Abstract
This study determined the normal ECG patterns and values for Bama miniature pigs. Standard limb-lead ECG were recorded from 120 clinically healthy, unanesthetized piglets (age, 2 to 4 mo). The values for the ECG parameters (mean ± 1 SD) were: heart rate, 125.56 ± 18.80 bpm; P amplitude, 0.11 ± 0.03 mV; QRS amplitude, 0.63 ± 0.31 mV; P duration, 43.99 ± 5.98 ms; QRS complex, 55.27 ± 7.02 ms; RR interval, 487.55 ± 77.32 ms; PR interval, 90.72 ± 11.94 ms; QT interval, 244.72 ± 25.27 ms; and mean electrical axis, 22.2 ± 80.3°. The P waves were predominantly positive in leads I and II and in the augmented unipolar limb aVF lead; by comparison, the QRS patterns were less uniform. The T waves were slightly positive in leads II, III, and aVF. The determination and publication of the normal ECG patterns and values of Bama minipigs facilitates understanding of the electrocardiographic changes that arise under experimental conditions.
Minipigs play an essential role in biomedical and pharmaceutical research.8 General toxicology studies can be performed in minipigs by using the oral, cutaneous, parenteral, and inhalant routes of administration.1 These animals are advantageous for safety pharmacology studies, particularly for studies involving the cardiovascular system, because their morphology and functional, hemodynamic, and metabolic values are similar to those of humans.8,9,11
Although more than 20 minipig breeds exist, only a few have been bred for use as standard laboratory animals.7 Among laboratory minipigs, Göttingen minipigs have been widely used in regulatory toxicity studies in Europe, North America, and Japan.1,6,7 However, in China, Bama minipigs are used more frequently than are Göttingen minipigs. The Bama minipigs are native to the Guangxi province of China, has been bred as a laboratory animal, and is widely used in Chinese medical and pharmaceutical research.10
During pharmaceutical research, particularly during safety pharmacology studies, serial ECG recordings are necessary components of the cardiovascular system monitoring process.5,16 In addition, ECG recordings are well-established tools for evaluating animals with cardiac diseases.15 The reported ECG values and patterns of pigs vary somewhat,3,4 possibly due to differences in breed, age, and body weight of the experimental animals. To our knowledge, standard ECG values have not been published for Bama minipigs, making the evaluation of study-derived ECG changes difficult. Therefore, the current study describes the normal ECG patterns and values of Bama minipigs under conventional, controlled conditions.
Materials and Methods
This study involved 120 Bama minipigs (58 male, 62 female; weight, 7.9 to 14.4 kg [mean ± 1 SD, 11.5 ± 2.3 kg]; age, 2 to 4 mo) procured from the Department of Laboratory Animal Science, College of Basic Medical Sciences, Third Military Medical University, Chongqing, China (Experimental Animal Manufacture license no., SCXK [Chongqing] 2013-0001; Experimental Animal Use license no., SYXK [Chongqing] 2013-0001). The minipigs were housed in pens and fed a commercial diet (Hope, Chongqing, China), with unlimited access to fresh water. The air-conditioned animal rooms were maintained at 18 to 26 °C, with a relative humidity of 50% to 60%. The experiments were approved and supervised by the Ethics Committee of the Third Military Medical University.
ECG were recorded by using a 6-channel ECG processor ( model EP-2B, Softron, Beijing, China); neither sedation or anesthesia was used during recording. Before ECG recording, physical examination, routine pathology screening, and clinical observations were performed to confirm the health of the animals. Each pig was suspended, in a fixed hammock, in a standing position. The lower thirds of the front and back legs, where the electrodes were attached, were shaved, and the skin was cleaned with alcohol. Alligator-clip electrodes were attached to the skin by using disposable electrode patches. After optimal immobilization and sufficient contact were obtained and while the pig remained calm, standard bipolar (I, II, and III) and augmented unipolar (aVR, aVL, and aVF) limb-lead recordings began simultaneously. According to the heart rate and wave amplitude, the electrocardiographic recorders were calibrated to 50 to 100 mm/s and 10 to 20 mm/mV. All procedures were performed in an isolated room to minimize the stress to the animals.
ECG recordings were analyzed by using a computerized waveform-analysis program (SP 2006, Softron, Beijing, China). In each tracing, 9 beats were selected for high quality; the wave values and the P–QRS–T deflection intervals were determined by using a magnifying glass. Lead II waveforms were analyzed to determine the amplitudes and durations of the waves and intervals. The rate-corrected QT interval was calculated by using the Bazzett formula:
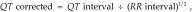 |
where QT interval is expressed in milliseconds and the RR interval in seconds. The QRS complex amplitude was calculated by using the following formula:
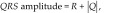 |
where R is the R-wave amplitude, and Q is the Q-wave amplitude. The mean electrical axis of the ventricular depolarization in the frontal plane was calculated by using the vector method and lead II.15 The morphologic patterns of the P–QRS–T deflections were evaluated for each lead.
Statistical analyses were performed by using SPSS 14.0 for Windows (SPSS, Chicago, IL). All measurements are expressed as mean ± 1 SD, and a P value less than 0.05 was considered statistically significant.
Results
Examples of the standard, augmented limb lead tracings, taken sequentially and simultaneously, are shown in Figure 1. All of the minipigs presented normal sinus rhythms; sinus arrhythmia was evident in 5.8% of the tracings (that is, 7 of the 120 minipigs evaluated). The mean durations and ranges of the P, PR, QRS, and QT intervals, calculated from lead II, are presented in Table 1. None of these measures differed between sexes.
Figure 1.
The ECG of an apparently healthy and unanesthetized Bama miniature pig (Sus scrofa) in the standard bipolar and augmented unipolar limbs.
Table 1.
Lead II ECG values in Bama miniature pigs (n= 120)
| Mean ± 1 SD | Range | |
| Heart rate (bpm) | 125.56 ± 18.80 | 82 to 172 |
| P amplitude (mV) | 0.11 ± 0.03 | 0.04 to 0.18 |
| R (mV) | 0.63 ± 0.31 | 0.11 to 1.51 |
| P duration (ms) | 43.99 ± 5.98 | 22 to 58 |
| QRS duration (ms) | 55.27 ± 7.02 | 34 to 76 |
| RR interval (ms) | 487.55 ± 77.32 | 323 to 788 |
| PR interval (ms) | 90.72 ± 11.94 | 34 to 117 |
| QT interval (ms) | 244.72 ± 25.27 | 126 to 319 |
| QT corrected (ms) | 349.90 ± 38.77 | 176 to 447 |
| QRS amplitude (mV) | 75.48 ± 34.00 | 18 to 173 |
| Mean electrical axis (°) | 22.2 ± 80.3 | −160 to 170 |
The morphologic patterns of the P and T deflections are presented in Table 2. The P wave was completely positive in leads I, II, and aVF but negative in the aVR lead. In leads III and aVL, most of the P waves were positive. Compared with the P wave, the T-wave deflection in leads I, II, III, and aVR was more irregular, with positives, negatives, and diphasics observed. Lead I had almost equal numbers of negative and positive T waves, with only a few diphasic (positive–negative) waves evident. In leads II, III, and aVF, positive waves predominated; in the aVR and aVL leads, negative waves were predominant. In all leads, the T wave was discordant with the major QRS deflection.
Table 2.
Polarity of P and T waves in Bama miniature pigs (%)
| P wave |
T wave |
||||
| Lead | Positive | Negative | Positive | Negative | Biphasic |
| I | 100.0 | 58.3 | 40.8 | 0.8 | |
| II | 100.0 | 92.5 | 5.0 | 2.5 | |
| III | 69.2 | 30.8 | 85.8 | 11.7 | 2.5 |
| aVR | 100.0 | 16.7 | 81.7 | 1.7 | |
| aVL | 73.3 | 26.7 | 23.3 | 76.7 | |
| aVF | 100.0 | 90.8 | 9.2 | ||
Table 3 reflects the frequency of each type of QRS complex pattern recorded. All leads showed a wide range of QRS morphologies, with decreased pattern uniformity. The most frequent morphology in leads I, II, and III was qr, qRs, and RS, respectively. In the aVR lead, the most frequent morphologies were qr and qR; rs and rS were most frequent in the aVL lead. There was no predominant pattern in the aVF lead.
Table 3.
Electrocardiographic patterns of QRS complexes in Bama miniature pigs (%)
| Lead | q | qr | qR | Qr | QR | qrs | qrS | qRs | qRS | Qrs | QRs | r | R | rs | rS | Rs | RS |
| I | 47.5 | 22.5 | 5.0 | 2.5 | 1.7 | 6.7 | 1.7 | 0.8 | 7.5 | 1.7 | 1.7 | 0.8 | |||||
| II | 6.7 | 5.8 | 0.8 | 0.8 | 12.5 | 6.7 | 32.5 | 7.5 | 1.7 | 0.8 | 9.2 | 4.2 | 5.0 | 5.8 | |||
| III | 1.7 | 0.8 | 2.5 | 0.8 | 0.8 | 5.0 | 14.2 | 0.8 | 2.5 | 13.3 | 10.0 | 11.7 | 35.8 | ||||
| aVR | 0.8 | 23.3 | 28.3 | 13.3 | 14.2 | 4.2 | 1.7 | 0.8 | 1.7 | 5.0 | 2.5 | 1.7 | 2.5 | ||||
| aVL | 3.3 | 3.3 | 0.8 | 5.0 | 0.8 | 0.8 | 1.7 | 55.8 | 28.3 | ||||||||
| aVF | 1.7 | 3.3 | 0.8 | 0.8 | 10.0 | 2.5 | 20.8 | 10.0 | 16.7 | 5.8 | 10.0 | 17.5 |
Discussion
Only a few English language publications have described the normal ECG of minipig breeds. One study of conventional and miniature pigs obtained varied ECG values and patterns, possibly related to differences in the breeds, ages, and body weight of the experimental animals.3 Such breed-associated variations support the need for obtaining normal ECG patterns for each breed of minipigs to have meaningful reference values.
Routinely, limb lead II is used for the clinical evaluation of ECG parameters. A study similar to the present one except for using adult Göttingen minipigs (mean weight, 12.4 kg) reported a similar mean PR interval (88 ms), a lower mean heart rate (111 bpm) and QRS complex duration (36 ms), and higher means for the QRS total amplitudes (0.9 mV) and QT intervals (252 ms).4
In the present Bama minipig study, the P waves were mainly positive in leads I, II, III, aVL, and aVF but negative in the aVR lead. These findings are generally consistent with other results from conventional and miniature pigs.3,4 T waves show great variability in their shape and polarity from subject to subject. In addition, the positions of the thoracic limbs are important factors in determining the T-wave configuration,2 and another study that involved placing the electrodes on the thorax demonstrated that the thoracic limb effect can be reduced but not eliminated.3 In comparison, ECG recordings in Göttingen minipigs that involved use of the triangular leads led the authors to conclude that the electrode position has no effect on the amplitude of the T wave, due to the lability of the T wave and pronounced interindividual variations.13 Another author suggested that the transmural and apicobasal heterogeneities of final repolarization of the action potential within ventricular myocardium are responsible for inscription of the T wave.17 Our current study similarly demonstrated that the T waves of Bama minipigs are discordant with the QRS complex duration in all limb leads, a finding that is characteristic of ruminants and chickens also.12,14
In the present study, considerable variation in voltages, especially for the QRS complexes, was present between minipigs, as was reported previously.3 Such variability might reflect factors such as differences in the topographic anatomy of the heart within the thorax, the heart position relative to the limbs, and the mechanism of ventricle activation.12
From the current study, we have established the standard values and patterns for ECG parameters in normal, healthy Bama minipigs. These results will facilitate a better understanding of the ECG changes associated with unhealthy or experimentally treated Bama animals during toxicology and safety pharmacology studies.
Acknowledgment
This study was funded by the Major Scientific Problem-Oriented Project Topics (2011CBA01006) of the National Key Basic Research and Development Program (973 program).
References
- 1.Bode G, Clausing P, Gervais F, Loegsted J, Luft J, Nogues V, Sims JSteering Group of the RETHINK Project. 2010. The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods 62:196–220. [DOI] [PubMed] [Google Scholar]
- 2.Dubois M. 1961. On the electrocardiograms of some quadrupeds. C R Seances Soc Biol Fil 155:599–602. [Article in French]. [PubMed] [Google Scholar]
- 3.Dukes TW, Szabuniewicz M. 1969. The electrocardiogram of conventional and miniature swine (Sus scrofa). Can J Comp Med 33:118–127. [PMC free article] [PubMed] [Google Scholar]
- 4.Eckenfels A, Schuler S. 1988. The normal electrocardiogram of miniature swine. Arzneimittelforschung 38:253–259.[Article in German]. [PubMed] [Google Scholar]
- 5.Eckenfels A, Trieb G. 1979. The normal electrocardiogram of the conscious beagle dog. Toxicol Appl Pharmacol 47:567–584. [DOI] [PubMed] [Google Scholar]
- 6.Forster R, Ancian P, Fredholm M, Simianer H, Whitelaw B, Project R. 2010. The minipig as a platform for new technologies in toxicology. J Pharmacol Toxicol Methods 62:227–235. [DOI] [PubMed] [Google Scholar]
- 7.Ganderup NC, Harvey W, Mortensen JT, Harrouk W. 2012. The minipig as nonrodent species in toxicology—where are we now? Int J Toxicol 31:507–528. [DOI] [PubMed] [Google Scholar]
- 8.Jordan HL, Register TC, Tripathi NK, Bolliger AP, Everds N, Zelmanovic D, Poitout F, Bounous DI, Wescott D, Ramaiah SK. 2014. Nontraditional applications in clinical pathology. Toxicol Pathol 42:1058–1068. [DOI] [PubMed] [Google Scholar]
- 9.Liedtke AJ, Hughes HC, Neely JR. 1975. An experimental model for studying myocardial ischemia. Correlation of hemodynamic performance and metabolism in the working swine heart. J Thorac Cardiovasc Surg 69:203–211. [PubMed] [Google Scholar]
- 10.Liu Y, Zeng B-H, Shang H-t, Cen Y-Y, Wei H. 2008. Bama miniature pigs (Sus scrofa domestica) as a model for drug evaluation for humans: comparison of in vitro metabolism and in vivo pharmacokinetics of lovastatin. Comp Med 58:580–587. [PMC free article] [PubMed] [Google Scholar]
- 11.McKenzie JE, Scandling DM, Ahle NW, Bryant HJ, Kyle RR, Abbrecht PH. 1996. Effects of soman (pinacolyl methylphosphonofluoridate) on coronary blood flow and cardiac function in swine. Fundam Appl Toxicol 29:140–146. [DOI] [PubMed] [Google Scholar]
- 12.Mohan NH, Niyogi D, Singh HN. 2005. Analysis of normal electrocardiograms of Jamunapari goats. J Vet Sci 6:295–298. [PubMed] [Google Scholar]
- 13.Nahas K, Baneux P, Detweiler D. 2002. Electrocardiographic monitoring in the Goettingen minipig. Comp Med 52:258–264. [PubMed] [Google Scholar]
- 14.Sturkie PD. 1949. The electrocardiogram of the chicken. Am J Vet Res 10:168–175. [PubMed] [Google Scholar]
- 15.Tilley LP. 1981. Basic canine and feline electrocardiography. Can Vet J 22:23–24. [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. 1975. Guidelines for evaluation of drugs for use in man. Report of a WHO scientific group. World Health Organ Tech Rep Ser 563:1–59. [PubMed] [Google Scholar]
- 17.Yan GX, Antzelevitch C. 1998. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation 98:1928–1936. [DOI] [PubMed] [Google Scholar]



