Abstract
In accompanying papers (P. L. Acosta, M. T. Caballero, and F. P. Polack, Clin Vaccine Immunol 23:189–195, 2016, http://dx.doi.org/10.1128/CVI.00609-15; M. Vissers, I. M. L. Ahout, M. I. de Jonge, and G. Ferwerda, Clin Vaccine Immunol 23:243–245, 2016, http://dx.doi.org/10.1128/CVI.00590-15) in this issue of Clinical and Vaccine Immunology, the history of and immune mechanisms underlying vaccine-enhanced respiratory syncytial virus (RSV) disease and of investigations of mucosal antibodies and their association with viral load in RSV-infected children, respectively, are described. This commentary discusses RSV vaccine candidates, target populations, and the challenges associated with achieving a safe and effective vaccine.
TEXT
Respiratory syncytial virus is the leading cause of serious lower respiratory disease in young children throughout the world. An estimated 3.4 million children younger than 5 years of age are hospitalized each year with severe respiratory syncytial virus (RSV) lower respiratory tract infection, with the highest incidence in children younger than 6 months of age. Up to 200,000 deaths occur annually, with most deaths occurring in children younger than 1 year of age and in developing-country settings (1). Unfortunately, options for prevention and control are limited. No RSV vaccine is licensed anywhere in the world. While prophylactic treatment with RSV-specific neutralizing antibody is effective in reducing RSV morbidity in infants, its use is currently limited to select populations in high-resource settings because of its expense and because of challenges with its delivery (2). Prevention of severe RSV disease through active immunization of infants would be optimal but has been extremely challenging to implement, given the young age by which immunity is necessary and the legacy of vaccine-enhanced illness leading to deaths in a number of young children after receipt of a formalin-inactivated RSV vaccine in the 1960s.
In this issue of Clinical and Vaccine Immunology, two articles address important obstacles in the path to a successful RSV vaccine (3, 4). Acosta and colleagues provide a history and perspective on the vaccine-enhanced RSV illness that occurred in the 1960s. The devastating results from those trials have thwarted vaccine efforts for the past 5 decades. The authors summarize the large and important body of work that has informed our understanding of immune responses in seronegative infants. The authors caution that RSV vaccines triggering high levels of interleukin-4 (IL-4) and/or IL-13 are associated with enhanced disease in animal models and should be excluded as potential candidates for infant immunization. Likewise, vaccines eliciting nonneutralizing antibody may also be quite risky in seronegative individuals (3).
The second article addresses the important issue of identifying a correlate of protection, which if established could make vaccine licensure easier to achieve. Vissers et al. studied a cohort of infants hospitalized with RSV infection in the Netherlands to determine the importance of serum versus mucosal antibody, presumed to be maternally derived given the young age of the infants (mean, 53 days). The authors found that RSV-specific mucosal IgG concentrations, but not plasma IgG concentrations, inversely correlated with viral load (4). While the results are provocative, the study was limited by its small sample size and cross-sectional design. It will be important to replicate these results in larger populations in other locales. Nonetheless, such innovative work is needed to inform measures of vaccine-induced immunity and accelerate RSV vaccine development.
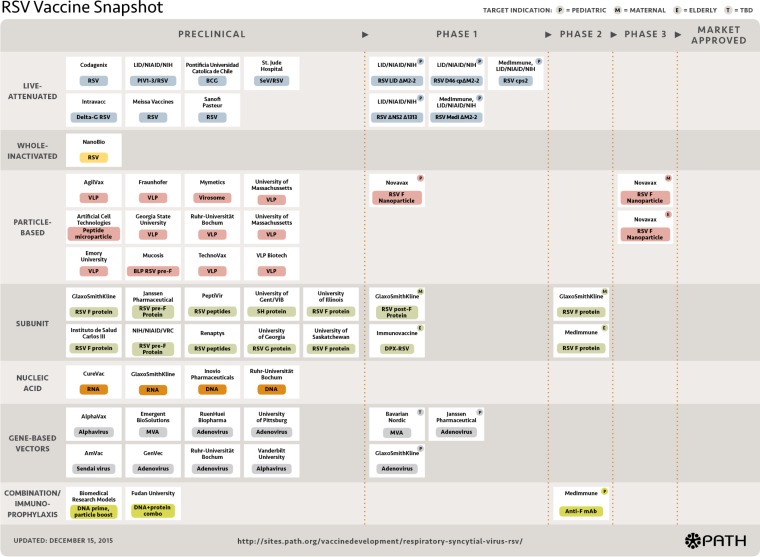
The RSV experience reminds us that vaccine development is by nature a prolonged, risky, and increasingly expensive process. Laboratory experiments and animal models are invaluable in deciphering the immune response and mechanism of action of new vaccine candidates, understanding the potential for adverse events, and identifying promising new vaccines. However, the in vitro and animal model experience can never fully predict the response in humans. The good news is that the current RSV vaccines in the development pipeline are diverse and robust (5) (Fig. 1). The vaccine constructs are varied and include live-attenuated vaccines as well as a variety of nonreplicating constructs—including particle-based, subunit-based, and gene-based vector approaches—that are designed to elicit broadly protective and safe immune responses. Multiple candidates are now in the human testing phase, targeting diverse populations—young children, older adults, and pregnant women (5).
FIG 1.
RSV vaccine snapshot (reproduced from the PATH website at http://sites.path.org/vaccinedevelopment/respiratory-syncytial-virus-rsv/ [accessed 19 January 2016]). BCG, Mycobacterium bovis BCG; mAb, monoclonal antibody; MVA, modified vaccinia Ankara virus; PIV, parainfluenza virus; SeV, Sendai virus; TBD, to be determined; VLP, virus-like particle.
The type of vaccine used will be dependent on the target population. For example, live-attenuated vaccines are mainly targeted to the pediatric population, and a number of such vaccines are in early clinical testing (Fig. 1). Live vaccines could be used in older children to protect them from RSV infection and to reduce the transmission of RSV to the youngest, most vulnerable infants. A modeling study based on data gathered in rural Kenya supported this approach, estimating that household transmission is responsible for 39% of infant infections and that school-age children are the main source of infection within the household, causing around 55% of cases (6). An alternative or complementary strategy would be to directly vaccinate the youngest infants, which will require constructs with optimal attenuation to balance safety and efficacy. Newer constructs are being designed with this balance in mind. A recent study demonstrated that a construct with deletion of the coding sequence for the viral M2-2 protein downregulated viral RNA replication and upregulated gene transcription and antigen synthesis. Evaluated in RSV-seronegative children, vaccine virus shedding was significantly more restricted, and the postvaccination RSV-neutralizing serum responses were superior to those seen after receipt of a more traditional live attenuated construct (7).
Even with a safe vaccine, infant vaccination likely will result in a gap in protection during all or part of the high-risk young-infancy period. An alternative and promising approach to safeguard against severe RSV disease in early infancy is vaccinating pregnant women. Both monoclonal and polyclonal RSV antibodies delivered prophylactically to children clearly reduce the incidence of severe RSV disease and document the impact of antibody to RSV on disease prevention (2). In healthy populations, RSV-specific IgG transfer from pregnant women to infants is an active process resulting in higher antibody titers in the infant than in the mother (8). Several studies have demonstrated a reduced incidence of RSV disease during the first several months after birth that correlates with higher concentrations of RSV-specific maternal antibody (9–12). Further, prevention of RSV in the mother resulting in reduced transmission to the infant is another potential benefit of vaccinating pregnant women.
Importantly, a strategy of vaccinating pregnant women to prevent infant disease is likely feasible. As adult women are already primed, a single immunization in late pregnancy could be sufficient to boost RSV antibody concentrations to protective levels. There are existing platforms for delivery of vaccines to pregnant women worldwide that take advantage of the fact that even in the least-developed countries, the majority of women have some antenatal health care contact. The successful global Maternal and Neonatal Tetanus Elimination Initiative and the increasing number of countries recommending influenza and pertussis vaccines for pregnant women provide important precedents for acceptance/justification of a maternal immunization approach.
Any vaccine administered to pregnant women will need to meet high tolerability and safety standards. RSV vaccines targeted for pregnant women that are currently in late stages of development are particle-based or subunit-based vaccines. One such candidate, an RSV F nanoparticle vaccine with alum, is undergoing phase 3 testing in women in the third trimester of pregnancy, with the primary endpoint of prevention of severe RSV disease in infants (13).
A vaccine to protect infants from RSV disease is a high public health priority. Such a vaccine has unique challenges, including the young age at peak onset of severe disease and the legacy of vaccine-enhanced illness and death. However, a better understanding of the adverse immune responses to a crude inactivated vaccine in the 1960s, combined with improvements in manufacturing, a better understanding of the structure of neutralizing RSV antibody, and improved vaccine design, has created a resurgence in RSV vaccine development. A number of pivotal studies are under way that will inform whether a licensed RSV vaccine will be a near-term reality.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Infectious Diseases and Bronchiolitis Guidelines Committee. 2014. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 134:415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 3.Acosta PL, Caballero MT, Polack FP. 2016. Brief history and characterization of enhanced respiratory syncytial virus disease. Clin Vaccine Immunol 23:189–195. doi: 10.1128/CVI.00609-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vissers M, Ahout IML, de Jonge MI, Ferwerda G. 2016. Mucosal IgG levels correlate better with respiratory syncytial virus load and inflammation than plasma IgG levels. Clin Vaccine Immunol 23:243–245. doi: 10.1128/CVI.00590-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PATH. 2016. Respiratory syncytial virus. Vaccine development against a major cause of childhood respiratory illness. http://sites.path.org/vaccinedevelopment/respiratory-syncytial-virus-rsv/ Accessed 19 January 2016.
- 6.Poletti P, Merler S, Ajelli M, Manfredi P, Munywoki PK, Nokes D, Melegaro A. 2015. Evaluating vaccination strategies for reducing infant respiratory syncytial virus infection in low-income settings. BMC Med 13:49. doi: 10.1186/s12916-015-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karron RA, Luongo C, Thumar B, Loehr KM, Englund JA, Collins PL, Buchholz UJ. 2015. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med 7:312ra175. doi: 10.1126/scitranslmed.aac8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu HY, Steinhoff MC, Magaret A, Zaman K, Roy E, Langdon G, Formica MA, Walsh EE, Englund JA. 2014. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. J Infect Dis 210:1582–1589. doi: 10.1093/infdis/jiu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eick A, Karron R, Shaw J, Thumar B, Reid R, Santosham M, O'Brien KL. 2008. The role of neutralizing antibodies in protection of American Indian infants against respiratory syncytial virus disease. Pediatr Infect Dis J 27:207–212. doi: 10.1097/INF.0b013e31815ac585. [DOI] [PubMed] [Google Scholar]
- 10.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. 1981. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 98:708–715. doi: 10.1016/S0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 11.Ochola R, Sande C, Fegan G, Scott PD, Medley GF, Cane PA, Nokes DJ. 2009. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One 4:e8088. doi: 10.1371/journal.pone.0008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piedra PA, Jewell AM, Cron SG, Atmar RL, Glezen WP. 2003. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 21:3479–3482. doi: 10.1016/S0264-410X(03)00355-4. [DOI] [PubMed] [Google Scholar]
- 13.U.S. National Institutes of Health. 2015. A study to determine the safety and efficacy of the RSV F vaccine to protect infants via maternal immunization. https://www.clinicaltrials.gov/ct2/show/NCT02624947?term=RSV+vaccines+and+pregnant+women&rank=2 Accessed 18 January 2016.