Highlight
Copy number variation at the F-M locus plays a driving role in flesh texture diversification in peach.
Key words: Copy number variation, flesh texture, melting flesh, peach, polygalacturonase, stone adhesion.
Abstract
Texture is an important attribute affecting consumer perception of fruit quality. Peach melting flesh and flesh adhesion to stone (endocarp) are simply inherited and controlled by the F-M locus on linkage group (LG) 4. Here, we report that two genes encoding endopolygalacturonase (endoPG) in the F-M locus, designated PpendoPGF and PpendoPGM, are associated with the melting flesh and stone adhesion traits. PpendoPGM controls melting flesh while PpendoPGF has pleiotropic effects on both melting flesh and stone adhesion. The F-M locus has three allelic copy number variants of endoPG, H1 (PpendoPGF and PpendoPGM), H2 (PpendoPGM), and H3 (null). The H2 haplotype represents the ancestral one while the H1 and H3 haplotypes are two variants due to duplication and deletion of PpendoPGM, respectively. Accessions with H1H1, H1H2, or H1H3 genotypes show the freestone or semi-freestone and melting flesh phenotype, while both H2H2 and H2H3 accessions have the clingstone and melting flesh phenotype. The H3H3 accessions have the clingstone and non-melting flesh phenotype. Our study not only demonstrates a driving role of gene copy number variations in flesh texture diversification in fruit trees, but also provides a useful diagnostic tool for early seedling selection in peach breeding programmes.
Introduction
Texture is a sensory property that involves a variety of traits such as crispness, firmness, meltiness, and juiciness, therefore, it has an important direct influence on the consumer’s perception of fruit quality (Brookfield et al., 2011). Major changes in fruit texture occur during ripening and are usually associated with softening. Fruit softening is primarily a result of the decline in cell wall strength and cell-to-cell adhesion. Numerous hydrolases have been suggested as being critical to cell wall disassembly in a variety of fruits such as tomato (Brummell and Harpster, 2001), strawberry (Quesada et al., 2009), apple (Tacken et al., 2010), and apricot (Leida et al., 2011). However, increasing evidence showed that cell wall hydrolases related to fruit softening differ among species and their specific contribution to softening are still not clear (Prasanna et al., 2007; Tomassen et al., 2007).
Peaches are climacteric fruits and can be divided into melting flesh (MF) and non-melting flesh (NMF) types according to fruit softening behaviour. MF peaches lose flesh firmness gradually during early ripening and then soften rapidly (melting phase) in the late stages of ripening, whereas NMF peaches lack the melting phase and retain flesh firmness when fully ripe. Both MF and NMF peaches show considerable variation in firmness and texture although MF is completely dominant over NMF (Bailey and French, 1949; Monet, 1989). Based on flesh adhesion to the stone (endocarp), peaches are also classified as either freestone (F) or clingstone (C). However, the degree of adhesion can be varied as some peaches show semi-freestone or semi-clingstone (Bailey and French, 1949). Based on both flesh softening and stone adhesion, all peaches can be classified into three phenotypes, freestone melting flesh (FMF), clingstone melting flesh (CMF), and clingstone non-melting flesh (CNMF). The phenotype of freestone non-melting flesh (FNMF) has not been reported (Van Der Heyden et al., 1997).
Both flesh texture (melting/non-melting) and stone adhesion (clingstone/freestone) in peach are simply inherited and controlled by the Freestone (F) and Melting flesh (M) loci, respectively. Several studies show that M and F are at the same locus which is designated F-M and mapped to a 3.5 cM interval on the bottom of linkage group (LG) 4 (Dettori et al., 2001; Dirlewanger et al., 2006; Ogundiwin et al., 2009). A number of studies have been conducted to identify potential candidate genes for melting flesh and stone adhesion in peach. Initially, biochemical studies revealed that an endopolygalacturonase (endoPG) is highly expressed in ripe MF peaches, but extremely low in NMF peaches (Pressey and Avants, 1973; Lester et al., 1996). Thus, the endoPG gene is deemed to be a candidate for the M locus in peach (Peace et al., 2005; Morgutti et al., 2006; Ghiani et al., 2011). However, recombination between M and the endoPG gene was observed in three progeny derived from a cross between NMF and MF cultivars (Lester et al., 1996). To reconcile this inconsistency, a hypothesis that the F-M locus may contain at least two copies of the endoPG gene was proposed (Peace et al., 2007). One is responsible for melting flesh texture and another for stone adhesion. The clingstone and non-melting flesh phenotypes result from deletions in an endoPG gene cluster (Callahan et al., 2004; Peace et al., 2007). Overall, evidence for an endoPG cluster controlling melting flesh and stone adhesion in peach is strong but not conclusive.
Digital gene expression (DGE), a novel approach to profiling gene expression at the genome-wide level using next generation sequencing technology, has been widely used to identify genes for important horticultural traits in fruit trees such as citrus (Xu et al., 2009), grape (Sweetman et al., 2013), and strawberry (Kang et al., 2013). The draft of the peach genome sequence was released in 2010 (Verde et al., 2013) which provides an opportunity to perform DGE analysis. In this study, DGE profiling was conducted to analyse differential gene expression between different flesh phenotypes of peaches and candidate genes for the melting flesh and stone adhesion traits were validated using a candidate gene-based association strategy. Allelic variation of the endoPG gene cluster in the F-M locus was also investigated. Our goal is to clarify the genetic basis of stone adhesion and melting flesh in peach. Our study not only demonstrates that gene copy number variations play a driving role in phenotypic diversification in plants but also provides a simple diagnostic PCR test for assisted selection of stone adhesion and flesh softening in peach breeding programmes.
Materials and methods
Plant materials
All peach cultivars used in this study are maintained at the Wuhan Botanical Garden, Chinese Academy of Science (Wuhan, Hubei Province, China). Three peach cultivars, Nanshantiantao (FMF), Zhaohui (CMF), and Myojo (CNMF), were selected for DGE analysis. Fruit samples were collected at four developmental stages, fruitlet (S1), stone hardening (S2), pre-ripening (S3), and the ripening stage (S4), and the detail of each sample collection is listed in Supplementary Table S1 at JXB online. For each sample, ten fruits were collected, cut into small pieces, and mixed. Leaf samples were collected at a young stage in the spring season and used for genomic DNA extraction using the Universal Plant genomic DNA Extraction Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. Both fruit and leaf samples were immediately frozen in liquid nitrogen and stored at −75 °C until use.
DGE library preparation and Illumina sequencing
DEG libraries were constructed from fruit samples of each cultivar at four different development stages, S1–S4. Total RNA was extracted using the Total RNA Rapid Extraction Kit (Zomanbio, Beijing, China) according to the manufacturer’s instructions. The purification of poly(A) mRNAs was performed using oligo-dT attached to magnetic beads. The purified mRNAs were fragmented using super sonication and then subjected to first- and second-strand cDNA synthesis using random hexamer primers. The DGE library was prepared using the Illumina gene expression sample preparation kit, and sequenced using the Illumina Hiseq2000 sequencer according to the manufacturer’s instructions.
Sequence analysis and mapping of DGE reads
Sequencing-received raw image data was transformed by base calling into sequence data. These raw reads were stored in fastq format and then processed using in-house perl script. The frequency of error rate for RNA-Seq reads was calculated based on Phred score (Qphred). RNA-Seq reads were mapped to the peach reference genome v.1.0 (Verde et al., 2013) and only 1bp mismatch was allowed. An index of the reference genome was built using Bowite V2.0.6 (Langmead et al., 2009) and the mapping of RNA-Seq reads was performed using TopHat v 2.0.9 (Trapnell et al., 2009).
Differential gene expression analysis
Gene expression levels were calculated based on reads per kilobase per million mapped reads (RPKM). The number of clean reads mapped to each gene was counted using the software HTSeq v0.5.4p3. The read counts were standardized between samples by scaling the number of reads in a given library to a common value across all sequenced libraries using the edgeR program, version 2.6.10 (Robinson et al., 2010). Differential expression analysis was performed using DEGSeq R package (1.12.0; TNLIST, Beijing, China). The P values were adjusted using the Benjamini and Hochberg method. A threshold Q-value of 0.005 and a log2-fold change of 1 was used to separate differentially expressed genes from non-differentially expressed genes. The sequences of the differentially expressed genes were compared against the NCBI RefSeq nucleotide database and the Swiss-Prot and UniPro protein databases. Differentially expressed genes were sequentially annotated according to the Blast results, followed by the pathway annotation pipelines, including GO (http://www.geneontology.org) and KEGG (www.genome.jp/kegg/).
Quantitative real-time RT-PCR (qRT-PCR)
Total RNA was isolated using the Universal Plant Total RNA Extraction Kit (BioTeke, Beijing, China) according to the manufacturer’s instructions. The RNA samples were treated with DNase I (Takara, Dalian, China) to remove any contamination of genomic DNA. One microgram of total RNA per sample was subjected to cDNA synthesis using cDNA Synthesis SuperMix (TransGen, Beijing, China) according to the manufacturer’s instructions. A SYBR Green-based real-time PCR assay was carried out in a total volume of 25 μl reaction mixture containing 12.5 μl of 2× SYBR Green I Master Mix (Takara, Dalian, China), 0.2 μM of each primer, and 100ng of template cDNA. Melting curve analysis was performed at the end of 40 cycles to ensure proper amplification of the target fragments. Fluorescence readings were consecutively collected during the melting process from 60–90 °C at a heating rate of 0.5 °C s−1. Reaction mixtures without cDNA templates were also run as a negative control. All analyses were repeated three times using biological replicates. The difference in cycle threshold (Ct) between target and actin genes corresponded to the level of gene expression. A peach GAPDH gene was used as a constitutive control (Tong et al., 2009), and all primer sequences are listed in Supplementary Table S2.
Phylogenetic analysis of endoPG genes
Nucleotide sequences of endoPG genes in plants were used for phylogenetic analysis. DNA sequences were aligned using ClustalX and adjusted manually, as necessary. The resulting data were analysed using equally weighted Neighbor–Joining (NJ). The NJ trees were constructed using the heuristic search strategies of MEGA version 5. Bootstrap values were calculated from 1 000 replicate analyses.
Thermal asymmetric interlaced PCR (TAIL-PCR) for unknown flanking sequences
Tail-PCR was performed according to a previously reported protocol (Liu and Chen, 2007). To recover the nucleotide sequences flanking the deletion of the PpendoPGF gene, three specific primers, designated DW-SP1, DW-SP2-adptor, and DW-SP3, were designed based on the sequences flanking the left side of the F-box gene in the F-M locus. Likewise, three specific primers, designated FBX-SP1, FBX-SP2-adptor, and FBX-SP3, were designed based on the coding sequence of the F-box gene to recover the nucleotide sequences flanking the deletion of the PpendoPFM and PpendoPGF genes. Tail-PCR was initially performed with DW-SP1 or FBX-SP1 and one of the four arbitrary degenerate primers containing an adaptor (LAD1-1 to LAD1-4) using genomic DNA as the template. The products from each first-round PCR were diluted 40-fold in double-distilled water (ddH2O) and then subjected to two sequential rounds of PCR amplification using two pairs of primers, DW-SP2-adptor or FBX-SP2-adptor/AC1 (an adaptor), and DW-SP3 or FBX-SP3/AC1, respectively. PCR amplification was conducted according to the same cycle parameters as reported by Liu and Chen (2007). All primer sequences are listed in Supplementary Table S3.
Results
Global gene expression patterns in different developmental stages of peach fruits
In this study, 12 DGE libraries were constructed and sequenced for fruit samples of three peach varieties, i.e. Nanshantiantao (NS), Zhaohui (ZH), and Myojo (MJ) at four developmental stages, i.e. fruitlet (S1), stone hardening (S2), pre-ripening (S3), and the ripening stage (S4). The raw reads were trimmed by removing adapter, empty reads, and low quality sequences. On average, approximately 8.8 million clean reads were generated for each library, with 0.44 Gb in size, and over 95% of the clean reads were mapped to the peach genome (Table 1). Reads mapped to unique or multiple locations of the reference genome were designated uni- or multi-reads, respectively. For each library, 91.3% and 4.5% of the clean reads were identified to be uni-reads and multi-reads, respectively.
Table 1.
RNA-Seq reads and their physical mapping result in peach
| Cultivar | Stage | No. of raw reads (Million) | No. of clean reads (Million) | Clean bases (Gb) | GC content (%) | |
|---|---|---|---|---|---|---|
| Single mapped | Multiple mapped | |||||
| NS | S1 | 10.61 | 9.51 | 0.67 | 0.53 | 45.62 |
| S2 | 8.26 | 7.53 | 0.31 | 0.41 | 45.82 | |
| S3 | 8.76 | 8.06 | 0.26 | 0.44 | 46.22 | |
| S4 | 8.36 | 7.80 | 0.23 | 0.42 | 46.03 | |
| ZH | S1 | 10.84 | 9.55 | 0.75 | 0.54 | 45.21 |
| S2 | 10.48 | 9.47 | 0.43 | 0.52 | 45.30 | |
| S3 | 7.83 | 7.20 | 0.26 | 0.39 | 45.48 | |
| S4 | 7.59 | 6.97 | 0.28 | 0.38 | 45.57 | |
| MJ | S1 | 8.18 | 7.18 | 0.60 | 0.41 | 45.24 |
| S2 | 7.66 | 6.94 | 0.35 | 0.38 | 46.55 | |
| S3 | 9.39 | 8.65 | 0.30 | 0.47 | 45.60 | |
| S4 | 7.95 | 7.33 | 0.29 | 0.4 | 45.77 | |
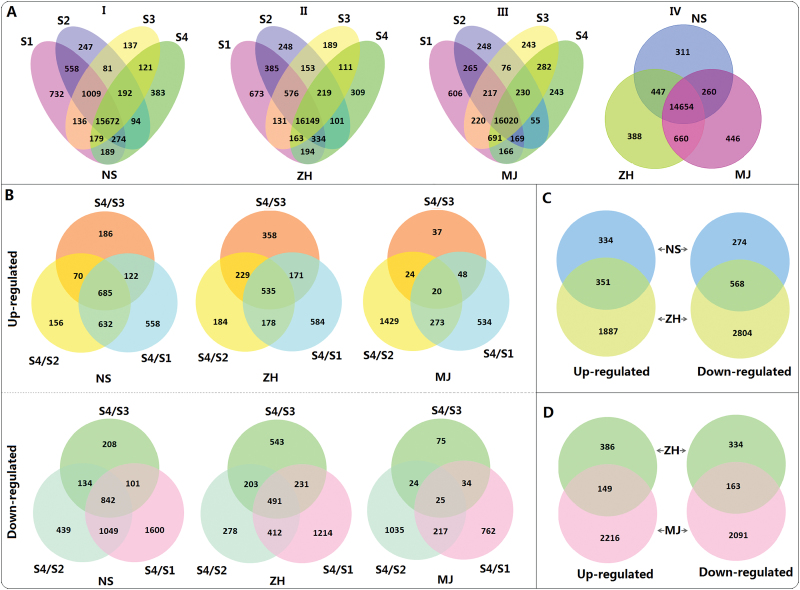
The clean reads were subjected to DGE analysis and the result revealed that 20 004, 19 908, and 19 731 genes were expressed in flesh tissues of cvs NS (FMF), ZH (CMF), and MJ (CNMF), respectively. For cv. NS, 18 749, 18 127, 17 527, and 17 104 genes were identified in flesh tissues of fruits at S1, S2, S3, and S4, respectively (Fig. 1A, I). A total of 15 672 genes were commonly expressed in all four stages, while 732, 247, 137, and 383 genes were exclusively expressed in S1, S2, S3, and S4, respectively. For cv. ZH, 18 605, 18 165, 17 691, and 17 580 genes were found in flesh tissues of fruits at S1, S2, S3, and S4, respectively (Fig. 1A, II). A total of 16 149 genes were commonly expressed in all four stages, while 673, 248, 189, and 309 genes were exclusively expressed at S1, S2, S3, and S4, respectively. For cv. MJ, 18 354, 17 280, 17 979, and 17 856 genes were identified in flesh tissues of fruits at S1, S2, S3, and S4, respectively (Fig. 1A, III). A total of 16 020 genes were commonly expressed in all four developmental stages, while 606, 248, 243, and 243 genes were exclusively expressed in S1, S2, S3, and S4, respectively. In addition, a total of 14 654 genes were commonly expressed in flesh tissues of fruits during the whole process of development in all three cultivars tested, whereas 311, 388, and 446 genes were exclusively expressed in flesh tissues of cvs NS, ZH, and MJ, respectively, during the whole process of fruit development (Fig. 1A, IV).
Fig. 1.
Identification of candidate genes responsible for stone adhesion and flesh melting in peach RNA-Seq based transcriptome analysis. (A) Venn diagrams showing the numbers of commonly and exclusively expressed genes in fruits throughout development (S1–S3) and ripening (S4) in cvs NS (I), ZH (II), and MJ (III), respectively, and the overlap between the commonly expressed genes in fruits of the three cultivars tested (IV). (B) Venn diagrams showing the numbers of genes commonly and exclusively up- or down-regulated in fruits of each cultivar tested between the developmental (S1–S-3) and ripening (S4) stages. (C) Comparison of up- and down-regulated genes in ripening fruits of clingstone cv. ZH with commonly up- and down-regulated genes, respectively, in ripening fruits of freestone cv. NS. (D) Comparison of up- and down-regulated genes in ripening fruits of non-melting cv. MJ with commonly up- and down-regulated genes, respectively, in ripening fruits of melting cv. ZH.
Identification of candidate genes for stone adhesion and melting flesh in peach
Fruit stone adhesion and melting flesh occur in the ripening stage so DGE analysis was performed to identify genes differentially expressed between the fruit developmental (S1–3) and ripening (S4) stages (Fig. 1B). For cv. NS, 2 409 and 4 373 genes were up- and down-regulated, respectively, in flesh tissues of fruits during ripening. Of these genes, 685 and 842 were commonly up- and down-regulated in the S4 versus S1, S4 versus S2, and S4 versuss S3 comparisons, respectively. For cv. ZH, 2 238 and 3 372 genes were up- and down-regulated in flesh tissues of fruits during ripening, respectively. Among these genes, 535 and 491 were commonly up- and down-regulated, respectively, when compared between the ripening and developmental stages. For cv. MJ, 2 365 and 2 182 genes were up- and down-regulated in flesh tissues of fruits during ripening, respectively. Of these genes, 20 and 25 were commonly up- and down-regulated, respectively, when the ripening and developmental stages were compared.
Overall, 1 527 genes were commonly differentially expressed in flesh tissues of fruits of freestone cv. NS between the developmental and ripening stages, thus, these genes were expected to contain the F gene for freestone in peach. To narrow down the candidate gene list, the 1 527 differentially expressed genes were also compared with the differentially expressed genes between the developmental and ripening stages in clingstone fruits of cv. ZH. As a result, 334 up-regulated and 274 down-regulated genes were exclusively differentially expressed in the fruits of cv. NS (Fig. 1C; Supplementary Table S4). The F locus has been mapped to an interval flanked by two SSR markers EPPCU8503 and CPSCT005 on linkage group (LG) 4 (Dirlewanger et al., 2006; Ogundiwin et al., 2009). We checked the marker resources in the Genome Database for Rosaceae (GDR, http://www.rosaceae.org/search/markers) and found that the F interval is about 9.8Mb in physical size, ranging from 20.1Mb to 29.9Mb on LG4. Of the 334 up-regulated and 274 down-regulated genes, five (ppa000311m, ppa006857m, ppa025466m, ppa007187m, and ppa012362m) are located in the F locus.
The five genes in the F interval were subsequently subjected to qRT-PCR analysis. It is worth noting that one gene (ppa006857m) has a paralogue (ppa006839m, which will be described later) in the F locus. The two homologues are almost identical in coding sequences and they show only five single nucleotide polymorphisms (SNPs) in coding region (Supplementary Fig. S1). Three SNPs in the middle of the coding sequences were successfully used to develop qRT-PCR primers specific to ppa006857m and ppa006839m, and the primer specificity was validated by direct sequencing of qRT-PCR products. The qRT-PCR analysis showed that the expression profiles of all five candidate genes were consistent with the results of RNA-Seq based comparative transcriptome analysis (Fig. 2). However, only one gene (ppa006857m) encoding polygalacturonase was exclusively expressed in freestone fruits of cv. NS in the ripening stage and so it was deemed the candidate for stone adhesion.
Fig. 2.
qRT-PCR validation of expression profiles of six genes in the F locus of peach. Red and blue colours represent cvs NS and ZH, respectively. S1 to S4 represent the four stages of fruit development.
Similarly, 1 026 genes were commonly differentially expressed in flesh tissues of fruits of melting flesh cv. ZH between the developmental and ripening stages and they were expected to contain the M gene for melting flesh in peach. The 1026 differentially expressed genes were also compared with the differentially expressed genes between the developmental and ripening stages in non-melting flesh fruits of cv. MJ and the result revealed that 386 up-regulated and 334 down-regulated genes were exclusively differentially expressed in the fruits of cv. ZH (Fig. 1D; Supplementary Table S5). Like the F locus, the M locus has also been mapped to the interval flanked by SSR markers EPPCU8503 and CPSCT005 on LG4 (Dirlewanger et al., 2006; Ogundiwin et al., 2009). Of the 386 up-regulated and 334 down-regulated genes, seven (ppa006653m, ppa000307m, ppa1027150m, ppa006839m, ppa007811m, ppa009438m, and ppa003222m) are located in the M locus. qRT-PCR analysis showed that the expression profiles of these seven genes in the M locus were consistent with the results of RNA-Seq based comparative transcriptome analysis (Fig. 3). However, only one gene (ppa006839m) was relatively highly expressed in ripening fruits of cv. ZH, but its transcripts were almost undetectable in fruits during development. Since the ppa006839m gene encodes polygalacturonase, it was considered as the candidate for melting flesh.
Fig. 3.
qRT-PCR validation of expression profiles of seven genes in the M locus of peach. Blue and green colours represent cvs ZH and MJ, respectively. S1 to S4 represent the four stages of fruit development.
Validation of the candidate genes for stone adhesion and melting flesh in peach
To determine whether ppa006857m and ppa006839m are strong candidates for stone adhesion and melting flesh, respectively, we conducted the following two experiments. Firstly, qRT-PCR analysis indicated that ppa006857m was exclusively expressed in ripening fruits of seven freestone cultivars, while its transcripts were almost undetectable in either ripening fruits of nine clingstone cultivars or immature fruits of all the 16 cultivars tested (Fig. 4). Similarly, the ppa006839m gene was expressed in ripening fruits of seven CMF cultivars, but its expression was extremely low to undetectable in either ripening fruits of FMF and CNMF cultivars or immature fruits of all the 16 cultivars tested (Fig. 4).
Fig. 4.
Expression profiling of two genes, Ppa006857m and Ppa006839m, in immature (S2) and ripening fruits of three types (FMF, CMF, and CNMF) of peach cultivars. The cultivars are as follows: 1, Early red 2; 2, Zhihebaitao; 3, F097NB; 4, Dalihehuangrou; 5, Ruiguangmeiyu; 6, Okitsu; 7, F725NB; 8, Huyou 002; 9, Hongfeng; 10, Jinxiang; 11, Reddomun; 12, Dubaifeng; 13, Hakuto; 14, Hujinmilu; 15, Xizhuan 1; 16, Long 124.
Secondly, two pairs of primers, P13 and P29 (Supplementary Table S6), flanking the whole genomic DNA sequences of ppa006857m and ppa006839m, respectively, were designed to amplify genomic DNA of the germplasm of 95 peach cultivars. A DNA fragment with an expected size of 2.9kb was present in freestone and semi-freestone cultivars but it was absent in clingstone cultivars (Fig. 5A). Similarly, a DNA fragment with an expected size of 3.0kb was detected in melting flesh cultivars but it was absent in non-melting cultivars (Fig. 5B). This demonstrated that the presence/absence of ppa006857m and ppa006839m is associated with stone adhesion and melting flesh, respectively.
Fig. 5.
Agarose gel electrophoresis of the PCR products of the full-length genomic DNA of Ppa006857m (A) and Ppa006839m (B) genes in peach germplasm. The cultivars are indicated with the same numbers as listed in Table 2, and M indicates DNA ladders.
Taken together, these results suggest that ppa006857m and ppa006839m are responsible for stone adhesion and melting flesh, respectively. In addition, phylogenetic analysis showed that ppa006857m and ppa006839m have diverged from previously reported PG genes for fruit softening in tomato (Chun and Huber, 1998) and Rosaceae species such as apple (Tacken et al., 2010), pear (Hiwasa et al., 2003), and strawberry (Quesada et al., 2009), and they were designated PpendoPGF and PpendoPGM, respectively (Fig. 6).
Fig. 6.
A phylogenetic tree derived from the nucleotide acid sequence of PG genes in both monocots and eudicots. Numbers near branches represent bootstrap values. The PG genes were named following the previously reported nomenclature system (Kim et al., 2006). The GenBank accession numbers or the annotation accession numbers in the Phytozome v5.0 database (http:// www.phytozome.net) are indicated in brackets. The two endoPG genes indentified in this study are highlighted in red and the PG genes for fruit softening that have previously been reported in tomato, pear, strawberry, and apple are highlighted in blue.
Table 2.
Characteristics of the stone adhesion and melting flesh traits in 95 peach cultivars
| No. | Cultivar | Phenotype | No. | Cultivar | Phenotype | No. | Cultivar | Phenotype |
|---|---|---|---|---|---|---|---|---|
| 1 | Myojo | CNMF | 33 | Honghuatao | CNMF | 65 | Jinhualu | Semi-FMF |
| 2 | Nanshantiantao | FMF | 34 | R8A01 | FMF | 66 | Zhaoxia | CMF |
| 3 | Zhaohui | CMF | 35 | SUNRAYCER | Semi-FMF | 67 | Beijing 2–7 | CMF |
| 4 | Early red 2 | FMF | 36 | 754PS | Semi-FMF | 68 | F702NJ | Semi-FMF |
| 5 | NECTARED 4 | FMF | 37 | Tiejing 1 | FMF | 69 | F122NB | Semi-FMF |
| 6 | MARAVIHA | Semi-FMF | 38 | C243NB | Semi-FMF | 70 | Chuqiu | CMF |
| 7 | Jinxiu | CMF | 39 | Yixianbai | FMF | 71 | C227NS | FMF |
| 8 | Huaguang 2 | CMF | 40 | Shengzhenbaitao | FMF | 72 | SUPPRISE | CMF |
| 9 | C209NB | Semi-FMF | 41 | Nagasawa Hakuho | CMF | 73 | Xinfeng | CMF |
| 10 | Huyou 002 | CMF | 42 | Shimizu Hakuto | CMF | 74 | Hakuho | CMF |
| 11 | Xizhuan 1 | CNMF | 43 | Dalihehuangrou | FMF | 75 | F098NB | FMF |
| 12 | FLORDAKING | CMF | 44 | Zaoyoutao | FMF | 76 | NECTARED 6 | FMF |
| 13 | GREATDIAM | FMF | 45 | Dayebaitao | CNMF | 77 | VEGA | FMF |
| 14 | Hongfeng | CMF | 46 | Gailiangbaifeng | CMF | 78 | Hujinmilu | CMF |
| 15 | Zhihebaitao | FMF | 47 | F111PB | FMF | 79 | R6A09 | FMF |
| 16 | H793NB | Semi-FMF | 48 | Reddomun | CMF | 80 | SUNRED | Semi-FMF |
| 17 | FLARDAGUARD | FMF | 49 | Tiejing 2 | FMF | 81 | Yingshuang | CMF |
| 18 | Qianqu | CMF | 50 | F084PS | Semi-FMF | 82 | Tiejing 3 | FMF |
| 19 | Jinxiang | CMF | 51 | Zaoyu | FMF | 83 | Okitsu | FMF |
| 20 | F718NB | Semi-FMF | 52 | Gangshanhong | CMF | 84 | Long 124 | CNMF |
| 21 | R3PBPB | CMF | 53 | Dubaifeng | CMF | 85 | Jingyu | FMF |
| 22 | Yixianhong | FMF | 54 | Ruiguangmeiyu | FMF | 86 | F763NB | Semi-FMF |
| 23 | Xiahui 5 | CMF | 55 | C226PB | FMF | 87 | Yanfeng | CNMF |
| 24 | Shanyibaitao | CMF | 56 | Zaohuangguan | CNMF | 88 | Qiubaimi | CMF |
| 25 | Dahongpao | FMF | 57 | Hakuto | CMF | 89 | NECTAROSS | FMF |
| 26 | Sunago wase | Semi-FMF | 58 | F127NB | Semi-FMF | 90 | FANTASIA | FMF |
| 27 | DISERFRED | FMF | 59 | Shenzhenhongmi | CMF | 91 | F084PS | FMF |
| 28 | Datuanmilu | CMF | 60 | Qiumi | CMF | 92 | F725NB | Semi-FMF |
| 29 | Shuho | Semi-FMF | 61 | F100NJ | Semi-FMF | 93 | Shinokubo | Semi-FMF |
| 30 | Ruiguang 27 | CMF | 62 | Tuanchengzaosheng | Semi-FMF | 94 | F729NB | FMF |
| 31 | F097NB | FMF | 63 | Baihuashuimi | CMF | 95 | Jinyan | CNMF |
| 32 | C203NJ | FMF | 64 | Yingqing | CMF |
Genomic structure variation of the endoPG gene cluster in the F-M locus in peach
The coding DNA sequences of PpendoPGF/PpendoPGM were blasted against the draft genome of peach cv. Lovell (Verde et al., 2013), and the result revealed that PpendoPGF/PpendoPGM and their two homologues, termed PpendoPG1 (ppa021953m) and PpendoPG2 (ppa025787m), were located together within a 61kb region in the F-M locus (Supplementary Fig. S2). PpendoPG1 and PpendoPG2 showed 81.5% and 86.5% identities in coding DNA sequences with PpendoPGF/PpendoPGM, respectively. DGE analysis showed that both PpendoPG1 and PpendoPG2 were not expressed in fruits of the three tested cultivars, NS, ZH, and MJ, which suggests that PpendoPG1 and PpendoPG2 are not responsible for stone adhesion and flesh melting in peach.
As mentioned above, both PpendoPGF and PpendoPGM show presence/absence variation. Thus, we investigated the genomic structure variation of the endoPG gene cluster in the F-M locus. Thirty-seven pairs of primers covering the F-M locus (Supplementary Fig. S2, Table S6) were designed based on the reference sequences of cv. Lovell and they were used to amplify cvs NS (FMF), ZH (CMF), and MJ (CNMF). For cv. NS, all these primers generated PCR fragments with the expected sizes. However, eight (P26–P33) and 27 (P2–P36) pairs of primers failed to generate any PCR products with the expected sizes using template DNA from cvs ZH and MJ, respectively. This indicates that a DNA fragment between primers P26 and P33 is lacking in cv. ZH, while a large-scale deletion is likely to occur between PpendoPG1 and F-box genes in cv. MJ. Subsequently, PCR-based genome walking was conducted to identify the nucleotide sequence flanking each side of the deletion. As a result, a 12.8-kb gap covering PpendoPGF was detected in cv. ZH, and a large-scale gap of 70.5kb, which covers PpendoPG2, PpendoPGF, and PpendoPGM, was identified in cv. MJ (Fig. 7A). For ease of description, the allelic variants of the endoPG gene cluster in cvs NS, ZH, and MJ were designated H1, H2, and H3 haplotypes, respectively. The H1 haplotype consists of both PpendoPGF and PpendoPGM. The H2 haplotype contains PpendoPGM, but lacks PpendoPGF. The H3 haplotype lacks both PpendoPGF and PpendoPGM.
Fig. 7.
Genomic structure of the F-M locus in Prunus. (A) Three haplotypes in peach. The homologous regions are shown in the same colour. (B) Colinear genomic regions in P. persica and P. mume.
To facilitate genotyping the F-M locus, two pairs of primers, P38 and P39 (Supplementary Table S1), were designed to detect H2 and H3 variants, respectively. P38 and P39 were used to screen the germplasm of 95 peach cultivars (Supplementary Fig. S3). This result, together with those of the P13 and P29 primers as described above, revealed that all the accessions tested were grouped into three homozygous (H1H1, H2H2, and H3H3) and three heterozygous (H1H2, H1H3, and H2H3) genotypes. This suggests that four pairs of primers, i.e. P13, P29, P38, and P39, are enough for genotyping the F-M locus using a PCR-based diagnostic test. The majority of germplasm (55%) had the H1H2 genotype, while both H1H1 and H1H3 genotypes were detected in only one accession. The H2H2, H2H3, and H3H3 genotypes accounted for 21%, 14%, and 8% of all tested accessions, respectively. Moreover, the copy number of PpendoPGF and/or PpendoPGM in each accession was also quantified using qPCR-based copy number analysis (D’haene et al., 2010), and the result (Supplementary Fig. S4) was consistent with that of the PCR-based genotyping method.
Moreover, we checked the genome of Prunus mume (Zhang et al., 2012) and found a colinear genomic region to the peach F-M locus (Fig. 7B). The colinear genomic region in P. mume contains one copy of the endoPG gene, which is an orthologue of PpendoPG. Thus, the H2 haplotype in the F-M locus is likely to be an ancestral one, while the H1 and H3 haplotypes represent its two variants that result from duplication and deletion of the PpendoPGM gene, respectively.
In summary, the peach F-M locus consists of three allelic copy number variants, H1, H2, and H3. All accessions with H1H1, H1H2, or H1H3 genotypes show the freestone or semi-freestone and melting flesh phenotype, while both H2H2 and H2H3 accessions have the clingstone and melting flesh phenotype. The H3H3 accessions have the clingstone and non-melting flesh phenotype.
Discussion
Copy number variation of the endoPG gene in the F-M locus mediates the flesh texture and stone adhesion phenotypes in peach
Copy number variation (CNV) is defined as DNA segments ~1kb or larger that vary in copy number among haplotypes (Feuk et al., 2006). CNV has been demonstrated to contribute substantially to the genetic diversity and to account for a significant proportion of phenotypic variation in humans (Sebat et al., 2004; Stankiewicz and Lupski, 2010), animals (She et al., 2008; Yalcin et al., 2011), and plants (Cook et al., 2012; Aliyu et al., 2013; Iovene et al., 2013; Maron et al., 2013). Here, we provide an example of copy number variation of the endoPG gene in the F-M locus, which accounts for the diversification of flesh texture and stone adhesion in peach. The two-copy (H1), single-copy (H2), and null (H3) alleles of the F-M locus are present in FMF/semi-FMF, CMF, and CNMF cultivars, respectively, indicating a link between CNV and phenotype in peach. To our knowledge, our study represents the first report of important horticultural traits that are associated with gene copy number variations in fruit trees.
Of all the peach cultivars tested, 57%, 35%, and 8% carry two-copy, single-copy, and null alleles at the F-M locus, respectively. The low frequency of the null allele is mainly attributed to the fact that peach cultivars are mainly bred for fresh consumption. Freshly consumed fruits tend to be of melting flesh and, to a lesser extent, non-melting flesh (Peace et al., 2007). The null allele conferring an undesired trait is likely to be eliminated by purifying selection in the breeding programme.This is consistent with a previous finding that CNV variants are under different levels of selection with deletions being under stronger purifying selection than duplications (Emerson et al., 2008).
Screening peach germplasm reveals three alleles in the F-M locus. Interestingly, the single-copy allele consists exclusively of PpendoPGM which suggests that the F-M locus is a hot spot of mutation and the two-copy and null alleles are probably derived from duplication and deletion of PpendoPGM, respectively. PpendoPGM and PpendoPGF share 99% and 97% identity in coding and genomic DNA sequences, respectively, and the polymorphisms are mainly due to single nucleotide substitutions and small insertions and deletions. In other words, structural variation between PpendoPGM and PpendoPGF occurs at a similar frequency to that of allelic variation observed in the peach genome (Aranzana et al., 2012). In addition, PpendoPGM and PpendoPGF share homologous sequences in both upstream and downstream regions. Thus, copy number variants in the F-M locus are likely to have arisen from replication slippage or retrotransposition (Hastings et al. 2009; Conrad et al., 2010). The duplicated endoPGM gene has evolved into the endoPGF gene through synonymous and non-synonymous substitutions in coding sequences. Subsequently, chromosome recombination during self- and/or cross-fertilization results in six different genotypes in the F-M locus detected in this study.
The freestone and melting flesh phenotype is also present in other Prunus species such as apricot, plum, and sweet cherry (Peace et al., 2007). Given the high level of synteny amongst the genome of Prunus species (Dirlewanger et al., 2004; Jung et al., 2009), it is possible that the flesh texture and stone adhesion phenotype in other Prunus species is also regulated by copy number variation of the endoPG gene.
Functional divergence of two tandem-duplicated genes PpendoPGF and PpendoPGM in peach
As mentioned above, PpendoPGF is exclusively expressed in ripening fruits of freestone and semi-freestone cultivars and it co-segregates with the freestone and semi-freestone phenotype in all tested cultivars. This clearly suggests that PpendoPGF is responsible for stone adhesion in peach. It is worth noting that the expression level of PpendoPGF in ripening fruits of freestone cultivars is more than 5-fold higher than that of semi-freestone cultivars. Thus, the variation in degree of stone adhesion is probably related to the change of expression level of PpendoPGF, with a low expression level corresponding to the semi-freestone or semi-clingstone phenotype.
Similarly, PpendoPGM co-segregates with the melting flesh phenotype in all of the cultivars tested and its expression is associated with the melting flesh phenotype in clingstone cultivars. Moreover, temporal expression analysis shows that PpendoPGM is mainly expressed in the ripening stage which is in accordance with the fruit softening process. These results strongly suggest that PpendoPGM is a good candidate for melting flesh in peach. However, the transcripts of PpendoPGM are extremely low or undetectable in ripening fruits of melting flesh and freestone/semi-freestone cultivars where a high level expression is detected for PpendoPGF. Given the fact that freestone non-melting flesh has not been reported (Bailey and French, 1949; Van Der Heyden et al., 1997), we speculate that PpendoPGF has a pleiotropic effect on flesh melting in peach. In summary, these findings suggest that the two tandemly duplicated genes PpendoPGF and PpendoPGM have diverged functionally. This is slightly different from a previous report that two endoPG genes in the F-M locus control melting flesh texture and stone adhesion, respectively (Peace et al., 2007).
PpendoPGF and PpendoPGM show five nucleotide differences between their coding regions, of which only two at positions 146 (T or C) and 806 (A or G) result in amino acid substitutions at residues 19 (F or S) and 269 (T or S), respectively. These two amino acid substitutions lie outside the conserved region of endoPGs in plants (Supplementary Fig. S5), and are thus unlikely to be responsible for the functional divergence between PpendoPGF and PpendoPGM. Previous studies demonstrate that the diverse functions of endoPGs may be a consequence of differential expression (Torki et al., 2000; Kim et al., 2006). PpendoPGF shows a significantly higher level of expression in ripening fruits when compared with PpendoPGM. Therefore, it seems that the pleiotropic effects of PpendoPGF may be attributed to its high level of expression.
In plants, divergence in expression occurs frequently between tandemly duplicated endoPGs (Kim et al., 2006). This expression divergence is also observed for tandemly duplicated endoPGs on peach LG4 in this study. Among the four endoPGs at the F-M locus, PpendoPG1 and PpendoPG2 are not expressed in fruits, while PpendoPGF and PpendoPGM are expressed in ripening fruits. Interestingly, the expression of PpendoPGM is extremely low or undetectable when it is clustered with PpendoPGF. As mentioned above, PpendoPGF has the functionality of PpendoPGM. Thus, it seems plausible to suppose that the high expression of PpendoPGF provides a negative feedback to inhibit the transcription of PpendoPGM. In addition, the transcription of endoPGs is known to be ethylene-dependent in peach (Hayama et al., 2006; Ziliotto et al., 2008). The promoter sequences are quite different between PpendoPGF and PpendoPGM (Supplementary Fig. S6). Therefore, it cannot be excluded that the ethylene-related transcription factors involved in the regulation of endoPG transcription prefer to bind to the promoter region of PpendoPGF, resulting in the divergence in expression between PpendoPGF and PpendoPGM.
Taken together, we propose a model to account for stone adhesion and fruit softening in peach (Fig. 8). The diversification of the stone adhesion and melting flesh phenotype is mainly due to the presence/absence of two functional divergent endoPG genes, PpendoPGF and PpendoPGM, in the F-M locus of LG4 in peach. However, more studies are needed to identify the regulators controlling the transcription of endoPGs and the mechanism underlying the divergence in expression between PpendoPGF and PpendoPGM in peach.
Fig. 8.
A proposed model underlying the diversification of flesh texture and stone adhesion in peach. The blue and red balls indicate PpendoPGF and PpendoPGM, respectively.
Multiple classes of genes encoding polygalacturonase involved in the regulation of fruit softening in Rosaceae fruit trees
Fruit softening is a result of modifications of cell wall polysaccharides’ architecture including the solubilization and depolymerization of pectin, the main cell wall component (Levy et al., 2002; Brummell, 2006). During fruit softening, the activity of several enzymes increases significantly, including endoPG, endo-1,4-β-mannanase, α-l-arabinofuranosidase, and β-galactosidase (Brummell et al., 2004; Hayama et al., 2006). Of these enzymes, endoPG, a cell-wall degrading hydrolytic enzyme (Hatfield and Nevins, 1986), is well known to play an important role in fruit softening (Lester et al., 1996; Callahan et al., 2004; Peace et al., 2005). In this study, we demonstrate that two endoPG genes are also responsible for flesh softening in peach.
Peach belongs to the Rosaceae family in which PG genes, such as MdPG1 and FaPG1, have also been identified to be responsible for apple and strawberry fruit softening, respectively (Quesada et al., 2009; Tacken et al., 2010). Phylogenetic analysis indicates that the PG genes from peach, apple, and strawberry have diverged and belong to different clades. The plant PG genes are classified into three major groups (A, B, and C) and multiple clades (Kim et al., 2006). PpendoPGF/PpendoPGM, MdPG1, and FaPG1 are grouped into the same group A, but belong to A3, A15, and A1 clades, respectively. Apple, peach, and strawberry fruits represent different types of fruits which are pome, drupe, and berry fruits, respectively. Thus, the divergence of PG genes for fruit softening in various genus of the Rosaceae family is associated with fruit types. More studies are needed to clarify whether the mechanism underlying fruit softening has diverged amongst different types of fruit in Rosaceae.
Supplementary data
Supplementary data can be found at JXB online.
Table S1. Fruit samples collected from three peach cultivars.
Table S2. Primers used for qRT-PCR analysis in peach.
Table S3. Primers used for DNA walking PCR in the F-M locus.
Table S4. Genes significantly differentially expressed between ripening stage (S4) and the three developmental stages (S1–S3) in fruits of two peach cultivars ‘NS’ and ‘ZH’.
Table S5. Genes significantly differentially expressed between ripening stage (S4) and the three developmental stages (S1–S3) in fruits of two peach cultivars ‘ZH’ and ‘MJ’.
Table S6. Primers used for screening allelic genomic variation at the F-M locus.
Fig. S1. Comparison of coding region sequences between the Ppa006839m and Ppa006857m genes. Fig. S2. Schematic diagrams of three types of endoPG gene clusters in the F-M locus on LG4 of peach.
Supplementary Fig. S3. Agarose gel electrophoresis shows the deletion of PpendpPGF (A) and PpendoPGM (B) in peach germplasm.
Fig. S4. Quantifying copy number of PG genes for melting flesh and/or stone adhesion in the F-M locus in peach using qRT-PCR.
Fig. S5. Alignment of the amino acid sequence of PG genes in plants. Fig. S6. Alignment of the promoter sequences of PpendoPGM and PpendoPGF genes in peach.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31201604), the Overseas Construction Plan for Science and Education Base, China–Africa Center for Research and Education, Chinese Academy of Sciences (Grant No. SAJC201327), and the National 863 programme of China (Grant No. 2011AA100206).
References
- Aliyu OM, Seifert M, Corral JM, Fuchs J, Sharbel TF. 2013. Copy number variation in transcriptionally active regions of sexual and apomictic Boechera demonstrates independently derived apomictic lineages. The Plant Cell 25, 3808–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranzana MJ, Illa E, Howad W, Arús P. 2012. A first insight into peach [Prunus persica (L.) Batsch] SNP variability. Tree Genetics & Genomes 8, 1359–1369. [Google Scholar]
- Bailey JS, French AP. 1949. The inheritance of certain fruit and foliage characteristics in the peach. Massachusetts Agricultural Experimental Station Bulletin 452, University of Massachusetts. [Google Scholar]
- Brookfield PL, Nicoll S, Gunson EA, Harker FR, Wohlers M. 2011. Sensory evaluation by small postharvest tems and the relationship with instrumental measurements of apple texture. Postharvest Biology and Technology 59, 179–186. [Google Scholar]
- Brummell DA. 2006. Cell wall disassembly in ripening fruit. Functional Plant Biology 33, 103–119. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Dal Cin V, Lurie S, Crisosto CH, Labavitch JM. 2004. Cell wall metabolism during the development of chilling injury in cold-stored peach fruit: association of mealiness with arrested disassembly of cell wall pectins. Journal of Experimental Botany 55, 2041–2052. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH. 2001. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Molecular Biology 47, 311–340. [PubMed] [Google Scholar]
- Callahan AM, Scorza R, Bassett C, Nickerson M, Abeles FB. 2004. Deletions in an endopolygalacturonase gene cluster correlate with non-melting flesh texture in peach. Functional Plant Biology 31, 159–168. [DOI] [PubMed] [Google Scholar]
- Chun JP, Huber DJ. 1998. Polygalacturonase-mediated solubilization and depolymerization of pectic polymers in tomato fruit cell walls. Regulation by pH and ionic conditions. Plant Physiology 117, 1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DE, Lee TG, Guo X, et al. 2012. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science 338, 1206–1209. [DOI] [PubMed] [Google Scholar]
- Conrad DF, Pinto D, Redon R, et al. 2010. Origins and functional impact of copy number variation in the human genome. Nature 464, 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettori MT, Quarta R, Verde I. 2001. A peach linkage map integrating RFLPs, SSRs, RAPDs, and morphological markers. Genome 44, 783–790. [PubMed] [Google Scholar]
- D’haene B, Vandesompele J, Hellemans J. 2010. Accurate and objective copy number profiling using real-time quantitative PCR. Methods 50, 262–270. [DOI] [PubMed] [Google Scholar]
- Dirlewanger E, Graziano E, Joobeur T, Garriga-Calderé F, Cosson P, Howad W, Arús P. 2004. Comparative mapping and marker-assisted selection in Rosaceae fruit crops. Proceedings of the National Academy of Sciences, USA 101, 9891–9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirlewanger E, Cosson P, Boudehri K, Renaud C, Capdeville G, Tauzin Y, Laigret F, Moing A. 2006. Development of a second-generation genetic linkage map for peach [Prunus persica (L.) Batsch] and characterization of morphological traits affecting flower and fruit. Tree Genetics & Genomics 3, 1–13. [Google Scholar]
- Emerson JJ, Cardoso-Moreira M, Borevitz JO, Long M. 2008. Natural selection shapes genome-wide patterns of copy number polymorphism in Drosophila melanogaster . Science 320, 1629–1631. [DOI] [PubMed] [Google Scholar]
- Feuk L, Carson AR, Scherer SW. 2006. Structural variation in the human genome. Nature Reviews Genetics 7, 85–97. [DOI] [PubMed] [Google Scholar]
- Ghiani A, Onelli E, Aina R, Cocucci M, Citterio S. 2011. A comparative study of melting and non-melting flesh peach cultivars reveals that during fruit ripening endo-polygalacturonase (endo-PG) is mainly involved in pericarp textural changes, not in firmness reduction. Journal of Experimental Botany 62, 4043–4054. [DOI] [PubMed] [Google Scholar]
- Hastings PJ, Lupski JR, Rosenberg SM, Ira G. 2009. Mechanisms of change in gene copy number. Nature Reviews Genetics 10, 551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield R, Nevins D. 1986. Characterization of the hydrolytic activity of avocado cellulose. Plant andCell Physiology 27, 541–552. [Google Scholar]
- Hayama H, Shimada T, Fujii H, Ito A, Kashimura Y. 2006. Ethylene-regulation of fruit softening and softening-related genes in peach. Journal of Experimental Botany 57, 4071–4077. [DOI] [PubMed] [Google Scholar]
- Hiwasa K, Kinugasa Y, Amano S, Hashimoto A, Nakano R, Inaba A, Kubo Y. 2003. Ethylene is required for both the initiation and progression of softening in pear (Pyrus communis L.) fruit. Journal of Experimental Botany 54, 771–779. [DOI] [PubMed] [Google Scholar]
- Iovene M, Zhang T, Lou Q, Buell CR, Jiang J. 2013. Copy number variation in potato – an asexually propagated autotetraploid species. The Plant Journal 75, 80–89. [DOI] [PubMed] [Google Scholar]
- Jung S, Jiwan D, Cho I, Lee T, Abbott A, Sosinski B, Main D. 2009. Synteny of Prunus and other model plant species. BMC Genomics 10, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Darwish O, Geretz A, Shahan R, Alkharouf N, Liu Z. 2013. Genome-scale transcriptomic insights into early-stage fruit development in woodland strawberry Fragaria vesca . The Plant Cell 25, 1960–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Shiu SH, Thoma S, Li WH, Patterson SE. 2006. Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biology 7, R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leida C, Ríos G, Soriano JM, Pérez B, Llácer G, Crisosto CH, Badenes ML. 2011. Identification and genetic characterization of an ethylene-dependent polygalacturonase from apricot fruit. Postharvest Biology and Technology 62, 26–34. [Google Scholar]
- Lester DR, Sherman WB, Atwell BJ. 1996. Endopolygalacturonase and the Melting flesh (M) locus in peach. Journal of the American Society for Horticultural Science 121, 231–235. [Google Scholar]
- Levy I, Shani Z, Shoseyov O. 2002. Modification of polysaccharides and plant cell wall by endo-1,4-beta-glucanase and cellulose-binding domains. Biomolecular Engineering 19, 17–30. [DOI] [PubMed] [Google Scholar]
- Liu YG, Chen Y. 2007. High-efficiency thermal asymmetric interlaced PCR for amplication of unknown flanking sequences. Biotechniques 43, 649–656. [DOI] [PubMed] [Google Scholar]
- Maron LG, Guimarães CT, Kirst M, et al 2013. Aluminum tolerance in maize is associated with higher MATE1 gene copy number. Proceedings of the National Academy of Sciences, USA 110, 5241–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monet R. 1989. Peach genetics: past, present and future. Acta Horticulturae 254, 49–53. [Google Scholar]
- Morgutti S, Negrini N, Nocito FF, Ghiani A, Bassi D, Cocucci M. 2006. Changes in endopolygalacturonase levels and characterization of a putative endo-PG gene during fruit softening in peach genotypes with nonmelting and melting flesh fruit phenotypes. New Phytologist 171, 315–328. [DOI] [PubMed] [Google Scholar]
- Ogundiwin EA, Peace CP, Gradziel TM, Parfitt DE, Bliss FA, Crisosto CH. 2009. A fruit quality gene map of Prunus . BMC Genomics 10, 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace C, Crisosto C, Gradziel T. 2005. Endopolygalacturonase: a candidate gene for freestone and melting flesh in peach. Molecular Breeding 16, 21–31. [Google Scholar]
- Peace CP, Callahan A, Ogundiwin EA, Potter D, Gradziel TM, Bliss FA, Crisosto CH. 2007. Endopolygalacturonase genotypic variation in Prunus . Acta Horticulturae 738, 639–646. [Google Scholar]
- Prasanna V, Prabha TN, Tharanathan RN. 2007. Fruit ripening phenomena—an overview. Critical Reviews in Food Science and Nutrition 47, 1–19. [DOI] [PubMed] [Google Scholar]
- Pressey R, Avants JK. 1973. Separation and characterization of endoPG and exopolygalacturonase from peaches. Plant Physiology 52, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada MA, Blanco-Portales R, Posé S, García-Gago JA, Jiménez-Bermúdez S, Muñoz-Serrano A, Caballero JL, Pliego-Alfaro F, Mercado JA, Muñoz-Blanco J. 2009. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiology 150, 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Troge J, et al. 2004. Large-scale copy number polymorphism in the human genome. Science 305, 525–528. [DOI] [PubMed] [Google Scholar]
- She X, Cheng Z, Zöllner S, Church DM, Eichler EE. 2008. Mouse segmental duplication and copy number variation. Nature Genetics 40, 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz P, Lupski JR. 2010. Structural variation in the human genome and its role in disease. Annual Review of Medicine 61, 437–455. [DOI] [PubMed] [Google Scholar]
- Sweetman C, Wong DCJ, Ford CM, Drew DP. 2013. Transcriptome analysis at four developmental stages of grape berry (Vitis vinifera cv. Shiraz) provides insights into regulated and coordinated gene expression. BMC Genomics 13, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacken E, Ireland H, Gunaseelan K, et al. 2010. The role of ethylene and cold temperature in the regulation of the apple POLYGALACTURONASE1 gene and fruit softening. Plant Physiology 153, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassen MM, Barrett DM, van der Valk HC, Woltering EJ. 2007. Isolation and characterization of a tomato non-specific lipid transfer protein involved in polygalacturonase-mediated pectin degradation. Journal of Experimental Botany 58, 1151–1160. [DOI] [PubMed] [Google Scholar]
- Tong Z, Gao Z, Wang F, Zhou J, Zhang Z. 2009. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Molecular Biology 10, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torki M, Mandaron P, Mache R, Falconet D. 2000. Characterization of a ubiquitous expressed gene family encoding polygalacturonase in Arabidopsis thaliana . Gene 242, 427–436. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Heyden CR, Holford P, Richards GD. 1997. A new source of peach germplasm containing semi-freestone non-melting flesh types. HortScience 32, 288–289. [Google Scholar]
- Verde I, Abbott AG, Scalabrin S, et al. 2013. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nature Genetics 45, 487–494. [DOI] [PubMed] [Google Scholar]
- Xu Q, Yu K, Zhu A, Ye J, Liu Q, Zhang J, Deng X. 2009. Comparative transcripts profiling reveals new insight into molecular processes regulating lycopene accumulation in a sweet orange (Citrus sinensis) red-flesh mutant. BMC Genomics 10, 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin B, Wong K, Agam A, et al. 2011. Sequence-based characterization of structural variation in the mouse genome. Nature 477, 326–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chen W, Sun L, et al. 2012. The genome of Prunus mume . Nature Communications 3, 1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziliotto F, Begheldo M, Rasori A, Bonghi C, Tonutti P. 2008. Transcriptome profiling of ripening nectarine (Prunus persica L. Batsch) fruit treated with 1-MCP. Journal of Experimental Botany 59, 2781–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.