Abstract
Epithelial cells lining the prostate acini release, in a regulated manner (exocytosis), nanosized vesicles called prostasomes that belong to the exosome family. Prostate cancer cells have preserved this ability to generate and export exosomes to the extracellular space. We previously demonstrated that human prostasomes have an ATP-forming capacity. In this study, we compared the capacity of extracellular vesicles (EVs) to generate ATP between normal seminal prostasomes and exosomes secreted by PC3 cells (PC3 exosomes), a prostate cancer cell line. Proteomic analyses identified enzymes of the glycolytic chain in both prostasomes and PC3 exosomes, and we found that both of them were capable of generating ATP when supplied with substrates. Notably, the net production of extracellular ATP was low for prostasomes due to a high ATPase activity contrary to an elevated net ATP level for PC3 exosomes because of their low ATPase activity. The uptake of the 2 types of EVs by normal prostate epithelial cells (CRL2221) and prostate cancer cells (PC3) was visualized and measured, demonstrating differential kinetics. Interestingly, this uptake was dependent upon an ongoing glycolytic flux involving extracellular ATP formation by EVs and/or intracellular ATP produced from the recipient cells. We conclude that the internalization of EVs into recipient cells is an energy-requiring process also demanding an active V-ATPase and the capacity of EVs to generate extracellular ATP may play a role in this process.
Keywords: extracellular ATP, prostate cancer, extracellular vesicles, exosomes, prostasomes, ATPase, glycolysis, energy metabolism, exosome uptake
Prostasomes are exosome-like vesicles that were described for the first time in 1977 (1). They represent a population of extracellular vesicles (EVs), the majority of which have a diameter of 30–200 nm (2). They have their intracellular origin in so-called storage vesicles (equivalent to multivesicular bodies, or MVBs) of prostate epithelial cells and have been generated by multiple invaginations of the endosomal membrane giving rise to intraluminal vesicles that in their extracellular context (after fusion between the membrane of MVBs and the plasma membrane of the prostate epithelial cells, i.e. exocytosis) are termed prostasomes/exosomes (2–4). Prostasomes are believed to function as messengers between the immobile acinar epithelial cells of the prostate gland and the mobile spermatozoa in favour of a successful fertilization (2,5).
Prostasomes bear specific protein markers such as CD10, CD13, CD26, CD38, CD59 and tetraspanins (CD9, CD63) as well as heat-shock proteins (HSP70 and HSP71) (6–10). They also contain raft-associated integral membrane proteins of the MAL family that are involved in membrane trafficking events (9,11). The prostasome membrane exhibits extraordinary properties with an exceptionally high cholesterol/phospholipid ratio (12,13). It is noteworthy that most of the protein markers mentioned were not unique for human prostasomes, but mass spectrometry revealed their presence in bovine, canine and equine prostasomes as well (14). Importantly, the majority of these markers identified were preserved in exosomes secreted by prostate cancer cells (e.g. DU145, LNCaP and PC3) (15,16). Several studies have shown that prostate cancer-derived exosomes have the capacity of moulding the tumour microenvironment by inducing the transformation of fibroblasts to cancer-associated fibroblasts and also angiogenesis (17).
Glycolysis is a fundamental bioenergetic process during which glucose is degraded to lactate, yielding a net gain of 2 moles of ATP per mole of glucose. Under normal physiological conditions, glycolysis is anaerobic and provides the cells with energy under conditions where oxygen is limited. However, in the cancer setting, an aberrant metabolic behaviour known as the Warburg effect makes cancer cells utilize glycolysis as the main source of energy even in conditions in which oxygen supply is not limited and should be capable of generating energy via the oxidative phosphorylation (so-called aerobic glycolysis) (18). Glycolysis takes place in the cytoplasm and is mediated by a series of biochemical reactions that are catalysed by the well-characterized glycolytic enzymes.
Exosomes have been recognized to possess intrinsic biological activity. We reported that human seminal prostasomes contain glycolytic enzymes with capacity for ATP production (19) and this capability was shared by bovine, canine and equine seminal prostasomes (14). It should be mentioned in this context that some of the involved glycolytic enzymes (glyceraldehyde 3-phosphate dehydrogenase, enolase-1 and pyruvate kinase) are among the 10 top proteins found in most exosomes, once again linking prostasomes to the exosome family (7,13,20). Furthermore, our finding of the ATP-forming ability by the different types of seminal prostasomes was balanced by a well-expressed ATPase activity in all of them, meaning that the extracellular net ATP availability was limited (14,19). Moreover, all of these aforementioned glycolytic enzymes were also identified in the proteomic analysis of exosomes derived from DU145, castration-resistant prostate cancer cells (15). However, the biological significance of this is not known nor is the potential of prostate cancer-derived exosomes to synthesize extracellular ATP via glycolysis; therefore, we sought to address these questions in the present study.
In summary, we compared the net formation of extracellular ATP by prostasomes and PC3 prostate cancer cell-derived exosomes (PC3 exosomes). The significantly higher level of extracellular ATP associated with PC3 exosomes was assignable to their obviously lower ATPase activity. We also examined the role of glucose as a fuel for prostasome and PC3 exosome uptake into prostate non-malignant and malignant cells and found this process to be energy-requiring.
Material and methods
Ethical permissions
This study was approved by the Ethics Committee of the University of Uppsala and has been performed according to the Declaration of Helsinki.
Antibodies and reagents
The primary antibodies used in this study against Alix (#21715) and Rab5 (#3547) were from Cell Signalling Technology (Danvers, MA, USA); GAPDH (#9485), hexokinase II (HK2) (#104856) and glucose transporter GLUT1 (#652) were from Abcam (Cambridge, UK); extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) (sc-53693), MCT4 (sc-50329) and V-ATPase A1 (sc-28801) were from Santa Cruz (Dallas, TX, USA); phosphoglycerate kinase 1 (PGK1) (AP13821PU-N) was from Acris Antibodies (San Diego, CA, USA). Bafilomycin A1, oleic acid, 2-deoxyglucose, adenosine and glucose were purchased from Sigma-Aldrich (St Louis, MO, USA).
Cell lines and culturing conditions
PC3 cells were cultured in RPMI 1640 medium (Hyclone, South Logan, UT, USA) enriched with 10% foetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin with 100 µg/ml streptomycin (all from Gibco, Waltham, MA, USA). CRL2221 cells were cultured in keratinocyte-SFM medium containing keratinocyte-SFM supplement (5 ng/ml human recombinant epidermal growth factor and 0.05 mg/ml bovine pituitary extract) and 100 U/ml penicillin with 100 µg/ml streptomycin (Gibco). Cells were cultured at 37°C in 5% CO2 and humidified atmosphere. For exosome isolation, the cells were cultured for 48 h in exosome-depleted medium as described below.
To prepare exosome-depleted medium, we centrifuged RPMI 1640 enriched with 30% FBS overnight at 120,000g at 4°C and then filtered the supernatant through a 0.22 µm disposable filter. This vesicle-depleted medium was further diluted with RPMI 1640 to reach the 10% FBS final concentration which was used for the subsequent culturing of the cells.
Exosome preparation and purification from PC3 cells
For isolation of PC3 exosomes, PC3 cells were cultured in 500 ml FBS exosome-depleted medium and when reaching 70% confluency (after 48 h) the supernatant was collected, centrifuged (600 g for 10 min) and filtered through a 0.22 µm disposable filter. The filtered supernatant was stored at −20°C. After thawing, the supernatant was centrifuged for 2 h at 120,000 g at 4°C. The pellet was washed once in phosphate-buffered saline (PBS), and the new pellet was resuspended in an appropriate volume of PBS and stored in aliquots at −80°C.
Exosome purification from blood plasma of prostate cancer patients
For exosome isolation from patient samples, 3 ml plasma of each patient was used. The plasma was centrifuged for 10 min at 1,500g at 4°C and the supernatant was collected, and centrifuged for 30 min at 12,000g at 4°C. The new supernatant was collected and filtered through a 0.22 µm disposable filter. The filtered supernatant was diluted in cold PBS to a final volume of 4 ml and was centrifuged for 2 h at 120,000g at 4°C. The pellet was washed once in PBS, and the new pellet was adjusted with PBS to a concentration of 2 mg/ml (protein content) and stored in aliquots at −80°C.
Exosome measurement
For each exosome sample, protein content was measured by BCA assay (Thermo Fisher Scientific, Waltham, MA, USA). The particle size was measured for 1 min captures in triplicates by using the Nanosight System LM10 (Malvern Instruments, Worcestershire, UK) with the CMOS camera (camera level on video capturing: 14; threshold limit on video analysis: 7; each sample was run 5 times with 1 min each run) and analysed using nanoparticle tracking analysis (NTA) software 2.3.
Preparation and purification of seminal prostasomes
Seminal plasma from the Fertility Clinic (Uppsala University Hospital) was obtained following well-established routines and stored at −20°C (6). Pooled seminal plasma was collected from 10 to 20 donors. Thawed seminal plasma was centrifuged for 15 min at 3,000g at 4°C. The supernatant was subjected to another centrifugation for 30 min at 10,000g at 4°C to avoid cell debris and larger vesicles. The new supernatant was then subjected to an ultracentrifugation for 2 h at 100,000g at 4°C, using rotor 90ti (Beckman Coulter, Brea, CA, USA). The obtained pellet was resuspended in PBS and the suspension was loaded on an XK16/70 Superdex 200 gel column (GE Healthcare, Uppsala, Sweden), to separate prostasomes from amorphous material (3). The flow rate for collected fractions was 5 ml/h, resulting in fraction volumes of approximately 1.3 ml. Fractions with elevated absorbances at 260 nm and 280 nm (reflecting nucleic acid and proteins, respectively) were pooled and ultracentrifuged for 2 h at 100,000g at 4°C. The pellet was resuspended in PBS and subjected to a density gradient containing 1, 1.5 and 2 M sucrose for 21 h at 185,000g at 4°C using rotor SW28.1 (Beckman Coulter). The main prostasome fraction on top of 1.5 M (density range 1.13–1.19 g/ml) was collected and pelleted by ultracentrifugation for 2 h at 100,000g at 4°C. The pellet was resuspended in an appropriate volume of PBS; protein was measured by the BCA assay kit, and stored in aliquots at −80°C.
Sucrose density gradient fractionation of prostasomes and PC3 exosomes visualized by SDS-PAGE
Two sucrose density gradients were built in tubes by 0.17, 0.5, 0.8, 1.1, 1.4, 1.7, 2, and 2.4 M sucrose, and 2 mg of prostasomes and 2 mg of PC3 exosomes were loaded respectively on top of each of the 2 gradients. Both tubes were ultracentrifuged for 20 h at 185,000g at 4°C. Fractions on top of each density layer of sucrose were aspirated and transferred to new tubes and diluted by PBS, and EVs were pelleted after ultracentrifugation for 2 h at 100,000g at 4°C. Pellets were generally solved in 300 µl PBS, and 16 µl of each were loaded in wells of a Novex NuPAGE 4–12% Bis-Tris gel (Thermo Fisher Scientific) and separated at 200 V for 35 min. The gel was stained using the SilverQuest staining kit (Thermo Fisher Scientific) and analysed by Molecular Imager ChemiDoc XRS+ imaging system using the software Image Lab 5.1 (BioRad Laboratories, Hercules, CA, USA).
Transmission electron microscopy
PC3 exosomes were added to a grid with a carbon supporting film for 5 min. The grid was rinsed with distilled water, soaked off, and stained with 1% uranyl acetate in water for 10 sec and then air-dried. The samples were examined in a Tecnai 12 Spirit Bio TWIN transmission electron microscope (FEI Company, Eindhoven, The Netherlands) at 100 kV. Digital images were captured by using a Veleta camera (Olympus Soft Imaging Solutions, GmbH, Münster, Germany). Prostasomes were fixed with 2.5% glutaraldehyde in PBS for 24 h and thereafter embedded in Epon according to conventional techniques. The embedded prostasome block was cut into 40–60 nm sheets. Sheets were placed on slot grids with 0.5% Formvar film, contrasted with lead citrate and uranyl acetate, and examined in a Zeiss Supra 35-VP (Carl Zeiss SMT, Oberkochen, Germany) field emission scanning electron microscope, equipped with a STEM detector for transmission electron microscopy.
General framework for ATP determination
Experiments were performed in a reaction buffer prepared by adding 1 mM of MgCl2, KCl and DTT, respectively, and 0.1 mM of NAD+ (final concentrations) into PBS. Most experiments included ADP, at a final concentration of 5 µM (ADP reaction buffer). Exosomal enzymatic reactions were performed at 37°C for 10 min in separate 1 ml tubes. Aliquots of 10 µl from each tube were added to columns of microtiter plates (polystyrene 96-weel*M, Sigma-Aldrich) and luciferin/luciferase assay kit components (90 µl/well) were added according to manufacturer's instructions (Thermo Fisher Scientific). The plates were incubated for 15 min at 37°C before registration of luminescence (Victor 1420 Multilabel counter, Perkin-Elmer, Santa Clara, CA, USA). ATP determination by luminescence was expressed in arbitrary units. The starting concentration of prostasomes/exosomes (EVs) was 0.4 mg/ml, in case of prostasomes recovered from 3.5 ml seminal plasma and in case of PC3 exosomes from typically 500–1,000 ml of conditioned medium.
Assays of ATPase activity of prostasomes and PC3 exosomes
The ATP ladder was prepared in reaction buffer of 8 standards of 1:2 dilutions spanning the range between 5 and 0.05 µM. A corresponding ATP ladder prepared in 8 standards of 1:2 dilutions (10 µL) in the range between 10 and 0.1 µM was mixed with 10 µL of each EV-type, with or without 3.3 mM vanadate, and incubated for 10 min at 37°C. ATP levels were determined according to general framework for ATP determination.
Glycolysis experiments involving prostasomes and PC3 exosomes
The ADP reaction buffer (720 µl) was divided equally into 2 tubes and 90 µl of seminal prostasomes or PC3 exosomes were added. The EV-enriched tubes were pre-incubated at 37°C for 10 min to allow prostasomal/exosomal ATPases to degrade some of the contaminating ATP present in the commercial ADP chemical (Sigma-Aldrich). Four compositions were prepared for EVs: 1) 90 µl of the pre-incubated ADP reaction buffer containing EVs and enriched with glucose (G) (5 mM, final concentration); 2) 90 µl of the pre-incubated ADP reaction buffer containing EVs and enriched with G and vanadate (V) (3.3 mM, final concentration); 3) 90 µl of the pre-incubated ADP reaction buffer containing EVs and enriched with G, V, and iodoacetate (IA) (1 mM, final concentration) and sodium fluoride (NaF) (1 mM, final concentration); 4) 90 µl of the pre-incubated ADP reaction buffer containing EVs and enriched with G, IA and NaF. ATP levels were determined according to general framework for ATP determination.
Western blot analysis
Cells were harvested and homogenized in RIPA lysis buffer (10 mM Tris, pH 7.2, 150 mM NaCl, 1% deoxycholate, 1% TritonX-100, 0.1% SDS, 5 mM EDTA) containing complete protease inhibitor cocktail, phospho-stop (Roche Diagnostics, Meylan, France), DTT (Sigma-Aldrich) and vanadate (Thermo Fisher Scientific). Exosomes were centrifuged for 2 h at 100,000g at 4°C. The supernatants were discarded and the pellets were lysed by complemented RIPA lysis buffer (10 mM Tris, pH 7.2, 150 mM NaCl, 1% deoxycholate, 1% TritonX-100, 0.1% SDS, 5 mM EDTA), phospho-stop, (Roche Diagnostics) and vanadate (Thermo Fisher Scientific). After 30 min on ice, protein content was measured by the BCA assay. Equal amounts of soluble protein (15–25 µg) were denatured by heating at 95°C for 5 min, resolved in SDS-PAGE and transferred to PVDF membranes. The membranes were blocked in 5% non-fat dry milk in TBS-Tween for 1 h and probed initially with specific primary antibody followed by horseradish peroxidase-conjugated secondary antibody. Blotted protein bands were detected by chemiluminescence (Supersignal, Thermo Fisher Scientific) exposure on X-ray films (Kodak, Rochester, NY, USA).
Proteinase K treatment
Isolated prostasomes/exosomes were treated with 50 µg/ml of proteinase K (Sigma-Aldrich) for 1 h at 37°C. The proteolytic reaction was stopped by the addition of 2 mM PMSF (Sigma-Aldrich) for 10 min at 4°C. The samples were then processed for western blotting (see above).
PKH67 labelling
EVs were labelled with PKH67 green fluorescent dye according to the manufacturer's instructions (PKH67 Green Fluorescent Cell Linker Midi Kit for General Cell Membrane Labelling, Sigma-Aldrich). Briefly, EVs were labelled with 2.5 µM of PKH67 dye in 400 µl of diluent C for 5 min, and then blocked for 1 min in 1% bovine serum albumin (BSA), after which EVs were washed with a high volume of PBS by ultracentrifugation for 2 h at 120,000g and at 4°C. PKH67-labelled EVs were then resuspended in PBS and stored at −80°C.
Uptake of labelled EVs estimated by flow cytometry
PC3 and CRL2221 cells were incubated with the 5 µg PKH67-labelled prostasomes and PC3 exosomes for 1, 3 and 6 h at 37°C. Then cells were washed 3 times with PBS containing 0.5% BSA, harvested and transferred to polystyrene tubes. After 1 PBS wash, fluorescence was determined using BD FACSCaliburTM flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). To determine the effect of treatment on EV uptake, EVs were pre-incubated in complete medium with bafilomycin A1 (BafA1, 10 nM), 2-deoxyglucose (2-DG, 10 mM) and/or oleic acid (100 µM) for 30 min at 37°C, prior to incubation with the cells for 3 h at 37°C.
Confocal fluorescence microscopy
Cells were incubated with PKH67-labelled exosomes for 3 h at 37°C. After PBS washes, cells were fixed in 4% paraformaldehyde for 15 min and then washed 3 times in PBS. The slides were mounted in DAPI-containing fluorescent mounting medium (Vector Laboratories, Burlingame, CA, USA). The images were recorded on a DAS Leitz DM RB microscope with a Hamamatsu C4880 dual-mode cooled CCD camera (Leica Microsystems, Wetzlar, Germany) and further processed using Photoshop software.
Statistical analysis
Statistical analyses were performed with Excel software, using the paired Student t-test, and p-values <0.05 were considered significant (*<0.05; **<0.01; ***<0.001).
Results
Characterization of prostasomes and PC3 cell-derived exosomes
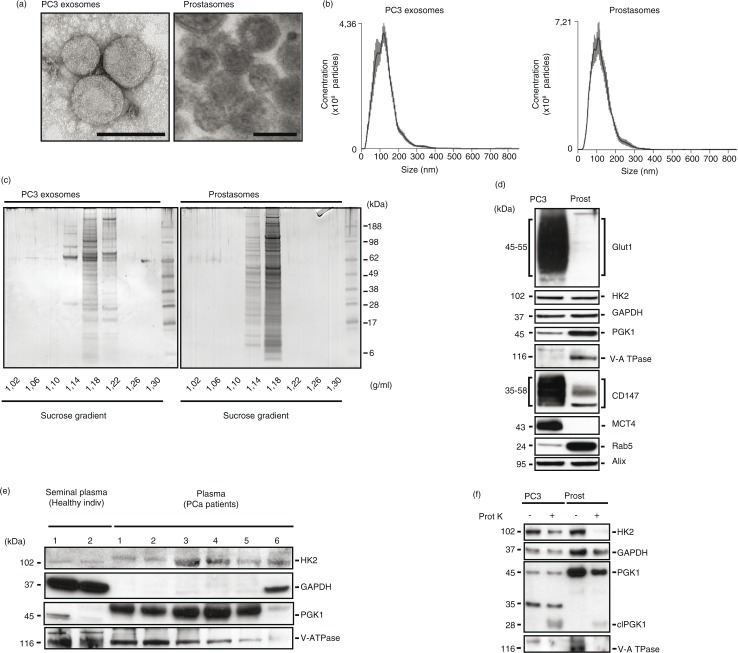
We performed a morphological and molecular comparison of human seminal prostasomes and exosomes derived from PC3 cells (PC3 exosomes), a castration-resistant prostate cancer cell line. Transmission electron microscopy revealed similar morphological features of spherical, bilayered, membrane-bound EVs with a mean diameter of about 100 nm (Fig. 1a). NTA confirmed the observed size distribution with an average peak of 110 and 120 nm for PC3 exosomes and prostasomes, respectively (Fig. 1b). SDS-PAGE was used for separation of proteins on the basis of their molecular weight (Fig. 1c). We have previously shown the consistent banding pattern of seminal prostasomes obtained from different preparations over time (6). The banding pattern of purified PC3 exosomes (density 1.18–1.22 g/ml) was, to a certain extent, similar to that of seminal prostasomes (density 1.14–1.18 g/ml). Additionally, minor fractions were discerned at density of 1.14 g/ml and in case of PC3 exosomes, even at density of 1.22 g/ml (Fig. 1c). This heterogeneity of exosomes was confirmatory of previous investigations on seminal prostasomes (7,8). The kindred nature of the 2 types of exosomes regarding protein composition was in line with identification of common proteins involving 130 molecules taken together (Supplementary Table I).
Fig. 1.
Comparative characterization of prostasomes and PC3 exosomes. (a) Representative transmission electron microscopy images of PC3 exosomes and prostasomes (scale bar: 100 nm). (b) Nanoparticle tracking analysis of PC3 exosomes and prostasomes (means of 5×1 min runs, error indicate ±standard error of the mean). (c) Representative image of an colloidal blue-stained SDS-PAGE gel demonstrating the separation of proteins found in seminal prostasomes and PC3 exosomes. The values beneath the gels indicate the density of the recovered fractions from the sucrose density gradient centrifugation. (d) Western blot analysis of seminal prostasomes and PC3 exosomes probed for the indicated proteins. (e) Western blot analysis of seminal plasma-derived prostasomes from healthy individuals and prostate cancer patient plasma-derived exosomes probed for the indicated proteins. (f) Western blot analysis of seminal prostasomes and PC3 exosomes with or without treatment with proteinase K and probed for the indicated proteins.
Among the concordantly identified proteins were members of the glycolytic machinery (Table I). To confirm the proteomics data, we performed western blot analyses for selected proteins associated with energy metabolism (Fig. 1d). We identified in prostasomes and PC3 exosomes the glycolytic enzymes hexokinase-2 (HK2), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and phosphoglycerate kinase 1 (PGK1). Additionally, we found enrichment of the glucose transporter-1 (Glut1), the monocarboxylate transporter-4 (MCT4) and its accessory protein CD147 only in PC3 exosomes. Importantly, we found that a core component of the vacuolar ATPase-1 was enriched in prostasomes while not detected in PC3 exosomes. Interestingly, we were able to detect core glycolytic enzymes (i.e. HK2, GAPDH, PGK1) in the EVs isolated from the plasma of prostate cancer patients (Fig. 1e). We found that HK2, GAPDH and PGK1 were differentially enriched in EVs isolated from prostate cancer patient plasma samples in comparison to the prostasomes isolated from the seminal plasma of healthy individuals.
Table I.
Identification of 29 common prostasomal proteins from human seminal plasma (14) and PC3 cells (9) subdivided in actins, annexins, CD antigens, HSP proteins, glycolytic enzymes and other proteins
| Actins | HSP Proteins |
| Actin, cytoplasmic 2 | Heat-shock 90kDa protein 1, alpha |
| Actin-related protein | Heat-shock cognate 71 kDa protein isoform 1 |
| Actin-related protein 2/3 complex subunit 4 isoform | HSP70-2 |
| Annexins | Glycolytic Enzymes |
| Annexin A1 | 5’-Nucleotidase precursor |
| Annexin A11 | ENO3, muscle enolase 3 beta |
| Annexin A2 isoform 2 | Aldolase A |
| Annexin A3 | Glucose-6-phosphate dehydrogenase |
| Annexin A7 isoform 1 | Glyceraldehyde-3-phosphate dehydrogenase |
| CD Antigens | l-Lactate dehydrogenase A chain isoform 1 |
| CD63 antigen isoform A | l-Lactate dehydrogenase B chain |
| CD81 antigen | Phosphoglycerate kinase 1 |
| CD9 antigen | Pyruvate kinase |
| Dipeptidyl peptidase 4 | |
| Leukocyte surface antigen CD47 isoform 1 precursor | Other Proteins |
| Basigin (Ok blood group), isoform CRA_d | ADP-ribosylation factor 4 |
| ADP-ribosylation factor 6 | |
| Clathrin heavy chain 1 |
We have previously demonstrated that a non-membrane-permeable glycolytic substrate is functional in a metabolic context on incubation with prostasomes, indicating the presence of glycolytic enzymes on the outer surface of prostasomes (19). To further verify such a location, we treated prostasomes and PC3 exosomes with proteinase K and examined by western blotting whether the protein expressions of these enzymes were affected or not (Fig. 1f). We found that proteinase K treatment of the EVs reduced the expressions of the glycolytic enzymes examined. It should be noted in this context that a functional test was not possible to carry out because the ATP assay was enzymatically based (luciferase), meaning that an eradication of the luciferase enzyme by remnants of proteinase K could not be ruled out.
Prostasomal and PC3 exosomal ATPase activities and glycolytic ATP formation
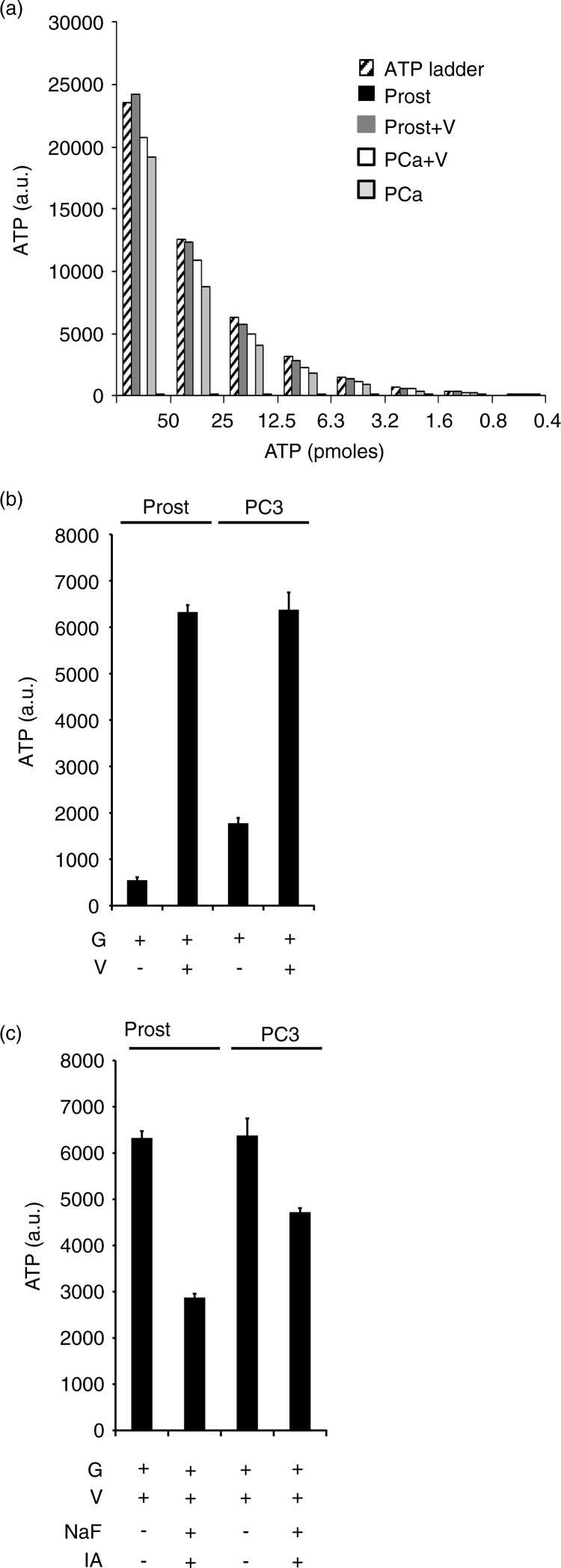
Following our recent publication that prostasomes can produce extracellular ATP by glycolysis, we wanted to compare this endogenous exosomal activity between prostasomes and PC3 exosomes (19). A distinct difference between prostasomes and PC3 exosomes was identified regarding ATPase activities at the surfaces of these 2 EVs. Prostasomes displayed a high ATPase activity when supplied with 1–50 pmoles ATP sharply contrasting to the weak ATPase activity demonstrated by PC3 exosomes under corresponding incubation conditions (Fig. 2a). We found that both EV types had the ability to produce ATP by glycolysis in similar amounts when fuelled with glucose in presence of ADP and vanadate (Fig. 2b). Vanadate was found in a previous work to be inhibitory of the seminal prostasome ATPase activity (14,19,21). Under basal conditions, the ATP yield of PC3 exosomes was higher due to their low ATPase activity contrary to that of prostasomes. In the presence of vanadate (the ATPase inhibitor), the levels of ATP produced by both types of EVs were equal. In order to demonstrate that the ATP produced by the prostasomes and the PC3 exosomes was by glycolysis we utilized the known glycolytic inhibitors, iodoacetate and sodium fluoride with satisfactory outcome. Iodoacetate is an irreversible inhibitor of GAPDH, and sodium fluoride is an inhibitor of alpha-enolase. A significant inhibition of glycolytic flux and therewith ATP formation was achieved on incubation of prostasomes and PC3 exosomes by these 2 inhibitors of glycolysis (Fig. 2c) (best illustrated for seminal prostasomes in the presence of vanadate). It should be noted that there is a rather high “background” ATP level (most evident in the case of PC3 exosomes because of their low ATPase activity) that is pertaining to an ATP contamination of ADP discussed in previous publications (14,19). In this study, we used a 10 times lower ADP concentration to better govern this type of complication. Additionally, there is an inherent adenylate kinase (AK) reaction of both types of EVs (14,19) most probably contributing to this background. We previously observed that diadenosinepentaphosphate (an inhibitor of the AK reaction) was contaminated as well with ATP (14,19). In summary, these data further support our observation of an ongoing glycolytic flow accomplished by the 2 types of EVs.
Fig. 2.
Glycolysis inhibition leads to decreased ATP formation by exosomes. ATP ladder (50 to 0.4 pmole) illustrating ATPase activities of prostasomes and PC3 exosomes in absence and presence of 3.3 mM vanadate (V, ATPase inhibitor) (a). Please note the minute amounts of ATP for prostasomes in absence of vanadate (V) in sharp contrast to PC3 exosomes. Estimation of ATP formation (incubation medium always containing glucose, G), in prostasomes (1 µg protein) and PC3 exosomes (1 µg protein), in (b) absence or (c) presence of glycolytic inhibitors (IA: iodoacetate; NaF: sodium fluoride) (mean values±SD, n ≥ 3).
Differential uptake of prostasomes and PC3 exosomes by non-malignant and malignant prostate cells
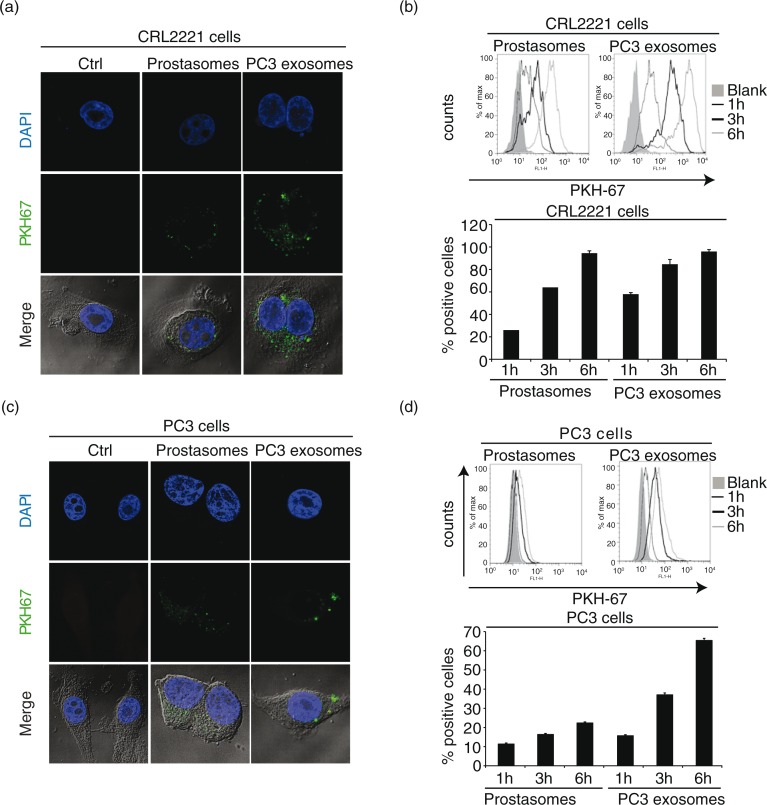
We next examined the uptake of prostasomes and PC3 exosomes into both a non-malignant, prostate epithelial cell line, CRL2221, and a malignant prostate cancer cell line, PC3. Non-malignant prostate cells accumulated higher levels of PC3 exosomes compared to the uptake of prostasomes at 3 h of incubation (Fig. 3a). However, at 6 h of incubation, both sets of EVs were equally taken up by the CRL2221 cells (Fig. 3b). Similar incubation experiments involving both sets of EVs using malignant PC3 cells demonstrated a somewhat different behaviour. An unambiguous uptake of PC3 exosomes was observed although at a retarded rate reaching an apparent level that was lower than that achieved by non-malignant prostate cells. Moreover, the uptake of prostasomes into malignant PC3 cells was markedly reduced in comparison with benign CRL2221 cells, even though a time-dependency was discernible (Fig. 3c and 3d). To sum up, the cellular uptake of PC3 exosomes seemed to be faster than that of prostasomes and the capacity of benign prostate cells to take up EVs seemed to be higher than that of malignant cells.
Fig. 3.
EVs are taken up by prostate healthy and cancer cells. Uptake of PKH67-labelled (a) prostasomes (representing 1 µg protein) and (c) PC3 exosomes (representing 1 µg protein) by prostate epithelial CRL2221 cells and PC3 cells, illustrated by confocal microscopy after 3 h of incubation (scale bar: 5 µm). Estimation of PKH67-labelled (b) prostasomes and (d) PC3 exosomes. EV uptake (measured by flow cytometry) after 1 h, 3 h and 6 h at 37°C and results are presented as percentage of positive cells and fluorescence intensity (mean values±SEM, n=3)
EV uptake by recipient cells is energy-requiring
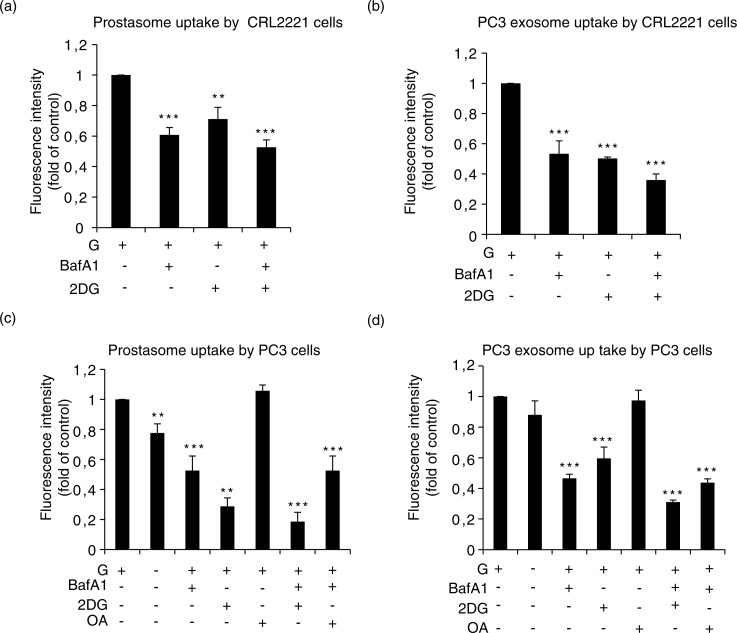
We incubated prostasomes and PC3 exosomes (together with recipient cells) with glucose in presence and absence of either a V-ATPase inhibitor (BafA1) or a glycolysis inhibitor 2-deoxyglucose (2-DG). In these series of experiments, we have used BafA1 instead of vanadate as ATPase inhibitor because of the high toxicity of the latter observed on recipient cells. 2-DG is a glucose analogue that inhibits glycolysis via its direct action on hexokinase and indirectly on phosphoglucose isomerase (18). We found that the uptake of prostasomes and PC3 exosomes by CRL2221 was decreased by inclusion of either BafA1 or 2-DG and the inhibitory action was augmented by the combination of the V-ATPase inhibitor and 2-DG (Fig. 4a and 4b). In PC3 cells, the endogenous energy substrates were sufficient for uptake of prostasomes and PC3 exosomes and the addition of exogenous glucose did not substantially enhance the uptake. Still, the importance of glycolysis for the uptake of prostasomes became evident by use of the glycolysis inhibitor 2-DG. Again, the attenuation of the EV uptake was more evident by the combination of BafA1 and 2-DG where prostasome and PC3 exosome uptake was completely abrogated. Similar results, although less pronounced, were obtained in presence of these 2 inhibitors for uptake of PC3 exosomes by PC3 cells (Fig. 4c and 4d). Oleic acid at a low concentration (2.5–5 µM) is a potent inhibitor of the Mg2+- and Ca2+-ATPase activity of seminal prostasomes (22). Inclusion of this inhibitor, opposite to the V-ATPase inhibitor (BafA1), had no effect on the uptake of either prostasomes or PC3 exosomes by PC3 cells. Nor did oleic acid work in synergy with other effectors (Fig. 4c and 4d). These results suggest that V-ATPase (but not Mg2+-and Ca2+-ATPase) activity is required for the uptake process by PC3 cells of both prostasomes and PC3 exosomes. Hence, functionality of V-ATPase and glycolysis seems to be obligatory for prostasome/exosome uptake into both normal and malignant prostate cells.
Fig. 4.
Estimation of prostasome and exosome uptake by recipient cells being dependent on V-ATPase. (a) CRL2221 cells and (b) PC3 cells were incubated with PKH67-labelled prostasomes (representing 1 µg protein) or PC3 exosomes (representing 1 µg protein) in absence or presence of bafilomycin (BafA1, 10 nM), 2-deoxyglucose (2-DG, 10 mM) and/or oleic acid (OA) (100 µM) for 3 h at 37°C. Fluorescence intensity was analysed by flow cytometry. Results are expressed as a fold relationship to the control (exosomes without treatment) (mean values±SEM, n=3).
Discussion
The present study addresses some aspects of energy metabolism involving non-malignant- and malignant-cell-derived exosomes. Normalcy is represented by exosomes (seminal prostasomes) obtained from patients visiting a fertility clinic with no suspicion of prostate cancer. Prostasomes are secreted by the acinar, epithelial cells of the prostate gland. These cells, although being in majority, are not alone in the epithelial lining of prostatic ducts, because there are also minor elements of basal and endocrine-paracrine cells. Using a highly specific monoclonal antibody against purified seminal prostasomes, immunohistochemical staining demonstrated that all prostate epithelial cells contained prostasomes with most intense staining in the apical area of the cells (23). Immunohistochemistry (using the same monoclonal antibody) did not reveal any positive staining in paraffin sections of human epididymides and testes, although a weak staining was observed in apical parts of seminal gland epithelium (23). The non-malignant prostate epithelial cell line, CRL2221, was used for uptake experiments.
Malignancy was represented by PC3 cells grown in traditional 2-dimensional monolayers (RPMI 1640 medium enriched with 10% FBS). We are well aware that in such a model many properties may be lost including cell–cell communication, polarization, and extracellular contacts, whereas properties like wound healing, inflammation responses and proliferation are artificially promoted in a similar fashion with what is found in in vivo situations (24,25). PC3 cells typically release exosomes (prostasomes) containing MAL, caveolin-1, CD59 and neuroendocrine markers including chromogranin B and A previously found in prostasomes (9,11,26). The human PC3 cell line was used as a target and mycophenolic acid, tiazofurin or ribavirin, which are inhibitors of IMP dehydrogenase and clinically adopted as anticancer drugs, as inducers (27). Interestingly, Floryk et al. found that these inhibitors evoked replication arrest, caused an increase in cell size, and triggered vacuolization of the cytoplasm. Moreover, this treatment resulted in the induction of the expression of 12 PC3-exosomal proteins typically found in seminal (non-malignant) prostasomes (28). These data corroborate our view of PC3 cells as being a relevant representative model of malignancy. Still, the present study restricts itself preferably to a comparison between seminal prostasomes (representing normalcy) and PC3 cell-derived exosomes (representing malignancy).
In this study, we confirm our previous observation that prostasomes are capable of producing extracellular ATP by glycolysis and provide additional evidence that the PC3 exosomes are equally potent in the formation of glycolytic ATP. In addition, we observed a clear-cut difference between the high ATPase activity of prostasomes and the low ATPase activity of PC3 exosomes, rendering these latter exosomes a higher net gain of ATP. The question arises whether this discrepant manifestation of ATPase activities is a general phenomenon, highlighting the difference between EVs derived from normal and cancer cells, or a peculiar phenomenon associated with PC3 cells and their exosomes. A previous study carried out in our laboratory dealt with a similar problem that was enacted at the (outer) plasma membrane surface of human glia (normal) and glioma (malignant) cells in culture. A comparison demonstrated that growing or stationary lines of normal glia cells showed high ATPase activity, whereas the neoplastic glioma cells exhibited very low surface-located enzyme activity. Of special interest in the latter group was the SV40-transformed line which was derived from glia-like cells with a high ATPase activity. After transformation, they behaved like glioma cells with again a markedly low ATPase activity (29). The ATPases of cell surfaces and prostasomes/exosomes may be kindred natures, keeping in mind the origin of prostasomes/exosomes as a result of double invaginations (first one involving the plasma membrane and the second one involving the late endosomal membrane), meaning that these EVs are “right-side-out.” Therefore, we cannot exclude the possibility that the discrepant ATPase activities exhibited by EVs originating from prostate acinar epithelial cells (non-malignant) and PC3 cells (malignant) may represent a more general phenomenon. Additionally, normal and malignant prostate cells had the capacity to incorporate EVs provided that a glycolytic flux and a functional V-ATPase were at hand. However, from these studies we cannot conclusively state whether this glycolytic flux was solitarily crucial for the recipient cells (intracellularly) or the EVs (extracellularly).
There are several mechanisms by which cells incorporate exosomes. There is agreement that uptake of exosomes is mediated by endocytic mechanisms, either clathrin- and/or caveolin-dependent, and also by other mechanisms such as micropinocytosis and phagocytosis. Experimental evidence exists that the uptake of EVs is an energy-dependent process that in addition requires a functioning cytoskeleton (30). Bafilomycin A1 is a specific inhibitor of the vacuolar type H(+)-ATPase (V-ATPase) in cells, and inhibits the acidification of organelles containing this enzyme, such as endosomes and lysosomes with inhibitory effects on the internalization of EVs (31,32). It is therefore tempting to speculate that by blocking the V-ATPase on the exosomes, we raise the microenvironmental pH that prevents the uptake (that needs acidic conditions) of the EVs.
The blockade of EV (prostasomes and PC3 exosomes) uptake into normal and malignant prostate cells by the glycolysis inhibitor 2-DG was conspicuous. We provide evidence suggesting that a prerequisite for the uptake of EVs into normal and malignant prostate cells required an ongoing glycolytic flux implying ATP presence extracellularly. This ATP may originate from the prostasome/exosome and/or intracellularly from the recipient cells. Extracellular ATP might act as a substrate for protein kinase occurring at the surface of prostasomes/exosomes or at the surface of recipient cells (33,34). Hence, surface-membrane phosphorylation reactions might be important initial steps in an internalization process as evidenced by ultrastructurally provable membranous changes (33). Elevated extracellular ATP levels might also stimulate EV-internalization perhaps via ionotropic purinergic (P2X) receptors in similarity with what has been reported for the glycoprotein, pannexin 1 (35). At the cellular level, purinergic receptors are strategic regulators of cell activation, proliferation, differentiation, migration and apoptosis. Indeed, in presence of ATP, purinergic receptors expressed by tumour cells are activated and apparently able to modulate both tumour growth and metastasis (36,37).
In conclusion, we have demonstrated the ability of metastatic prostate cancer cell line PC3 exosomes to form extracellular ATP similarly to human seminal prostasomes. The net ATP gain of PC3 exosomes was high due to their down-regulated ATPase activity in sharp contrast to prostasomes. We also demonstrated an energy-dependent uptake of EVs (prostasomes and PC3 exosomes) by normal and prostate cancer cells. This uptake process was dependent on an ongoing glycolytic flux and therewith ATP formation extracellularly by EVs and/or intracellularly by recipient cells in concert with a functional V-ATPase present.
Supplementary Material
Acknowledgements
TP is generously supported by Cancerfonden, Radiumhemmets, forskningsfonder and Vetenskapsrådet. CS was supported by a research grant obtained from Cancerfonden. DC is a recipient of a Hellenic Association for Molecular Cancer Research (HAMCR). KGR, LD, AL and GR is supported by Lions Cancerfond, Uppsala, and Erik, Karin och Gösta Selanders Stiftelse, Uppsala
Conflict of interest and funding
The authors declare no conflict of interest.
References
- 1.Ronquist G, Hedstrom M. Restoration of detergent-inactivated adenosine triphosphatase activity of human prostatic fluid with concanavalin A. Biochim Biophys Acta. 1977;483:483–6. doi: 10.1016/0005-2744(77)90078-x. [DOI] [PubMed] [Google Scholar]
- 2.Ronquist G. Prostasomes are mediators of intercellular communication: from basic research to clinical implications. J Intern Med. 2012;271:400–13. doi: 10.1111/j.1365-2796.2011.02487.x. [DOI] [PubMed] [Google Scholar]
- 3.Ronquist G, Brody I. The prostasome: its secretion and function in man. Biochim Biophys Acta. 1985;822:203–18. doi: 10.1016/0304-4157(85)90008-5. [DOI] [PubMed] [Google Scholar]
- 4.Sahlen GE, Egevad L, Ahlander A, Norlen BJ, Ronquist G, Nilsson BO. Ultrastructure of the secretion of prostasomes from benign and malignant epithelial cells in the prostate. Prostate. 2002;53:192–9. doi: 10.1002/pros.10126. [DOI] [PubMed] [Google Scholar]
- 5.Park KH, Kim BJ, Kang J, Nam TS, Lim JM, Kim HT, et al. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci Signal. 2011;4:ra31. doi: 10.1126/scisignal.2001595. [DOI] [PubMed] [Google Scholar]
- 6.Ronquist GK, Larsson A, Ronquist G, Isaksson A, Hreinsson J, Carlsson L, et al. Prostasomal DNA characterization and transfer into human sperm. Mol Reprod Dev. 2011;78:467–76. doi: 10.1002/mrd.21327. [DOI] [PubMed] [Google Scholar]
- 7.Ronquist GK, Larsson A, Stavreus-Evers A, Ronquist G. Prostasomes are heterogeneous regarding size and appearance but affiliated to one DNA-containing exosome family. Prostate. 2012;72:1736–45. doi: 10.1002/pros.22526. [DOI] [PubMed] [Google Scholar]
- 8.Aalberts M, van Dissel-Emiliani FM, van Adrichem NP, van Wijnen M, Wauben MH, Stout TA, et al. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol Reprod. 2012;86:82. doi: 10.1095/biolreprod.111.095760. [DOI] [PubMed] [Google Scholar]
- 9.Llorente A, de Marco MC, Alonso MA. Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. J Cell Sci. 2004;117(Pt 22):5343–51. doi: 10.1242/jcs.01420. [DOI] [PubMed] [Google Scholar]
- 10.Ronquist KG, Carlsson L, Ronquist G, Nilsson S, Larsson A. Prostasome-derived proteins capable of eliciting an immune response in prostate cancer patients. Int J Cancer. 2006;119:847–53. doi: 10.1002/ijc.21895. [DOI] [PubMed] [Google Scholar]
- 11.Dubois L, Ronquist KG, Ek B, Ronquist G, Larsson A. Proteomic profiling of detergent resistant membranes (lipid rafts) of prostasomes. Mol Cell Proteomics. 2015;14:3015–22. doi: 10.1074/mcp.M114.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arvidson G, Ronquist G, Wikander G, Ojteg AC. Human prostasome membranes exhibit very high cholesterol/phospholipid ratios yielding high molecular ordering. Biochim Biophys Acta. 1989;984:167–73. doi: 10.1016/0005-2736(89)90212-5. [DOI] [PubMed] [Google Scholar]
- 13.Brouwers JF, Aalberts M, Jansen JW, van Niel G, Wauben MH, Stout TA, et al. Distinct lipid compositions of two types of human prostasomes. Proteomics. 2013;13:1660–6. doi: 10.1002/pmic.201200348. [DOI] [PubMed] [Google Scholar]
- 14.Ronquist KG, Ek B, Morrell J, Stavreus-Evers A, Strom Holst B, Humblot P, et al. Prostasomes from four different species are able to produce extracellular adenosine triphosphate (ATP) Biochim Biophys Acta. 2013;1830:4604–10. doi: 10.1016/j.bbagen.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Drake RR, Kislinger T. The proteomics of prostate cancer exosomes. Expert Rev Proteomics. 2014;11:167–77. doi: 10.1586/14789450.2014.890894. [DOI] [PubMed] [Google Scholar]
- 16.Kharaziha P, Chioureas D, Rutishauser D, Baltatzis G, Lennartsson L, Fonseca P, et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget. 2015;6:21740–54. doi: 10.18632/oncotarget.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 18.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 19.Ronquist KG, Ek B, Stavreus-Evers A, Larsson A, Ronquist G. Human prostasomes express glycolytic enzymes with capacity for ATP production. Am J Physiol Endocrinol Metab. 2013;304:E576–82. doi: 10.1152/ajpendo.00511.2012. [DOI] [PubMed] [Google Scholar]
- 20.Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 21.Ronquist G. Zinc ion stimulation of ATP cleavage by prostasomes from human seminal plasma. Urol Int. 1988;43:334–40. doi: 10.1159/000281386. [DOI] [PubMed] [Google Scholar]
- 22.Ronquist G. Effect of modulators on prostasome membrane-bound ATPase in human seminal plasma. Eur J Clin Invest. 1987;17:231–6. doi: 10.1111/j.1365-2362.1987.tb01241.x. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson BO, Jin M, Einarsson B, Persson BE, Ronquist G. Monoclonal antibodies against human prostasomes. Prostate. 1998;35:178–84. doi: 10.1002/(sici)1097-0045(19980515)35:3<178::aid-pros3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Harma V, Virtanen J, Makela R, Happonen A, Mpindi JP, Knuuttila M, et al. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One. 2010;5:e10431. doi: 10.1371/journal.pone.0010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyga A, Cheema U, Loizidou M. 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signal. 2011;5:239–48. doi: 10.1007/s12079-011-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois L, Stridsberg M, Kharaziha P, Chioureas D, Meersman N, Panaretakis T, et al. Malignant cell-derived extracellular vesicles express different chromogranin epitopes compared to prostasomes. Prostate. 2015;75:1063–73. doi: 10.1002/pros.22990. [DOI] [PubMed] [Google Scholar]
- 27.Pankiewicz KW. Novel nicotinamide adenine dinucleotide analogues as potential anticancer agents: quest for specific inhibition of inosine monophosphate dehydrogenase. Pharmacol Ther. 1997;76:89–100. doi: 10.1016/s0163-7258(97)00092-2. [DOI] [PubMed] [Google Scholar]
- 28.Floryk D, Tollaksen SL, Giometti CS, Huberman E. Differentiation of human prostate cancer PC-3 cells induced by inhibitors of inosine 5’-monophosphate dehydrogenase. Cancer Res. 2004;64:9049–56. doi: 10.1158/0008-5472.CAN-04-1553. [DOI] [PubMed] [Google Scholar]
- 29.Agren G, Ponten J, Ronquist G, Westermark B. Demonstration of an ATPase at the cell surface of intact normal and neoplastic human cells in culture. J Cell Physiol. 1971;78:171–6. doi: 10.1002/jcp.1040780203. [DOI] [PubMed] [Google Scholar]
- 30.Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–12. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–12. [PubMed] [Google Scholar]
- 32.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124(Pt 3):447–58. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 33.Stegmayr B, Brody I, Ronquist G. A biochemical and ultrastructural study on the endogenous protein kinase activity of secretory granule membranes of prostatic origin in human seminal plasma. J Ultrastruct Res. 1982;78:206–14. doi: 10.1016/s0022-5320(82)80024-5. [DOI] [PubMed] [Google Scholar]
- 34.Agren G, Ronquist G. (32-P) Phosphoryl transfer by endogenous protein kinase at the glia and glioma cell surface in culture into extrinsic acceptor proteins. Acta physiol Scand. 1974;92:430–2. doi: 10.1111/j.1748-1716.1974.tb05763.x. [DOI] [PubMed] [Google Scholar]
- 35.Boyce AK, Kim MS, Wicki-Stordeur LE, Swayne LA. ATP stimulates pannexin 1 internalization to endosomal compartments. Biochem J. 2015;470:319–30. doi: 10.1042/BJ20141551. [DOI] [PubMed] [Google Scholar]
- 36.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27:211–7. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Roger S, Pelegrin P. P2X7 receptor antagonism in the treatment of cancers. Expert Opin Investig Drugs. 2011;20:875–80. doi: 10.1517/13543784.2011.583918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.