Abstract
Maternal vaccination is currently considered a strategy against respiratory syncytial virus (RSV) infections. In RSV-infected infants, high mucosal IgG levels correlated better with reduced RSV load and lower mucosal CXCL10 levels than plasma IgG levels. For future vaccination strategies against RSV, more focus should be on the mucosal humoral immune response.
TEXT
The first description of respiratory syncytial virus (RSV) was published 60 years ago (1, 2). Despite the long awareness of the virus and the morbidity it causes, vaccines are still unavailable. This may partly be due to the results of one of the first vaccination trials in the 1960s, which had a devastating effect on RSV vaccine development. However, there are more hurdles to be encountered in RSV vaccine development. An important difficulty is the fact that the largest target group consists of very young infants (<6 months of age), who may respond inadequately to vaccination. Also, RSV is very efficient in evading the immune response, as is shown by the occurrence of reinfections throughout life, and last, there is no animal model that is fully permissive to human RSV infection.
Most vaccines aim to induce pathogen-specific IgGs. Palivizumab, a passively administered neutralizing monoclonal antibody, is able to protect infants from severe RSV disease (3–5). This shows that antibodies are able to prevent severe RSV infections and that induction of neutralizing antibodies by vaccination could potentially work. A vaccination route that is often considered for protection against RSV infection and which resembles passive immunization is maternal vaccination. High levels of maternally derived RSV-specific antibody, measured in the sera of infants, protect against RSV infection during the first months of life (6–8). Maternal vaccination aims to enhance the maternally derived IgG antibody levels in the infant.
However, it is unknown if plasma IgGs also reach the mucosal locations, if plasma and mucosal IgG levels are correlated with each other, and if plasma and mucosal IgGs are equally protective. To address these issues, we studied maternally derived preexisting RSV-specific plasma and mucosal antibody titers and their correlation with RSV load and RSV-associated inflammation, i.e., CXCL10, in a clinical pediatric cohort.
A total of 23 hospitalized children less than 3 months of age with laboratory-confirmed RSV infections were prospectively included during two consecutive winter seasons (November to April in 2010 to 2011 and 2011 to 2012). Patients with congenital heart or lung disease, immunodeficiency, or glucocorticoid use were excluded. Written informed consent was obtained from all parents of patients. The study was approved by the Central Committee on Research involving Human Subjects of the Radboud university medical center (Radboudumc). Demographics and clinical parameters were collected from questionnaires and medical records. Within 24 h after admission, a blood sample and a nasopharyngeal aspirate (NPA) sample were collected as previously described (9). The mean age of the patients was 53 days, the average gestational age was 38 weeks, and 48% of the patients were male (Table 1). Regarding their disease status, the mean duration of hospitalization was almost 10 days and the average RSV load gave a threshold cycle (CT) value of 25. The young age of the infants enhanced the chance that this was their primary RSV infection; therefore, only maternal antibodies were studied. Moreover, it is known from the literature that infants do not mount significant neutralizing antibody responses before the age of 4 months (10).
TABLE 1.
Patient characteristics
| Characteristic of RSV-infected patients (n = 23) | Value |
|---|---|
| Median days of age (IQRa) | 53 (31–70) |
| No. (%) of males | 11 (48) |
| Median gestational wks of age (IQR) | 38.4 (38.3–38.5) |
| Median RSV load (CT value) (IQR) | 24.8 (24.6–25.0) |
| Median days of hospitalization (IQR) | 9.6 (9.4–9.7) |
IQR, interquartile range.
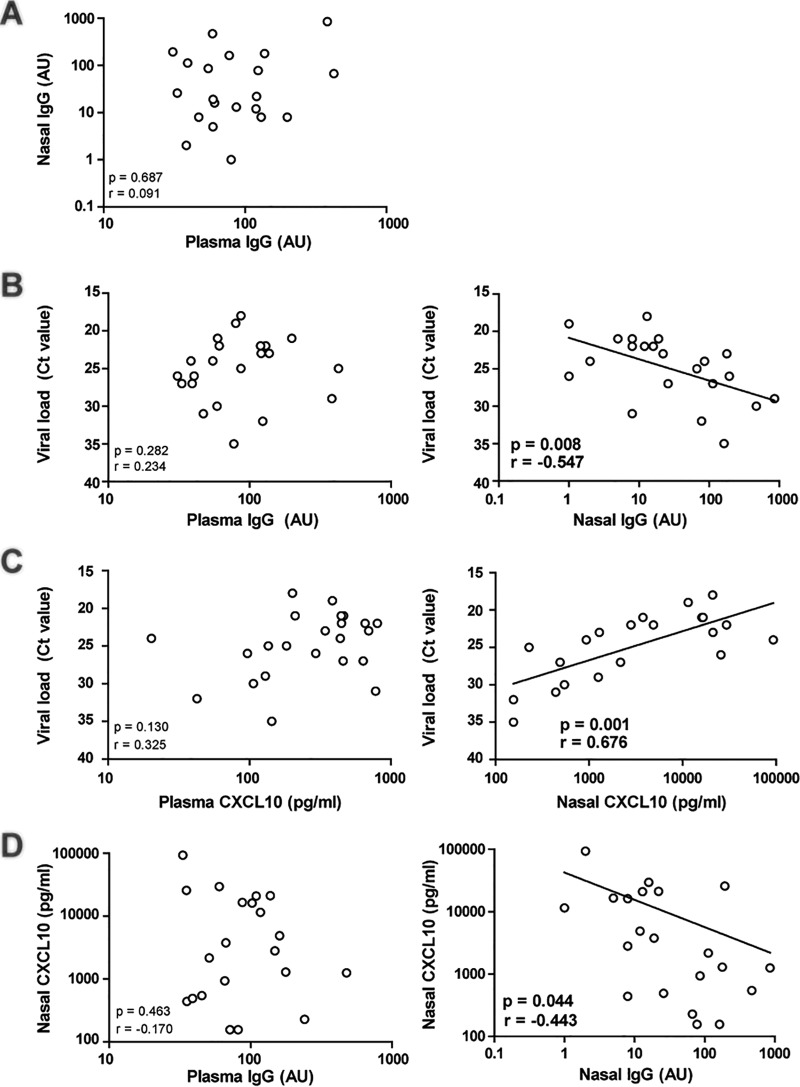
Maternally derived RSV-specific IgGs were measured in both plasma and nasal aspirate samples from patients by enzyme-linked immunosorbent assay (ELISA). Whole RSV-A2 (4 × 107 fluorescein isothiocyanate [FITC]-detected infectious particles/ml) (diluted 1:200 in phosphate-buffered saline [PBS]; Lonza) was used to coat 96-well plates (Nunc Maxisorp). RSV-A2 was cultured and quantified as described previously (11). Plates were incubated for 5 h at 4°C, washed (PBS–0.05% Tween 20), and blocked for at least 2 h with 100 μl PBS–1% bovine serum albumin (BSA) (Sigma-Aldrich). A standard for IgG determination was prepared using two healthy volunteers. Samples were diluted 1:10 two times, as technical replicates, and incubated for 2 h at room temperature. After washing, alkaline phosphatase (AP)-conjugated antibody against human IgG (Southern Biotech) (diluted 1:10,000 in 1% BSA) was added and incubated for 2 h at room temperature. After washing, substrate buffer (10 mM diethanolamine–0.5 mM MgCl2) was added. Absorbance was measured at 450 nm and 690 nm after 30 min and 60 min. Background binding was substracted from each sample measurement, and results were calculated using arbitrary units (AU). In our pediatric cohort, no correlation was found between the levels of mucosal and plasma IgGs (Fig. 1A), suggesting that, in addition to passive transudation, other mechanisms are at play during infection. It has been shown that, due to an infection, IgG antibodies can be actively secreted to the lumen (12).
FIG 1.
Mucosal IgG levels correlate better with respiratory syncytial virus load than plasma IgG levels. Mucosal and plasma samples were taken from RSV-infected infants. Viral load, mucosal and plasma IgG levels, and mucosal and plasma CXCL10 levels were determined. Correlation analyses were performed using a Pearson correlation test. GraphPad Prism 5.03 was used for statistics (GraphPad Software). P values of <0.05 were considered statistically significant. (A) Mucosal and plasma IgG levels were not correlated. (B) Viral load was negatively correlated with mucosal IgG levels but not with plasma IgG levels. (C) Viral load is positively correlated with CXCL10 levels in the nose but not in plasma. (D) Finally, mucosal CXCL10 levels were negatively correlated with mucosal IgG levels but not plasma IgG levels.
For viral diagnostics, samples were analyzed by multiplex PCR, quantifying 15 different viral pathogens, as previously described (13). In contrast to plasma IgG levels (Fig. 1B), mucosal IgG levels were correlated with the RSV load; higher mucosal IgG levels resulted in a lower RSV load (Fig. 1B). This shows that mucosal IgG levels are a better correlate than plasma IgG levels for viral load.
High CXCL10 plasma levels are indicative of RSV-associated inflammation (14). Therefore, we tested whether mucosal and plasma CXCL10 levels correlated with RSV load. The concentration of CXCL10 was determined using a cytometric bead array (CBA), according to the manufacturer's protocol (BD Biosciences). Briefly, CXCL10 levels in individual plasma samples (50 μl) were analyzed, in duplicate, using the CBA kit and an LSR II flow cytometer. We found that a higher viral load resulted in higher mucosal CXCL10 levels but not in higher plasma CXCL10 levels (Fig. 1C). Therefore, mucosal CXCL10 is a better correlate for viral load than plasma CXCL10.
Finally, mucosal CXCL10 levels were correlated with plasma and mucosal IgG levels. We found a significant correlation showing that higher mucosal IgG levels resulted in lower mucosal CXCL10 levels (Fig. 1D). No correlation was found between mucosal CXCL10 and plasma IgG levels. This suggests that mucosal antibodies also reduce RSV-associated inflammation. As a control for confounders, none of the measured parameters were correlated with the age or gender of the infants (data not shown).
IgA is the predominant immunoglobulin present in the mucosa; therefore, not many studies have focused on the presence and function of IgG at this location. However, as maternal vaccination aims to enhance mainly the IgG levels of the infant, it is of importance to study whether maternally derived IgGs are present on the nasopharyngeal mucosa of the infant and, if so, whether that presence correlates with viral load and the immune response. Our data suggest that high levels of IgG on the nasal mucosa are able to protect against RSV infections. Although plasma IgG levels are often used as a readout for vaccine development, it should be taken into account for future vaccine development that mucosal IgG levels are potentially of greater importance than plasma IgG levels. These results suggest that mucosal (intranasal) vaccination which aims to evoke a strong mucosal immune response (15) may be a more effective vaccination strategy. For future studies, a group with very mild RSV infection should be included to determine what level of mucosal IgGs may protect against severe infection. Moreover, correlating maternal plasma IgG levels with mucosal IgG levels of the infant would give insight into the potential of maternal vaccination. Also, more knowledge has to be generated as to how IgG molecules are transported to the nasopharynx and whether this can be enhanced. This could lead to novel immunization strategies to improve mucosal protection by maintaining long-lasting higher IgG levels in the nasopharynx.
ACKNOWLEDGMENTS
We thank all of the parents and children for their participation in the study. We are very grateful to Mariëtte Las for all her help and assistance during the collection of the clinical samples. We thank the medical staff of the Department of Pediatrics and Intensive Care Unit at the Radboudumc and Canisius Wilhelmina Hospital for their help with the collection of the clinical samples. We thank F. Brouwer and E. Simonetti for setting up the different assays used. We thank J. Heldens and R. de Groot for the fruitful discussions and their constructive comments. We also thank M. E. Peeples, Ohio State University, for kindly providing us with a transgenic RSV strain expressing renilla-green fluorescent protein (GFP).
This work was supported by the Virgo consortium.
We declare that we have no conflicts of interest.
Funding Statement
Funders had no role in study design, collection, analysis and interpretation of data, the writing of the manuscript or the decision to submit the article for publication.
REFERENCES
- 1.Blount RE Jr, Morris JA, Savage RE. 1956. Recovery of cytopathogenic agent from chimpanzees with goryza. Proc Soc Exp Biol Med 92:544–549. doi: 10.3181/00379727-92-22538. [DOI] [PubMed] [Google Scholar]
- 2.Chanock RM. 1956. Association of a new type of cytopathogenic myxovirus with infantile croup. J Exp Med 104:555–576. doi: 10.1084/jem.104.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The IMpact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531–537. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. 2013. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 4:CD006602. doi: 10.1002/14651858.CD006602.pub4. [DOI] [PubMed] [Google Scholar]
- 6.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. 1981. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 98:708–715. doi: 10.1016/S0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 7.Glezen WP, Taber LH, Frank AL, Kasel JA. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 140:543–546. [DOI] [PubMed] [Google Scholar]
- 8.Ogilvie MM, Vathenen AS, Radford M, Codd J, Key S. 1981. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol 7:263–271. doi: 10.1002/jmv.1890070403. [DOI] [PubMed] [Google Scholar]
- 9.van den Kieboom CH, Ahout IM, Zomer A, Brand KH, de Groot R, Ferwerda G, de Jonge MI. 2015. Nasopharyngeal gene expression, a novel approach to study the course of respiratory syncytial virus infection. Eur Respir J 45:718–725. doi: 10.1183/09031936.00085614. [DOI] [PubMed] [Google Scholar]
- 10.Sande CJ, Cane PA, Nokes DJ. 2014. The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine 32:4726–4729. doi: 10.1016/j.vaccine.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vissers M, Habets MN, Ahout IM, Jans J, de Jonge MI, Diavatopoulos DA, Ferwerda G. 2013. An in vitro model to study immune responses of human peripheral blood mononuclear cells to human respiratory syncytial virus infection. 2013:e50766. doi: 10.3791/50766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E. 2006. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest 116:2142. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. 2004. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol 42:1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roe MF, Bloxham DM, Cowburn AS, O'Donnell DR. 2011. Changes in helper lymphocyte chemokine receptor expression and elevation of IP-10 during acute respiratory syncytial virus infection in infants. Pediatr Allergy Immunol 22:229–234. doi: 10.1111/j.1399-3038.2010.01032.x. [DOI] [PubMed] [Google Scholar]
- 15.De Magistris MT. 2006. Mucosal delivery of vaccine antigens and its advantages in pediatrics. Adv Drug Deliv Rev 58:52–67. doi: 10.1016/j.addr.2006.01.002. [DOI] [PubMed] [Google Scholar]