Abstract
Aim
Gastric cancer is etiologically related to interactions between Helicobacter pylori infection, environmental, and host factors. Gastric carcinoma is associated with a cascade of increasing atrophic gastric mucosal damage. Prostate stem cell antigen polymorphisms have been associated with an increased risk of gastric cancer. Here, we examined the interaction between prostate stem cell antigen polymorphisms and H. pylori in the progression of H. pylori gastritis.
Methods
Prostate stem cell antigen polymorphisms (TT, TC and CC) among H. pylori infected and uninfected Bhutanese were compared with the severity of H. pylori gastritis (neutrophils, monocytes, atrophy scores, H. pylori density, and the presence and extent of intestinal metaplasia) using the updated Sydney system.
Results
Biopsies from 339 patients were included. The proportion of biopsies with intestinal metaplasia was also significantly (P<0.05) greater among those with the TT genotype than with either the CT or CC genotype. Despite no significant differences in inflammation or H. pylori density scores, the scores for the premalignant condition, intestinal metaplasia in both the gastric corpus and antrum, among H. pylori infected with the TT genotype was significantly (P <0.05) greater than C allele carriers.
Conclusions
Prostate stem cell antigen TT genotype was associated with more than a 3-fold increase in the prevalence and extent of gastric mucosal intestinal metaplasia compared to C allele carriers among H. pylori infected Bhutanese.
Keywords: Helicobacter pylori, Gastric cancer, Prostate stem cell antigen gene, Intestinal metaplasia, Single nucleotide polymorphism
Introduction
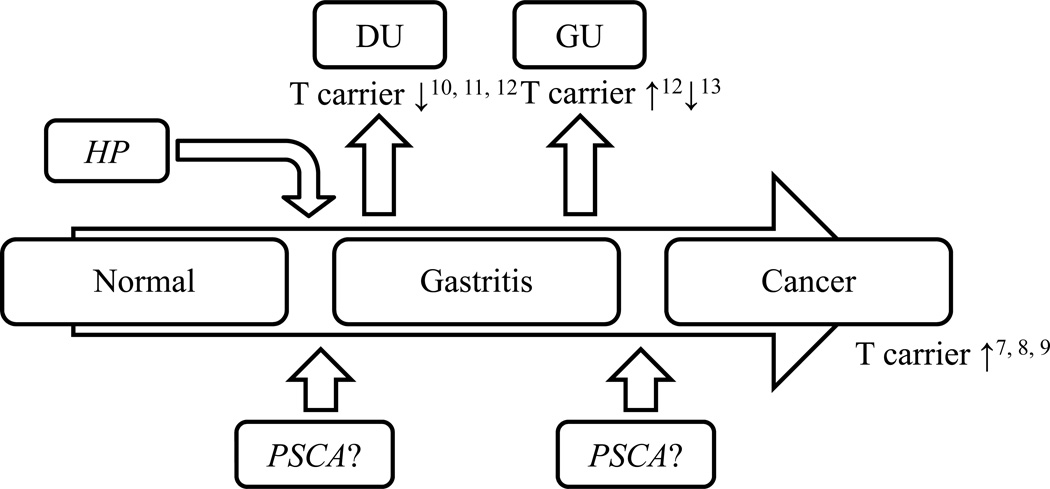
Helicobactor pylori (H. pylori) is an important human pathogen etiologically associated with atrophic gastritis and gastric cancer1. H. pylori related gastric carcinogenesis is thought to involve an interplay between bacterial-, environment- and host factors2, 3. H. pylori virulence factors such as CagA, VacA, OipA and DupA associated with an increased host inflammatory response are also associated with an enhanced risk of clinically significant outcomes including peptic ulcer and cancer4. Host factors identified as related to gastric cancer have often been recognized on the basis of genetic polymorphisms (e.g. single nucleotide polymorphisms; SNPs) and primarily involve genes related to an enhanced inflammatory response5. Genome wide association studies (GWAS) identified that prostate stem cell antigen (PSCA, rs2294008), a cell surface marker overexpressed in prostate cancer6, was associated with an increased risk of gastric cancer7. This PSCA SNP is located in exon 1 of chromosome 8q24.2 and is thought to possibly have tumor suppressor-like role in the stomach.7 GWAS identified the TT genotype as a possible important genetic risk factor for both diffuse type gastric cancer7 and for non-cardia gastric cancer8, 9. The C allele of this SNP was however associated with an increased risk for duodenal ulcer10–12 which is interesting in that gastric cancer is associated with atrophic pangastritis whereas duodenal ulcer is associated with corpus sparing antral predominant gastritis. The role of PSCA polymorphisms in the pathogenesis of gastric cancer and different patterns of gastritis remains unclear (Figure 1)12, 13.
Figure 1. Relationship between PCSA genotypes and gastroduodenal disease.
Although the relation between gastric cancer, gastroduodenal ulcer and PSCA genotypes have been shown, the relationship between gastric histological changes and PSCA genetic polymorphism in H. pylori infection remains unclear. Superscript on figure means reference number.
Bhutan is a landlocked country surrounded in the north by China and to the south, east and west by India. The prevalence of H. pylori infection in Bhutanese is high (eg, >70%)14. The H. pylori in Bhutanese is typically cagA positive with the cagA of the more virulent East Asian-type15. The age-standardized rate (ASR) of gastric cancer in Bhutan has been reported to be 17.2/100,000 which is higher than in adjacent India (GLOBOCAN 2012: http://globocan.iarc.fr/).
Here, we compared PSCA polymorphisms with the histological grading of gastritis among H. pylori-infected Bhutanese to assess the possible role of PSCA in the different histologic patterns of gastritis in a non-east Asian population.
Methods
This study is part of a series of studies designed to examine the importance of H. pylori in Bhutan14, 15. Approval for the samples and the protocol including this study was obtained in advance form the Ethics Committee of Jigme Dorji Wangchuk National Referral Hospital, Bhutan and of Oita University Faculty of Medicine, Japan. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration.
Subjects
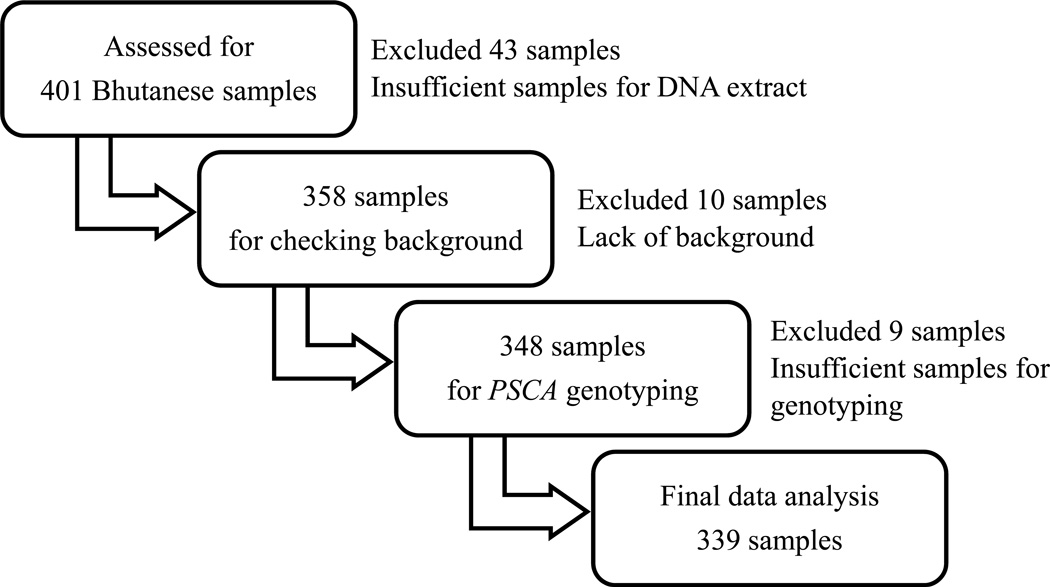
As previously described, we recruited 401 Bhutanese volunteers with no symptom or mild dyspeptic symptoms in December 201014. Exclusion criteria were past treatment for H. pylori infection, history of abdominal operation, regular use of nonsteroidal anti-inflammatory drugs, low-doses of aspirin, or potent acid inhibitors. Additionally, we excluded volunteers who took any antibiotics for the previous 4 weeks. Informed consent was obtained from all individual participants included in the study. We excluded 62 samples for lack of insufficient samples or lack of background data resulting in a total of 339 samples (Figure 2).
Figure 2. Design of study.
We recruited 401 Bhutanese volunteers. After excluding samples where background data was unknown or the samples were insufficient for DNA extraction or genotyping, a total of 339 samples remained.
Gastroscopy and histological examination
At gastroscopy, one biopsy from the gastric corpus and three biopsies from the antrum were obtained. The two samples from the antrum were used for rapid urease test and for H. pylori culture. The gastric biopsy specimens from the antrum and corpus were fixed in 10% buffered formalin and serial sections were stained with hematoxylin and eosin and with May-Giemsa stain and scored using the updated Sydney system by a single Japanese pathologist who was blinded to the background16. The updated Sydney system scores H. pylori density, neutrophils, monocytes, atrophy, intestinal metaplasia in the antrum and corpus into one of 5 grades (0 to 4). Samples with a grade 1 or more were considered positive17. Furthermore, H. pylori density was also evaluated by immunohistochemistry with polyclonal anti-H. pylori antibody as described previously18.
H. pylori diagnosis and PSCA polymorphism genotyping
H. pylori infection was diagnosed using combinations of rapid urease test, culture, histology and serological examination. H. pylori was identified on the basis of colony morphology, Gram staining and positive reactions for oxidase, catalase and urease. Serological examination was evaluated with a commercially available ELISA kit (Eiken Co., Ltd., Tokyo, Japan) and rapid urease test was also evaluated with a commercially available kit (CLO or Campylobactor-like organism test; Kimberly-Clark Ballard Medical products, Roswell, GA). The samples with least two positive results were diagnosed to be H. pylori-positive. H. pylori negative was defined as negative by all tests.
Genomic DNA was extracted from gastric mucosal biopsy specimens using a commercially available kit (QIAamp DNA Mini kit; QIAGEN, Hilden, Germany). The samples were classified into 3 SNPs of PSCA (T/T, T/C and C/C) by PCR-Restriction Fragment Length Polymorphism (PCR-RFLP). The primers were used 5’-AGG TGG AAA GAA GGA CAA AGG G-3’ (forward) and 5’-GGC CAA GCC TGC CAT CAA-3’(reverse) and the PCR products were digested with the restriction enzyme NlaIII (New England BioLabs, Beverly, MA)19.
Statistical Analyses
Hardy-Weinberg equilibrium of allele frequencies at individual assessed by comparing the observed and expected genotype frequencies using the chi squared test. Ages were given as mean and standard deviation, histological score were given as mean and standard error. Chi-square test, Fisher’s exact test, Wilcoxon’s signed-rank test, Kruskal-Wallis test and Spearman’s rank correlation coefficient were used as appropriate for statistical analysis using JMP 10.0 software (SAS, Cary, NC). All P < 0.05 were considered to be statistically significant.
Results
Subject Characteristics
Gastric biopsy samples from 339 Bhutanese were included in this study. No significant differences were observed in sex (Table 1). The age of H. pylori negative subjects was significantly greater than that of positive subjects (H. pylori positives; 37 ± 14 years old, and negatives; 43.4 ± 14 years) (Table 1). The prevalence of PSCA genotypes was similar between H. pylori positive and negative subjects (Table 1). HapMap project data showed that the prevalence of PSCA genotypes and allele frequency of Bhutanese was relatively similar to that of Chinese but dissimilar to that of Japanese and Gujarati Indians living in Texas (Table 2). Clinical presentation included 19 subjects with duodenal ulcer (DU), 22 with gastric ulcer (GU) and 3 with gastric cancer; DU, GU, and gastric cancer were identified by endoscopy. Gastric cancer was confirmed by histopathology. The remaining 295 subjects had endoscopically normal appearing mucosa or endoscopic gastritis without ulcers or cancer. The prevalence of H. pylori infection was 94.7% (18/19) in DU, 90.9% (20/22) in GU, 33.3% (1/3) in gastric cancer, and 71.9% (212/295) in subjects without peptic ulcers or gastric cancer.
Table 1.
Patients characteristics
| Total N = 339 (%) |
H. pylori positive N = 251 (%) |
H. pylori negative N = 88 (%) |
P value | |
|---|---|---|---|---|
| Man Female |
150 : 189 (44.2 : 55.8) |
118 : 133 (47.0 : 53.0) |
32 : 56 (36.4 : 63.6) |
P = 0.10 |
| Age | 38.7 ± 14.5 | 37.0 ± 13.9 | 43.4 ± 15.0 | P < 0.01 |
|
PSCA CC CT TT |
176 : 127 : 36 (52.0 : 37.5 : 10.6) |
131 : 90 : 30 (52.2 : 35.8 : 12.0) |
45 : 37 : 6 (51.1 : 42.0 : 6.8) |
P = 0.32 |
Prostate stem cell antigen; PSCA
No significantly differences in sex and prevalence of PSCA genotypes between H. pylori positive and negative subjects. Age of H. pylori negative subjects was significantly greater than that of positive subjects.
Table 2.
The prevalence of PSCA genotypes in Asia
| Genotypes (%) | Allele (%) | ||||
|---|---|---|---|---|---|
| CC | CT | TT | C | T | |
| Bhutanese | 52.0 | 37.5 | 10.6 | 70.7 | 29.3 |
| HapMap-HCB | 53.5 | 41.9 | 4.7 | 74.4 | 25.6 |
| HapMap-JPT | 12.8 | 50 | 37.2 | 37.8 | 62.2 |
| HapMap-CHD | 48.2 | 44.6 | 7.2 | 70.5 | 29.5 |
| HapMap-GIH | 25.0 | 52.3 | 22.7 | 51.1 | 48.9 |
Hap-Map is quoted from International HapMap Project site http://hapmap.ncbi.nlm.nih.gov/.
HCB; Han Chinese in Beijing, China, JPT; Japanese in Tokyo, Japan, CHD; Chinese in Metropolitan Denver, Colorado, GIH; Gujarati Indians in Houston, Texas.
HapMap project data shows that the prevalence of genotypes and allele frequency of Bhutanese was relatively similar to that of Chinese.
The updated Sydney System score between H. pylori positives and negatives
We compared histological scores between H. pylori infected vs. uninfected at each gastric site. As expected, neutrophils, monocytes and atrophy scores in H. pylori positives were significantly higher than these in negatives in both the antrum and corpus (all P <0.01) (Table 3). Intestinal metaplasia was overall uncommon and the differences in severity and proportion with intestinal metaplasia were not different irrespective of H. pylori infection (Table 3). The scores of neutrophils and monocytes had significant correlations with H. pylori density of H. pylori positives (Table 4). There was no correlation between inflammatory cell scores and the score of intestinal metaplasia (Table 4).
Table 3.
The updated Sydney System score between H. pylori positive and negative in each gastric site
| Region | Histology |
H. pylori positive |
H. pylori negative |
P value |
|---|---|---|---|---|
| Corpus | Neutrophils | 0.86 ± 0.04 (1) | 0.08 ± 0.06 (0) | < 0.01 |
| Monocytes | 1.10 ± 0.04 (1) | 0.35 ± 0.06 (0) | < 0.01 | |
| Atrophy | 0.51 ± 0.04 (0) | 0.23 ± 0.02 (0) | < 0.01 | |
| IM | 0.02 ± 0.01 (0) | 0.03 ± 0.02 (0) | = 0.69 | |
| Antrum | Neutrophils | 1.36 ± 0.04 (1) | 0.18 ± 0.07 (0) | < 0.01 |
| Monocytes | 1.63 ± 0.04 (2) | 0.59 ± 0.07 (1) | < 0.01 | |
| Atrophy | 1.36 ± 0.04 (1) | 0.97 ± 0.07 (1) | < 0.01 | |
| IM | 0.16 ± 0.03 (0) | 0.11 ± 0.05 (0) | = 0.47 | |
Intestinal metaplasia; IM
Values are shown as mean±SE (median).
Comparing histological scores between H. pylori infected vs. uninfected, neutrophils, monocytes and atrophy scores in H. pylori positives were significantly higher than these in negatives in both the antrum and corpus. The differences in severity and proportion with intestinal metaplasia were not different irrespective of H. pylori infection.
Table 4.
The correlation between the updated Sydney System score and H. pylori density in each gastric site.
| Histology | Corpus | Antrum | ||
|---|---|---|---|---|
| P value | R | P value | R | |
| Neutrophils | < 0.01 | 0.43 | < 0.01 | 0.45 |
| Monocytes | < 0.01 | 0.28 | < 0.01 | 0.37 |
| Atrophy | < 0.01 | 0.22 | < 0.01 | 0.16 |
| IM | 0.77 | 0.02 | 0.52 | −0.04 |
Intestinal metaplasia; IM, Correlation coefficient; R
However the scores of neutrophils and monocytes had significant correlations with H. pylori density of H. pylori positives, there was no correlation between inflammatory cell scores and the score of intestinal metaplasia.
The updated Sydney System score separated by PSCA genotypes
Because the gastric mucosa of H. pylori-negative subjects was typically normal (Table 3), we focused on possible relationships between H. pylori infection and PSCA genotypes. Separating each histological score by PSCA genotypes, we found that the intestinal metaplasia score among those with TT genotype was significantly greater than that of C allele carriers in both the gastric corpus and antrum (corpus; TT vs CT; P = 0.02 and TT vs CC; P <0.01, antrum; TT vs CT; P = 0.01 and TT vs CC; <0.01) (Table 5). There was no relationship between PSCA genotype and gastric mucosal damage as expressed by scores for neutrophils, monocytes or atrophy (Table 5). Although, the numbers with clinical disease were low, we found no relationship between PSCA genotype and clinical presentation; CC:CT:TT (number [%]) = 8 (44.4%): 6 (33.3%): 4 (22.2%) for DU, 11 (55%): 8 (40%): 1 (5%) for GU, 1 (100%):0:0 for gastric cancer and 104 (52.3%): 71 (37.2%): 24 (12.0%) for subjects without ulcers or cancer).
Table 5.
The updated Sydney System score separated by PSCA genotypes with H. pylori positive in each gastric site
| Histology | CC | CT | TT |
P value (TT vs CT) |
P value (TT vs CC) |
|
|---|---|---|---|---|---|---|
| Corpus | Neutrophils | 0.79 ± 0.06 (1) |
0.99 ± 0.07 (1) |
0.77 ± 0.12 (1) |
0.16 | 0.70 |
| Monocytes | 1.06 ± 0.05 (1) |
1.19 ± 0.06 (1) |
0.97 ± 0.11 (1) |
0.11 | 0.40 | |
| Atrophy | 0.44 ± 0.06 (0) |
0.61 ± 0.07 (1) |
0.47 ± 0.12 (0) |
0.37 | 0.71 | |
| IM | 0.00 ± 0.02 (0) |
0.02 ± 0.02 (0) |
0.13 ± 0.04 (0) |
= 0.02 | < 0.01 | |
| Antrum | Neutrophils | 1.34 ± 0.07 (1) |
1.38 ± 0.08 (1) |
1.40 ± 0.14 (1) |
0.65 | 0.61 |
| Monocytes | 1.61 ± 0.06 (2) |
1.69 ± 0.07 (2) |
1.53 ± 0.13 (1) |
0.26 | 0.42 | |
| Atrophy | 1.36 ± 0.05 (1) |
1.34 ± 0.07 (1) |
1.43 ± 0.11 (1) |
0.49 | 0.56 | |
| IM | 0.12 ± 0.04 (0) |
0.14 ± 0.05 (0) |
0.33 ± 0.09 (0) |
= 0.01 | < 0.01 | |
Intestinal metaplasia; IM, Values are shown as mean ± SE (median).
Separating each histological score by PSCA genotypes, the intestinal metaplasia score among those with TT genotype was significantly greater than that of C allele carriers in both the gastric corpus and antrum.
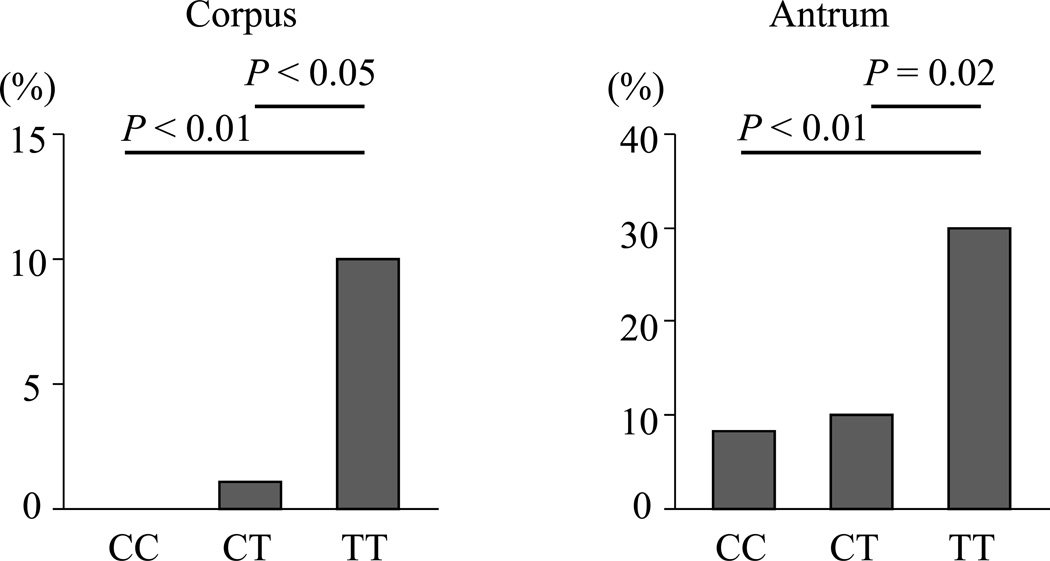
Figure 3 shows the comparison of intestinal metaplasia among each PSCA genotype. In the corpus, the proportion of intestinal metaplasia in TT genotype was significantly greater than in either the CT or CC genotype (TT genotype: 10%, CT genotype: 1.1%, CC genotype: 0%, P <0.01 and <0.05) (Figure 3). In the antrum, the pattern was similar (TT genotype: 30%, CT genotype: 10%, CC genotype: 8.4%, P <0.01 and = 0.02) (Figure 3). The proportion with intestinal metaplasia in the antrum was significantly greater than that in the corpus consistent with intestinal metaplasia starting in the antrum and then progressing into the corpus (CC; P <0.01, CT; P = 0.02, TT; P = 0.04) (Figure 3).
Figure 3. The proportion of intestinal metaplasia with H. pylori infection in each gastric site.
The proportion with intestinal metaplasia was significantly greater with the TT genotype than with the CT or CC genotypes in both the corpus and antral mucosa (P <0.05). In all genotypes, the proportion of intestinal metaplasia in the antrum was significantly higher than that in the corpus (P < 0.05).
As shown in Table 5 and Figure 3, the histological scores for intestinal metaplasia and the proportion with intestinal metaplasia with the TT genotype of PCSA with H. pylori positive was significantly greater than these among C allele carriers without other mucosal changes in each genotype.
Discussion
H. pylori infection results in progressive gastric mucosal damage that may advance to atrophic gastritis, increasing intestinal metaplasia and gastric carcinoma20. In this study, we aimed to investigate whether the PSCA genotype was linked to the progression of H. pylori-associated gastric mucosal damage as evidenced by the presence of atrophy and intestinal metaplasia. Intestinal metaplasia is considered a premalignant condition. For example, in a cohort study of 4,655 healthy asymptomatic subjects over 7 years, the risk of gastric cancer in severe chronic atrophic gastritis with extensive intestinal metaplasia was higher than that in chronic atrophic gastritis without intestinal metaplasia21. Overall, gastric cancer risk correlates with atrophy/intestinal metaplasia, rather than with neutrophil/monocyte infiltration22.
PSCA is a member of the lymphocyte antigen 6 (LY-6) family of glycosylphosphatidylnositol (GPI) –anchored cell surface proteins and one of the most abundant transcripts in differentiated tumors (ex. urothelial tumor)23. Although the gene was first found as a prostate-specific antigen overexpressed in prostate cancer, it was subsequently shown that other normal organs such as stomach, colon, and trophoblast6, 24. PSCA expression in cancer depends on each organ (e.g. prostate, pancreas, kidney and ovary are up-regulated, whereas, stomach and esophagus are down-regulated)23, 25–27. In the stomach PSCA protein is mainly expressed in neuroendocrine cells 24. PSCA is thought to have an inhibitory role in proliferation of differentiating gastric epithelial cells and leads the down-regulation in gastric cancer tissues as a tumor suppresser gene7, 23. There are several hypotheses about the differences of pathogenicity among each genotype. In one of the reasonable hypotheses, T allele carrier’s express a PSCA protein with an additional fragment of nine amino acid at the N-terminal protein (long PSCA, 123 amino acids), whereas C allele carrier’s express shorter a PSCA protein (114 amino acids)11. Although long PSCA has N- terminal protein localizing to the plasma membrane, short PSCA resides in the cytoplasm28. This genetic variation is thought to result in the differences in biological function of PSCA.
A number of studies have confirmed that the PSCA TT genotype is associated with an increased risk of atrophic gastritis and gastric cancer and a reduced risk of duodenal ulcer7,8–12. Conversely, the CC genotype is related to an increased risk of duodenal ulcer and a reduced risk of gastric cancer. To date the focus on gastric carcinogenesis has largely been on inflammation2, 3. The presence of atrophy and intestinal metaplasia are currently thought to be end products of the inflammatory response. However, the details of the mechanisms remain unclear and include both the aggressive feature of inflammation and those factors acting to limit the inflammatory response. The fact that the presence of severity and location of gastric inflammation did not differ in relation to PSCA genotype suggests the role of PSCA in gastric cancer involves factors other than those directly related to the type and severity of inflammation. However, the presence and location of intestinal metaplasia did relate to PSCA genotype suggesting factors more directly related to progression of intestinal metaplasia are involved. The available data suggest that the T genotypes predispose to corpus atrophy whereas the C genotype is either not conducive to the development of corpus atrophy or is actually related to mechanisms protecting the corpus from H. pylori-induced inflammation and its consequences. Gastric cancer is associated with low to absent gastric acid secretion. In contrast, duodenal ulcer disease is associated with the combination of minimal corpus inflammation with marked antral inflammation which allows robust acid secretion and the high duodenal acid load required for development of duodenal ulcer disease29. .The potential mechanisms that differ in relation to PSCA genotype include those responsible for blunting the effects of H. pylori associated inflammation and/or its ability to damage genes which result in a reduction of acid secretion or those associated with mucosal repair 2. For example, duodenal ulcer disease is also associated with a high parietal cell mass and a high acid output. The gastric pits in the corpus transmit 140–160 mmol hydrochloric acid from the parietal cells to the lumen. This high acidity prevents deep colonization of the corpus mucosa by H. pylori and H. pylori-related mucosal inflammatory cell-derived IL-1β from inhibiting parietal cell secretion1. However, a reduction in acid secretion such as associated with parietal cell vagotomy or the use of a proton pump inhibitor rapidly can rapidly result in with extension of H. pylori into the corpus pits and development of corpus gastritis 30, 31. It is possible that the CC genotype may be related in some why to parietal cell mass or to the regulation of gastric acid secretion. However, this study was not designed to comprehensively evaluate either the structure or function of the corpus mucosa. Subsequent studies should consider a more comprehensive evaluation of gastric acid secretion, mapping of the gastric corpus as well as studies of genes involved in repair of mucosal and DNA damage.
Our study confirms and extends prior studies relating PSCA genotypes and H. pylori-related diseases8, 32. We evaluated a non-east Asian population with a relatively uniform population, diet, and environment. However, our study has the several limitations. First, the sample size was relatively small and the range of mucosal damage among the participants was limited. Second, the virulence of the H. pylori infecting Bhutanese is uniform which did not allow any analysis of the potential effects of different H. pylori virulence factors. Finally, the protocol allowed only two biopsy specimens for histology so that the full histologic evaluation by the by updated Sydney system could not he obtained. However, the results suggest that the possibilities for the differences in outcome with the different genotypes need to be expanded to include both protective factors and those related to the control of gastric acid secretion.
In summary, PSCA polymorphism TT was associated with an increased prevalence of intestinal metaplasia in H. pylori-infected Bhutanese. The patients with TT genotype of PSCA increased the risk of development of intestinal metaplasia more than 3-fold compared with C allele carrier. Gastric atrophy/intestinal metaplasia is final stage of mucosal change of the gastric inflammatory response resulting in gastric cancer and the data suggest that PSCA polymorphisms may be related to the development of intestinal metaplasia in the H. pylori infected stomach. We failed to find a relation between PSCA polymorphisms and intensity of acute and chronic cellular inflammation suggesting the additional mechanisms need to also be reconsidered such as mechanisms related to control of acid secretion. Gastric cancer is related to gastric atrophy and genetic instability. It is possible that PSCA genotype influences H. pylori induced genetic instability by interacting with repair mechanism such as those involved in DNA methylation, microRNA expression, or H. pylori induced DNA breakage. This study was not designed to evaluate gastric acid secretion or the expression of genes involved in repair of mucosal or DNA damage. Such studies might be useful to clarify the links between increased risk of gastric inflammation intestinal metaplasia and genetic polymorphisms of PSCA.
Acknowledgments
We thank Lotay Tshering (Department of Surgery, Jigme Dorji Wangchuck National Referral Hospital, Thimphu, Bhutan) providing us the gastric biopsy samples.
Funding
This report is based on work supported in part by grants from the National Institutes of Health (DK62813), and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (24406015, 24659200, 25293104, 26640114 and 15H02657). This work was also supported by the Japan Society for the Promotion of Science (JSPS) Institutional Program for Young Researcher Overseas Visits and the Strategic Funds for the Promotion of Science and Technology from Japan Science and Technology Agency (JST).
Abbreviations
- PSCA
Prostate stem cell antigen
- H. pylori
Helicobacter pylori
- cagA
Cytotoxin-associated gene A
- vacA
Vacuolating cytotoxin A
- oipA
Outer inflammatory protein
- DupA
duodenal ulcer promoting gene A product
- SNP
Interleukin Single nucleotide polymorphism
- GWAS
Genome wide association studies
Footnotes
Disclosure of funding and conflicts of interest
Conflict of Interest
TU has received research grants from Institute for Fermentation, Osaka (IFO), Japan.
DYG is a consultant for RedHill Biopharma and has received research support. He is also a consultant for Otsuka Pharmaceuticals and for BioGaia.
References
- 1.Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983–1991. doi: 10.1016/s0016-5085(97)70019-2. [DOI] [PubMed] [Google Scholar]
- 2.Hanada K, Graham DY. Helicobacter pylori and the molecular pathogenesis of intestinal-type gastric carcinoma. Expert Rev Anticancer Ther. 2014;14:947–954. doi: 10.1586/14737140.2014.911092. [DOI] [PubMed] [Google Scholar]
- 3.Graham DY. Helicobacter pylori Update: Gastric Cancer, Reliable Therapy, and Possible Benefits. Gastroenterology. 2015;148:719–731. doi: 10.1053/j.gastro.2015.01.040. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 6.Reiter RE, Gu Z, Watabe T, et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 1998;95:1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Study Group of Millennium Genome Project for C. Sakamoto H, Yoshimura K, et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008;40:730–740. doi: 10.1038/ng.152. [DOI] [PubMed] [Google Scholar]
- 8.Lochhead P, Frank B, Hold GL, et al. Genetic variation in the prostate stem cell antigen gene and upper gastrointestinal cancer in white individuals. Gastroenterology. 2011;140:435–441. doi: 10.1053/j.gastro.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocellin S, Verdi D, Pooley KA, et al. Genetic variation and gastric cancer risk: a field synopsis and meta-analysis. Gut. 2015;64:1209–1219. doi: 10.1136/gutjnl-2015-309168. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Gonzalez MA, Bujanda L, Quintero E, et al. Association of PSCA rs2294008 gene variants with poor prognosis and increased susceptibility to gastric cancer and decreased risk of duodenal ulcer disease. Int J Cancer. 2015;137:1362–1373. doi: 10.1002/ijc.29500. [DOI] [PubMed] [Google Scholar]
- 11.Tanikawa C, Urabe Y, Matsuo K, et al. A genome-wide association study identifies two susceptibility loci for duodenal ulcer in the Japanese population. Nat Genet. 2012;44:430–434. doi: 10.1038/ng.1109. S1–2. [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa H, Sugimoto M, Uotani T, et al. Influence of prostate stem cell antigen gene polymorphisms on susceptibility to Helicobacter pylori-associated diseases: a case-control study. Helicobacter. 2015;20:106–113. doi: 10.1111/hel.12183. [DOI] [PubMed] [Google Scholar]
- 13.Tanikawa C, Matsuo K, Kubo M, et al. Impact of PSCA variation on gastric ulcer susceptibility. PLoS One. 2013;8:e63698. doi: 10.1371/journal.pone.0063698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilaichone RK, Mahachai V, Shiota S, et al. Extremely high prevalence of Helicobacter pylori infection in Bhutan. World J Gastroenterol. 2013;19:2806–2810. doi: 10.3748/wjg.v19.i18.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagashima H, Iwatani S, Cruz M, et al. Differences in interleukin 8 expression in Helicobacter pylori-infected gastric mucosa tissues from patients in Bhutan and the Dominican Republic. Hum Pathol. 2015;46:129–136. doi: 10.1016/j.humpath.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Bornschein A, Paz-Filho G, Graf H, et al. Treating primary hypothyroidism with weekly doses of levothyroxine: a randomized, single-blind, crossover study. Arq Bras Endocrinol Metabol. 2012;56:250–258. doi: 10.1590/s0004-27302012000400006. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda A, Uchida T, Nguyen LT, et al. A novel diagnostic monoclonal antibody specific for Helicobacter pylori CagA of East Asian type. APMIS. 2009;117:893–899. doi: 10.1111/j.1600-0463.2009.02548.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Chen J, Ding Y, et al. Genetic variation of PSCA gene is associated with the risk of both diffuse- and intestinal-type gastric cancer in a Chinese population. Int J Cancer. 2010;127:2183–2189. doi: 10.1002/ijc.25228. [DOI] [PubMed] [Google Scholar]
- 20.Correa P, Haenszel W, Cuello C, et al. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–4740. [PubMed] [Google Scholar]
- 21.Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138–143. doi: 10.1002/ijc.11680. [DOI] [PubMed] [Google Scholar]
- 22.Shimoyama T, Fukuda S, Tanaka M, et al. Evaluation of the applicability of the gastric carcinoma risk index for intestinal type cancer in Japanese patients infected with Helicobacter pylori. Virchows Arch. 2000;436:585–587. doi: 10.1007/s004289900179. [DOI] [PubMed] [Google Scholar]
- 23.Bahrenberg G, Brauers A, Joost HG, et al. Reduced expression of PSCA, a member of the LY-6 family of cell surface antigens, in bladder, esophagus, and stomach tumors. Biochem Biophys Res Commun. 2000;275:783–788. doi: 10.1006/bbrc.2000.3393. [DOI] [PubMed] [Google Scholar]
- 24.Gu Z, Thomas G, Yamashiro J, et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19:1288–1296. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- 25.Argani P, Rosty C, Reiter RE, et al. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320–4324. [PubMed] [Google Scholar]
- 26.Cao D, Ji H, Ronnett BM. Expression of mesothelin, fascin, and prostate stem cell antigen in primary ovarian mucinous tumors and their utility in differentiating primary ovarian mucinous tumors from metastatic pancreatic mucinous carcinomas in the ovary. Int J Gynecol Pathol. 2005;24:67–72. [PubMed] [Google Scholar]
- 27.Elsamman EM, Fukumori T, Tanimoto S, et al. The expression of prostate stem cell antigen in human clear cell renal cell carcinoma: a quantitative reverse transcriptase-polymerase chain reaction analysis. BJU Int. 2006;98:668–673. doi: 10.1111/j.1464-410X.2006.06350.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakai K, Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191–5204. doi: 10.3748/wjg.v20.i18.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigneri S, Termini R, Savarino V, et al. Review article: is Helicobacter pylori status relevant in the management of GORD? Aliment Pharmacol Ther. 2000;14(Suppl 3):31–42. doi: 10.1046/j.1365-2036.2000.00398.x. [DOI] [PubMed] [Google Scholar]
- 31.Graham DY, Opekun AR, Yamaoka Y, et al. Early events in proton pump inhibitor-associated exacerbation of corpus gastritis. Aliment Pharmacol Ther. 2003;17:193–200. doi: 10.1046/j.1365-2036.2003.01400.x. [DOI] [PubMed] [Google Scholar]
- 32.Rizzato C, Kato I, Plummer M, et al. Genetic variation in PSCA and risk of gastric advanced preneoplastic lesions and cancer in relation to Helicobacter pylori infection. PLoS One. 2013;8:e73100. doi: 10.1371/journal.pone.0073100. [DOI] [PMC free article] [PubMed] [Google Scholar]