrogen gas. Then, samples were dissolved in 210 µl methanol and diluted to 700 µl d.i. H2O with 0.1% formic acid. The sample was filtered with a syringe filter (0.2 µm).
The iPA was analyzed using an indirect enzyme-linked immunosorbent assay (ELISA) as described by Zhang and Ervin (2004). Briefly, wells of a 96-unit plate were coated with 100 µl per well of iPA conjugated to bovine serum albumin (BSA) (1:10 000 dilution), incubated overnight at 4ºC, emptied, and washed three times with phosphate buffered saline (PBS, 50mM, pH 7.2)-Tween-20 (PBS containing 0.05% Tween 20). The reaction was ‘blocked’ with 200 µl of 1% BSA in PBS (37ºC, 30min) to prevent nonspecific protein absorption. After the plate was washed twice with PBS-Tween, 50 µl of the iPA extract or iPA standard and 50 µl of the antibody iPA (1:200 dilution) were added to the wells and the plate was incubated at 37ºC for 60min, emptied, and washed three times with PBS-Tween. A series of iPA concentrations (0, 3.13, 6.25, 12.5, 25, and 50ng ml-1) were made for a standard curve. To each well, 100 ul of alkaline phosphatase-labeled goat anti-mouse IgG (1:1 000 dilution; Sigma, St Louis, MO) was added and the plate were incubated at 37ºC for 60min. After three washes with PBS Tween, 100 µl of substrate solution (3mg ml-1 of p-nitrophenyl phosphate in 10% diethanolamine with 0.5mM MgCl2, pH 9.8) were added to each well and the plate was incubated at 37ºC for 30min. The color reaction in each well was determined by measuring absorbance at 405nm with a microplate reader. Isopentenyl adenosine concentration was calculated on the basis of the standard curve after logarithmic conversion of the data.
trans-ZR analysis using liquid chromatography-tandem mass spectrometry
The content of trans-zeatin riboside (trans-ZR) was analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Alvarez et al., 2008). Root tissues (2.0g) were freeze-dried and ground to powder using mortar and pestle in liquid nitrogen. To each ground sample, 900 µl of ice-cold methanol/acetonitrile (MeOH/ACN, 1:1 [v/v]) and 10 µl of a 2.5 µM deuterium-labeled standard ([d5]-trans-zeatin riboside) was added, and the sample was homogenized with the TissueLyser II (QIAGEN, Valencia, CA) for 5min at a frequency of 20 Hz/s, then centrifuged at 16 000 ×g for 10min at 4°C. The supernatant was transferred to a new 2ml tube and the pellet was re-extracted as previously described. The second supernatant was combined to the first one and dried down. The dried pellet was dissolved in 200 μl of 30% [v/v] methanol, then centrifuged again to remove un-dissolved material and the supernatant was transferred to vial for LC-MS/MS analysis. The injected volume of the sample was 50 µl.
The LC-MS/MS system used is composed of a Shimadzu LC system with two Shimadzu solvent delivery pumps (LC20AD) and autosampler (SIL20AC) with a 100 ul sample loop (Shimadzu, Kyoto, Japan), and a Valco two-position diverter valve (VICI, Houston, TX). This LC system is interfaced with an AB Sciex 4 000 QTRAP mass spectrometer equipped with a TurboIonSpray (TIS) electrospray ion source (SCIEX, Framingham, MA). Source parameters were set as follows: curtain gas, 20 arbitrary units (a.u.); source gas 1, 50 a.u.; source gas 2, 50 a.u.; collision activated dissociation, high; interface heater, on; temperature, 550°C; ionspray voltage, +5500. Both quadruples (Q1 and Q3) were set to unit resolution. Analyst software (version 1.5) was used to control sample acquisition and data analysis. The 4 000 QTRAP mass spectrometer was tuned and calibrated according to the manufacturer’s recommendations. The hormone contents were detected using MRM transitions that were previously optimized using a standard and a deuterium-labeled standard. All data were presented as the mean (the average content in g DW) ±SE (the standard error) of four biological replicates.
Root growth analysis
Following 21 d of drought treatment, roots were washed free of fritted clay to measure total root length and root-to-shoot ratio. For root length measurement, roots were stained with 1% crystal violet solution and scanned with a digital scanner (Epson Expression 1680, US Epson, Inc., Long Beach, CA) to generate high-definition digital images. Images were analyzed using WinRHIZO Basic V.2002 software (Regent Instruments Inc., Quebec, QC, Canada) for root length. To determine root-to-shoot (R/S) biomass ratio, shoots were severed from roots, both tissue types were dried in an oven at 80°C for 7 d, weight determined on a mass balance, and ratio of roots to shoots was calculated. All data were presented as the mean (the average content in g dry weight (DW) or ratio) ±SE of four biological replicates.
Root physiological analysis
Following 21 d of drought treatment, roots free of fritted clay were collected to quantify root electrolyte leakage (EL) and malondialdehyde (MDA) content. EL was measured according to the procedure described by Blum and Ebercon (1981) and used to indicate cellular membrane stability or membrane status following treatment (Merewitz et al., 2011b; Burgess and Huang, 2014). Roots were rinsed with deionized water to remove exogenous solutes and placed in a test tube containing 30ml deionized water. Tubes were agitated in a conical flask shaker for 12h and the initial conductance (Ci) of incubation solution measured using a conductivity meter (YSI Model 32, Yellow Springs, OH). Tubes containing root tissue were then autoclaved at 121°C for 20min and again agitated for 12h. The maximal conductance (Cmax) of incubation solution was then measured and EL (%) was calculated as [(Ci/Cmax)×100].
MDA is the final product of lipid peroxidation in plant tissue and was quantified according to the procedure described by Zhang and Kirkham (1996) with slight modifications. Roots (0.5g) were homogenized in 6ml 0.1% trichloroacetic acid (TCA) and the homogenate was centrifuged at 10 000 ×g for 10min. 1ml supernatant was added to 4ml 10% TCA containing 0.5% thiobarbituric acid. The mixture was incubated at 95°C for 30min, quickly cooled on ice, and centrifuged at 10 000 ×g for 10min at 4°C. The absorbance of supernatant was measured at 532 and 600nm using a spectrophotometer (Spectronic Instruments, Rochester, NY). The concentration of MDA was calculated using an extinction coefficient of 155mM−1 cm−1 (Heath and Packer, 1968). All data were presented as the mean (the average content in g DW) ±SE of four biological replicates.
Histochemical staining for hydrogen peroxide and superoxide in roots
Histochemical staining for the presence of hydrogen peroxide and superoxide was performed following 21 d stress treatment according to procedures described in Thordal-Christensen et al. (1997) and Dunand et al. (2007), respectively, each with slight modifications. To evaluate the presence of H2O2, roots were stained with 1% (w/v) 3-diaminobenzinidine (DAB; pH 3.8) for 2h and subsequently rinsed with deionized water. To evaluate the presence of O2 -, roots were stained with 2mM nitroblue tetrozolium (NBT) in 20mM phosphate-buffered saline (PBS; pH 6.8) for 30min and subsequently rinsed with deionized water. DAB- or NBT-stained roots were visually observed using an Olympus FSX100 Bio-imaging navigator (Central Valley, PA) and pictures were captured using bright-field single-shot mode at ×4.2 magnification.
Quantification of reactive oxygen species in roots
The production rate of O2 - was measured according to the procedure described by Bian and Jiang (2009) with slight modifications. Root tissues (0.5g) were ground to powder in liquid nitrogen, homogenized in 1ml 50mM Tris-HCl (pH 7.5), and centrifuged at 5 000g for 10min at 4°C. 200 μl supernatant was added to 800 μl 0.5mM 3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrozolium-5-carboxanilide inner salt (XTT). XTT reduction was recorded once per minute for 3min at 470nm using a spectrophotometer (Spectronic Instruments, Rochester, NY) and the background absorbance was corrected with 50 units of superoxide dismutase (SOD). The O2 - production rate was calculated using a 2.16×104 M−1 cm−1 extinction coefficient and expressed as µmol O2 - min−1 g−1 DW (Sutherland and Learmonth, 1997).
The content of H2O2 content was measured according to the procedure described by Zhou et al. (2006) with slight modifications. Ground root tissues (0.5g) were homogenized in 5ml 5% (w/v) TCA and the homogenate was centrifuged at 10 000 ×g for 20min at 4°C. The supernatant was adjusted to pH 8.4 with 17M ammonia solution, briefly centrifuged to remove large particles, and divided into 1ml aliquots. To a single aliquot, 8 μg catalase was added, to serve as the blank. To each aliquot was added 1ml colorimetric reagent solution containing 10mg 4-aminoantipyrine, 10mg phenol and 5mg peroxidase in 100mM acetic acid buffer (pH 5.6), and the color reaction was incubated for 10min at 30°C. Following incubation, the absorbance was measured at 505nm using a spectrophotometer (Spectronic Instruments, Rochester, NY) and H2O2 content determined based on standard curve generated with known H2O2 concentrations. All data were presented as the mean (the average content in g DW) ±SE of four biological replicates.
Quantification of non-enzymatic antioxidant content in roots
Glutathione (GSH) content was quantified according to the procedure described in Guri (1983) with slight modifications. Frozen root powder (0.5g) was ground to a powder in liquid nitrogen, homogenized with 5ml of 5% ice-cold TCA, and centrifuged at 16 000 ×g for 20min at 4°C. The homogenate was titrated to a pH range of 6–8 with 1.5ml 0.1M NaOH. 2.0ml titrated homogenate was added to 0.5ml of 0.2M PBS (pH 7.0), 0.4ml of distilled water, and 0.1ml of dithiobis-2-nitrobenzoic acid (DTNB) and absorbance measured at 412nm using a spectrophotometer (Spectronic Instruments, Rochester, NY). Titrated homogenate containing sodium phosphate buffer, deionized water, but lacking DTNB served as the blank. GSH content was determined based on standard curves generated with known concentrations of GSH.
Free and total ascorbate (ASA) content were quantified according to the procedure described in Ma et al. (2008) with slight modifications. Frozen root powder (0.5g) was ground to a powder in liquid nitrogen, homogenized in 8ml 5% (w/v) TCA on ice, centrifuged at 10 000 ×g for 10min at 4°C, and the resulting supernatant was used immediately for analysis. For total ASA quantification, the supernatant was incubated in 200mM sodium phosphate buffer (pH 7.4) and 1.5mM dithiothreitol (DTT) for 50min to reduce all dehydroascorbic acid to ASA. Following incubation, 200 μl 0.5% (w/v) N-ethylmaleimide (NEM) was added to remove excess DTT. The resulting solution (0.8ml) was then added to a reaction mixture containing 1ml 10% (w/v) TCA, 800 μl 42% (w/v) o-phosphoric acid, 800 μl 65mM 2,2’-dipyridyl in 70% (v/v) ethanol, and 400 μl 3% (w/v) iron (III) chloride. The reaction was incubated at 42°C for 1h, and absorbance measured at 525nm using a spectrophotometer (Spectronic Instruments, Rochester, NY). Free ASA was measured using the procedure described above with DTT and NEM substituted with 400 μl deionized water. Free and total ASA content were determined based on standard curves generated with known ASA concentrations. All data were presented as the mean (the average content in g DW) ±SE of four biological replicates.
Quantification of enzymatic antioxidant activity in roots
Enzyme activity of CAT, POD, SOD, APX, DR, MR, and GR was measured according to the procedures described by Zhang and Kirkham (1996). For each CAT, POD, and SOD assay, 0.5g ground root tissue was homogenized in 6ml 50mM sodium phosphate buffer (pH 7.0) containing 0.2mM ethylenediaminetetraacetic acid (EDTA) and 1% (w/v) polyvinylpyrrolidone (PVP) on ice and the homogenates were centrifuged at 15 000 ×g for 20min at 4°C. Absorbances were measured at 240, 470, and 560nm for CAT, POD, and SOD, respectively, using a spectrophotometer (Spectronic Instruments, Rochester, NY). For each APX, DR, MR, and GR assay, 0.5g ground root tissue was homogenized in 6ml 25mM sodium phosphate buffer (pH 7.8) containing 0.2mM EDTA and 1% (w/v) PVP and the homogenates were centrifuged at 15 000 ×g for 20min at 4°C. Absorbances were measured at 290, 265, 340, and 340nm for APX, DR, MR, and GR, respectively, using a spectrophotometer (Spectronic Instruments, Rochester, NY). All data were presented as the mean (the average content in mg protein) ±SE of four biological replicates.
Gene expression analysis of enzymatic antioxidants in roots
Gene expression analysis was performed by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). Total RNA was isolated from root tissue using TRIzol reagent (Life Technologies, Grand Island, NY) and treated with DNase (TURBO DNA-free kit; Life Technologies, Grand Island, NY) to remove contaminating genomic DNA. Using a high-capacity cDNA reverse transcription kit (Life Technologies, Grand Island, NY), 2 μg total RNA was reverse-transcribed and the synthesized cDNA was amplified in a StepOnePlus Real-Time PCR system (Life Technologies, Grand Island, NY) using the following parameters: pre-heat cycle of 95°C for 3min, 40 cycles of 95°C denaturation for 30s, and 60°C annealing/extension for 60s. Power SYBR Green PCR Master Mix (Life Technologies, Grand Island, NY) was the intercalating dye used to detect gene expression level. Gene name, accession number, forward and reverse primer sequences are provided in Table 1. A melting curve analysis was performed for each primer pair to confirm its specificity. Actin was used as the reference gene, since its expression was constant throughout treatments. A ΔΔCt method was used to calculate the relative expression level between genes of interest and reference gene, respectively. All transcript levels were expressed as the mean (the average relative expression level) ±SE of four biological replicates.
Table 1.
Primer sequences of genes used in qRT-PCR
Proposed gene names, GenBank accession numbers, best BLAST hit names, E-values, and cellular localizations are also listed.
| Gene | Accession number | Best BLAST hit | E-value | Localization | Primer sequence | |
|---|---|---|---|---|---|---|
| CuZn-SOD | DV867103 | JQ269675.1 (Triticum aestivum) | 3e-161 | Cytosolic | Forward | CACTGGACCTCACTTCAAC |
| Reverse | GTAGCAACACCATCCACTC | |||||
| POD2 | DV867327 | XM_010230345.1 (Brachypodium distachyon) | 6e-153 | Cytosolic | Forward | CTTCGACAACGCCTACTAC |
| Reverse | TTTGCCCATGTTCACCA | |||||
| CAT1 | DY543619 | AJ634002.1 (Schedonorus arundinaceus) | 0 | Chloroplast | Forward | TTGCCAATAAGAGGGAGAATG |
| Reverse | CGAAGCCGAGCATGTAAG | |||||
| APX2 | GR281667 | KP852178.1 (Beckmannia syzigachne) | 0 | Cytosolic | Forward | AGGACATTGTTGCCCTTTC |
| Reverse | GCTCCGTGAAGTAAGAGTTG | |||||
| GR | AB277097 | AB277097 (Hordeum vulgare) | 0 | Cytosolic | Forward | GATGGAGGCTACTTGCTTTG |
| Reverse | GCTAAGACCCACGACAGATA | |||||
| MR | DV865077 | KC884831.1 (Triticum aestivum) | 5e-160 | Cytosolic | Forward | CCATGAAGCTCTACAACGAG |
| Reverse | GTAGAAGTAGGGCAGGTAGT | |||||
| DR | DV853556 | HM125046.1 (Puccinellia tenuiflora) | 0 | Cytosolic | Forward | GAAAGGTGCCTGTGTTTAATG |
| Reverse | GTGATGGAGTTGGGTACTTC | |||||
| ACT2 | DY543529 | XM_003578821.2 (Brachypodium distachyon) | 0 | Cytosolic | Forward | CCTTTTCCAGCCATCTTTCA |
| Reverse | GAGGTCCTTCCTGATATCCA | |||||
Analysis of root respiration rate
Root respiration rate was measured according to the procedure described by Rachmilevitch et al. (2006) with slight modifications. A subset of roots was detached from shoots, washed free of fritted clay, and immediately transferred into 500ml Buchner flasks containing 400ml half-strength Hoagland’s nutrient solution (Hoagland and Arnon, 1950). To mimic drought stress conditions, polyethylene glycol (PEG) 8 000 was added to nutrient solution to adjust the osmotic potential to −1.0MPa (Lagerwerff et al., 1961; Janes 1974). Subsequently, the nutrient solution with or without PEG 8 000 contained either 200 μM sodium nitroprusside (SNP) to inhibit the cytochrome respiratory pathway or 10mM salicylhydroxamic acid (SHAM) to inhibit the alternative respiratory pathway. Solutions containing SNP or SHAM were maintained as an open-flow system by aerating with circulating pumps (Apollo Enterprises Inc., Oxnard, CA) for 30min, after which time a closed-flow system was created by connecting the terminal air tube to the circulating pump inlet. Vacuum grease and Teflon tape were used to maintain an airtight seal around the rubber stoppers. CO2 evolution rate was measured every 30min for 2h by extracting 1ml air samples from the flasks using airtight syringes and resealable septa affixed to flask side arms. Air samples were then injected into a Shimadzu GC-8AIT gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a thermal conductivity detector and a stainless steel column (length: 6 ft; I.D.: 0.08''; O.D.: 1/8'') packed with Porapack Q (80/100 mesh). The temperatures for injector, column, and detector were set at 30°C, 150°C and 150°C, respectively. Helium was used as a carrier gas at a flow rate of 30ml min−1. Remaining root tissue was dried in an oven at 80°C for 72h and subsequently weighed on a mass balance. Root respiration rates were expressed as CO2 evolution rate in mmol h−1 g−1 DW.
Statistical analysis
Significant effects of treatment, plant line and their interactions were determined by two-way analysis of variance (ANOVA) using a statistical program (JMP11, SAS Institute, Cary, NC). The effects of transgene, drought stress and the interaction of the above two factors on the parameters tested were provided as P-values in Supplementary Table S1 available at JXB online. Differences between treatment mean values were distinguished by Student’s t-test at the 0.05 probability level.
Results
Differential root cytokinin content, root growth and physiological responses to drought stress
Cytokinin content varied between the WT and transgenic plants under both well-watered and drought conditions. iPA content was not significantly different between WT and S41 under well-watered conditions (Fig. 1A). Root iPA content decreased under drought stress in WT whereas it increased in S41 and S41 roots had significantly higher amounts of iPA than the WT under drought stress. The trans-ZR content was significantly higher (2.4-fold) in roots of S41 than the WT under well-watered conditions (Fig. 1B). Under drought stress, both WT and S41 had significantly higher ZR contents (2.6 fold and 3.25 fold, respectively), while S41 root also had significantly greater (25%) ZR content than WT roots (Fig. 1B).
Fig. 1.
Cytokinin (iPA and trans-ZR) content in roots of WT and transgenic S41 plants under control or drought stress conditions: (A) iPA content, (B) trans-ZR content. Data shown are the mean ±SE of four biological replicates. Letters above bars indicate significant differences at the P≤0.05 level.
The R/S ratio (Fig. 2A) and total length (Fig. 2B) did not differ between the WT and S41 under well-watered conditions. At 21 d of drought stress, root total length and R/S ratio was significantly higher (25% for root length and 12% for R/S) in S41 compared to WT. Under drought stress, WT plants had more elongated root systems, while S41 had more abundant root systems, compared with respective controls. (Fig. 2C).
Fig. 2.
The dry weight (DW) ratio of root to shoot (R/S) (A), total root length per plant (B) and representative plants (C) of WT and S41 under control or drought stress. Data shown are the mean ±SE of four biological replicates. Letters above bars indicate significant differences exist at the P≤0.05 level.
Root EL did not differ between plant lines under well-watered conditions (Fig. 3A). Root EL increased by 74 and 66% for WT and S41, respectively, at 21 d of drought stress compared to their respective well-watered control. Root EL was significantly lower (9%) in S41 compared to WT under drought stress.
Fig. 3.
Root electrolyte leakage (A) and MDA content (B) of WT and S41 following control or drought stress. Data shown are the mean ±SE of four biological replicates. Letters above bars indicate significant differences exist at the P≤0.05 level.
Root MDA content was not significantly different between plant lines under well-watered conditions (Fig. 3B). In response to drought stress, root MDA content increased significantly in both WT (by 50%) and S41 (by 12%) compared to their respective well-watered controls (Fig. 3B). Root MDA content was significantly lower (19%) in S41 compared to WT following drought stress.
Differential levels of ROS production under drought stress
Under well-watered conditions, root O2 - production rate in S41 was significantly higher than that in WT. Root O2 - production rate increased 2.7- and 1.6-fold for WT and S41, respectively, at 21 d of drought stress compared to their respective well-watered controls (Fig. 4A). Roots of S41 maintained significantly lower O2 - production rate (23%) in S41 compared to WT under drought stress.
Fig. 4.
O2- production rate (A) and H2O2 content (B) of WT and S41 following control or drought stress. Data shown are the mean ±SE of four biological replicates. Letters above bars indicate significant differences exist at the P≤0.05 level.
Bar, 100 μm.
Under well-watered conditions, root H2O2 content in S41 was significantly higher than that in WT. Root H2O2 content increased significantly in WT (by 98%) but did not change significantly under drought stress compared to well-watered conditions (Fig. 4B). Roots of S41 maintained significantly lower (22%) O2 - production rate in S41 compared to WT under drought stress.
Non-enzymatic antioxidant content and antioxidant enzyme activities
Root free ASA content was significantly higher (33%) in S41 than WT under well-watered conditions, and remained significantly higher (28%) in S41 under drought stress (Fig. 5A). Root total ASA content did not differ between WT and S41 under well-watered conditions but was significantly higher (14%) in S41 than that in WT under drought stress (Fig. 5B). No significant differences GSH content in roots between WT and S41 under well-watered or drought conditions (Fig. 5C).
Fig. 5.
Free ascorbate (A), total ascorbate (B) and glutathione (C) content in WT and S41 roots under control or drought stress. Data shown are the mean ±SE of four biological replicates. Letters above bars indicate significant differences exist at the P≤0.05 level.
Root SOD activity was significantly lower in S41 than WT under well-watered conditions, but decreased by 81 and 57% for WT and S41, respectively, under drought stress compared to their respective well-watered controls (Fig. 6A). Roots of S41 exhibited significantly higher (66%) SOD activity than WT roots under drought stress. Root CAT activity was significantly higher in S41 than WT under well-watered conditions, and increased by 2.5- and 2.0-fold for WT and S41, respectively, under drought stress compared to their respective well-watered controls (Fig. 6B). Roots of S41 had significantly higher (31%) CAT activity than WT roots at 21 d of drought stress. Root POD activity was significantly lower in S41 than WT under well-watered conditions, and decreased by 63 and 54%, respectively, due to drought stress treatment (Fig. 6C), while there was no significant difference in POD activity between WT and S41 under drought stress.
Fig. 6.
Enzymatic activity of SOD (A), CAT (B) and POD (C) in roots of WT and S41 under control or drought stress. Data shown are the mean ±SE of four biological replicates. Letters above bars indicate significant differences exist at the P≤0.05 level.
Under well-watered conditions, root APX, GR, MR and DR activity in S41 were significantly higher than in WT. Root APX activity decreased by 79 and 62% for WT and S41, respectively, due to drought stress (Fig. 7A). Root APX activity was significantly higher (2.0-fold) in S41 compared to WT under drought stress. Root GR activity did not change significantly in response to drought stress for both WT and S41 while it was significantly higher (14%) in S41 than in WT at under both well-watered and drought stress conditions (Fig. 7B). Root MR activity increased by 38% for WT, but did not change in S41 during drought stress and did not differ between WT and S41 at 21 d of drought stress (Fig. 7C). Root DR activity decreased by 24% for WT but increased by 15% for S41 due to drought stress treatment (Fig. 7D). Root DR activity was significantly higher (79%) in S41 compared to WT under drought stress.
Fig. 7.
Enzymatic activity of APX (A), GR (B), MR (C) and DR (D) in roots of WT and S41 under control or drought stress. Data shown are the mean ±SE of four biological replicates. Letters above bars indicate significant differences exist at the P≤0.05 level.
Antioxidant enzyme gene expression
Antioxidant enzyme gene transcript levels exhibited significant differences between WT and S41 in response to drought stress treatment. Root SOD expression level was significantly down-regulated for WT (by 97%) and S41 (by 98%) due to drought-stress treatment compared to respective well-watered controls (Fig. 8A). Root SOD expression level did not differ between WT and S41 under well-watered conditions or drought stress. Root POD expression was significantly up-regulated for WT (by 1.9-fold) and S41 (by 6.0-fold) due to drought stress treatment compared to respective well-watered controls and was significantly higher (7.4-fold) in S41 compared to WT under drought stress (Fig. 8B). Root CAT expression was significantly down-regulated for WT (by 31%) and up-regulated for S41 (by 4.5-fold) due to drought stress treatment compared to respective well-watered controls (Fig. 8C). Root CAT expression was significantly higher (4.1-fold) in S41 compared to WT at 21 d of drought.
Fig. 8.
Transcript levels of SOD (A), POD (B) and CAT (C) in roots of control or drought-stressed WT and S41. Data shown are the mean ±SE of four biological replicates. Letters above bars indicate significant differences exist at the P≤0.05 level.
Root APX expression was significantly down-regulated for WT (by 89%) and S41 (by 95%) due to drought stress treatment compared to respective well-watered controls (Fig. 9A). Root APX expression was significantly lower (49%) in S41 compared to WT following drought stress. Root GR expression did not change in response to drought stress for WT or S41 while it was significantly lower (56%) in S41 compared to WT following drought stress (Fig. 9B). Root MR expression decreased by 68 and 54% for WT and S41, respectively, due to drought stress treatment, and did not differ between WT and S41 (Fig. 9C). Root DR expression level decreased by 48 and 53% for WT and S41, respectively, due to drought stress treatment. It was significantly higher (1.5-fold) in S41 compared to WT following drought stress (Fig. 9D).
Fig. 9.
Transcript levels of APX (A), GR (B), MR (C) and DR (D) in roots of WT and S41 under control or drought stress. Data shown are the mean ±SE of four biological replicates. Letters above bars indicate significant differences exist at the P≤0.05 level.
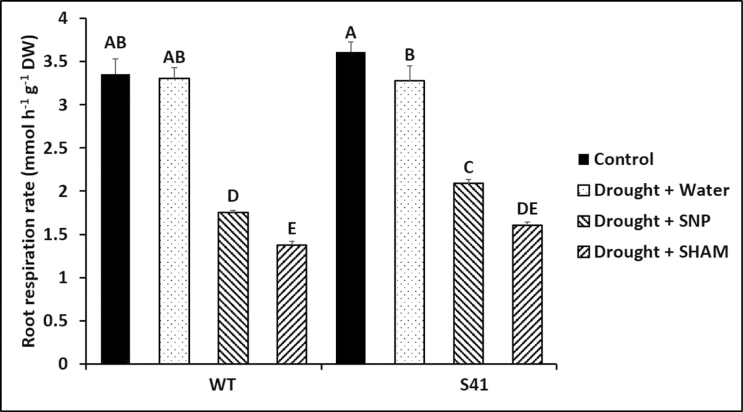
ROS production in relation to root respiration
The addition of SNP to root incubation solutions reduced respiration rates of drought-stressed roots by 47 and 36% in WT and S41, respectively, compared to non-SNP treatment (Fig. 10). The addition of SHAM to incubation solutions reduced respiration rates of drought-stressed roots by 58 and 51% in WT and S41, respectively, compared to non-SHAM treatment (Fig. 10). Roots of S41 exhibited higher alternative respiration rate (SNP-resistant, SHAM-inhibited) under drought stress, compared to WT roots (Fig. 10).
Fig. 10.
Root respiration rate for WT and S41 under drought stress condition as affected by SNP or SHAM. Data shown are the mean ±SE of four biological replicates. Letters above bars indicate significant differences exist at the P≤0.05 level.
Histochemical staining for O2 - and H2O2 in SNP- and SHAM-incubated roots visually depicted the differences in ROS accumulation resulting from changes in respiration rates. Root tips displayed O2 - accumulation in drought-stressed roots, as shown by increased stain intensity with NBT for O2 - in WT or S41 roots under drought stress without SNP treatment but stain intensity decreased significantly and was barely visible for root tips treated with SNP treatment; however, SHAM treatment did not appear to be effective to reduce staining for O2 - in both WT and S41 roots (Fig. 11A–L). DAB staining for H2O2 (Fig. 12A–L) depict that H2O2 was accumulated in the vascular tissues of drought-stressed roots of both WT and S41, but the staining intensity decreased in roots of WT and S41 treated with either SNP or SHAM which inhibited cytochrome and alternative respiration, respectively.
Fig. 11.
Histochemical staining of water-, SNP- and SHAM-incubated WT (A–F) and S41 (G–L) root tips under control and drought stress conditions using NBT. Bar, 100 μm.
Fig. 12.
Histochemical staining of water-, SNP- and SHAM-incubated WT (A–F) and S41 (G–L) root tips under control and drought stress conditions using DAB. Bar, 100 μm.
Discussion
The capacity for water uptake by the root system is a key determinant of plant adaptation to drought stress, but severe drought stress typically restricts root production and proliferation, and accelerates root mortality (Sharp et al., 1988; Davies and Zhang, 1991; Davies et al., 2000; Suralta and Yamauchi, 2008). Understanding metabolic factors regulating root growth responses to drought stress is critically important for improving plant drought tolerance. Cytokinins are known to regulate plant responses to drought stress (Hare et al., 1997; Zhang and Ervin, 2004; Rivero et al., 2007, 2009). While multiple types of CK may be present in plants, the predominant types of CK in grasses are isopentenyl adenine (iPA) and zeatin riboside (ZR), which are related to plant stress responses (Xu et al., 2009). In our previous studies, transgenic creeping bentgrass overexpressing SAG12-ipt showed strong ipt expression in leaves and roots under drought stress, which corresponded to an increase in endogenous iPA and ZR content in leaves and roots (Merewitz, 2010, 2011a). In the present study, increases in both iPA and ZR content were also detected in roots of SAG12-ipt transgenic creeping bentgrass. Increasing endogenous CK content has been positively associated with improved plant drought tolerance in bentgrass species based on evaluating shoot growth and physiological activities (DaCosta and Huang, 2007; Merewitz et al., 2010).
In this study, SAG12-ipt transgenic creeping bentgrass not only had higher total root length but also greater R/S biomass ratio compared to the WT under drought stress, suggesting that increasing CK in the transgenic plants preferentially promoted root growth over shoot growth when plants were exposed to drought stress and may be associated with CK-regulation of biomass partitioning between shoots and roots. In contrast, the vast majority of research suggests increasing CK content negatively affects root growth of dicot species, such as for Arabidopsis and tobacco (Nicotiana tabacum) (Li et al. 2006; Laplaze et al. 2007; Kuderová et al. 2008). Morphology of the fibrous root system in monocots (such as grass species) differs from that of tap root systems of dicots (such as Arabidopsis) which may exhibit unique responses to CK. Several other studies in grass species also reported positive effects of CKs on root growth (Merewitz et al., 2010; Zalewski et al., 2010). For example, RNAi silencing of HvCKX1 encoding cytokinin oxidase controlling CK degradation in barley (Hordeum vulgare) increased root weight and root length and the lower CKX activity or higher CK content is correlated with greater root mass (Zalewski et al., 2010). The regulatory roles of CK for controlling root growth in grass species may differ from the dicot tap root system but underlying mechanisms of CK promoting grass root growth, particularly in response to drought stress, deserves further investigation
Our previous studies found that roots of SAG12-ipt plants with increasing CK content maintained significantly higher root viability and metabolic activities as measured by the triphenyl tetrazolium chloride reduction method during prolonged drought stress (Merewitz et al., 2011a). In the present study, enhanced cellular membrane stability (lower EL) and decreased membrane lipid peroxidation (lower MDA content) were observed during drought stress, confirming that increasing endogenous CK content through overexpression of SAG12-ipt mitigated cellular damages for creeping bentgrass by maintaining membrane integrity. Although transgenic S41 roots had significantly higher H2O2 and O2 - production than WT roots under well-watered conditons, the opposite occurred under drought stress (Fig. 4). This suggests that increasing CK content in the transgenic plants may have suppressed ROS accumulation and mitigated cellular membrane damages (lower EL and MDA content) in roots exposed to drought stress. Most previous studies reported CK involvement in stress-induced oxidative defense in leaves, regulating leaf senescence and shoot growth (Chaloupková and Smart 1994; Kurepa et al. 1997; Petit-Paly et al. 1999; Zhang and Ervin, 2004; Synkova et al., 2006; Rivero et al., 2007, 2009; Zavaleta-Mancera et al., 2007; Merewitz et al., 2010, 2011a). Our study is one of the first to demonstrate increasing CK production could protect roots from drought-induced oxidative damage and maintain better root growth by suppressing ROS levels in root systems due to CK-activation of antioxidant defense mechanisms.
Antioxidant defense encompasses various antioxidant compounds (i.e. ascorbate and glutathione) and enzymes for dismutation of O2 - (i.e. SOD) and reduction of H2O2 by CAT, the ascorbate-glutathione cycle (i.e. APX, MR, DR), or peroxidase cycle (POD) (Bowler et al., 1992; Asada 1992; Foyer and Noctor, 2003; Mittler, 2002). Proteomic analysis of leaves and roots for SAG12-ipt transgenic creeping bentgrass revealed a maintenance or increased abundance of several ROS-related enzymes, including CAT-1, APX4, and glutathione S-transferase, under drought stress (Merewitz et al., 2011b). The ipt-transgenic tobacco plants exhibited differential elevations of antioxidant transcripts and enzyme activity in different plant organs (Rivero et al., 2007). In the current study, the activities of SOD, CAT, APX and DR and transcript levels of CAT, POD, and DR were all significantly greater in S41 roots compared to WT roots exposed to drought stress, suggesting that the antioxidant effects of CK may be due to activating SOD to transform O2 - to H2O2 and then converting H2O2 to H2O through CAT and ascorbate-glutathione cycle enzymes (APX and DR). In addition, free and total ASA content were also significantly higher in S41 roots exposed to drought stress, further confirming the activation of ascorbate-glutathione cycle for H2O2 scavenging. Moreover, CK promoted CAT activity, but decreased APX activity in Morinda citrifolia roots (Baque et al., 2010). The greater antioxidant enzyme activities and free and total ASA content along with up-regulated transcript levels of antioxidant enzymes in SAG12-ipt transgenic creeping bentgrass suggests that superior antioxidant capabilities involving both enzymatic and non-enzymatic pathways activated by CK could facilitate efficient ROS scavenging and reducing ROS accumulation, thereby mitigating cellular damage to maintain active root growth under drought stress.
Coinciding with essential energy production and carbon metabolism functions, plant respiration also serves as a major source of ROS production in root tissues (Juszczuk et al., 2001; Gill and Tuteja, 2010). In addition to enzymatic and non-enzymatic pathways mentioned above, alternative respiration regulated by alternative oxidase (AOX) also prevents over-production of ROS during cytochrome respiration caused by excessive NADH supply from cell metabolism (Purvis, 1997, Ribas-Carbo et al. 2005). Plant species which exhibit superior drought tolerance may acclimate to unfavorable environments by maintaining lower cytochrome respiration rates and higher alternative respiration rate, thereby avoiding excessive ROS production (Burton et al., 1998; Huang and Fu, 2000; Liu and Li, 2005). Nitric oxide (NO) induces AOX activity and alternative respiration by inhibiting cytochrome oxidase activity, which itself is involved in ROS-facilitated stress responses (Millar and Day, 1996). AOX can be an antioxidant enzyme involved in ROS scavenging (Purvis, 1997). In the current study, S41 roots maintained lower cytochrome respiration rates estimated through SHAM-inhibition of alternative respiration but higher alternative respiration rate induced by the treatment of SNP (NO donor) under drought stress. Roots with the SNP treatment also accumulated less O2 - in root tips due to the suppression of cytochrome respiration and activation of alternative respiration and either SNP or SHAM treatments also produced less H2O2 under drought stress. Thus, maintaining greater alternative respiration could suppress ROS production in roots of ipt-transgenic creeping bentgrass exposed to drought stress, as shown by lower staining intensity of ROS in roots by DAB or NBT. Rivero et al. (2009) reported overexpressing ipt in tobacco increased the level of transcripts coding for enzymes in the photorespiration pathway in leaves, which subsequently resulted in increased metabolites generated by photorespiration for antioxidant functions. Several studies investigated the exogenous application of synthetic CKs and the inhibition of both cytochrome and alternative respiration in plant species (Musgrave and Siedow, 1985; Musgrave et al., 1987; Vankova et al., 1991). More recently, an activation of AOX1 in the presence of exogenous CKs was observed in tobacco cells, but its direct relationship to ROS scavenging mechanisms remain unclear. (Mlejnek, 2013). To our knowledge, there are no previous studies relating CK-regulation of alternative respiration in roots to antioxidant defense mechanisms. Our study demonstrated that CK could activate the alternative respiration pathway of roots under drought stress, which along with enhanced activities of SOD, CAT, APX, and DR could mitigate H2O2 and O2 - production and drought-induced oxidative stress in roots.
In summary, overexpressing SAG12-ipt and the associated increased cytokinin content promoted root growth of creeping bentgrass under drought stress and mitigated drought-induced cellular damages, as manifested by greater root length, root-to-shoot ratio, less membrane lipid peroxidation and greater cellular membrane stability of roots. CK could mitigate drought damages in roots by suppressing ROS accumulation and accelerating ROS detoxification by promoting non-enzymatic antioxidant (increased ascorbate content) production, and activating SOD, CAT and ascorbate-glutathione cycle enzymes (APX and DR), as well as enhancing alternative respiration pathways. Further investigation is needed regarding the specific mechanisms and signaling pathways for CK interaction with antioxidant enzymes and genes.
Supplementary data
Supplementary data are available at JXB online.
Table S1. P-values for the effect of S41 transgene, drought treatment and S41 transgene × drought treatment by two-way ANOVA.
Acknowledgements
The authors thank Dr Sophie Alvarez and the Proteomics Facility at The Donald Danforth Plant Science Center (St Louis, MO) for assistance with the mass spectrometry work which is based upon work supported by the National Science Foundation under Grant No. DBI-0521250 for acquisition of the QTRAP LC-MS/MS.
References
- Alvarez S, Marsh EL, Schroeder SG, Schachtman DP. 2008. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant, Cell & Environment 31, 325–340. [DOI] [PubMed] [Google Scholar]
- Anjum SA, Xie XY, Wang LC, Saleem MF, Man C, Lei W. 2011. Morphological, physiological and biochemical responses of plants to drought stress. African Journal of Agricultural Research 6, 2026–2032. [Google Scholar]
- Asada K. 1992. Ascorbate peroxidase – a hydrogen peroxide-scavenging enzyme in plants. Physiologia Plantarum 85, 235–241. [Google Scholar]
- Baque MA, Hahn E-J, Paek K-Y. 2010. Growth, secondary metabolite production and antioxidant enzyme response of Morinda citrifolia adventitious root as affected by auxin and cytokinin. Plant Biotechnology Reports 4, 109–116. [Google Scholar]
- Bian S, Jiang Y. 2009. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Scientia Horticulturae 120, 264–270. [Google Scholar]
- Blum A, Ebercon A. 1981. Cell-membrane stability as a measure of drought and heat tolerance in wheat. Crop Science 21, 43–47. [Google Scholar]
- Bowler C, Montagu MV, Inze D. 1992. Superoxide dismutase and stress tolerance. Annual Review of Plant Physiology and Plant Molecular Biology 43, 83–116. [Google Scholar]
- Burgess P, Huang B. 2014. Effects of sequential application of plant growth regulators and osmoregulants on drought tolerance of creeping bentgrass (Agrostis stolonifera). Crop Science 54, 837–844. [Google Scholar]
- Burton AJ, Pregitzer KS, Zogg GP, Zak DR. 1998. Drought reduces root respiration in sugar maple forests. Ecological Applications 8, 771–778. [Google Scholar]
- Chaloupková K, Smart CC. 1994. The abscisic acid induction of a novel peroxidase is antagonized by cytokinin in Spirodela polyrrhiza L. Plant Physiology 105, 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaCosta M, Huang B. 2007. Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. Journal of the American Society for Horticultural Science 132, 319–326. [Google Scholar]
- Davies WJ, Bacon MA, Stuart Thompson D, Sobeih W, González Rodríguez L. 2000. Regulation of leaf and fruit growth in plants growing in drying soil: exploitation of the plants’ chemical signalling system and hydraulic architecture to increase the efficiency of water use in agriculture. Journal of Experimental Botany 51, 1617–1626. [DOI] [PubMed] [Google Scholar]
- Davies WJ, Zhang J. 1991. Root signals and the regulation of growth and development of plants in drying soil. Annual Review of Plant Physiology and Plant Molecular Biology 42, 55–76. [Google Scholar]
- Dunand C, Crèvecoeur M, Penel C. 2007. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytologist 174, 332–341. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2003. Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia Plantarum 119, 355–364. [Google Scholar]
- Ghanem ME, Albacete A, Smigocki AC, et al. 2011. Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. Journal of Experimental Botany 62, 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48, 909–930. [DOI] [PubMed] [Google Scholar]
- Guri A. 1983. Variation in glutathione and ascorbic acid content among selected cultivars of Phaseolus vulgaris prior to and after exposure to Ozone. Canadian Journal of Plant Science 63, 733–737. [Google Scholar]
- Halliwell B. 2006. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology 141, 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J. 1999. The chemistry of free radicals and related ‘reactive species’. Free Radicals in Biology and Medicine 3, 1–7. [Google Scholar]
- Hare PD, Cress WA, van Staden J. 1997. The involvement of cytokinins in plant responses to environmental stress. Plant Growth Regulation 23, 79–103. [Google Scholar]
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics 125, 189–198. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. California Agricultural Experiment Station 347. Berkeley: University of California. [Google Scholar]
- Huang B, Fu J. 2000. Photosynthesis, respiration, and carbon allocation of two cool-season perennial grasses in response to surface soil drying. Plant and Soil 227, 17–26. [Google Scholar]
- Jain M, Nandwal AS, Kundu BS, Kumar B, Sheoran IS, Kumar N, Mann A, Kukreja S. 2006. Water relations, activities of antioxidants, ethylene evolution and membrane integrity of pigeonpea roots as affected by soil moisture. Biologia Plantarum 50, 303–306. [Google Scholar]
- Janes BE. 1974. The effect of molecular size, concentration in nutrient solution, and exposure time on the amount and distribution of polyethylene glycol in pepper plants. Plant Physiology 54, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszczuk I, Malusà E, Rychter AM. 2001. Oxidative stress during phosphate deficiency in roots of bean plants (Phaseolus vulgaris L.). Journal of Plant Physiology 158, 1299–1305. [Google Scholar]
- Kuderová A, Urbánková I, Válková M, Malbeck J, Brzobohatý B, Némethová D, Hejátko J. 2008. Effects of conditional IPT-dependent cytokinin overproduction on root architecture of Arabidopsis seedlings. Plant and Cell Physiology 49, 570–582. [DOI] [PubMed] [Google Scholar]
- Kudoyarova GR, Vysotskaya LB, Cherkozyanova A, Dodd IC. 2007. Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. Journal of Experimental Botany 58, 161–168. [DOI] [PubMed] [Google Scholar]
- Kültz D. 2005. Molecular and evolutionary basis of the cellular stress response. Annual Review of Physiology 67, 225–257. [DOI] [PubMed] [Google Scholar]
- Kurepa J, Hérouart D, Van Montagu M, Inzé D. 1997. Differential expression of CuZn-and Fe-superoxide dismutase genes of tobacco during development, oxidative stress, and hormonal treatments. Plant and Cell Physiology 38, 463–470. [DOI] [PubMed] [Google Scholar]
- Lagerwerff JV, Ogata G, Eagle HE. 1961. Control of osmotic pressure of culture solutions with polyethylene glycol. Science 133, 1486–1487. [DOI] [PubMed] [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB. 2007. Cytokinins act directly on lateral root founder cells to inhibit root initiation. The Plant Cell 19, 3889–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mo X, Shou H, Wu P. 2006. Cytokinin-mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant and Cell Physiology 47, 1112–1123. [DOI] [PubMed] [Google Scholar]
- Li Y, Hagen G, Guilfoyle T. 1992. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Developmental Biology 53, 386–395. [DOI] [PubMed] [Google Scholar]
- Liu HS, Li FM. 2005. Root respiration, photosynthesis and grain yield of two spring wheat in response to soil drying. Plant Growth Regulation 46, 233–240. [Google Scholar]
- Ma Y-H, Ma F-W, Zhang J-K, Li M-J, Wang Y-H, Liang D. 2008. Effects of high temperature on activities and gene expression of enzymes involved in ascorbate–glutathione cycle in apple leaves. Plant Science 175, 761–766. [Google Scholar]
- Merewitz EB, Du H, Yu W, Liu Y, Gianfagna T, Huang B. 2012. Elevated cytokinin content in ipt transgenic creeping bentgrass promotes drought tolerance through regulating metabolite accumulation. Journal of Experimental Botany 63, 1315–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merewitz EB, Gianfagna T, Huang B. 2010. Effects of SAG12-ipt and HSP18.2-ipt expression on cytokinin production, root growth, and leaf senescence in creeping bentgrass exposed to drought stress. Journal of the American Society for Horticultural Science 135, 230–239. [Google Scholar]
- Merewitz EB, Gianfagna T, Huang B. 2011a Photosynthesis, water use, and root viability under water stress as affected by expression of SAG12-ipt controlling cytokinin synthesis in Agrostis stolonifera . Journal of Experimental Botany 62, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merewitz EB, Gianfagna T, Huang B. 2011b Protein accumulation in leaves and roots associated with improved drought tolerance in creeping bentgrass expressing an ipt gene for cytokinin synthesis. Journal of Experimental Botany 62, 5311–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Day DA. 1996. Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS letters 398, 155–158. [DOI] [PubMed] [Google Scholar]
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7, 405–410. [DOI] [PubMed] [Google Scholar]
- Mlejnek P. 2013. Cytokinin-induced cell death is associated with elevated expression of alternative oxidase in tobacco BY-2 cells. Protoplasma 250, 1195–1202. [DOI] [PubMed] [Google Scholar]
- Musgrave ME, Miller CO, Siedow JN. 1987. Do some plant responses to cytokinins involve the cyanide-resistant respiratory pathway? Planta 172, 330–335. [DOI] [PubMed] [Google Scholar]
- Musgrave ME, Siedow JN. 1985. A relationship between plant responses to cytokinins and cyanide‐resistant respiration. Physiologia Plantarum 64, 161–166. [Google Scholar]
- Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T. 2011. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. The Plant Cell 23, 2169–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology 49, 249–279. [DOI] [PubMed] [Google Scholar]
- Peres LEP, Kerbauy GB. 1999. High cytokinin accumulation following root tip excision changes the endogenous auxin-to-cytokinin ratio during root-to-shoot conversion in Catasetum fimbriatum L. (Orchidaceae). Plant Cell Reporter 18, 1002–1006. [Google Scholar]
- Petit-Paly G, Franck T, Brisson L, Kevers C, Chénieux J-C, Rideau M. 1999. Cytokinin modulates catalase activity and cournarin accumulation in in vitro cultures of tobacco. Journal of Plant Physiology 155, 9–15. [Google Scholar]
- Purvis AC. 1997. Role of the alternative oxidase in limiting superoxide production by plant mitochondria. Physiologia Plantarum 100, 165–170. [Google Scholar]
- Purvis AC, Shewfelt RL. 1993. Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiologia Plantarum 88, 712–718. [DOI] [PubMed] [Google Scholar]
- Rachmilevitch S, Lambers H, Huang B. 2006. Root respiratory characteristics associated with plant adaptation to high soil temperature for geothermal and turf-type Agrostis species. Journal of Experimental Botany 57, 623–631. [DOI] [PubMed] [Google Scholar]
- Ribas-Carbo M, Taylor NL, Giles L, Busquets S, Finnegan PM, Day DA, Lambers H, Medrano H, Berry JA, Flexas J. 2005. Effects of water stress on respiration in soybean leaves. Plant Physiology 139, 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E. 2007. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proceedings of the National Academy of Sciences, USA 104, 19631–19636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Shulaev V, Blumwald E. 2009. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiology 150, 1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, Silk WK, Hsiao TC.1988. Growth of the maize primary root at low water potentials I. Spatial distribution of expansive growth.Plant Physiology 87, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. 1993. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist 125, 27–58. [DOI] [PubMed] [Google Scholar]
- Suralta RR, Yamauchi A. 2008. Root growth, aerenchyma development, and oxygen transport in rice genotypes subjected to drought and waterlogging. Environmental and Experimental Botany 64, 75–82. [Google Scholar]
- Sutherland MW, Learmonth BA. 1997. The tetrazolium dyes MTS and XTT provide new quantitative assays for superoxide and superoxide dismutase. Free Radical Research 27, 283–289. [DOI] [PubMed] [Google Scholar]
- Synkova H, Semoradova S, Schnablova R, Witters E, Husak M, Valcke R. 2006. Cytokinin-induced activity of antioxidant enzymes in transgenic Pssu-ipt tobacco during plant ontogeny. Biologia Plantarum 50, 31–41. [Google Scholar]
- Takei K, Sakakibara H, Taniguchi M, Sugiyama T. 2001. Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: implication of cytokinin species that induces gene expression of maize response regulator. Plant and Cell Physiology 42, 85–93. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. 1997. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. The Plant Journal 11, 1187–1194. [Google Scholar]
- Vankova R, Hsiao K-C, Bornman CH, Gaudinova A. 1991. Effects of synthetic cytokinins on levels of endogenous cytokinins and respiration patterns of Beta vulgaris cells in suspension. Journal of Plant Growth Regulation 10, 197–199. [Google Scholar]
- Wagner AM, Krab K. 1995. The alternative respiration pathway in plants: role and regulation. Physiologia Plantarum 95, 318–325. [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell 15, 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W. 1997. Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. The EMBO Journal 16, 4806–4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tian J, Gianfagna T, Huang B. 2009. Effects of SAG12-ipt expression on cytokinin production, growth and senescence of creeping bentgrass (Agrostis stolonifera L.) under heat stress. Plant Growth Regulation 57, 281–291. [Google Scholar]
- Zalewski W, Galuszka P, Gasparis S, Orczyk W, Nadolska-Orczyk A. 2010. Silencing of the HvCKX1 gene decreases the cytokinin oxidase/dehydrogenase level in barley and leads to higher plant productivity. Journal of Experimental Botany 61, 1839–1851. [DOI] [PubMed] [Google Scholar]
- Zavaleta-Mancera HA, López-Delgado H, Loza-Tavera H, Mora-Herrera M, Trevilla-García C, Vargas-Suárez M, Ougham H. 2007. Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. Journal of Plant Physiology 164, 1572–1582. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ervin EH. 2004. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Science 44, 1737–1745. [Google Scholar]
- Zhang X, Ervin EH. 2008. Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Science 48, 364–370. [Google Scholar]
- Zhang X, Ervin EH, Evanylo GK, Li J, Harich K. 2013. Corn and soybean hormone and antioxidant metabolism responses to biosolids under two cropping systems. Crop Science . 53, 2079–2089. [Google Scholar]
- Zhang J, Kirkham MB. 1996. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytologist , 361–373. [DOI] [PubMed] [Google Scholar]
- Zhou B, Wang J, Guo Z, Tan H, Zhu X. 2006. A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regulation 49, 113–118. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.