Abstract
Background
Global glomerulosclerosis is characteristic of chronic kidney disease and also occurs with normal aging. Our goal was to determine the upper limit of normal for number of globally sclerotic glomeruli.
Methods
Core-needle biopsies of the renal cortex were obtained at the time of living kidney transplantation at three centers between 1998 and 2011. The number of globally sclerotic glomeruli was averaged across two biopsy sections. Quantile regression was used to estimate the 95th percentile for globally sclerotic glomeruli as the upper reference limit. There were 2052 donors (mean age 43 years, 41% male, 10% hypertensive), with a mean (SD) of 16.0 (9.7) glomeruli and 0.47 (0.99) globally sclerotic glomeruli on biopsy; only 2.6% had >5% fibrosis.
Results
In a multivariable model excluding hypertensive donors, independent predictors of the number of globally sclerotic glomeruli were age, total number of glomeruli and cortex area. A simplified model was used to estimate the 95th percentile for number of globally sclerotic glomeruli by total number of glomeruli and age. For a biopsy section with 17–32 total glomeruli, the 95th percentile ranged from 1 for a 20-year old to 5.5 for a 70-year old donor. Hypertensive donors were more likely to have an abnormal number of globally sclerotic glomeruli (OR = 1.79, P = 0.035).
Conclusions
We have derived the 95% reference limit for number of globally sclerotic glomeruli in ostensibly healthy individuals accounting for age and the biopsy characteristics. Numbers of globally sclerotic glomeruli in a kidney biopsy that exceed these thresholds suggest chronic pathological injury in excess of that expected with normal aging.
Keywords: kidney biopsy, glomerulosclerosis, kidney transplantation, fibrosis, age
INTRODUCTION
Chronic kidney disease (CKD), defined by a reduced glomerular filtration rate or elevated urinary albumin excretion, markedly increases with age [1]. Underlying histological abnormalities of CKD include global glomerulosclerosis, tubular atrophy, interstitial fibrosis and arteriosclerosis [2–7]. Some of these histological abnormalities are also observed in the absence of CKD and correlate significantly with older age, e.g. the prevalence of at least two of these histological abnormalities increases from 2.7% in living kidney donors aged 18–29 years to 75% among those aged 70–77 years [8]. In particular, glomerulosclerosis with normal aging has been well described in numerous autopsy studies [9–11].
Pathologic features, particularly those seen on immunofluorescence or electron microscopy, can help distinguish disease-related from age-related glomerulosclerosis, but this is not always the case. A globally sclerotic glomerulus, particularly those due to obsolescence [12], can lack features to determine etiology. Since the presence of glomerulosclerosis increases with normal aging, even in persons with preserved kidney function [8], any determination of the clinical significance of number of globally sclerotic glomeruli on a renal biopsy should account for age. Similarly, the amount of cortex actually biopsied (i.e. number of glomeruli biopsied) will influence the amount of glomerulosclerosis detected [13].
Because of its invasive nature, kidney biopsies are generally not available among individuals with normal kidney function. One exception is the kidney transplant donor population, since many centers obtain an implant biopsy of the donated kidney during the transplant surgery [14]. Kidney donors undergo an extensive screening to determine their overall health and health of their kidneys prior to donation. Therefore, these kidney biopsies are a unique and valuable source to determine reference limits for number of globally sclerotic glomeruli in a healthy kidney.
We systematically analyzed kidney biopsies from a large cohort of living kidney donors obtained across three separate transplant sites to define the upper reference limit for number of globally sclerotic glomeruli as a function of age and clinical and biopsy characteristics. These thresholds can be used to help identify chronic damage exceeding that expected from aging alone in patients who undergo a diagnostic renal biopsy.
MATERIALS AND METHODS
Setting and participants
The Aging Kidney Anatomy study includes living kidney donors who have undergone core-needle biopsy of the donated kidney cortex during the transplant surgery at one of three participating sites (Mayo Clinic, MN; Mayo Clinic, AZ; Cleveland Clinic, OH) between 1998 and 2011. All kidney donors at the three sites had a thorough medical evaluation with a prescheduled battery of tests [15]. Information on donor demographics, clinical characteristics and medical history were obtained from the pre-donation medical records. Hypertension was defined by an office blood pressure >140/90 mmHg or the use of one anti-hypertensive medication (combination pills with thiazide diuretics permitted). Family history of end-stage renal disease (ESRD) was defined by the recipient being biologically related to the donor.
Renal biopsies
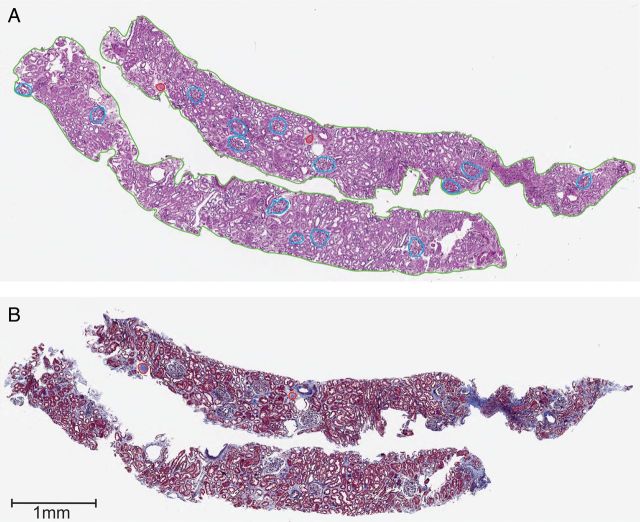
The study sample was limited to donors with available renal biopsies of the donated kidney obtained at the time of transplantation. Details of processing and histological examination of renal biopsies were described previously [15]. Briefly, two paraffin-embedded sections (2–3 µm thickness) were stained with periodic acid-Schiff and Masson's trichrome and scanned into a high-resolution image file (Figure 1). These two sections were chosen to be closest to the middle of the core and consecutive, as available. The number of globally sclerotic glomeruli was counted on each of the two sections, and averaged. Since the number of non-sclerotic glomeruli on each of the two sections did not change meaningfully, they were only counted on the periodic acid-Schiff-stained section. Non-sclerotic glomeruli on the section edge (bisected by the biopsy needle) were counted as half a glomerulus. The number of glomeruli per section was the sum of globally sclerotic glomeruli and non-sclerotic glomeruli per section. The number of ischemic non-sclerotic glomeruli (capillary wrinkling with or without pericapsular fibrosis) was also counted. Cortical area was determined by manually drawing regions of interest around the entire cortex on the biopsy sections. All biopsy sections were reviewed by one renal pathologist (V.K.) masked to clinical characteristics to score the proportion (%) of interstitial fibrosis in the cortex of all biopsy specimens.
FIGURE 1:
Example of two renal biopsy sections stained with periodic acid-Schiff (A) and Masson's trichrome (B) that were scanned into high-resolution images. Annotations for cortical area (green) in the periodic acid-Schiff section, 14 non-sclerotic glomeruli (blue) in the periodic acid-Schiff section, 2 globally sclerotic glomeruli in the periodic acid-Schiff section and in the Masson's trichrome section (red) are shown.
Statistical analysis
Numerical values were summarized by means and standard deviations and categorical data by counts and percentages. Linear regression was used to predict mean number of globally sclerotic glomeruli based on demographic, clinical and biopsy characteristics. Quantile regression was used to estimate the 95th percentile for number of globally sclerotic glomeruli based upon those predictors suggested by the linear regression. In multivariable analyses, we examined interactions between the significant main effects and considered them for inclusion in the models. Since hypertension is a disease linked to CKD, donors with hypertension were excluded in the model used to determine the 95th percentiles for number of globally sclerotic glomeruli. However, the full dataset was used to assess the association of an elevated number of globally sclerotic glomeruli (>95th percentile) with hypertension as well as other pathological characteristics (ischemic non-sclerotic glomeruli, interstitial fibrosis and cortical volume). We used procedures GLM, QUANTREG, FREQ and LOGISTIC of SAS, version 9.3 (SAS Institute Inc., Cary, NC) for the analysis.
RESULTS
The study sample consists of 2052 donors from three participating sites (1447, 420 and 185 donors from Mayo Clinic, MN; Mayo Clinic, AZ and Cleveland Clinic, OH, respectively). Donor and biopsy characteristics are described in Table 1 by the presence or absence of hypertension. The three sites differed in the amount of tissue biopsied, and the mean (SD) number of glomeruli on a sectioned renal biopsy was 16.9 (8.6) for Mayo Clinic MN, 11.0 (7.5) for Mayo Clinic, AZ and 20.2 (16.0) for Cleveland Clinic, OH (P < 0.001).
Table 1.
Living kidney donor clinical characteristics and biopsy findings at the time of donation
| No hypertension (n = 1847) | Hypertension (n = 205) | Total (n = 2052) | |
|---|---|---|---|
| Donor characteristicsa | |||
| Age, years | 41.9 (11.4) | 53.6 (9.8) | 43.1 (11.7) |
| Men | 756 (40.9%) | 91 (44.4%) | 847 (41.3%) |
| Race-ethnicity | |||
| Non-Hispanic white | 1570 (85%) | 184 (90%) | 1754 (85%) |
| Black | 49 (2.7%) | 3 (1.5%) | 52 (2.5%) |
| Hispanic | 87 (4.7%) | 6 (2.9%) | 93 (4.5%) |
| Native American | 30 (1.6%) | 1 (0.5%) | 28 (1.4%) |
| Asian | 26 (1.4%) | 2 (1.0%) | 31 (1.5%) |
| Unknown | 85 (4.6%) | 9 (4.4%) | 94 (4.6%) |
| Body mass index (kg/m2) | 27.3 (4.8) | 29.5 (5.0) | 27.5 (4.8) |
| Family history of ESRD | 990 (53.7%) | 92 (44.9%) | 1082 (52.8%) |
| 24-h urine albumin >10 mg | 194 (13.7%) | 42 (23.2%) | 236 (14.7%) |
| Measured GFR, mL/min/1.73 m2 | 105 (21) | 98 (25) | 104 (21) |
| Measured GFR < 80 mL/min/1.73 m2 | 97 (5.9%) | 34 (17.7%) | 131 (7.1%) |
| Systolic BP (mmHg) | 118 (14) | 132 (17) | 119 (15) |
| Diastolic BP (mmHg) | 72 (9) | 79 (11) | 73 (9) |
| Renal biopsy and CT scan findings | |||
| Cortical area (mm2) | 6.07 (3.03) | 5.96 (2.61) | 6.06 (2.99) |
| At least one globally sclerotic glomeruli | 561 (30.4%) | 110 (53.7%) | 671 (32.7%) |
| Number of glomeruli | 16.15 (9.84) | 14.73 (7.76) | 16.00 (9.66) |
| Number of globally sclerotic glomeruli | 0.42 (0.87) | 0.95 (1.62) | 0.47 (0.99) |
| Number of non-sclerotic glomeruli | 15.73 (9.70) | 13.78 (7.37) | 15.53 (9.51) |
| Number of ischemic non-sclerotic glomeruli | 0.091 (0.34) | 0.15 (0.46) | 0.096 (0.36) |
| Presence of capsule | 91 (4.9%) | 6 (2.9%) | 97 (4.7%) |
| Presence of corticomedullary junction | 382 (20.7%) | 40 (19.5%) | 422 (20.6%) |
| Interstitial Fibrosis >1% | 204 (11.2%) | 56 (27.5%) | 260 (12.8%) |
| Interstitial Fibrosis > 5% | 35 (1.9%) | 17 (8.3%) | 52 (2.6%) |
ESRD, end-stage renal disease; BP, blood pressure.
aFor quantitative measures, entries are mean (standard deviation) and for categorical measures, entries are count (percent).
The association of clinical and histological characteristics with number of globally sclerotic glomeruli is shown in Supplementary Table 1. Inspection of the data suggested that an association between age with number and globally sclerotic glomeruli was not evident among donors younger than 30 years. There was also an interaction between number of glomeruli and age in donors older than 30 years. Thus, we included an interaction term between age and number of glomeruli and treated age <30 years as age 30 years in subsequent models. The multivariable model estimating mean number of globally sclerotic glomeruli excluded patients with hypertension but considered all other significant terms from the unadjusted analysis. Statistically significant predictors of mean number of globally sclerotic glomeruli in a multivariable model were number of glomeruli, cortical area and the interaction term between age and number of glomeruli (P < 0.01 for all). Transplant center site, systolic blood pressure, corticomedullary junction and donor sex were not statistically significant after adjustment for age and number of glomeruli.
The 95th percentile was derived using quantile regression after restricting the model to include terms for age, total number of glomeruli and their respective interaction. Cortical area was not included because it is not routinely measured in clinical practice. The quadratic term for age was significant (P = < 0.001) and therefore included in the model. Age was discretized into groups of 18–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74 and 75–77 years old. Total number of glomeruli was similarly discretized into groups of 1, 2, 3–4, 5–8, 9–16, 17–32, 33–48 and 49–64 glomeruli and the model refit. Supplementary Figure 1 illustrates the 95th percentile for number of globally sclerotic glomeruli per biopsy section by age and total number of glomeruli per biopsy section. From this model fit, we constructed Table 2 which uses age (rows) and total number of glomeruli (columns) to determine the 95th percentile, or upper limit of normal, for number of globally sclerotic glomeruli. Goodness of fit with respect to age and total number of glomeruli was confirmed with the χ2 goodness of fit test (P = 0.3 and P = 0.4).
Table 2.
Upper reference limit (95th percentile) for number of globally sclerotic glomeruli per section, based upon age and number of glomeruli (GN) per section
| Age (years) | Number of glomeruli |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3–4 | 5–8 | 9–16 | 17–32 | 33–48 | 49–64 | |
| 18–29 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 1 |
| 30–34 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 1.5 |
| 35–39 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1.5 | 2 | 2 |
| 40–44 | 0.5 | 0.5 | 0.5 | 1 | 1 | 2 | 2.5 | 3 |
| 45–49 | 0.5 | 0.5 | 1 | 1 | 1.5 | 2 | 3 | 4 |
| 50–54 | 1 | 1 | 1 | 1.5 | 2 | 3 | 4 | 5 |
| 55–59 | 1 | 1 | 1.5 | 1.5 | 2 | 3.5 | 4.5 | 6 |
| 60–64 | 1 | 1.5 | 1.5 | 2 | 2.5 | 4 | 5.5 | 7 |
| 65–69 | 1 | 2 | 2 | 2.5 | 3 | 4.5 | 6.5 | 8 |
| 70–74 | 1 | 2 | 2.5 | 3 | 4 | 5.5 | 7.5 | 9 |
| 75–77 | 1 | 2 | 2.5 | 3 | 4 | 6 | 8 | 9.5 |
An electronic version of these reference limits are available on the QxMD app “Calculate” (iOS: http://qx.md/qx and web tool: http://qxmd.com/glomerulosclerosis).
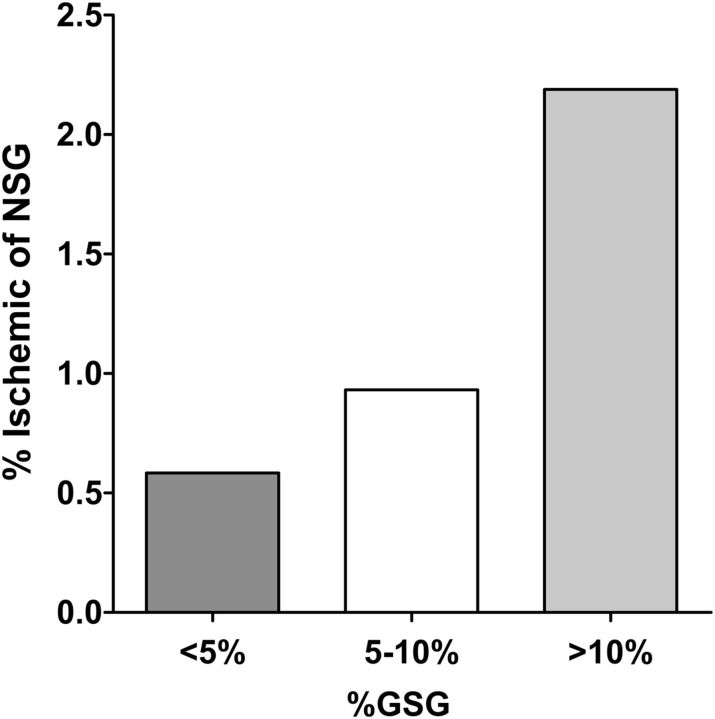
We investigated pathological associations with an abnormal number of globally sclerotic glomeruli (>95th percentile) in the full sample of donors. Among the 106 (5.2%) donors with an abnormal number of globally sclerotic glomeruli, 36 (35%) had interstitial fibrosis >1%, 12 (12%) had interstitial fibrosis >5% and 17 (16%) had hypertension, whereas overall these rates were 12.8, 2.6 and 10.0%. An abnormal number of globally sclerotic glomeruli associated with interstitial fibrosis >1% (OR = 4.1, P ≤ 0.001), interstitial fibrosis >5% (OR = 6.2, P ≤ 0.001) and hypertension (OR = 1.79, P = 0.03). The association between hypertension and an abnormal number of globally sclerotic glomeruli remained of similar magnitude, although was no longer statistically significant, after excluding donors with >1% fibrosis (OR = 1.75, P = 0.1). Figure 2 shows that globally sclerotic glomeruli associated with ischemic non-sclerotic glomeruli. The presence of an abnormal number of globally sclerotic glomeruli also associated with the presence of any ischemic non-sclerotic glomeruli (OR = 2.75, P < 0.001), even after excluding donors with hypertension (OR = 2.44, P < 0.001). Neither 24-h urine albumin >10 mg (OR = 0.87, P = 0.7) nor measured GFR < 80 mL/min/1.73 m2 (OR = 1.22, P = 0.32) was associated with an abnormal number of globally sclerotic glomeruli.
FIGURE 2:
The mean proportion of non-sclerotic glomeruli (NSG) that are ischemic increases with a higher percentage globally sclerotic glomeruli (GSG) on biopsy among living kidney donors (P < 0.001 for trend).
DISCUSSION
Both normal aging and CKD lead to glomerulosclerosis [16]. We quantified the mean number of globally sclerotic glomeruli expected as the result of aging apart from disease. We next derived the 95th percentiles for number of globally sclerotic glomeruli on a sectioned renal biopsy as a function of age and total number of glomeruli. These 95th percentiles were consistent across three different sites and did not differ by gender or race. These percentiles for an abnormal number of globally sclerotic glomeruli were strongly associated with interstitial fibrosis and hypertension. Used as thresholds, these 95th percentiles can help clinicians distinguish glomerulosclerosis due to disease rather than normal aging among patients who undergo a diagnostic renal biopsy.
This study demonstrates that it is important to account for age when identifying an abnormal number of globally sclerotic glomeruli. For example, 31% of the oldest (>65 years) normotensive donors would have been considered ‘abnormal’, if the 95th percentile for number of globally sclerotic glomeruli were based on the youngest (<35 years) normotensive donors. Age-specific thresholds can thus be used to help identify glomerulosclerosis due to disease. For example, suppose a 45-year-old man has a renal biopsy for glomerulonephritis that reveals 10 glomeruli per section, 3 of which are globally sclerotic glomeruli. Since the 95% reference limit for ages 45–49 years in a biopsy with 9–16 glomeruli is 1.5 globally sclerotic glomeruli, 3 globally sclerotic glomeruli would be concerning for chronic injury from the glomerulonephritis. If instead the patient was 65 years old, the three globally sclerotic glomeruli would be consistent with normal aging and less clearly due to damage from the glomerulonephritis.
We developed globally sclerotic glomeruli thresholds to be useful across different renal biopsy programs. The ratio of globally sclerotic glomeruli to total glomeruli biopsied will actually increase if each unique glomerulus is counted across (instead of averaged across) multiple consecutive sections due to the smaller size of globally sclerotic glomeruli [17]. However, the number of serial sections obtained from a core-needle biopsy can differ across renal biopsy programs, and counting the number of unique glomeruli across many sections may be time consuming. To be more easily applicable to clinical practice, we derived our reference thresholds using the mean number of globally sclerotic glomeruli across two sections and the number of non-sclerotic glomeruli on one section. More precise estimates in individual patients can still be made by averaging glomerular counts across multiple sections.
When deriving reference limits, it is critical to consider the total number of glomeruli in the biopsy section. For biopsies with very few glomeruli (e.g. four or less), the power of any inference is not as a strong as a biopsy with more glomeruli [13]. Still, for a patient 50 years or older, one globally sclerosed glomerulus of one glomerulus would not be unusual; however, in a younger patient, one globally sclerosed glomerulus of one glomerulus would exceed the upper limit of normal and is more suggestive of chronic pathology. While biopsies with more glomeruli distinguish normal from chronic pathology with more certainty, the clinical experience is that the amount of cortex obtained with needle core biopsies can be suboptimal [13]. Having a quantitative approach helps glean some information about chronicity in these suboptimal biopsies. In this study, 152 (8.2%) biopsies had 1–4 glomeruli, so we could meaningfully model 95th percentile reference limits in this range.
We also considered cortical area as an additional predictor, but this added very little in terms of improved model fit and is not routinely measured in clinical practice. A measure of biopsy depth into the kidney (corticomedullary junction) was significant in univariate analysis, but was no longer significant in the multivariable model after adjusting for the total number of glomeruli. Inspection of the data revealed that the number of glomeruli significantly correlated with cortical area, the presence of corticomedullary junction and the presence of capsule. In essence, the number of glomeruli sufficiently characterizes the relevant variation in renal biopsies.
Global glomerulosclerosis can sometimes be classified into two different patterns [12, 18]. One pattern is described by hypertrophy of the glomerular tuft leading to focal segmental glomerulosclerosis lesions and finally sclerosis via solidification of the tuft. We did not see evidence of this ‘hypertrophic’ or ‘solidification’ pattern of glomerulosclerosis in this population of normal adults. The other pattern is described as ‘ischemic’ with capillary wrinkling and pericapsular fibrosis of the glomerular tuft with eventual retraction of the tuft with sclerosis and collagen deposition in Bowman's space and finally ‘obsolescence’. This pattern describes the globally sclerotic glomeruli seen in this population. Evidence of this pattern was further supported by the presence of globally sclerotic glomeruli associating with the presence of ischemic-appearing non-sclerotic glomeruli. Thus, these reference limits are of use for evaluating globally sclerotic glomeruli of the ‘ischemic’ or ‘obsolescent’ pattern, whereas a globally sclerotic glomerulus of the ‘solidification’ pattern is always abnormal.
This study has several potential limitations. The use of a study population consisting of thoroughly evaluated living kidney donors guaranteed health, but limited the sample of individuals aged >70 years (since very few donors are elderly). Race differences in the reference thresholds were not detected, but the limited sample size for each non-white race group could have missed a small difference.
In conclusion, we have derived the 95% reference limit for number of globally sclerotic glomeruli in ostensibly healthy individuals accounting for age and the biopsy characteristics, which provide a practical tool, easily applicable to clinical practice. Further evaluation of these reference thresholds in diseased populations that undergo renal biopsies may help further delineate their clinical utility.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None of the authors have a conflict of interest in this study. The results presented in this paper have not been published previously in whole or in part, except in abstract form.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK 090358). The funding source had no role in the design, conduct, analysis or reporting of this study.
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 2.Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int 2012; 82: 270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhury D, Moshe L. Aging and kidney disease. In: Brenner BM, ed. Brenner and Rector’s the Kidneyy. 8th ed. Philadelphia: WB Saunders, 2007: 681–693 [Google Scholar]
- 4.Silva FG. The aging kidney: a review—part I. Int Urol Nephrol 2005; 37: 185–205 [DOI] [PubMed] [Google Scholar]
- 5.Silva FG. The aging kidney: a review—part II. Int Urol Nephrol 2005; 37: 419–432 [DOI] [PubMed] [Google Scholar]
- 6.Eknoyan G. Chronic kidney disease definition and classification: no need for a rush to judgment. Kidney Int 2009; 75: 1015–1018 [DOI] [PubMed] [Google Scholar]
- 7.Stevens LA, Coresh J, Levey AS. CKD in the elderly--old questions and new challenges: World Kidney Day 2008. Am J Kidney Dis 2008; 51: 353–357 [DOI] [PubMed] [Google Scholar]
- 8.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 2010; 152: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasiske BL. Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int 1987; 31: 1153–1159 [DOI] [PubMed] [Google Scholar]
- 10.Neugarten J, Gallo G, Silbiger S, et al. Glomerulosclerosis in aging humans is not influenced by gender. Am J Kidney Dis 1999; 34: 884–888 [DOI] [PubMed] [Google Scholar]
- 11.Anderson S, Brenner BM. Effects of aging on the renal glomerulus. Am J Med 1986; 80: 435–442 [DOI] [PubMed] [Google Scholar]
- 12.Hughson MD, Puelles VG, Hoy WE, et al. Hypertension, glomerular hypertrophy and nephrosclerosis: the effect of race. Nephrol Dial Transplant 2014; 29: 1399–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HJ, Kjellstrand CM, Cockfield SM, et al. On the influence of sample size on the prognostic accuracy and reproducibility of renal transplant biopsy. Nephrol Dial Transplant 1998; 13: 165–172 [DOI] [PubMed] [Google Scholar]
- 14.Mancilla E, Avila-Casado C, Uribe-Uribe N, et al. Time-zero renal biopsy in living kidney transplantation: a valuable opportunity to correlate predonation clinical data with histological abnormalities. Transplantation 2008; 86: 1684–1688 [DOI] [PubMed] [Google Scholar]
- 15.Elsherbiny HE, Alexander MP, Kremers WK, et al. Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol 2014; 9: 1892–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 2010; 152: 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weibel ER, Gomez DM. A principle for counting tissue structures on random sections. J Appl Physiol 1962; 17: 343–348 [DOI] [PubMed] [Google Scholar]
- 18.Hill GS. Hypertensive nephrosclerosis. Curr Opin Nephrol Hypertens 2008; 17: 266–270 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.