Abstract
Objective
The purpose of this study was to figure out the radiologic findings and risk factors related to adjacent segment degeneration (ASD) after anterior cervical discectomy and fusion (ACDF) using 3-year follow-up radiography, computed tomography (CT), and magnetic resonance image (MRI).
Methods
A retrospective matched comparative study was performed for 64 patients who underwent single-level ACDF with a cage and plate. Radiologic parameters, including upper segment range of motion (USROM), lower segment range of motion (LSROM), upper segment disc height (UDH), and lower segment disc height (LDH), clinical outcomes assessed with neck and arm visual analogue scale (VAS), and risk factors were analyzed.
Results
Patients were categorized into the ASD (32 patients) and non-ASD (32 patients) group. The decrease of UDH was significantly greater in the ASD group at each follow-up visit. At 36 months postoperatively, the difference for USROM value from the preoperative one significantly increased in the ASD group than non-ASD group. Preoperative other segment degeneration was significantly associated with the increased incidence of ASD at 36 months. However, pain intensity for the neck and arm was not significantly different between groups at any post-operative follow-up visit.
Conclusion
The main factor affecting ASD is preoperative other segment degeneration out of the adjacent segment. In addition, patients over the age of 50 are at higher risk of developing ASD. Although there was definite radiologic degeneration in the ASD group, no significant difference was observed between the ASD and non-ASD groups in terms of the incidence of symptomatic disease.
Keywords: Adjacent segment degeneration, Anterior, Cervical, Fusion, Spondylosis
INTRODUCTION
Anterior cervical discectomy and fusion (ACDF) is commonly used for the treatment of degenerative cervical disease, and is considered by some the gold standard for surgical treatment following failure of non-operative measures3,4,7,8,9,10,17,33). However, the development of adjacent segment degeneration (ASD) following ACDF is well established3,4,5,6,7,10,11,13,14,16,18,24,26,30,34,35). When discussing ASD, it is important to differentiate between radiological ASD and clinical ASD. The former involves radiological evidence of degeneration at the adjacent motion segment, whereas the latter involves the development of symptoms related to radiological changes at the adjacent motion segment12,35). In several studies, ASD rates varied from 25–92% during a long follow-up period10,12,32,35). Lawrence et al.18) estimated the rate of development of new symptomatic degeneration in the cervical spine after ACDF to be between 1.6% and 4.2% per year. Although magnetic resonance imaging (MRI) is the most sensitive imaging modality for evaluating disc degeneration, most previous studies regarding ASD used only using radiography or computed tomography (CT). In addition, previous studies have demonstrated that the development of ASD may be influenced by several factors, including the number and location of fusion segments, cervical spine sagittal alignment range of motion, spinal canal stenosis, age, smoking history, and pre-existing degenerative changes at adjacent segments. However, it remains controversial as to whether ASD represents an operative complication or a progression of the natural history of cervical spondylosis. Therefore, the objective of our study was to examine radiologic findings and risk factors related to ASD after single-level ACDF with a three-year follow-up using radiography, CT, and MRI.
MATERIALS AND METHODS
Study design
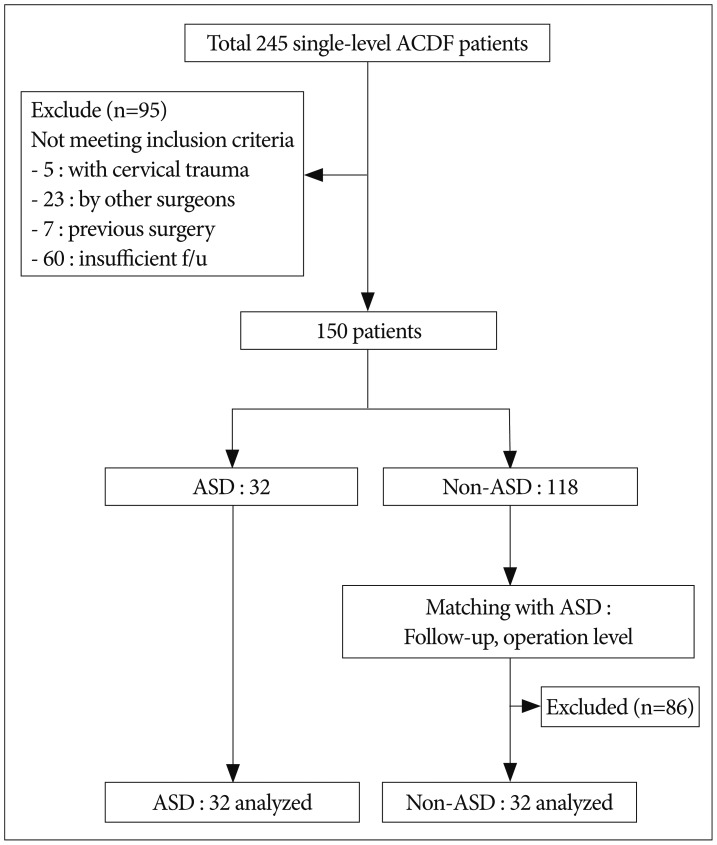
This retrospective, matched cohort study was conducted after obtaining institutional review board approval. A total of 245 consecutive patients with degenerative cervical disc disease who underwent single-level ACDF between January 2010 and July 2012 were considered for this study. Inclusion criteria were as follows : 1) single-level cervical degenerative disc disease on plain radiography, CT and MRI; 2) radiculopathy consistent with radiological findings (myelopathy patients were excluded due to more severe spondylotic characteristics); 3) unsuccessful conservative-therapy administration for at least 16 weeks; 4) all surgical procedures performed by two experienced spine surgeons; 5) cage and plate implantation; 6) clinical and radiological follow-up for at least 36 months; 7) operative levels C3–4, C4–5, C5–6, and C6–7; and 7) no previous history of cervical spine surgery. Patients were excluded if they met any of the following criteria : 1) operative levels including C2–3 and C7–T1; 2) follow-up of less than three years; 3) cervical trauma and/or infection; 4) history of cervical spine surgery; and 5) inability to provide accurate responses on pre- and post-operative questionnaires due to medical or other problems. Ultimately, 95 patients were excluded. Enrolled patients were divided into two groups, ASD and non-ASD, according to radiologic findings. The non-ASD group was matched 1 : 1 by follow-up time and operation level. Finally, 32 patients in the ASD group and 32 patients in the non-ASD group were analyzed (Fig. 1). All patients were Korean military serviceman at the time of operation. Before surgery, all patients were informed of the details of the surgery, including the method of anesthesia, potential complications, and benefits of the procedure.
Fig. 1. Flow chart depicting patient selection.
Surgical technique
All patients underwent surgery performed by two surgeons using the Smith-Robinson anterior approach. After applying a Caspar screw at the index level and cutting the anterior longitudinal ligament (ALL), complete discectomy, osteophyte removal, and gentle endplate preparation were performed. Subsequently, cage size was determined after inserting a trial cage (height 5, 6, or 7 mm). We used a polyetheretherketone (PEEK) cage filled with a mixture of hydroxyapatite/β-tricalcium phosphate (HA/TCP) (Cervios chronOS, Synthes Spine, West Chester, PA, USA). Next, the Skyline (Johnson and Johnson Professional, Inc., Raynham, MA, USA) anterior cervical plate system was utilized. The cage width was matched to the site of discectomy. Patients were typically discharged 5 to 7 days after surgery and advised to wear neck collars for 10 weeks after surgery.
Radiologic and clinical evaluation
Pre-operative work-up included plain X-rays (standing anterior-posterior, lateral neutral, lateral flexion, and lateral extension view), CT, and MRI. After the operation, plain X-rays were taken at each follow-up visit. CT scans were performed at 12, 24, and 36 months to assess bony fusion, and MRI was performed at 36 months to discern the presence of ASD.
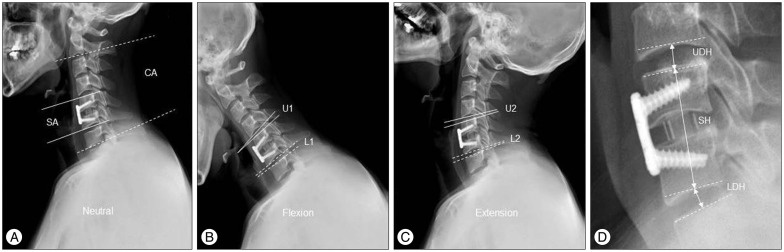
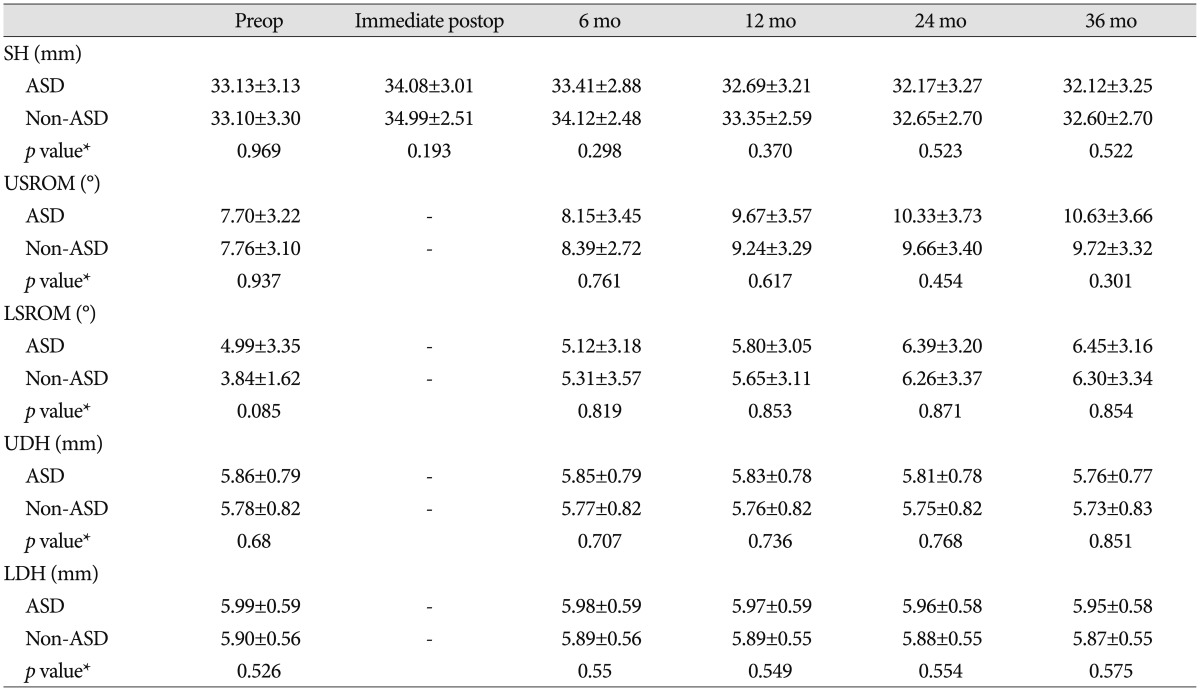
The parameters in Table 1 were measured at each time point. Cervical alignment (CA) and segmental angle (SA) were measured in order to compare to pre-operative baseline values. Segmental height (SH) was measured to assess the rate of subsidence. The upper segment range of motion (USROM), lower segment range of motion (LSROM), upper segment disc height (UDH), and lower segment disc height (LDH) were measured to discern the presence of ASD. Pre-operative USROM, LSROM, UDH, and LDH values were used as reference values, and post-operative changes at 6, 12, 24, and 36 months were compared between the two groups (Fig. 2).
Table 1. Measurement of radiologic parameters.
Fig. 2. Radiologic measurements. Cervical alignment (CA) and segmental angle (SA) was measured in neutral position using Cobb method (A). Upper segmental range of motion (USROM, U2–1) and lower segmental range of motion (LSROM, L2–1) was measured in both flexion and extension position (B and C). Segmental height (SA), upper segmental disc height (UDH), and lower segmental disc height (LDH) was measured along the line passing through the center of the vertebral bodies (D). For formulas and study time point, see Table 1.
We used two simultaneous methods to identify the presence of ASD. One was with the modified Hilibrand criteria on radiography, while the other utilized CT and MRI. For our purposes, ASD encompassed the following degenerative changes : disc signal change, anterior/posterior disc herniation, calcification of the anterior/posterior longitudinal ligament, anterior/posterior osteophytes, and a decrease in disc height of ≥25%12). Fusion was evaluated using the Bridwell fusion grading system. We defined fusion as grades 1 to 2 and motion <2 mm on flexion/extension lateral radiographs. Subsidence was defined as a ≥3 mm reduction in post-operative segmental height due to graft migration into adjacent endplates between the immediate post-operative period and the final follow-up visit. Measurements and evaluations based on radiography were performed by two independent radiologists using a picture archiving communication system (Marosis 5.0 PACS viewer, Marotech, Seoul, Korea).
We compared pre-operative fixed factors such as age, body mass index (BMI), operation level, smoking history, diabetes mellitus (DM), and follow-up duration between groups. Risk factors for ASD were examined, and included the following : age (>50 vs. ≤50), fusion rate, DM, BMI, smoking history, subsidence, pre-operative CA, pre-operative SA, pre-operative USROM, pre-operative LSROM, pre-operative SH, pre-operative UDH, pre-operative LDH, pre-operative other-segment degeneration, and preoperative ASD. Pain intensity was assessed using a visual analogue scale (VAS) score. Subjects completed a questionnaire consisting of a ten-point VAS for pre-operative neck and arm pain and at each follow-up visit. Follow-up visits occurred 6, 12, 24, and 36 months after surgery. VAS scores were collected and analyzed by one neurosurgeon who was not involved in the study. Patients were not allowed to review their previous questionnaire responses.
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Mean values±standard deviation or median values with interquartile ranges are shown. Student's t-tests were conducted to confirm intergroup differences in cases with normal distributions. Mann-Whitney U tests were used to compare variables with non-normal distributions between the two groups. Chi-square tests and Fischer's exact tests were performed for categorical variables. The degree of change in radiologic and clinical outcomes was assessed using repeated measures analysis of variance (RM-ANOVA), and post hoc comparisons between the two groups were performed. Risk factors for ASD were explored using binary logistic regression analysis. All p-values less than 0.05 were considered statistically significant.
RESULTS
Demographics
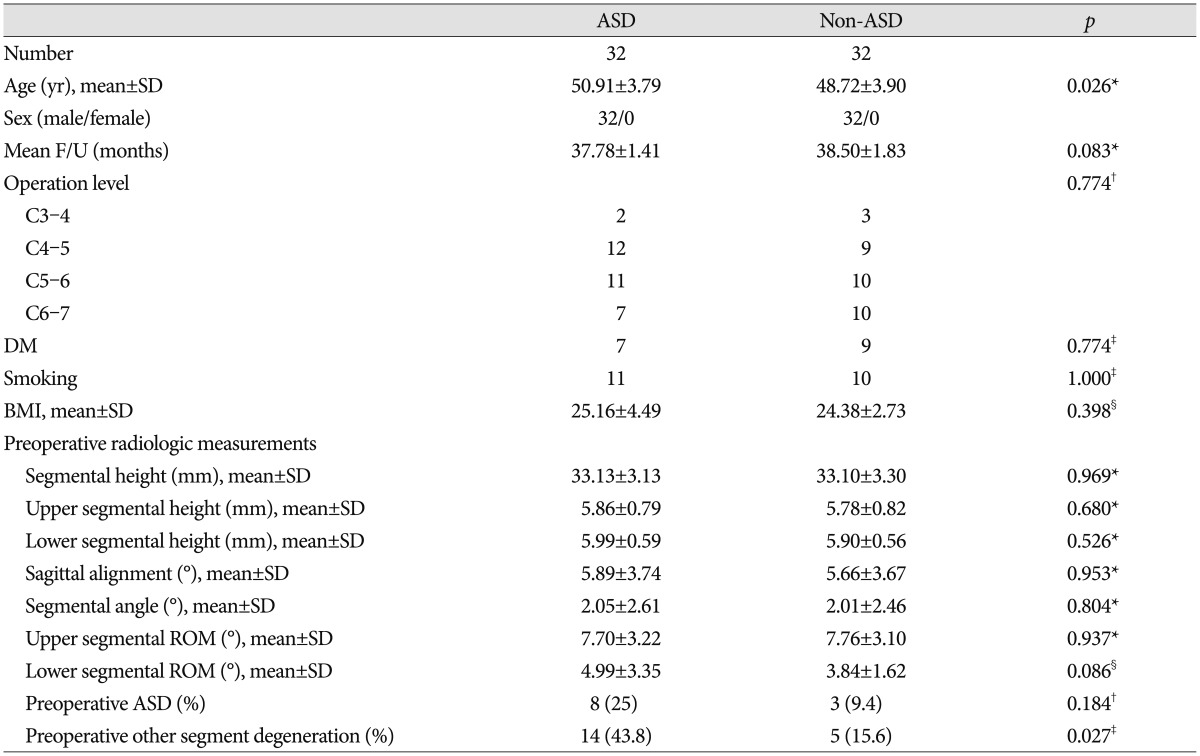
There were significant differences in age and pre-operative other-segment ASD between the ASD and non-ASD groups (p<0.05) (Table 2). ASD radiologic findings are summarized in Table 3.
Table 2. Comparison of the ASD and non-ASD group.
*Student t-test, †Fischer' exact test, ‡Chi-square test, §Mann-Whitney U test. ASD : adjacent segment degeneration, BMI : body mass index, DM : diabetes mellitus, ROM : range of motion
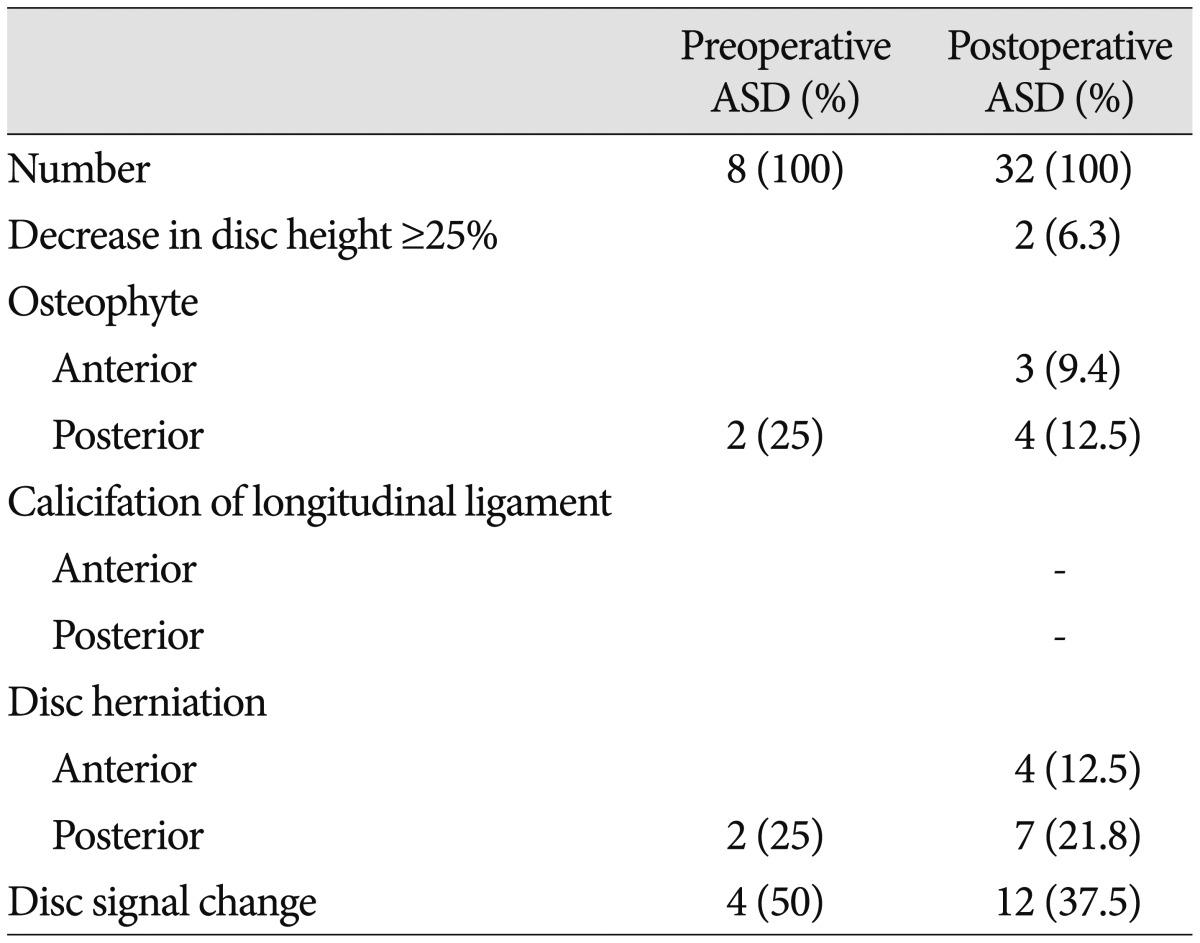
Table 3. The radiologic findings of ASD.
If there were more than one lesion, we counted only one finding, which was presented dominantly. ASD : adjacent segment degeneration
Radiologic and clinical outcomes
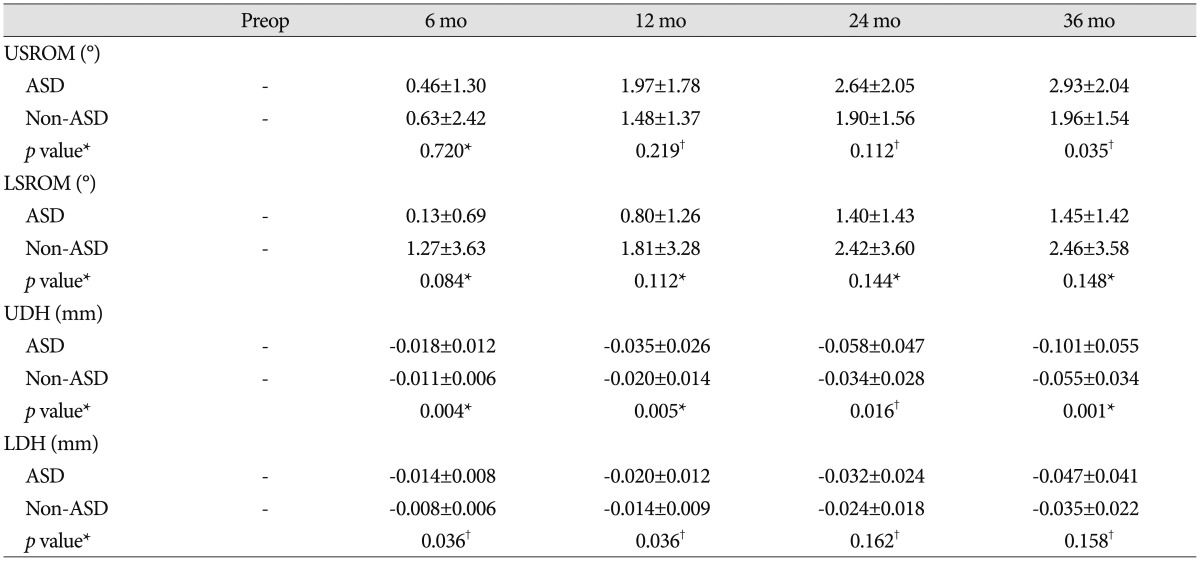
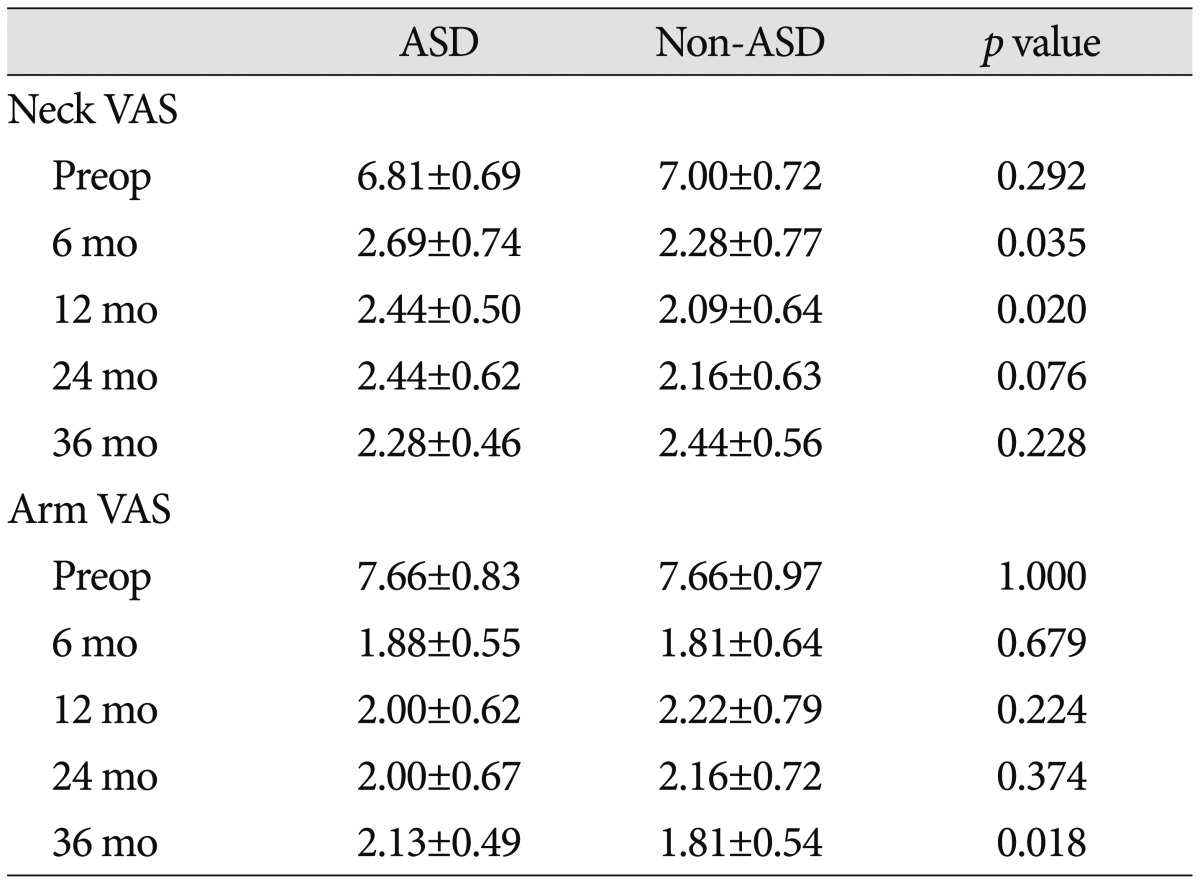
USROM and LSROM increased in both groups post-operatively. Although the difference was not statistically significant, the increase was more substantial in the ASD group (Table 4, 5). At 36 months post-operatively, the difference for value from the pre-operative one significantly increased in the ASD group than non-ASD group. Additionally, UDH and LDH gradually decreased in both groups over time (Table 4, 5). The decrease in UDH was significantly greater in the ASD group at each follow-up visit. The decrease in LDH was significantly greater in the ASD group at 6 and 12 months post-operatively (Table 5). However, pain intensity for the neck and arm was not significantly different between groups at any post-operative follow-up visit (Table 6). At the final follow-up visit, fusion rates were 84.4% (27/32) in the ASD group and 87.5% (28/32) in the non-ASD group. Cage subsidence was observed in seven cases in the ASD group (21.9%) and in 9 cases (28.1%) in the non-ASD group. However, these differences were not statistically significant.
Table 4. The radiologic parameters at pre- and postoperative period.
*Repeated measures analysis of variance. ASD : adjacent segment degeneration, LDH : lower segment disc height, LSROM : lower segment range of motion, Mo : month, Postop : postoperative, Preop : preoperative, SH : segmental height, UDH : upper segment disc height, USROM : upper segment range of motion
Table 5. Radiologic parameters of adjacent segments at each time point.
The differences for each value (obtained at 6, 12, 24, and 36 months) from the preoperative ones are calculated. *Mann-Whitney U test, †Student t-test. ASD : adjacent segment degeneration, LDH : lower segment disc height, LSROM : lower segment range of motion, UDH : upper segment disc height, USROM : upper segment range of motion
Table 6. Pre- and postoperative clinical outcomes.
All p values were calculated using repeated measures analysis of variance with post hoc using Student t-tests. All p<0.01 means significant difference. ASD : adjacent segment degeneration
Risk factors of ASD
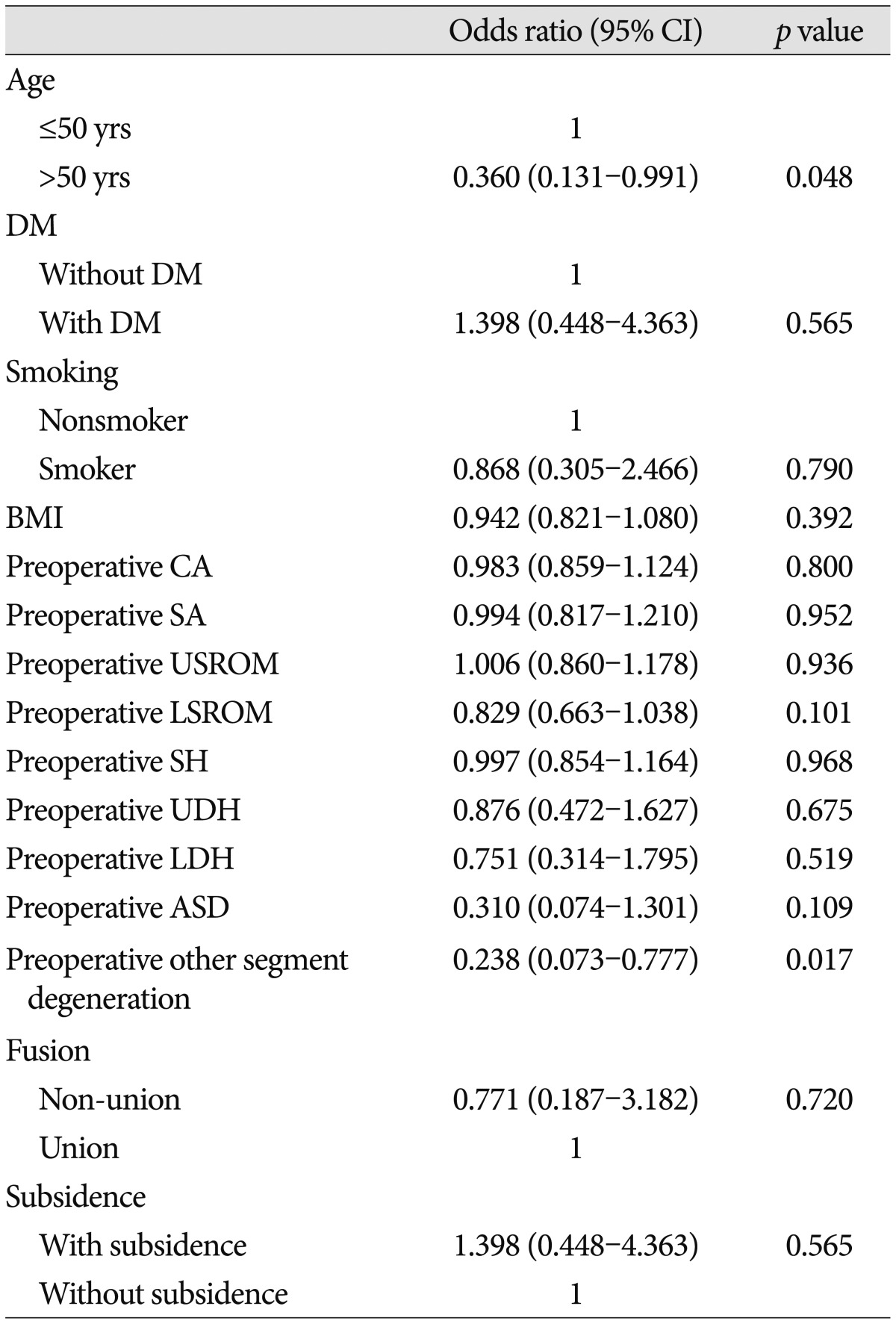
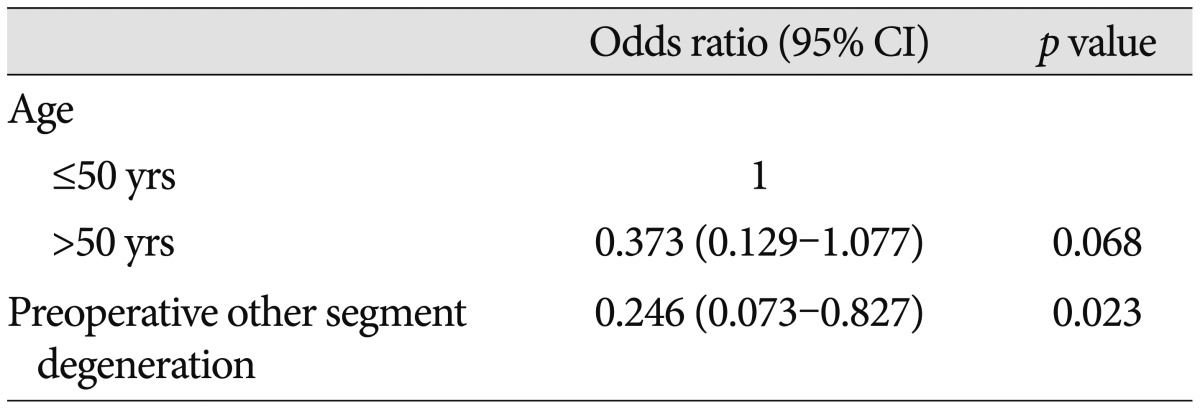
Univariate logistic regression analysis demonstrated that age >50 (p=0.048) and pre-operative other-segment degeneration (p=0.017) were significantly associated with an increased incidence of ASD at 36 months (Table 7). In multivariate logistic regression analysis, preoperative other-segment degeneration (p=0.023; OR, 0.246; 95% confidence interval, 0.073–0.827) was significantly associated with an increased incidence of ASD at 36 months (Table 8).
Table 7. Logistic regression analysis of risk factors for ASD.
ASD : adjacent segment degeneration, BMI : body mass index, CA : cervical alignment, DM : diabetes mellitus, LDH : lower segment disc height, LSROM : lower segment range of motion, SA : segmental angle, UDH : upper segment disc height, USROM : upper segment range of motion
Table 8. Multivariate logistic regression analysis of risk factors for ASD.
ASD : adjacent segment degeneration
DISCUSSION
ACDF is an effective method for the treatment of degenerative cervical disease1,2,4,7,15,19,20,21,24,27,28,29,35,36,37,38). However, it leads to excessive loading and additional motion at adjacent levels3,4,5,6,7,10,13,16,17,18,19,20,30,33,38). Reported rates of ASD following ACDF using radiographic criteria range from 25 to 92%10,12,32). Using a biomechanical model, Seo and Choi33) reported increased pressure within the intervertebral discs of adjacent segments. However, Matsunaga et al.25) showed that there was no increase in the strain adjacent to a single-level ACDF25). Again using biomechanical models, Rao et al.31) demonstrated that the pressure on intervertebral discs of adjacent segments and the movement between vertebrae did not change after fusion. The group maintained that symptomatic adjacent segment disease is the result of progressive cervical spondylosis, and is not caused by the arthrodesis itself. It remains unclear whether or not ASD following ACDF represents a true iatrogenic post-operative complication or a progression of the natural history of cervical spondylosis. Most previous studies examining ASD following ACDF were conducted with only radiographs and CT. Prior studies therefore did not provide sufficient information regarding the significance of ASD and its outcomes. Thus, we investigated risk factors associated with ASD after ACDF during a three-year follow-up period using MRI.
From our study, we can conclude that : 1) there were significant differences in age, and pre-operative other-segment ASD between the ASD and non-ASD groups; 2) adjacent disc height decreased in most of the upper and lower adjacent levels in both groups; 3) disc height change in the adjacent segment was more severe in the ASD group than the non-ASD group; 4) USROM and LSROM increased in both groups post-operatively; 5) pain intensity for the neck and arm was not significantly different between groups at any post-operative follow-up visit; and 6) pre-operative other-segment ASD was significantly associated with increased incidence of ASD at 36 months.
Matsumoto et al.22) reported that asymptomatic volunteers exhibited cervical disc degeneration in the range of 60% to 70% over a 10-year period, and natural disc degeneration rates were higher than those of ASD after ACDF. The group reported a 40–60% degenerative change in adjacent discs after ACDF using MRI. Another CT-based study reported that asymptomatic ASD was identified in up to 50% of patients22,23,24). However, there is currently no universally accepted radiologic measurement tool for the assessment of cervical spondylosis. From our 36-month follow-up using CT and MRI, we were able to discover a broad range of adjacent segment degenerative changes, including disc signal change, anterior/posterior disc herniation, calcification of the anterior/posterior longitudinal ligament, anterior/posterior osteophytes, and decreased disc height.
Changes in adjacent disc height were observed in most of the upper and lower adjacent levels in both groups. Moreover, the mean intervertebral disc height change in the adjacent segment was substantially more severe in the ASD group than the non-ASD group. In addition, USROM and LSROM increased in both groups as time passed after surgery. The mean USROM and LSROM changes tended to be greater in the ASD group than the non-ASD group. Although many previous studies reported similar results, it remains unclear whether this change represents a true iatrogenic post-operative complication or a progression of the natural history of cervical spondylosis. We hypothesize that ASD, especially proximal to the fused segment, occurs because of excessive mobility above the fused segments, which in turn causes premature degeneration of the facet joints. Facet hypertrophy and thickening of the ligamentum flavum may precede disc collapse, signal change, and disc herniation.
Park et al.30) reported that pre-existing ASD and other-segment post-operative degeneration out of the adjacent segment were associated with post-operative ASD, and they concluded that ASD is therefore related to the natural degenerative process instead of arthrodesis itself. Lundine et al.21) also suggested that adjacent segment disc degeneration is not primarily a complication of fusion surgery, and is at least in part due to the natural history of cervical spondylosis. Our study also found a significant, direct relationship between pre-operative other-segment degeneration and post-operative ASD as described earlier. Matsumoto et al.23) studied age-related changes in cervical intervertebral discs in asymptomatic subjects. In the present study, we identified an association between age (>50) and post-operative ASD. This finding may lend evidence to the hypothesis that ASD is at least in part due to the natural history of cervical spondylosis.
The correlation between ASD and clinical outcomes remains controversial. Previous studies reported no significant difference in clinical outcomes between patients with and without ASD14,30,35). In our study, the clinical outcome, based on the neck and arm VAS scores, suggests that improvements occurred after surgery (compared with pre-operative findings in both groups). In addition, there were no significant differences between groups at any post-operative follow-up visit. We can therefore hypothesize that radiographic ASD after ACDF is not associated with clinical outcome.
The current study has several limitations. First, it was a retrospective matched cohort study with a small sample size (32 patients vs. 32 patients) and short follow-up duration (3 years). Second, 2 different spine surgeons performed those operations. Even though we used same technique and same materials, there might have been subtle differences in the ACDF techniques based on a surgeon's experiences. Third, the study participants were limited to males who were recruited from an armed forces hospital, making our conclusions potentially invalid for other populations. To know the exact cause of ASD, a larger group of patients treated with ACDF and a multi-segment operative technique are required. A prospective multi-center study with long term follow-up would certainly provide more useful information. However, this study has a meaningful strength, 1) because it included a homogenous population of male patients who were military servicemen and therefore, performed similar degrees of training and physical activity. The enrollment of this population may reduce selection biases, resulting in positively influencing our study outcomes; and 2) CT and MRI was used for a more accurate diagnosis of ASD. MRI is most sensitive in assessing degenerative disc changes and CT displays more accurate bony changes.
CONCLUSION
According to the results, we can suggest that ASD is not primarily a complication of fusion surgery and is at least in part due to the natural course of cervical spondylosis. Clinical symptoms, especially increasing neck pain may be a clue of finding ASD. Additional studies should be performed in a larger sample population with an extended follow-up period using a prospective randomized design to assess the factor of ASD after ACDF.
References
- 1.Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298–1307. doi: 10.2106/00004623-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine(Phila Pa 1976) 1995;20:1410–1418. [PubMed] [Google Scholar]
- 3.Carrier CS, Bono CM, Lebl DR. Evidence-based analysis of adjacent segment degeneration and disease after ACDF : a systematic review. Spine J. 2013;13:1370–1378. doi: 10.1016/j.spinee.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 4.Cho SK, Riew KD. Adjacent segment disease following cervical spine surgery. J Am Acad Orthop Surg. 2013;21:3–11. doi: 10.5435/JAAOS-21-01-3. [DOI] [PubMed] [Google Scholar]
- 5.Eck JC, Humphreys SC, Lim TH, Jeong ST, Kim JG, Hodges SD, et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine(Phila Pa 1976) 2002;27:2431–2434. doi: 10.1097/00007632-200211150-00003. [DOI] [PubMed] [Google Scholar]
- 6.Elsawaf A, Mastronardi L, Roperto R, Bozzao A, Caroli M, Ferrante L. Effect of cervical dynamics on adjacent segment degeneration after anterior cervical fusion with cages. Neurosurg Rev. 2009;32:215–224. discussion 224. doi: 10.1007/s10143-008-0164-2. [DOI] [PubMed] [Google Scholar]
- 7.Faldini C, Miscione MT, Acri F, Leonetti D, Nanni M, Chehrassan M, et al. Single level cervical fusion by an anterior approach using autologous bone graft influences the adjacent levels degenerative changes : clinical and radiographic results at 10-year minimum follow-up. Eur Spine J. 2012;21(Suppl 1):S90–S93. doi: 10.1007/s00586-012-2215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faldini C, Pagkrati S, Leonetti D, Miscione MT, Giannini S. Sagittal segmental alignment as predictor of adjacent-level degeneration after a cloward procedure. Clin Orthop Relat Res. 2011;469:674–681. doi: 10.1007/s11999-010-1614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujibayashi S, Neo M, Nakamura T. Stand-alone interbody cage versus anterior cervical plate for treatment of cervical disc herniation : sequential changes in cage subsidence. J Clin Neurosci. 2008;15:1017–1022. doi: 10.1016/j.jocn.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Goffin J, Geusens E, Vantomme N, Quintens E, Waerzeggers Y, Depreitere B, et al. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17:79–85. doi: 10.1097/00024720-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hakalo J, Pezowicz C, Wronski J, Bedzinski R, Kasprowicz M. Comparative biomechanical study of cervical spine stabilisation by cage alone, cage with plate, or plate-cage : a porcine model. J Orthop Surg(Hong Kong) 2008;16:9–13. doi: 10.1177/230949900801600103. [DOI] [PubMed] [Google Scholar]
- 12.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Hilibrand AS, Fye MA, Emery SE, Palumbo MA, Bohlman HH. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Joint Surg Am 83-A. 2001:668–673. doi: 10.2106/00004623-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease : the consequences of spinal fusion? Spine J. 2004;4(6 Suppl):190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Ji GY, Oh CH, Shin DA, Ha Y, Kim KN, Yoon do, H, et al. Stand-alone cervical cages versus anterior cervical plates in 2-level cervical anterior interbody fusion patients : analysis of adjacent segment degeneration. J Spinal Disord Tech. 2015;28:E433–E438. doi: 10.1097/BSD.0b013e3182a355ad. [DOI] [PubMed] [Google Scholar]
- 16.Kaito T, Hosono N, Fuji T, Makino T, Yonenobu K. Disc space distraction is a potent risk factor for adjacent disc disease after PLIF. Arch Orthop Trauma Surg. 2011;131:1499–1507. doi: 10.1007/s00402-011-1343-0. [DOI] [PubMed] [Google Scholar]
- 17.Katsuura A, Hukuda S, Saruhashi Y, Mori K. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur Spine J. 2001;10:320–324. doi: 10.1007/s005860000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence BD, Hilibrand AS, Brodt ED, Dettori JR, Brodke DS. Predicting the risk of adjacent segment pathology in the cervical spine : a systematic review. Spine(Phila Pa 1976) 2012;37(22 Suppl):S52–S64. doi: 10.1097/BRS.0b013e31826d60fb. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Lee JS, Yi JS, Cho W, Zebala LP, Riew KD. Anterior cervical plating technique to prevent adjacent-level ossification development. Spine J. 2013;13:823–829. doi: 10.1016/j.spinee.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Li Y, Kong F, Zhang D, Zhang Y, Shen Y. Adjacent segment degeneration after single-level anterior cervical decompression and fusion : disc space distraction and its impact on clinical outcomes. J Clin Neurosci. 2015;22:566–569. doi: 10.1016/j.jocn.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Lundine KM, Davis G, Rogers M, Staples M, Quan G. Prevalence of adjacent segment disc degeneration in patients undergoing anterior cervical discectomy and fusion based on pre-operative MRI findings. J Clin Neurosci. 2014;21:82–85. doi: 10.1016/j.jocn.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto M, Fujimura Y, Suzuki N, Nishi Y, Nakamura M, Yabe Y, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998;80:19–24. doi: 10.1302/0301-620x.80b1.7929. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M, Okada E, Ichihara D, Watanabe K, Chiba K, Toyama Y, et al. Age-related changes of thoracic and cervical intervertebral discs in asymptomatic subjects. Spine(Phila Pa 1976) 2010;35:1359–1364. doi: 10.1097/BRS.0b013e3181c17067. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto M, Okada E, Ichihara D, Watanabe K, Chiba K, Toyama Y, et al. Anterior cervical decompression and fusion accelerates adjacent segment degeneration : comparison with asymptomatic volunteers in a ten-year magnetic resonance imaging follow-up study. Spine(Phila Pa 1976) 2010;35:36–43. doi: 10.1097/BRS.0b013e3181b8a80d. [DOI] [PubMed] [Google Scholar]
- 25.Matsunaga S, Kabayama S, Yamamoto T, Yone K, Sakou T, Nakanishi K. Strain on intervertebral discs after anterior cervical decompression and fusion. Spine(Phila Pa 1976) 1999;24:670–675. doi: 10.1097/00007632-199904010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Okada E, Matsumoto M, Ichihara D, Chiba K, Toyama Y, Fujiwara H, et al. Does the sagittal alignment of the cervical spine have an impact on disk degeneration? Minimum 10-year follow-up of asymptomatic volunteers. Eur Spine J. 2009;18:1644–1651. doi: 10.1007/s00586-009-1095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JB, Cho YS, Riew KD. Development of adjacent-level ossification in patients with an anterior cervical plate. J Bone Joint Surg Am. 2005;87:558–563. doi: 10.2106/JBJS.C.01555. [DOI] [PubMed] [Google Scholar]
- 28.Park JB, Watthanaaphisit T, Riew KD. Timing of development of adjacent-level ossification after anterior cervical arthrodesis with plates. Spine J. 2007;7:633–636. doi: 10.1016/j.spinee.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, Hyun SJ, Lee CH, Kim KJ, Yeom JS, Jahng TA, et al. Efficacy of a short plate with an oblique screw trajectory for anterior cervical plating : a comparative study with a two-year minimum follow-up. Clin Spine Surg. 2016 doi: 10.1097/BSD.0000000000000111. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Park JY, Kim KH, Kuh SU, Chin DK, Kim KS, Cho YE. What are the associative factors of adjacent segment degeneration after anterior cervical spine surgery? Comparative study between anterior cervical fusion and arthroplasty with 5-year follow-up MRI and CT. Eur Spine J. 2013;22:1078–1089. doi: 10.1007/s00586-012-2613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao RD, Wang M, McGrady LM, Perlewitz TJ, David KS. Does anterior plating of the cervical spine predispose to adjacent segment changes? Spine(Phila Pa 1976) 2005;30:2788–2792. discussion 2793. doi: 10.1097/01.brs.0000190453.46472.08. [DOI] [PubMed] [Google Scholar]
- 32.Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty : a prospective 2-year study. J Neurosurg Spine. 2005;3:417–423. doi: 10.3171/spi.2005.3.6.0417. [DOI] [PubMed] [Google Scholar]
- 33.Seo M, Choi D. Adjacent segment disease after fusion for cervical spondylosis; myth or reality? Br J Neurosurg. 2008;22:195–199. doi: 10.1080/02688690701790605. [DOI] [PubMed] [Google Scholar]
- 34.Song JS, Choi BW, Song KJ. Risk factors for the development of adjacent segment disease following anterior cervical arthrodesis for degenerative cervical disease : comparison between fusion methods. J Clin Neurosci. 2014;21:794–798. doi: 10.1016/j.jocn.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 35.Song KJ, Choi BW, Kim JK. Adjacent segment pathology following anterior decompression and fusion using cage and plate for the treatment of degenerative cervical spinal diseases. Asian Spine J. 2014;8:720–728. doi: 10.4184/asj.2014.8.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song KJ, Taghavi CE, Lee KB, Song JH, Eun JP. The efficacy of plate construct augmentation versus cage alone in anterior cervical fusion. Spine(Phila Pa 1976) 2009;34:2886–2892. doi: 10.1097/BRS.0b013e3181b64f2c. [DOI] [PubMed] [Google Scholar]
- 37.Sugawara T, Itoh Y, Hirano Y, Higashiyama N, Mizoi K. Long term outcome and adjacent disc degeneration after anterior cervical discectomy and fusion with titanium cylindrical cages. Acta Neurochir(Wien) 2009;151:303–309. discussion 309. doi: 10.1007/s00701-009-0217-5. [DOI] [PubMed] [Google Scholar]
- 38.Yang JY, Song HS, Lee M, Bohlman HH, Riew KD. Adjacent level ossification development after anterior cervical fusion without plate fixation. Spine(Phila Pa 1976) 2009;34:30–33. doi: 10.1097/BRS.0b013e318190d833. [DOI] [PubMed] [Google Scholar]