Abstract
Because surface disinfectants are an important means of pathogen control within laboratory animal facilities, these products must have an appropriate spectrum of antimicrobial activity. However, many other factors must also be considered, including effects on human health, environmental safety, and animal behavior. Aqueous solutions of sodium hypochlorite often are considered to be the ‘gold standard’ for surface disinfection, but these products can be corrosive, caustic, and aversive in odor. This study was designed to identify disinfectants that are as effective as hypochlorite solutions but more acceptable for use in a laboratory animal setting. An antiviral disinfectant-efficacy assay was developed by using viral vectors that expressed green fluorescence protein as surrogates for wild-type viruses of concern in laboratory animals. Efficacy testing revealed that most of the products were highly effective when used against viral vectors in suspension. However, when the disinfectants were challenged by buffering virus in protein or drying virus on nonporous surfaces, the hypochlorite and peroxymonosulfate products performed the best. Review of safety data sheets for the agents indicated that a peroxide-based product was considerably safer than the other products tested and that the pH of most products was not conducive to disposal down a drain. Behavioral testing of Swiss Webster, C57Bl/6, and BALB/c mice showed that the hypochlorite- and peroxide-based products were clearly aversive, given that the mice consistently avoided these products. All of these factors must be considered when choosing the appropriate disinfectant.
Abbreviations: CCM, complete culture medium; EPA, Environmental Protection Agency; HEK, human embryonic kidney; HMIS, Hazardous Material Information System; SDS, Safety Data Sheet
The selection of appropriate disinfectants for use in animal facilities requires consideration of multiple factors, including spectrum of activity, human safety, and effects on the health and behavior of animals exposed to these agents. Multiple engineering standards are rigidly enforced to limit the spread of pathogens within animal facilities.100,43 Chemical disinfectants are often the first line of defense against these pathogens; however, guidelines for their selection and usage often are defined less strictly. Because disinfectant use is critical in preventing the spread of adventitious disease within animal colonies and is an essential component of laboratory animal facility management,87 a fact-based approach is imperative when selecting an appropriate product.
Disinfection is the process of eliminating many or all pathogenic microorganisms, other than bacterial spores, on inanimate objects.83 A hierarchy originally designed by Earle H Spaulding defined disinfectants as either high-, intermediate-, or low-level, based on their ability to kill various microorganisms.92,93 According to the current usage of this hierarchy, high-level disinfectants are those capable of killing most pathogens, including all types of viruses, vegetative bacteria, mycobacteria, and bacterial spores, with the only exception being large numbers of spores.66,83 Intermediate-level disinfectants are usually able to inactivate most viruses, vegetative bacteria, mycobacteria, and fungi but are unlikely to eliminate most bacterial spores. Low-level disinfectants are capable of eliminating vegetative bacteria and most enveloped viruses but are ineffective against some nonenveloped viruses, mycobacteria, some fungi and most bacterial spores. Since the establishment of the Spaulding hierarchy, there have been multiple schemes designed to classify microorganisms by their susceptibility to particular chemicals, including work by Klein and Deforest, who specified 3 levels of viral sensitivity to disinfectants, based the presence or absence of viral envelopes, in addition to their solubility.51 According to the Klein–Deforest scheme, hydrophilic nonenveloped viruses, such as parvoviruses, are the least sensitive to disinfectants, whereas partially lipophilic nonenveloped viruses of intermediate solubility, such as adenoviruses and rotaviruses, are slightly more sensitive, and lipophilic, or enveloped viruses, such as retroviruses, herpesviruses, paramyxoviruses, and coronaviruses, are the most sensitive.51,80,87 Prince and colleagues further elaborated on this scale, categorizing the susceptibility of multiple human and animal pathogens.80 Based on extrapolations from previous work, hierarchies have been developed specifically to classify pathogens affecting laboratory animals, including bacterial spores and parasites, which are even less sensitive to disinfection than are hydrophilic nonenveloped viruses.87 These schemes are accepted guidelines for disinfectant differentiation and denote that chemicals capable of killing pathogens at a higher point in the spectrum are also capable of killing all of the more sensitive organisms. These general hierarchies may not provide fine distinctions between similar organisms,60 but to this day, the Spaulding hierarchy is still considered to be as applicable as when it was first established and is considered as new products are developed and tested.66
Chemical disinfectants can be further categorized into 3 classes according to their method of action—denaturants, reactants, and oxidants.80,87 Denaturants, such as quaternary ammonium compounds, phenolics, and alcohols, act by disrupting protein and lipid structures, making these products particularly effective against lipophilic enveloped viruses.4,54,83 These chemicals are widely available, cost-effective, and are generally considered to be bactericidal, fungicidal, and variably tuberculocidal. They are not usually sporocidal or virucidal against nonenveloped viruses and therefore cannot be used as high-level disinfectants. Disinfectants that are reactants, including aldehydes (formaldehyde and glutaraldehyde) and ethylene oxide, form and break covalent bonds, altering DNA, RNA, and protein structure and synthesis.4,54,83 These products are most commonly used as high-level disinfectants but are not typically applied as routine surface disinfectants because they are expensive and are considered relatively toxic, acting as both irritants and carcinogens. Oxidant disinfectants are the largest group and include halogens—hypochlorites, chlorine dioxide, and iodine—peroxides, and peroxymonosulfates. These disinfectants oxidize proteins, enzymes, and amino acids, making their spectrum of activity relatively broad.4,54,83 Oxidants are inexpensive and fast-acting and are often considered to be mycobacteriocidal, sporocidal, and fungicidal; they also have the ability to kill nonenveloped viruses. However, some oxidant disinfectants present considerable health hazards and can be corrosive to equipment.13,23,84 Given the obvious advantages and disadvantages of each disinfectant class, many variables must be considered as these disinfectants are assessed.
When selecting a disinfectant for use in a laboratory animal facility, its spectrum of activity is arguably the most important factor to consider. A disinfectant must be able to eliminate the most resistant pathogens under conditions that vary among facilities, depending on the biosafety level, barrier agent exclusions, and immunocompetency of the animals being housed.52 Animal pathogens of concern often include retroviruses, herpesviruses, adenoviruses, parvoviruses, coronaviruses, and coxsackieviruses, and bacterial organisms such as Mycobacterium spp., Staphylococcus spp., Clostridium spp., and Corynebacterium bovis among many others.15,16,37,87 Infectious diseases, whether experimentally induced or naturally occurring, can have profound effects on research animals,37 potentially leading to invalidation of research, extensive depopulation within vivaria, and significant economic loss.15,16,44 In addition, containment facilities house specific pathogenic agents that pose considerable risk to personnel and public health if proper controls are not in place.52,71,100 The Biosafety in Microbial and Biomedical Laboratories (BMBL) manual contains an overview of disinfection concepts and decontamination strategies, in addition to defining specific microbiologic practices, including the need for routine decontamination of work surfaces with an appropriate disinfectant.100 However, this manual does not specify the type or class of disinfectant that is to be used, limiting guidance to the statement that intermediate- and low-level disinfectants can be sufficient for most environmental surfaces. The ultimate decision is left to the facility, after an appropriate risk assessment.33,52
In our facility, as well as others, Environmental Health and Safety departments are taking a more prevalent role in overseeing the risk assessment and use of disinfectants, and in making their assessments, spectrum of activity is considered to be of great importance. If a disinfectant is intended for use in a facility that is biosafety level II or higher—and therefore contains pathogens capable of causing disease in healthy humans100—it must meet certain requirements for broad-spectrum activity. The first requirement for a disinfectant with public-health claims is inclusion in the US Environmental Protection Agency (EPA) list of Hospital Sterilants, Disinfectants, and Tuberculocides—a compilation of disinfectants that have been tested and shown through the Antimicrobial Testing Program to be effective against Pseudomonas aeruginosa and Staphylococcus aureus at minimum, as well as Mycobacterium bovis BCG when tuberculocidal claims are made.30 The Antimicrobial Testing Program follows performance standards developed by AOAC International (formerly, the Association of Official Analytical Chemists); these standards primarily focus on the specific bacterial organisms just listed but not on viruses or other pathogens that may be less sensitive to disinfection.29 In addition, when human bloodborne pathogens may be present, as is the case in situations involving human cells or humanized animals, a disinfectant must be proven effective against HIV and hepatitis B virus at least, and potentially against Mycobacterium tuberculosis as well, when tuberculocidal action is indicated.76 Still, agents known to require a higher level of disinfection must be treated with either diluted bleach or other disinfectants of appropriate spectrum.61,83,102 For some products, efficacy is also tested by the former American Society for Testing and Materials, ASTM International, an additional third-party organization that has developed standards for disinfectant-efficacy testing.6,7,75 Although these standards are well defined and accepted by many disinfectant companies, they are not requirements, given that enrollment in the organization is voluntary. With all of the potential testing agencies and methods considered, it is difficult to determine the degree of testing that is sufficient for a specific facility, and in some cases, even these listed standards may not be sufficiently stringent to allow use against less-sensitive pathogens. Lastly, contact times for products listed as appropriate for specific pathogens may be as long as 10 min,61 which does not reflect the short spray-and-wipe method of disinfection that is practiced in most animal facilities.83 It therefore becomes increasingly important to consider possibilities for quality control when assessing disinfectant efficacy.
Multiple methods have been developed to assess the efficacy of disinfectants.9,11,95,99,101 Virucidal activity has been a primary focus of researchers working not only in the veterinary field, but also in human medicine and in food and water safety.34,57,88 Although wild-type viruses are often used as the subjects of efficacy testing,1,26,57 these agents themselves may represent potential hazards to people and animals, and some are not easily cultured in a laboratory setting.96 Therefore, it is often ideal to use less pathogenic or more easily propagated viruses as surrogates24,31,45,97—for example, feline calicivirus is used as a surrogate for norovirus, another calicivirus that cannot be cultivated in vitro.46,69,104 One question to consider is whether modified, nonreplicating agents, such as viral vectors used in gene therapy, can serve as surrogates for agents that are similarly sensitive to disinfection. The benefits of this approach include a decreased risk to animals and personnel in the testing facility, the availability of high-titer viral stocks, the extensive use of these vectors in some facilities, and the possibility of using fluorescence markers for detection and quantification of surviving virus. The use of fluorescence expressing viral vectors allows easy and rapid assessment of viral survival, because microscopy or flow cytometry can be used to detect viral transduction and expression soon after exposure. The value of viral vectors as surrogates for wild-type viruses is further supported by the use of adenovirus types 5 and 6 to predict the inactivation of similarly structured adenovirus-based vectors.64
Virucidal and bactericidal test methods have included suspension tests and carrier tests. Suspension testing involves exposing virus in suspension to a disinfectant and monitoring for survival, and is often the primary means of efficacy testing.9,79,95,101,102 Carrier tests are used to mimic practical applications, such as virus dried on a hard surface, instruments, or hands.1,2,9,11,26 The value of carrier tests is that they represent typical challenges faced by disinfectants during normal use and therefore may be more informative than is suspension testing.95 Additional variables that can influence disinfectant efficacy include the amount of organic material present in the environment, the amount and degree of aggregation of pathogen, the complexity of the surface being disinfected, the amount of agitation once the disinfectant is applied, the age of the disinfectant, and the method of application.33,58,60,66
In addition to their focus on spectrum of activity, Environmental Health and Safety departments consider a multitude of regulations and recommendations from several different agencies and organizations. To be considered for use in a facility, a disinfectant must meet several specifications. The disinfectant must be registered as a pesticide on both a national and state level.32 According to both the EPA, which enforces the Federal Insecticide, Fungicide, and Rodenticide Act, and the California Department of Pesticide Regulation, a pesticide is any substance that is used to control, destroy, or mitigate any pest, including microorganisms such as viruses or bacteria.32 It is therefore necessary that a disinfectant meets the requirements for registration with the EPA, in addition to the state requirements, which may be more stringent, as is the case in California.32 Lastly, disinfectants that are disposed of down a drain (for example, as mop water or expired product) and through the sewer system to publically owned treatment works must meet appropriate disposal requirements. The product must have a pH between 2 and 12.5, or it is considered to be corrosive hazardous waste, according to the Resource Conservation and Recovery Act.28 Once that requirement is met, disinfectants often must meet additional, stricter regulations for disposal through publically owned treatment works that are enforced on a state or local level. For example, in the city of Los Angeles, a product that is poured down a drain must have a pH between 5.5 and 11, and dilution in water is not considered a valid means of achieving this goal.25
In addition, the importance of human occupational safety cannot be overstated, because this factor may be used as the basis for rejecting products with an ideal spectrum of activity. The hazards considered in the Safety Data Sheets (SDS) are reflected in guidelines set forth by the National Research Council,72 and the observation of appropriate precautions when using disinfectants in the laboratory is regulated on a federal level by OSHA.77 Having a knowledge of the potential health risks of disinfectants is vital, because the incorrect usage of products may lead to occupational illness.14 Adverse effects experienced by those exposed to a disinfectant could include skin sensitivity, ocular or nasal mucous membrane irritation, and reaction to strong odors.5,8 Thorough review of the SDS is necessary to understand the risks associated with using each product and the precautions that must be taken to prevent human exposure, including personal protective equipment and fume hoods for the reconstitution of various chemicals. The health hazards associated with these products can be compounded by inappropriate usage or toxic combinations of incompatible products.81 As noted previously, pH is a major consideration, not only for human safety but also in the context of environmental safety and disposal requirements. Corrosiveness is important because it relates to both human safety and damage to laboratory equipment.39,74
One final consideration that is often overlooked but that definitely should not be discounted is the effect of these disinfectants on the animals living in a facility. The potential health risks to humans pose similar threats to animals, including risk of contact dermatitis50,86 and irritation of mucous membranes.3 In addition, disinfectants have the potential to produce aversive odors that may have negative effects on animal behavior and wellbeing.35 Disinfectants may have a variety of uses within a facility, and those intended for treating only floors, walls, and lab benches may not be appropriate for situations where the potential for animal exposure exists—for example, when they are applied to forceps used transferring mice, hoods used for changing cages, or behavioral testing equipment. Whether a disinfectant is intended for use only on surfaces that come into intimate contact with animals or as an all-purpose product in a facility, which is often the goal, it is important that it be unlikely to cause irritation or aversion in animals when used at the recommended concentration.
The most widely used and highly regarded surface disinfectant is household bleach, an aqueous solution of 5.25% to 6.15% sodium hypochlorite, typically used after a 10:1 dilution in water.83,84,102 Bleach is therefore the standard recommendation made by most institutional Environmental Health and Safety departments.84 Household bleach has a broad spectrum of antimicrobial activity, and is relatively inexpensive, fast-acting, resistant to water hardness, and capable of penetrating biofilms and dried organisms.67,83,84 However, bleach is offensive in odor and relatively caustic, being capable of inducing ocular and respiratory irritation, electrolyte imbalances if ingested, and cutaneous, oropharyngeal, esophageal, and gastric burns.23,36,42,56,82,103 In addition, bleach releases toxic gas when mixed with ammonia-based products.70,81 Other disadvantages are that bleach is inactivated by organic matter, can discolor fabrics, and is corrosive to metals, even when compared with other chlorine-based products, such as chlorine dioxide.13,17,84
The current study was designed to determine suitable alternatives to bleach as a universal disinfectant and to discern whether any products might truly be used for all purposes in a given facility. The hypothesis was that alternative disinfectants are as effective and well-accepted as chlorine bleach for use against viral agents of concern within animal facilities. The expectation was that a disinfectant that could be used for all purposes in a given facility would be identified, with the understanding that this product would still be unlikely to be superior in all parameters being considered. We selected 4 disinfectants—3 oxidants and one denaturant—for comparison on the basis of several criteria, including mechanism of action, reported broad-spectrum activity, accessibility, and relevance to the testing facility.
Three specific aims were assessed. First, the spectrum of activity of the 4 disinfectants was compared with that of bleach. Comparative efficacy against GFP-expressing viral vectors was assessed under various conditions, including in suspension, buffered in fetal bovine serum, and dried on a nonporous surface. Second, human and environmental exposure risks associated with each of the disinfectants were assessed through interpretation of the SDS information. Lastly, the behavioral effect of disinfectants on mice was assessed by using an innate aversion test developed to monitor avoidance of potentially aversive odors.
Materials and Methods
Animals.
A total of 56 Swiss Webster Crl:CFW (SW), 42 C57Bl/6NCrl BR, and 40 BALB/cAnNCrl mice were purchased from Charles River Laboratory (Wilmington, MA). Mice were allowed at least 7 d to acclimate before the start of the study. All mice were housed in a SPF, AAALAC-accredited facility, where sentinel mice are tested quarterly and remain negative for mouse parvovirus, minute virus of mice, mouse norovirus, mouse hepatitis virus, Sendai virus, lymphocytic choriomeningitis virus, polyomavirus, K virus, pneumonia virus of mice, mouse adenovirus, epizootic diarrhea of infant mice, mouse encephalomyelitis virus, reovirus, ectromelia virus, Mycoplasma pulmonis, and Helicobacter spp. as well as endo- and ectoparasites. Mice were socially housed in polycarbonate cages (Lab Products, Seaford, DE) on corncob bedding (Bed-O'Cobs, The Andersons, Maumee, OH) and were given enrichment nesting material (Cotton squares, Ancare, Bellmore, NY). Mice had unrestricted access to Laboratory Rodent Diet 5001 (PMI Nutrition International, Richmond, IN). The animal room was environmentally controlled, with a temperature of 68 to 79 °F (20.0 to 26.1 °C), relative humidity between 30% and 70%, and 12:12-h light:dark cycle. The IACUC of the University of California–Los Angeles approved all animal use activity in this study. Due to the noninvasive nature of the current study, all animals were transferred to other protocols at the conclusion of behavioral testing.
Disinfectants and controls.
Five disinfectants were evaluated in this study. Disinfectant A was 10% bleach (The Clorox Company, Oakland, CA), an aqueous solution of 5.25% to 8% sodium hypochlorite diluted 1:10 in tap water. Disinfectant B was Accel TB (Virox Technologies, Oakville, Ontario, Canada), a ready-to-use formulation of 0.5% hydrogen peroxide combined with a surfactant accelerant. Disinfectant C was Vimoba (Quip Laboratories, Wilmington, DE), a chlorine-dioxide–based mixture diluted to 100 ppm in tap water. Disinfectant D was Virkon-S (DuPont, Wilmington DE), a 21.4% potassium peroxymonosulfate soluble concentrate diluted to 1% in tap water. Disinfectant E was A-456 II (Ecolab, St Paul, MN), a quaternary ammonium compound diluted automatically with tap water through a multiproduct dispenser (catalog no. 2011, QC Central Supply Dispenser Air Gap, Ecolab) set to produce a concentration between 886 and 3390 ppm. Disinfectants A through D were oxidants, whereas disinfectant E was a denaturant. Tap water was used to reconstitute and dilute the disinfectants and served as a negative control. Each disinfectant requiring reconstitution or dilution was made fresh on the day of exposure. The tap water, provided by the Los Angeles Department of Water and Power, had a hardness between 102 and 106 ppm CaCO3 and a pH between 7.5 and 7.6.65 All disinfectants used in this study are registered with the EPA, and all except for disinfectant D are currently registered for use in the State of California according to the California Department of Pesticide Regulation.
Viruses, cells, and culture media.
Three commonly used viral vectors expressing GFP were used for the antiviral testing. Those tested included the self-inactivating lentivirus RRL-CMV-GFP (4.3 × 108 Infectious Units (IU)/mL), adenovirus ad-CMV-GFP (2.1 × 1011 IU/mL), and adeno-associated viral vector AAV-2 IRES-GFP (2.7 × 107 IU/mL). The initial stock of adenovirus was diluted to a concentration of 4.2 × 109 IU/mL in PBS containing 2.5% glycerol, and aliquots of 200 to 300 µL were stored at –80 °C until use; the lentiviral and adeno-associated viral vectors were stored at –80 °C until use also.
Human embryonic kidney (HEK) 293T cells (ATCC, Vienna, VA) were propagated and maintained at 37 °C and 5% CO2 in a complete culture medium (CCM), which consisted of DMEM (Mediatech, Winchester, VA), containing 10% heat-inactivated FBS (Hyclone, Thermo Fischer Scientific, Waltham, MA), and 100 IU/mL penicillin–streptomycin (Cellgro, Mediatech). For experimentation, cells were plated as needed onto 12-well plates (Greiner–Bio One, Kremsmunster, Austria) at an initial count of 2 × 104 cells/well and incubated for approximately 24 h before use. In addition, CCM was used for dilution and drying of the virus and as an additional negative control for the experimental disinfectants.
In vitro efficacy testing.
Establishing a method for neutralizing disinfectants.
HEK-293T cells were plated at 2 × 104 cells per well on 12-well plates and incubated for approximately 24 h at 37 °C, as previously described. Cells were then exposed in triplicate to serial dilutions (1:10, 1:100, 1:1000, and 1:10,000 in CCM) of each test disinfectant or negative control. After 8 h of incubation, the diluted disinfectant mixture was aspirated from the plated cells and replaced with fresh CCM. The plates were then returned to incubation at 37 °C. At 24, 48, or 72 h after exposure, the cells were incubated in cell dissociation buffer (Gibco Invitrogen, Thermo Fisher Scientific, Waltham, MA) for 5 min at 37 °C and resuspended in PBS. To determine the percentage of live cells, the resuspended cells either were counted with a cell counter (TC 10, Bio-Rad, Hercules, CA) after the addition of trypan blue or analyzed by flow cytometry (FACS Canto II, Becton Dickinson, Franklin Lakes, NJ) after a 10-min incubation with 5 µL of propidium iodide.
Testing disinfectants against viruses in suspension.
HEK-293T cells were incubated for 24 h as previously described. We then placed 10-µL aliquots of viral vectors into 2-mL microfuge tubes for disinfectant exposure; 90 µL of a disinfectant at the recommended concentration was added to each tube, yielding a 90% concentration of disinfectant. The tubes were gently agitated by tapping 2 or 3 times and then left untouched for contact times of either 1, 5, or 10 min; 900 µL of CCM was then immediately added to each tube (a 1:10 dilution) and mixed. We added 40 µL of this diluted mixture to 3960 µL of CCM in a 50-mL conical centrifuge tube for an additional 1:100 dilution and mixed. This serial dilution achieved a 1:1000 dilution of the disinfectant overall, and negative controls were expected to yield viral concentrations of 4.2 × 105 IU/mL, 4.3 × 104 IU/mL, and 2.7 × 103IU/mL for adenoviral, lentiviral, and adeno-associated viral vectors, respectively.
Diluted virus–disinfectant mixture (1 mL) was added to the plated HEK-293T cells in triplicate, and the cells were incubated at 37 °C for 7 to 8 h to facilitate viral transduction. The mixture was then replaced with fresh CCM. All plates were incubated for another 60 to 70 h at 37 °C. The cells were visualized under fluorescence microscopy prior to resuspension in PBS as described previously. Flow cytometry was performed to determine the percentage of GFP-expressing cells (an indicator of viral survival and subsequent transduction) and assess cell survival by propidium iodide fluorescence. The data were analyzed by using FlowJo cytometric analytical software (Tree Star, Ashland, OR), and the GFP expression percentage among live cells was compared between the different experimental groups. A baseline of 0.5% GFP expression was established as a cutoff, below which the detected expression was attributed to machine error and considered to be zero.
Testing disinfectants against adenoviral vector in suspension after dilution in serum and water.
HEK-293T cells were incubated for 24 h as previously described. We placed 10-µL aliquots (4.2 × 107 IU) of adenovirus into 2-mL tubes for disinfectant exposure as done in previous experiments. Before disinfectant was added to virus, we added 15, 40, or 65 µL of heat-inactivated FBS (Hyclone, Thermo Fischer Scientific) to the 10-µL viral samples and vortexed, so that the addition of 75, 50, or 25 µL of disinfectant would respectively yield 75, 50, and 25% concentrations of the disinfectant. Immediately on addition of disinfectant, the tube was mildly agitated by gently tapping 3 times. After 1 min of exposure, the virus–disinfectant mixture was diluted 1000-fold in CCM, plated, incubated, and analyzed in a manner identical to that of the previous protocols, including all disinfectants and controls used previously, with the exception of disinfectant E, which was not tested in this or later trials. This protocol was repeated 3 times, by using a fresh sample of disinfectant and aliquot of virus each time. In addition, the protocol was completed once with water as the disinfectant diluent instead of serum.
Testing disinfectants against dried adenoviral vector on nonporous surfaces.
HEK-293T cells were incubated for 24 h as previously described. Concurrently, adenoviral vector was suspended at a ratio of 10 µL per 90 µL of CCM, and the resultant 100-µL aliquots of CCM containing 4.2 × 107 IU of adenovirus were placed in the centers of the wells of a 12-well plate.
The viral suspensions were allowed to dry onto the plates for approximately 24 h, after which they were exposed to recommended concentrations of disinfectants or controls for 1, 5, or 10 min. For each exposure, 300 µL of disinfectant (the quantity necessary to completely cover the 100 µL aliquot of dried virus in CCM) was added to each well for the required contact time and then immediately diluted by adding 700 µL CCM to the well, pipetting to mix, and removing 40 µL to be added to 3960 µL of CCM. The final result was an approximately 1:300 dilution of disinfectant, with an expected viral yield of 4.2 × 105 IU/mL for the negative controls. The samples were then analyzed in a manner identical to that in previous protocols.
SDS evaluation.
To obtain Hazardous Material Information System (HMIS) ratings for health, flammability, and reactivity–physical hazard and pH values, SDS were obtained from the manufacturer of each product.
In vivo behavioral testing.
A clear plastic, acrylic 3-chamber apparatus (Figure 1), custom built by the UCLA Psychology Technical Services, was used. The apparatus, measuring 25 cm high by 60 cm long by 40 cm wide, consisted of 3 identical chambers, one central and 2 side, separated by 2 walls. The central chamber could be isolated from the 2 side chambers by removable doors. The apparatus was placed in a dimly lit room, measuring 5 Lux. Each disinfectant agent was sprayed on one of the side chambers and tap water was sprayed on the other side. Using a standard spray bottle, 15 sprays were applied followed by gently wiping each surface with a paper towel to ensure even distribution. The surfaces were not wiped dry immediately but were left slightly damp and allowed to air dry. The disinfectant side was counterbalanced (alternated for different trials) to avoid any potential bias toward one side of the apparatus or the other. The mice were first placed in the central chamber with the side doors closed for a 10-min acclimation period. During this acclimation period, the disinfectant reached a point that was considered to be essentially dry. This method was designed to mimic an environment where the disinfectant would be dried immediately before coming into contact with the animal. The doors were then removed, and the mice were free to explore either side of the apparatus for 10 min. By using an overhead video, the amount of time spent in each chamber was recorded with a stopwatch. A preference score was calculated according to the following equation: (time on the agent side – time on the tap-water side) / (time on the agent side + time on the tap water side). Between mice, the apparatus was thoroughly rinsed in a large wash basin by using a hose and copious amounts of water and then cleaned with unscented soap (Seventh Generation, Burlington, VT), followed by another thorough rinsing. Disinfectants A through D were tested, all prepared as described above. Tap water was used as a negative control. This test was done with each of the 3 mouse lines listed earlier.
Figure 1.
The 3-chamber apparatus used for testing innate aversion. Experimental stimuli can be presented in either side chamber of the apparatus. The mouse begins the trial in the center chamber; after a 10-min acclimation period, removable door panels are lifted, allowing exploration of both side chambers. This image shows the apparatus with the door panels removed, exposing 2 holes in the interior walls that allow mice to travel freely between the chambers.
A follow-up experiment was conducted, in which C57Bl/6 mice were preexposed to either disinfectant A or B for 15 min each day for 3 consecutive days prior to the aversion test using the same disinfectant. For this passive preexposure, one of the disinfectants was placed in a metal dish on top of the wire grid of the home cage, and the lid was replaced. After 15 min, the metal dish was removed, and the cage was returned to its rack. After the preexposure phase was complete, the aversion testing was conducted as described previously, using the same disinfectant from the preexposure phase.
Statistical analysis.
For the cell-culture work, generalized linear mixed-effects models were run to assess the number of live GFP-expressing cells across different combinations of disinfectants, exposure time, and concentrations. The negative binomial distribution was used in these models with the log-link function. To assess the rate instead of the count, an offset for the total number of cells in the given slide was included in the models. Expected rates were computed and plotted with 95% confidence intervals. Statistical analyses were performed in SAS version 9.3 (SAS Institute, Cary, NC); P values less than 0.05 were considered statistically significant. Residual analysis was performed for the models to assess model fit, with no obvious departures from assumptions (homoscedasticity, normality).
Statistical analysis for the aversion testing results was conducted by using SPSS (version 20, IBM, Armonk, NY), with ANOVA of the preference ratio followed by least squares different posthoc comparisons when justified. For analysis across all 3 mouse strains, the factors of disinfectant, strain, and agent side were included in the ANOVA. For the follow-up experiment involving preexposure to disinfectants A and B, ANOVA was conducted with disinfectant as a factor and the following groups: tap-water control, disinfectant A, preexposure to disinfectant A, disinfectant B, and preexposure to disinfectant B. A P value less than 0.05 was considered statistically significant. In addition, 2 BALB/c mice were excluded from the behavior study for failing to spend more than 30 s exploring either of the side chambers.
Results
In vitro efficacy testing.
When each of the disinfectants was diluted in CCM at a ratio of 1:1000 and exposed to HEK-293T cells, none of the diluted disinfectants had negative effects on cell morphology or survival as detected using cell counting and flow cytometry. Therefore, after the viral vectors were exposed to disinfectants, the resultant mixture could be effectively neutralized by employing a 1:1000 dilution before cell exposure, without leading to disinfectant-induced cell toxicity. In contrast, when disinfectants were diluted at a 1:100 ratio and exposed to cells, results were more variable between disinfectants, with indication of decreased cell survival. In the absence of disinfectants, viral titers were still sufficiently high at a 1:1000 dilution of lentivirus and adenoviral vectors to transduce GFP into HEK-293T cells. This dilution factor was therefore used for all disinfection assays, with the exception of the dried adenovirus on a nonporous surface, where a slight variation in the protocol required a maximum dilution of 1:300. In addition, the initial titer of adeno-associated viral vector was too low to yield measurable numbers of GFP-expressing cells after a 1:1000 dilution, therefore this vector was excluded from the study.
Efficacy of disinfectants against lentiviral and adenoviral vectors in suspension.
When lentiviral vectors in suspension were treated with disinfectants at the recommended concentrations, all virus was effectively eliminated by all tested disinfectants at all contact times. After 1 min of contact time for all disinfectants, the percentage of HEK-293T cells expressing GFP was negligible and did not vary significantly from the baseline GFP expression of cells unexposed to viral vectors. When water and CCM (negative controls) were used in place of disinfectants, there was 10% to 20% GFP expression, a value that significantly differed (P < 0.0001) from the GFP expression for cells unexposed to the viral vectors.
Similar results were obtained when adenovirus in suspension was treated with disinfectants. All 4 oxidant disinfectants (A through D) effectively eliminated the adenoviral vector at all exposure times, yielding a negligible GFP expression percentage, which was not significantly different compared with unexposed cells (Figure 2) but did differ (P < 0.0001) from the GFP expression of approximately 100% for water and CCM controls. Disinfectant E did not effectively eliminate all virus, yielding an average GFP expression of 90.2% for 1 min of contact, which decreased to 39.2% for 10 min of contact (P < 0.0001). The GFP expression percentage for all contact times with disinfectant E was greater than that of other disinfectants (P < 0.0001) but less than that of the water and CCM controls (P < 0.01).
Figure 2.
Percentage of live HEK-293T cells expressing GFP after incubation with adenoviral vector that had undergone exposure to disinfectants A through E for contact times of either 1 or 10 min. Percentages for exposures to disinfectants A through D were negligible and equivalent to the GFP expression of cells unexposed to virus. Data are expressed as means ± SE; †, value significantly (P < 0.01) different from baseline fluorescence of cells unexposed to virus.
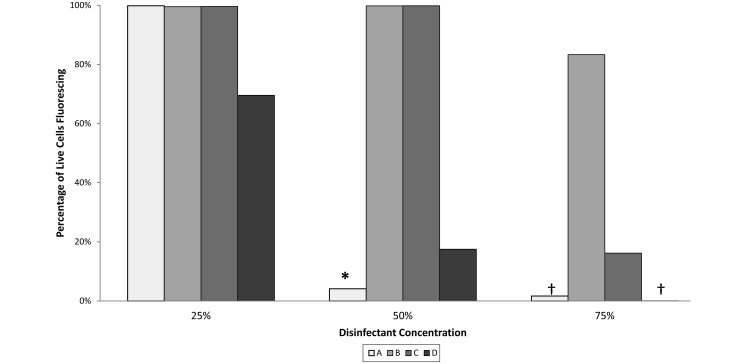
Effect of dilution of disinfectants with FBS on the elimination of adenoviral vector in suspension after 1-min contact times.
When suspended in serum before a 1-min disinfectant contact time, the adenovirus became less susceptible to all of the disinfectants. This effect was more pronounced as the dilution of disinfectant in serum increased (Figures 3 and 4). When serum was used to dilute disinfectants to 75% of the recommended concentration, significant differences in efficacy were noted. At 75% concentration, exposure to disinfectants A and D resulted in a GFP expression of 0%. Disinfectant B produced a GFP expression approaching 100%, which was equivalent to those of the water and CCM controls. The GFP expression after exposure to disinfectant C trended toward an intermediate level, being greater than those of disinfectants A and D (P < 0.01 for both) but not different from those of the water and CCM controls. At 50% concentration, disinfectants B and C resulted in 100% GFP expression, which was equivalent to those of the water and CCM controls. At 50% concentration, disinfectant A resulted in an average of 4% GFP expression, which was greater (P < 0.005) than that of undiluted disinfectant A but less (P < 0.05) than those of the water and CCM controls. In addition, 50% disinfectant D yielded 17% GFP expression, which was not different from those of the CCM and water controls but was greater (P < 0.005) than the result for undiluted disinfectant A. All disinfectants at 25% concentration yielded results that did not differ from that of the water and CCM controls. However, the 70% GFP expression for disinfectant D trended lower than those of the others, which were nearly 100%.
Figure 3.
Fluorescence microscopic images of HEK-293T cells previously incubated with adenoviral vector that had undergone disinfectant exposure. Adenovirus vector was applied onto cells after 1-min contact times in suspension with disinfectants A through D diluted with FBS to 75%, 50%, or 25% of the recommended concentration. GFP expression increases with decreasing disinfectant concentration. Microscopy is less sensitive than flow cytometry in detecting fluorescence. Magnification, 10× (initial images); bar, 100 µm.
Figure 4.
Percentage of live HEK-293T cells expressing GFP after incubation with adenoviral vector that had undergone 1 min of exposure in suspension with disinfectants A through D diluted with FBS to 75%, 50%, or 25% of the recommended concentration. Percentages after exposure to disinfectants A and D at 75% concentration were not significantly different from those of cells unexposed to virus. Data are expressed as means without error bars, due to the degree of variability; values significantly (*, P < 0.05; †, P < 0.01) different from those of the medium controls (virus unexposed to disinfectant) are indicated.
When water was used to dilute the disinfectants instead of serum, the same effect was not seen. All 4 of the tested disinfectants yielded negligible GFP expression after 1 min of contact at levels as low as 25% of the recommended concentration – a result that was significantly different from water and CCM controls (P < 0.005).
Efficacy of disinfectants against adenoviral vectors dried on a nonporous surface.
When adenovirus in CCM was dried onto wells and exposed to each of the disinfectants for a 1-min contact time, disinfectants A and D resulted in a negligible GFP expression percentage, which was again equivalent to the results for cells unexposed to virus (Figure 5). Conversely, after 1-min contact times, disinfectants B and C both resulted in a GFP expression percentage that was significantly (P < 0.001) higher than those of disinfectants A and D and that did not differ significantly from those of CCM and water controls. Disinfectants A and D continued to result in negligible GFP expression at 5- and 10-min exposure times, but there were no significant trends for disinfectants B or C, CCM, or water after 5- and 10-min exposures, likely because of the considerable variation among samples.
Figure 5.
Percentage of live HEK-293T cells expressing GFP after incubation with adenoviral vector that had undergone 1 min of exposure to disinfectants A through D after being dried in complete culture medium (CCM). Disinfectants B and C did not differ significantly from the medium control. Exposure to disinfectants A and D led to negligible GFP expression, equivalent to the expression of cells unexposed to virus. Data are expressed as means ± SE; †, value is significantly (P < 0.01) different from baseline fluorescence of cells unexposed to virus.
SDS evaluation.
The HMIS hazard ratings for disinfectant B were the lowest, with health, flammability, and reactivity–physical hazard ratings all of 0 (Figure 6). All other disinfectants had a health-hazard rating of 2 or 3. Disinfectant E had a flammability rating of 2, whereas all others had a rating of 0. The reactivity–physical hazard rating was 1 for disinfectants A and C and 0 for all others. The pH values of the disinfectants varied considerably between agents. Disinfectants B, C, and D were acidic, all being lower than the pH cutoff of 5.5 for disposal down a drain, whereas disinfectants A and E were basic and, in their concentrated form, exceeded a pH of 11.
Figure 6.
HMIS hazard ratings, pH, and incompatibility information for each tested disinfectant in its undiluted state. These data were obtained from Safety Data Sheets. Hazard ratings are as follows: 4, extreme or life-threatening; 3, high; 2, moderate; 1, slight; 0, insignificant.
In vivo behavioral testing.
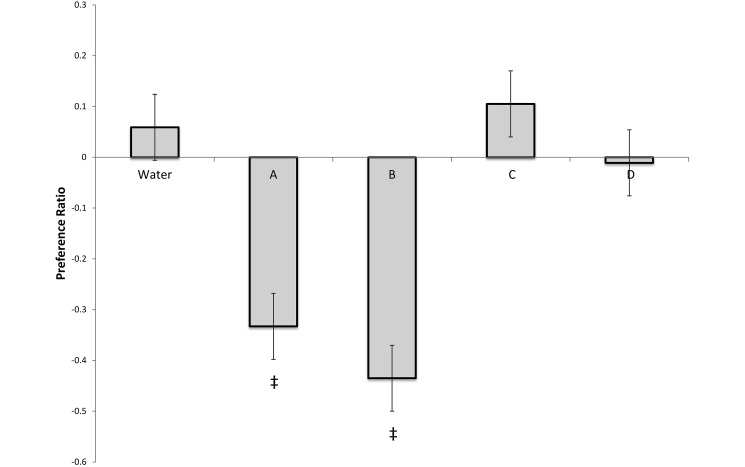
Relative to their responses to the tap-water controls, all mouse strains had significant (P < 0.001) aversion to disinfectants A and B but no aversion to disinfectants C and D (Figure 7). There was no overall effect of strain (P = 0.830) or strain–agent interaction (P = 1.61), indicating that all 3 mouse strains showed the same behavioral pattern. In C57Bl6/J mice, preexposure reduced aversion to both disinfectant A (P = 0.011) and disinfectant B (P = 0.025; Figure 8). However, even after preexposure, these mice still expressed significant aversion to both disinfectants A and B (P = 0.015 and P < 0.001, respectively) relative to tap-water controls.
Figure 7.
Preference scores for innate aversion testing of Swiss Webster, C57Bl/6, and BALB/c mice with disinfectants A through D, calculated by using the following equation: (time on the agent side – time on the tap-water side) / (time on the agent side + time on the tap-water side). A value of 0 indicates no preference. A positive value indicates that the mice spent more time on the agent side, whereas a negative value indicates that the mice spent more time on the tap-water side. All strains had a significant aversion to disinfectants A and B relative to tap water controls, but no aversion to disinfectants C and D. Data are expressed as means ± SE; ‡, value differs significantly (P < 0.001) from that for the water control.
Figure 8.
Preference scores for innate aversion testing of C57Bl/6 mice with disinfectants A and B, with either no prior exposure (A, B) or a history of previous exposure (Pre-A, Pre-B). Scores were calculated by using the following equation: (time on the agent side – time on the tap-water side) / (time on the agent side + time on the tap-water side). A value of 0 indicates no preference. A positive value indicates that the mice spent more time on the agent side, whereas a negative value indicates that the mice spent more time on the tap-water side. Preexposure reduced but did not eliminate aversion to both disinfectants A and B. Data are expressed as mean ± SE; values significantly (*, P < 0.05; †, P < 0.01) different from control values are indicated.
Discussion
This study investigated spectrum of activity, environmental and occupational safety, and animal behavior as criteria for the selection of an appropriate vivarium surface disinfectant. Under ideal conditions, all four oxidant disinfectants were sufficiently efficacious against multiple viruses, but the results clearly favored disinfectants A and D when conditions including increased organic load and viral drying were used to challenge these products. SDS evaluation established that hazards varied considerably between products, with disinfectant B proving to be the safest, and indicated that pH was a limiting factor in whether each product could be disposed of through drainage to publically owned treatment works.25 Finally, aversion testing indicated that disinfectants A and B caused significant aversion in mice, whereas disinfectants C and D caused no behavioral changes.
Possibly the most important outcome of this study was the refinement of an alternative assay for disinfectant efficacy testing and comparison. The use of nonreplicating, GFP-expressing viral vectors as surrogates for equivalent viral diseases has proven to be an effective way to verify and compare the efficacy of various disinfectants. The benefits of this approach include decreased biosafety precautions and health risks, compared with testing using wild-type viruses, and the convenience of using GFP expression as an indicator of viral survival. According to hierarchy schemes developed by Spaulding and others and still used currently, nonenveloped adenoviral vectors are representative of common viral and bacterial organisms of equal or greater sensitivity.51,66,80,87,93 Surrogates for more pathogenic or less easily cultivated organisms have been used for multiple studies of antiviral efficacy,24,46,69,96,97,104 but most of these studies still evaluated wild-type viruses. To date, GFP-expressing viral vectors have not been used as surrogates in this manner. The development of this assay allows similar testing to be repeated in any facility that uses GFP-expressing vectors but may not have the capability to cultivate wild-type viruses.
A major contributor to the success of this assay was the use of CCM to dilute disinfectants after exposure to viral vectors to render them nontoxic to HEK-293T cells. At the 1:1000 disinfectant dilution used for the suspension testing in this study, exposed cells showed no changes in morphology or increases in cell death, as indicated by microscopy and flow cytometry. In addition, a preliminary MTS assay performed early in the study showed that cell viability was retained after exposure to disinfectants diluted to this degree. Similar dilution of disinfectants after exposure has been used in other efficacy tests, with great success.24,27,41,47 The serial dilutions used after the disinfectant contact times were sufficient to arrest the antiviral activity of these disinfectants but maintained the surviving virus at a sufficiently high titer to transduce into cells. Consequently, viral isolation and propagation, which had been required in some previous studies,31,75,101 were unnecessary, thus making our modified assay considerably less complex than are other methods.
Our assay is particularly useful as a means of confirming efficacy of disinfectants, especially when they have not been tested in regard to specific exposure times or under certain conditions. It predominantly has been used to search for an all-or-nothing response. That is, complete elimination of viral transduction, and therefore GFP expression, provides confirmation of efficacy, whereas a GFP expression that is significantly different from 0 indicates that under the given conditions, the disinfectant is not completely effective.
All 4 oxidant disinfectants successfully eliminated lentiviral and adenoviral vectors in suspension (Figure 2), even at 1-min contact times. Therefore these disinfectants have an appropriate spectrum of activity against some of the most common viruses in a research facility, given that any organism of equal or greater sensitivity than that of adenoviruses likely also will be inactivated by these products.66,80,87 The quaternary ammonium compound, disinfectant E, eliminated all lentiviral vector in suspension, but it was not fully effective against the adenoviral vector. This finding was consistent with the manufacturer's recommendations, because the concentration used in this study was less than the 3390 ppm marketed for killing adenovirus types 5 and 7 (according to the product insert). In addition, this result was expected, because quaternary ammonium compounds are considered to be less effective than are most oxidant disinfectants, particularly against nonenveloped viruses.33,49 One study similarly found that sodium hypochlorite, chlorine dioxide, and potassium peroxymonosulfate were able to inactivate feline calicivirus, parvovirus (both nonenveloped), and herpesvirus (enveloped) in suspension, whereas a quaternary ammonium compound was effective against the herpesvirus only.27 Finally, the efficacy results for disinfectant E provided an additional validation of the methodology, by confirming that adenovirus was not eliminated by a product that was expected to be ineffective at the concentration used in this study.
Several methods were developed to challenge the oxidant disinfectants (A through D) and elicit differences in efficacy. Dilution in serum was chosen as the first means of challenging the disinfectants, because it was expected to simulate buffering of virus by organic material. The adenoviral vector was used for this and all subsequent viral studies because it was considered to have decreased susceptibility to disinfection. In addition, contact times were limited to 1 min, to further challenge the disinfectants. When tested in this way, disinfectants A and D performed better than did disinfectants B and C. When diluted in water instead of bovine serum, all 4 oxidant disinfectants effectively eliminated the virus, even at 25% concentration, indicating that the presence of organic material—and not dilution itself—was the factor responsible for the attenuation of efficacy. Another means of challenging the disinfectants was to apply them to adenovirus that had been dried onto a nonporous surface, because pathogens become more difficult to eliminate when they are dried in protein or protected by biofilms.2,67,99 Viral vectors were dried in CCM, because this substrate was deemed to be ideal for this assay, in light of the inability for the virus to consistently survive after drying in water or in a concentrated form, without any medium. Again disinfectants A and D performed better than did disinfectants B and C, killing 100% of the virus in a little as 1 min of contact time.
When challenged by either the serum dilution or the dried virus method, disinfectants A and D consistently outperformed the other products. This result was expected for disinfectant A, given that sodium hypochlorite represents the ‘gold standard’ against which the other products were compared. Sodium hypochlorite solutions generally are considered to be intermediate- to high-level disinfectants and therefore are expected, at minimum, to eliminate most viruses.100,83 Disinfectant D, a peroxymonosulfate, was expected to perform well in this assay, because it has previously been proven effective in eliminating hydrophilic, nonenveloped viruses in suspension and carrier tests.11,41 In addition, disinfectant D is marketed to be effective against many nonenveloped viruses, including mouse and canine parvoviruses, and is used extensively for farm biosecurity. Despite the results of the current study, this product is, at most, an intermediate-level disinfectant and is considered by some to be a low-level disinfectant, because it is ineffective against bacterial spores, some fungi, and some mycobacterial species.11,41
When challenged, disinfectants B and C did not perform as well disinfectants A and D. It is important to consider that disinfectant B contains a surfactant, which is expected to improve efficacy by increasing surface area of contact. A possible explanation for its unexpected decrease in performance with challenges is that the degree of dilution might have diminished the product's efficacy, given that it is formulated to have a certain ratio of disinfectant and surfactant to other components. Previous testing of this product showed it to be effective against several nonenveloped viruses when used at full-strength,75 but suspension testing of protein-buffered virus was not performed previously, and the maximal burden of FBS to be overcome in dry-surface carrier testing was 5% in the cited study, as compared with the 10% used in the current study. Previous efficacy testing has supported the virucidal activity of liquid formulations of chlorine dioxide,27,107 such as disinfectant C, in addition to chlorine dioxide in the gaseous form.69 In one study, sporocidal efficacy testing indicated that disinfectant C was very effective against Bacillus anthracis in suspension within a closed tube.17 However, when the product was applied in a thin film and exposed to air in that study, efficacy diminished significantly—an effect presumed to be due to rapid vaporization of chlorine dioxide gas from solution.17 A similar effect has likely occurred in the present study, as the application method was very similar. One additional challenge to disinfectant C in our study was its usage in the lower part of its recommended concentration range. This product is most commonly used at 100 to 200 ppm; we chose to use 100 ppm, as a means of challenging the disinfectant as much as possible. A dose-dependent decrease in efficacy was noted previously when this disinfectant was used in suspension.17
The serum and dry-surface carrier testing used in the current study correspond to possible real-life scenarios in which viruses may be less susceptible to disinfectants. Examples include viral contamination of any dried or wet organic substance, most notably blood, feces, or any bodily secretions. These materials have been shown to inactivate hypochlorites, quaternary ammonium compounds, and many other disinfectants12,33,94,102—an effect supported by the results of the current study (Figure 4), which indicate that even the best performing products became less effective as organic material increased. A much more literal translation of the current results would be the disinfection of high-titer viruses spilled during cell culture experiments in biomedical laboratories, many of which use the same composition of CCM as the current study. Organic material interferes with disinfectant function through chemical reactions, making pathogens less susceptible to disinfection.55,64,83 In addition, organic material acts as a physical barrier against disinfectant contact, making the pathogens less accessible to the products being used.10,40,57 This physical barrier mechanism is predominantly responsible for the decrease in disinfectant sensitivity when a pathogen is dried onto a surface in a protein-containing medium or a biofilm.58 In the current study, the adenoviral sample was protected when dried on a surface with CCM, a mixture containing 10% FBS (Figure 5).
The best way to overcome the described physical and chemical barriers is to apply the disinfectant in a manner that ensures appropriate contact. This goal typically requires a certain degree of mechanical agitation or friction.38,105,106 In some cases, it is vital that a surface is cleaned before disinfectant application, to remove excessive foreign material.22,83 This step is particularly important for surfaces that are more complex, porous, contaminated, or moist, because these factors provide additional protection from disinfection.59 In performing suspension and surface disinfection assays in the current study, physical agitation was kept to a minimum between the application of the disinfectants and subsequent neutralization, to present the greatest challenge to the disinfectants. Although not a focus of this study, viruses initially were found to be more susceptible to disinfectants when mixed more aggressively (that is, vortexing during contact times). The utility of mechanical agitation suggests that the act of wiping with a paper towel may have a similar effect, supporting the practice of wiping down surfaces after they are disinfected, rather than allowing them to dry. Alternatively, some suggest that wiping surfaces may decrease efficacy by allowing quicker evaporation and decreasing exposure volume.85
Another factor related to disinfectant application that may have an influence on efficacy is the way the disinfectant is initially applied to a surface. Disinfectants are often sprayed onto a surface and left to act for a period of time before being wiped away. The present study predominantly involved pouring disinfectants onto the surface or suspension of interest, but in most situations, the disinfectant is sprayed from a bottle. Spray bottles can have different settings, from a fine mist to a coarse stream, capable of directing dramatically different amounts of disinfectant to a wide range of areas, and different users may be more or less liberal with their application. One study found that the way a disinfectant was applied—sprayed or poured—did not have a significant effect as long as the same amount of disinfectant was ultimately present.17 However, the amount of disinfectant applied by different users cannot be standardized easily, because users do not directly measure the volume being applied.
Once the disinfectant is applied, contact time is a very important variable to address. The recommended contact time for most EPA-registered disinfectants is 10 min, unless additional confirmatory efficacy testing is performed and accepted.83,85 However, 10 min is often an unrealistic expectation in a standard animal facility in the context of how disinfectants are typically sprayed and wiped from a surface. In a human healthcare setting, products may be allowed to dry on a surface, limiting contact times to approximately 1 min.83 This report is in concordance with observations within our facility that the time from the first spray of a disinfectant to the completion of wiping all surfaces is typically between 15 and 45 s. For this reason, the 1-min exposure time in the current study is likely the most representative of what occurs in fact, and actually might not be sufficiently stringent. In the current study, disinfectants A and D were the only 2 that passed the dried, nonporous surface test for the 1-min time point.
The described variables were deemed to be the most relevant efficacy-related factors to consider when selecting a disinfectant, so they have been the focus of this study. One factor that is difficult to assess but that may decrease the efficacy of a disinfectant is human error in disinfectant application or reconstitution. If products are used incorrectly, mixed to inappropriate concentrations, or combined with chemicals that cause neutralizing reactions, they may not be as effective as when used according to manufacturer recommendations.83,98 Conversely, some products might have a synergistic effect when combined, for example, chlorine dioxide and sodium hypochlorite.17 Another critical factor in the preparation of disinfectants is the source of water used for reconstitution. Water hardness, pH, and temperature all influence the overall efficacy of disinfectants.60,78,83,91 The present study used tap water, as is typical in practical settings, but the quality of tap water can vary between locations, even on a local level.65
Finally, the characteristics of the pathogen itself may have considerable effects on disinfectant efficacy. As described previously, there is a general hierarchy of sensitivity among microorganisms,66,87 and the structure of a given pathogen may make it more or less sensitive to disinfection or environmental factors such as desiccation. The adenoviral vector, a partially lipophilic nonenveloped virus, was difficult to eliminate, but a hydrophilic nonenveloped pathogen, such as a parvovirus, is even less susceptible to disinfectants.31 The GFP-expressing adeno-associated viral vector is an example of a parvovirus that may be available for testing by using the described methods, but the titer available for this study was insufficient to allow its use. This finding brings to light another important factor—the amount of pathogen being disinfected. As the number and diversity of pathogens increases, the susceptibility to disinfection decreases, due to synergistic effects, such as aggregation and the formation of biofilms, which greatly increase the amount and complexity of the organic load that the disinfectant must overcome.58,59,63,67,90 Lastly, recent findings suggest that there may be differences in susceptibility among viruses of similar envelope structure when disinfectants that do not specifically damage the viral genome, including many oxidizing agents, are used89 and some pathogens may adapt to become less sensitive to disinfectants through repeated exposure.60
Another step in disinfectant selection is the consideration of safety—both to the people using the disinfectants and to the environment. Much of the information necessary to assess safety can be found in the SDS. These typically contain a scale of hazard ratings, designated according to the HMIS scheme, including health hazard, flammability, and reactivity–physical hazard. The hazard ratings range from 0, which is negligible, to 4, which is extreme or life threatening. The HMIS ratings for the disinfectants in this study identified disinfectant B as the safest overall, with health, flammability, and reactivity ratings of 0 (Figure 6), in contrast to the other disinfectants, which all had health hazard ratings of 2 or 3 and various, but typically negligible, ratings for flammability and reactivity–physical hazard. Despite efforts to control health hazards in a laboratory setting, occupational disinfectant-related illness is still fairly common among certain populations, especially when adequate training is not enforced.14 It is therefore critical to consider hazard ratings—and other SDS information—when choosing disinfectants, to limit the possibility of adverse reactions.
The disposal of disinfectants is addressed in the SDS, but the standard guidance provided is to follow federal, state, and local regulations regarding appropriate disposal. Local regulations vary, but in the city of Los Angeles, any chemical to be poured down a drain must have a pH between 5.5 and 11.25 A couple of the most common reasons for pouring a chemical down a drain are disposing mop water containing the disinfectant and discarding excess disinfectant remaining after it has passed an expiration date, which can vary from days to months after reconstitution. The pH values in the SDS of the tested products indicate that most of them are beyond the recommended range for disposal via the sewage system to publically owned treatment works. Disinfectants B, C, and D are all acidic solutions, with a pH lower than 5.5 at their active concentrations, although they still have a pH at or above 2, allowing them to avoid being classified as corrosive hazardous waste. Unfortunately, dilution is not “the solution to pollution” in the case of acidic disinfectant disposal, given that dilution in water is not considered an adequate means of achieving compliance with the discharge limitations set forth by the Los Angeles Municipal Code.25 However, pH might be neutralized by other means, such as buffering. One study found that the activity and pH of disinfectant D could be effectively neutralized by adding sodium thiosulfate and sodium bicarbonate, respectively.64 Similar methods might be used for the other products as needed, as long as risks of reactivity are taken into consideration. Another potential approach is to eliminate the need for disposing of chemicals down the drain. This goal might be accomplished by only using products with very long shelf-lives, so that only a minimal amount of product reaches expiration and thus can be removed as chemical waste. In the case of mop-water disposal, it might also be practical to use a different disinfectant for cleaning floors, given that these surfaces may be at decreased risk of coming into contact with pathogens and might accommodate the use of less efficacious disinfectants to achieve sufficient disinfection.
Mice were used to test the behavioral effects of disinfectants, because this species has a highly developed sense of smell20 and is often in intimate contact with recently disinfected surfaces. Aversion to disinfectants is particularly relevant in behavioral core facilities, where environmental factors may confound experimental results.19,20,62 Swiss Webster mice were chosen as a representative of a more diverse, outbred stock,18 while the 2 strains – C57Bl/6 and BALB/c – were chosen because they represent some of the most commonly used strains in biomedical research.21,68 In the current study, disinfectants A and B were aversive, whereas disinfectants C and D did not have significant effects on behavior. This effect was consistent among all strains and stocks tested, allowing the data for all 3 populations to be pooled (Figure 7). This consistency among strains that are known to have varying behavioral phenotypes,19,53,68 and an outbred stock, which is known to yield more variable results,18 suggests that the results are likely representative of the general laboratory mouse population. The lack of differences between strains allowed the following preexposure experiment to be performed in only the C57Bl/6 mice, in the interest of reducing animal numbers, because the results were expected to be similar among all mice tested. The preexposure experiment was designed to minimize the contribution of neophobia to the behavioral response. Although the degree of avoidance did decrease significantly with increased familiarity, the mice did not develop complete tolerance to the more aversive disinfectants with repeated exposures, supporting the conclusion that these disinfectants would have a lasting effect on behavior (Figure 8).
The avoidance trends in mice appear to be irrespective of subjective observations of personnel within the testing facility, many of whom find the scent of disinfectant B to be more pleasant than those of disinfectants A, C, and D. This statement suggests that mice perceive the odor of disinfectants differently than do humans—a conclusion that is supported by the fact that the olfactory system of mice is considerably more developed than those of many other species.48 It is plausible that a particular component of disinfectant B, although imperceptible to humans, was making it as aversive to mice as disinfectant A. It is worth noting that chemical reactions can occur between disinfectants when mixed, potentially causing offensive or even toxic odors.70,73 To combat this possibility, the testing apparatus was scrubbed thoroughly with unscented soap and flushed extensively with water between tests, as a means of removing previous residue and limiting unwanted interactions between different chemicals.
Each disinfectant we tested has specific advantages and disadvantages. Disinfectant A, a sodium hypochlorite suspension, is considered by many facilities to be the gold standard for disinfection. Although this disinfectant performed very well in efficacy testing, it has considerable health and reactivity hazards and caused aversion in mice. Disinfectants B and C were not as effective when challenged with extreme conditions, although they were effective when used at recommended concentrations in suspension. Disinfectant B was deemed the safest, based on its negligible hazard ratings, but caused aversion mice. Disinfectant C had a greater health risk than did disinfectant B, but disinfectant C did not cause any significant behavioral effects in mice. Compared with disinfectant A, disinfectant D was as effective against viruses and caused less aversion in mice, but health hazards still existed. Disinfectant E, the only denaturant disinfectant in the study, is a quaternary ammonium compound that was tested at a low concentration. At its given concentration, disinfectant E was not as effective as the other disinfectants; because the product tested is not marketed for use in animals, it was not used in aversion testing. However, quaternary ammonium compounds are used frequently in veterinary and human hospitals, and testing different concentrations and multiple products might yield more promising results. Although none of the tested disinfectants was clearly superior overall, any one of these might be acceptable for some or all purposes in a given facility, depending on its specific needs. In facilities with low pathogen risks or decreased chance of direct animal contact, finding a single product that can be used for every purpose likely is straightforward. Alternatively, in more diverse facilities, where disinfectants serve a multitude of purposes, having multiple products available may be practical.
In conclusion, the selection of an appropriate disinfectant for a laboratory animal facility is a complex, multifactorial decision requiring consideration of spectrum of activity, human safety, environmental safety, and behavioral effects on research animals. The present study explored several critical characteristics of surface disinfectants and validated the need for a comprehensive approach to product assessment. Although no single disinfectant is best for every purpose, consideration of the characteristics we tested likely will lead to the selection of a product or combination of products that effectively meets the needs of any given facility.
Acknowledgments
This work was supported by a grant from the UC Center for Laboratory Safety. Funding for the statistical analysis was supported by the NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI grant (no. UL1TR000124). We thank the members of the UCLA laboratory of Dr Noriyuki Kasahara, particularly Ms Brooke Bogan and Mr Kip Hermann, for their technical assistance and constructive input in assay development. We also thank Ms Stacey Kraemer and Dr Michael Fanselow for their assistance in the development of research objectives for this study and Dr Lisa Williams for her assistance in editing the manuscript.
References
- 1.Abad FX, Pinto RM, Bosch A. 1994. Survival of enteric viruses on environmental fomites. Appl Environ Microbiol 60:3704–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abad FX, Pinto RM, Bosch A. 1997. Disinfection of human enteric viruses on fomites. FEMS Microbiol Lett 156:107–111. [DOI] [PubMed] [Google Scholar]
- 3.Akamatsu A, Lee C, Morino H, Miura T, Ogata N, Shibata T. 2012. Six-month low-level chlorine dioxide gas inhalation-toxicity study with 2-week recovery period in rats. J Occup Med Toxicol 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Adham I, Haddadin R, Collier P. 2013. Types of microbicidal and microbistatic agents. In: Fraise AP, Maillard J-Y, Sattar SA. Russell, Hugo, and Ayliffe's principles and practice of disinfection, preservation, and sterilization. Hoboken (NJ): Wiley–Blackwell. [Google Scholar]
- 5.Arts JH, de Heer C, Woutersen RA. 2006. Local effects in the respiratory tract: relevance of subjectively measured irritation for setting occupational exposure limits. Int Arch Occup Environ Health 79:283–298. [DOI] [PubMed] [Google Scholar]
- 6.ASTM International. 2014. E1052-11. Standard test method to assess the activity of microbicides against viruses in suspension. In: Annual book of ASTM standards. West Conshohhocken (PA): ASTM International. [Google Scholar]
- 7.ASTM International. 2014. E1053-11. Standard test method to assess the activity of chemicals intended for disinfection of inanimate, nonporous environmental surfaces. In: Annual book of ASTM standards. West Conshohhocken (PA): ASTM International. [Google Scholar]
- 8.Bauer A. 2013. Contact dermatitis in the cleaning industry. Curr Opin Allergy Clin Immunol 13:521–524. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy K. 1995. A review of the test methods used to establish virucidal activity. J Hosp Infect 30 Suppl:389–396. [DOI] [PubMed] [Google Scholar]
- 10.Berman D, Hoff JC. 1984. Inactivation of simian rotavirus SA11 by chlorine, chlorine dioxide, and monochloramine. Appl Environ Microbiol 48:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Best M, Springthorpe VS, Sattar SA. 1994. Feasibility of a combined carrier test for disinfectants: studies with a mixture of 5 types of microorganisms. Am J Infect Control 22:152–162. [DOI] [PubMed] [Google Scholar]
- 12.Bloomfield SF, Miller EA. 1989. A comparison of hypochlorite and phenolic disinfectants for disinfection of clean and soiled surfaces and blood spillages. J Hosp Infect 13:231–239. [DOI] [PubMed] [Google Scholar]
- 13.Bohner HF, Bradley RL. 1991. Corrosivity of chlorine dioxide used as sanitizer in ultrafiltration systems. J Dairy Sci 74:3348–3352. [Google Scholar]
- 14.Brevard TA, Calvert GM, Blondell JM, Mehler LN. 2003. Acute occupational disinfectant-related illness among youth, 1993–1998. Environ Health Perspect 111:1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burr HN, Lipman NS, White JR, Zheng J, Wolf FR. 2007. Strategies to prevent, treat, and provoke Corynebacterium-associated hyperkeratosis in athymic nude mice. J Am Assoc Lab Anim Sci 50:378–388. [PMC free article] [PubMed] [Google Scholar]
- 16.Burr HN, Wolf FR, Lipman NS. 2011. Corynebacterium bovis: epizootiologic features and environmental contamination in an enzootically infected rodent room. J Am Assoc Lab Anim Sci 51:189–198. [PMC free article] [PubMed] [Google Scholar]
- 17.Chatuev BM, Peterson JW. 2010. Analysis of the sporicidal activity of chlorine dioxide disinfectant against Bacillus anthracis (Sterne strain). J Hosp Infect 74:178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chia R, Achilli F, Festing MF, Fisher EM. 2005. The origins and uses of mouse outbred stocks. Nat Genet 37:1181–1186. [DOI] [PubMed] [Google Scholar]
- 19.Crabbe JC, Wahlsten D, Dudek BC. 1999. Genetics of mouse behavior: interactions with laboratory environment. Science 284:1670–1672. [DOI] [PubMed] [Google Scholar]
- 20.Crawley JN. 2007. What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. Hoboken (NJ): John Wiley and Sons. [Google Scholar]
- 21.Currer JM, 2009. Handbook on genetically standardized mice. Bar Harbor (ME): The Jackson Laboratory. [Google Scholar]
- 22.Dancer SJ. 2009. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect 73:378–385. [DOI] [PubMed] [Google Scholar]
- 23.Das R, Blanc PD. 1993. Chlorine gas exposure and the lung: a review. Toxicol Ind Health 9:439–455. [DOI] [PubMed] [Google Scholar]
- 24.Dellanno C, Vega Q, Boesenberg D. 2009. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Am J Infect Control 37:649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Public Works. 2004. Industrial waste control ordinance. Los Angeles Municipal Code § 64. [Google Scholar]
- 26.Doerrbecker J, Friesland M, Ciesek S, Erichsen TJ, Mateu-Gelabert P, Steinmann J, Pietschmann T, Steinmann E. 2011. Inactivation and survival of hepatitis C virus on inanimate surfaces. J Infect Dis 204:1830–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eleraky NZ, Potgieter LN, Kennedy MA. 2002. Virucidal efficacy of 4 new disinfectants. J Am Anim Hosp Assoc 38:231–234. [DOI] [PubMed] [Google Scholar]
- 28.Environmental Protection Agency (EPA). 2005. Characteristic of corrosivity. 40 CFR 26122. [Google Scholar]
- 29.Environmental Protection Agency (EPA). 2013. Revisions to the performance standard for the AOAC use-dilution methods for Staphylococcus aureus (955.15) and Pseudomonas aeruginosa (964.02). Washington (DC): EPA. [Google Scholar]
- 30.Environmental Protection Agency (EPA). 2014. Antimicrobial testing program. Washington (DC): EPA. [Google Scholar]
- 31.Eterpi M, McDonnell G, Thomas V. 2005. Decontamination efficacy against Mycoplasma. Lett Appl Microbiol 52:150–155. [DOI] [PubMed] [Google Scholar]
- 32.Federighi V. 2011. A guide to pesticide regulation in California. Sacramento (CA): California Department of Pesticide Regulation. [Google Scholar]
- 33.Fraise AP. 1999. Choosing disinfectants. J Hosp Infect 43:255–264. [DOI] [PubMed] [Google Scholar]
- 34.Fraisse A, Temmam S, Deboosere N, Guillier L, Delobel A, Maris P, Vialette M, Morin T, Perelle S. 2011. Comparison of chlorine and peroxyacetic-based disinfectant to inactivate feline calicivirus, murine norovirus, and hepatitis A virus on lettuce. Int J Food Microbiol 151:98–104. [DOI] [PubMed] [Google Scholar]
- 35.Gagnaire F, Marignac B, Hecht G, Hery M. 2002. Sensory irritation of acetic acid, hydrogen peroxide, peroxyacetic acid, and their mixture in mice. Ann Occup Hyg 46:97–102. [DOI] [PubMed] [Google Scholar]
- 36.Gapany-Gapanavicius M, Yellin A, Almog S, Tirosh M. 1982. Pneumomediastinum. A complication of chlorine exposure from mixing household cleaning agents. JAMA 248:349–350. [DOI] [PubMed] [Google Scholar]
- 37.Gesellschaft fur Versuchstierkund–Society for Laboratory Animal Science Working Group on Hygiene. 1999. Implications of infectious agents on results of animal experiments. Report of the Working Group on Hygiene of the Gesellschaft fur Versuchstierkunde–Society for Laboratory Animal Science (GV-SOLAS). Lab Anim 33 Suppl 1:39–87. [PubMed] [Google Scholar]
- 38.Graziano MU, Graziano KU, Pinto FM, Bruna CQ, de Souza RQ, Lascala CA. 2013. Effectiveness of disinfection with alcohol 70% (w/v) of contaminated surfaces not previously cleaned. Rev Lat Am Enfermagem 21:618–623. [DOI] [PubMed] [Google Scholar]
- 39.Hartnett KM, Fulginiti LC, Di Modica F. 2011. The effects of corrosive substances on human bone, teeth, hair, nails, and soft tissue. J Forensic Sci 56:954–959. [DOI] [PubMed] [Google Scholar]
- 40.Hejkal TW, Wellings FM, LaRock PA, Lewis AL. 1979. Survival of poliovirus within organic solids during chlorination. Appl Environ Microbiol 38:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernndez A, Martro E, Matas L, Martin M, Ausina V. 2000. Assessment of in vitro efficacy of 1% Virkon against bacteria, fungi, viruses, and spores by means of AFNOR guidelines. J Hosp Infect 46:203–209. [DOI] [PubMed] [Google Scholar]
- 42.Ingram TA., 3rd 1990. Response of the human eye to accidental exposure to sodium hypochlorite. J Endod 16:235–238. [DOI] [PubMed] [Google Scholar]
- 43.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academic Press. [Google Scholar]
- 44.Janus LM, Bleich A. 2011. Coping with parvovirus infections in mice: health surveillance and control. Lab Anim 46:14–23. [DOI] [PubMed] [Google Scholar]
- 45.Jean J, Morales-Rayas R, Anoman MN, Lamhoujeb S. 2011. Inactivation of hepatitis A virus and norovirus surrogate in suspension and on food-contact surfaces using pulsed UV light (pulsed light inactivation of food-borne viruses). Food Microbiol 28:568–572. [DOI] [PubMed] [Google Scholar]
- 46.Jimenez L, Chiang M. 2006. Virucidal activity of a quaternary ammonium compound disinfectant against feline calicivirus: a surrogate for norovirus. Am J Infect Control 34:269–273. [DOI] [PubMed] [Google Scholar]
- 47.Kampf G, Steinmann J, Rabenau H. 2007. Suitability of vaccinia virus and bovine viral diarrhea virus (BVDV) for determining activities of 3 commonly used alcohol-based hand rubs against enveloped viruses. BMC Infect Dis 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kay LM, Stopfer M. 2006. Information processing in the olfactory systems of insects and vertebrates. Semin Cell Dev Biol 17:433–442. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy MA, Mellon VS, Caldwell G, Potgieter LN. 1995. Virucidal efficacy of the newer quaternary ammonium compounds. J Am Anim Hosp Assoc 31:254–258. [DOI] [PubMed] [Google Scholar]
- 50.King WW, Dial SM, Bivin WS. 1999. Quaternary ammonium-induced cutaneous and gastrointestinal mucosal lesions in a dog. Contemp Top Lab Anim Sci 38:69–73. [PubMed] [Google Scholar]
- 51.Klein M, Deforest A. 1983. Principles of viral inactivation. In: Block SS. Disinfection, sterilization, and preservation. Philadelphia (PA): Lea and Febiger. [Google Scholar]
- 52.Knudsen R. 2001. Risk assessment for working with infectious agents in the biological laboratory. Appl Biosaf 6:19–26. [Google Scholar]
- 53.Lad HV, Liu L, Paya-Cano JL, Parsons MJ, Kember R, Fernandes C, Schalkwyk LC. 2010. Behavioural battery testing: evaluation and behavioural outcomes in 8 inbred mouse strains. Physiol Behav 99:301–316. [DOI] [PubMed] [Google Scholar]
- 54.Lambert PA. 2013. Mechanisms of action of microbicides. In: Fraise AP, Maillard J-Y, Sattar SA. Russell, Hugo, and Ayliffe's: principles and practice of disinfection, preservation, and sterilization, 5th ed. Hoboken (NJ): Wiley–Blackwell. [Google Scholar]
- 55.Lambert RJ, Johnston MD. 2001. The effect of interfering substances on the disinfection process: a mathematical model. J Appl Microbiol 91:548–555. [DOI] [PubMed] [Google Scholar]
- 56.Lang C, Cox M. 2013. Pediatric cutaneous bleach burns. Child Abuse Negl 37:485–488. [DOI] [PubMed] [Google Scholar]
- 57.Lewis DL, Arens M. 1995. Resistance of microorganisms to disinfection in dental and medical devices. Nat Med 1:956–958. [DOI] [PubMed] [Google Scholar]
- 58.Mah TF, O'Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39. [DOI] [PubMed] [Google Scholar]
- 59.Maillard J-Y. 2013. Factors affecting the activities of microbicides. In: Fraise AP, Maillard J-Y, Sattar SA. Russel, Hugo, and Ayliffe's principles and practice of disinfection, preservation, and sterilization. Hoboken (NJ): Wiley–Blackwell. [Google Scholar]
- 60.Maillard J-Y, Sattar SA, Pinto F. 2013. Virucidal activity of microbicides. In: Fraise AP, Maillard J-Y, Sattar SA. Russell, Hugo, and Ayliffe's principles and practice of disinfection, preservation, and sterilization. Hoboken (NJ): Wiley–Blackwell. [Google Scholar]
- 61.Mallinger S. 1997. EPA-registered disinfectants for HIV/HBV. Washington (DC): Occupational Health and Safety Administration. [Google Scholar]
- 62.Mandillo S, Tucci V, Holter SM, Meziane H, Banchaabouchi MA, Kallnik M, Lad HV, Nolan PM, Ouagazzal AM, Coghill EL, Gale K, Golini E, Jacquot S, Krezel W, Parker A, Riet F, Schneider I, Marazziti D, Auwerx J, Brown SD, Chambon P, Rosenthal N, Tocchini-Valentini G, Wurst W. 2008. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol Genomics 34:243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mattle MJ, Crouzy B, Brennecke M, Wigginton KR, Perona P, Kohn T. 2011. Impact of virus aggregation on inactivation by peracetic acid and implications for other disinfectants. Environ Sci Technol 45:7710–7717. [DOI] [PubMed] [Google Scholar]
- 64.McCormick L, Maheshwari G. 2004. Inactivation of adenovirus types 5 and 6 by Virkon S. Antiviral Res 64:27–33. [DOI] [PubMed] [Google Scholar]
- 65.Los Angeles Department of Water and Power (LADWP). 2014. LA's drinking water quality report for the period of Jan 1–Dec 31, 2013. Los Angeles (CA): LADWP. [Google Scholar]
- 66.McDonnell G, Burke P. 2011. Disinfection: is it time to reconsider Spaulding? J Hosp Infect 78:163–170. [DOI] [PubMed] [Google Scholar]
- 67.Merritt K, Hitchins VM, Brown SA. 2000. Safety and cleaning of medical materials and devices. J Biomed Mater Res 53:131–136. [DOI] [PubMed] [Google Scholar]
- 68.Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. 2007. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav 6:192–200. [DOI] [PubMed] [Google Scholar]
- 69.Morino H, Fukuda T, Miura T, Lee C, Shibata T, Sanekata T. 2009. Inactivation of feline calicivirus, a norovirus surrogate, by chlorine dioxide gas. Biocontrol Sci 14:147–153. [DOI] [PubMed] [Google Scholar]
- 70.Mrvos R, Dean BS, Krenzelok EP. 1993. Home exposures to chlorine/chloramine gas: review of 216 cases. South Med J 86:654–657. [DOI] [PubMed] [Google Scholar]
- 71.National Research Council. 1997. Occupational health and safety in the care and use of research animals. Washington (DC): National Academy Press. [Google Scholar]
- 72.National Research Council. 2011. Prudent practices in the laboratory: Handling and management of chemical hazards. Washington (DC): National Academic Press. [PubMed] [Google Scholar]
- 73.Occupational Health and Safety Agency for Healthcare (OHSAH). 2007. Exposure hazards that may arise from the use of Caviwipes in combination with Virox 5 RTU wipes and/or Accel TB wipes. Vancouver (Canada): OHSAH. [Google Scholar]
- 74.Oliet S, Sorin SM. 1978. Inhibition of the corrosive effect of sodium hypochlorite on carbon steel endodontic instruments. J Endod 4:12–16. [DOI] [PubMed] [Google Scholar]
- 75.Omidbakhsh N, Sattar SA. 2006. Broad-spectrum microbicidal activity, toxicologic assessment, and materials compatibility of a new generation of accelerated hydrogen peroxide-based environmental surface disinfectant. Am J Infect Control 34:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.OSHA. 2012. Bloodborne pathogens. 29 CFR 19101030.
- 77.OSHA. 2012. Occupational exposure to hazardous chemicals in laboratories. 29 CFR 19101450.
- 78.Page MA, Shisler JL, Marinas BJ. 2009. Kinetics of adenovirus type 2 inactivation with free chlorine. Water Res 43: 2916–2926. [DOI] [PubMed] [Google Scholar]
- 79.Panteleeva LG. 2010. [New guidelines for investigating and evaluating the virucidal activity of disinfectants] Gig Sanit 5:36–39. [Article in Russian] [PubMed] [Google Scholar]
- 80.Prince HN, Prince DL, Prince RN. 1991. Principles of viral control and transmission. In: Block SS. Disinfection, sterilization, and preservation. Philadelphia (PA): Lea and Febiger. [Google Scholar]
- 81.Reisz GR, Gammon RS. 1986. Toxic pneumonitis from mixing household cleaners. Chest 89:49–52. [DOI] [PubMed] [Google Scholar]
- 82.Ross MP, Spiller HA. 1999. Fatal ingestion of sodium hypochlorite bleach with associated hypernatremia and hyperchloremic metabolic acidosis. Vet Hum Toxicol 41:82–86. [PubMed] [Google Scholar]
- 83.Rutala WA, Weber DJHealthcare Infection Control Practices Advisory Committee (HICPAC). 2008. Guideline for disinfection and sterilization in healthcare facilities. Atlanta (GA): Centers for Disease Control. [Google Scholar]
- 84.Rutala WA, Weber DJ. 1997. Uses of inorganic hypochlorite (bleach) in healthcare facilities. Clin Microbiol Rev 10:597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sattar SA. 2010. Assessing the microbial activities of disinfectants and antiseptics: making label claims more relevant and reliable. In: Rutala WA. Disinfection, sterilization, and antisepsis: principles, practices, current issues, new research, and new technologies. Washington (DC): Association for Practitioners in Infection Control. [Google Scholar]
- 86.Serrano LJ. 1972. Dermatitis and death in mice accidentally exposed to quaternary ammonium disinfectant. J Am Vet Med Assoc 161:652–655. [PubMed] [Google Scholar]
- 87.Shek WR, Gaertner DJ. 2002. Microbiological quality control for laboratory rodents and lagomorphs, p 365–393. In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine. San Diego (CA): Academic Press. [Google Scholar]
- 88.Shin GA, Sobsey MD. 2008. Inactivation of norovirus by chlorine disinfection of water. Water Res 42:4562–4568. [DOI] [PubMed] [Google Scholar]
- 89.Sigstam T, Gannon G, Cascella M, Pecson BM, Wigginton KR, Kohn T. 2013. Subtle differences in virus composition affect disinfection kinetics and mechanisms. Appl Environ Microbiol 79:3455–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sigstam T, Rohatschek A, Zhong Q, Brennecke M, Kohn T. 2014. On the cause of the tailing phenomenon during virus disinfection by chlorine dioxide. Water Res 48:82–89. [DOI] [PubMed] [Google Scholar]
- 91.Sirikanchana K, Shisler JL, Marinas BJ. 2008. Inactivation kinetics of adenovirus serotype 2 with monochloramine. Water Res 42:1467–1474. [DOI] [PubMed] [Google Scholar]
- 92.Spaulding EH. 1968Chemical disinfection of medical and surgical materials. In: Lawrence C, Block SS. Disinfection, sterilization, and preservation. Philadelphia (PA): Lea and Febiger. [Google Scholar]
- 93.Spaulding EH. 1977. Chemical disinfection of medical and surgical materials. In: Block SS. Disinfection, sterilization, and preservation. Philadelphia (PA): Lea and Febiger. [Google Scholar]
- 94.Springthorpe VS, Grenier JL, Lloyd-Evans N, Sattar SA. 1986. Chemical disinfection of human rotaviruses: efficacy of commercially available products in suspension tests. J Hyg (London) 97:139–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steinmann J. 2001. Some principles of virucidal testing. J Hosp Infect 48 Suppl A:S15–17. [DOI] [PubMed] [Google Scholar]
- 96.Steinmann J. 2004. Surrogate viruses for testing virucidal efficacy of chemical disinfectants. J Hosp Infect 56 Suppl 2: S49–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Su X, D'Souza DH. 2012. Inactivation of human norovirus surrogates by benzalkonium chloride, potassium peroxymonosulfate, tannic acid, and gallic acid. Foodborne Pathog Dis 9:829–834. [DOI] [PubMed] [Google Scholar]
- 98.Taylor GR, Butler M. 1982. A comparison of the virucidal properties of chlorine, chlorine dioxide, bromine chloride and iodine. J Hyg (Lond) 89:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terpstra FG, van den Blink AE, Bos LM, Boots AG, Brinkhuis FH, Gijsen E, van Remmerden Y, Schuitemaker H, van ’t Wout AB. 2007. Resistance of surface-dried virus to common disinfection procedures. J Hosp Infect 66:332–338. [DOI] [PubMed] [Google Scholar]
- 100.US Department of Health and Human Services, Centers for Disease Control, NIH. 2009. Biosafety in microbiological and biomedical laboratories. Washington (DC): Government Printing Office. [Google Scholar]
- 101.Valot S, Edert D, Le Faou A. 2000. A simple method for the in vitro study of the virucidal activity of disinfectants. J Virol Methods 86:21–24. [DOI] [PubMed] [Google Scholar]
- 102.Weber DJ, Barbee SL, Sobsey MD, Rutala WA. 1999. The effect of blood on the antiviral activity of sodium hypochlorite, a phenolic, and a quaternary ammonium compound. Infect Control Hosp Epidemiol 20:821–827. [DOI] [PubMed] [Google Scholar]
- 103.White CW, Martin JG. 2010. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc 7:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Whitehead K, McCue KA. 2010. Virucidal efficacy of disinfectant actives against feline calicivirus, a surrogate for norovirus, in a short contact time. Am J Infect Control 38:26–30. [DOI] [PubMed] [Google Scholar]
- 105.Wu YT, Zhu H, Willcox M, Stapleton F. 2011. The effectiveness of various cleaning regimens and current guidelines in contact lens case biofilm removal. Invest Opthalol Vis Sci 52: 5287–5292. [DOI] [PubMed] [Google Scholar]
- 106.Zeitler B, Rapp I. 2014. Surface-dried viruses can resist glucoprotamin-based disinfection. Appl Environ Microbiol 80:7169–7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zoni R, Zanelli R, Riboldi E, Bigliardi L, Sansebastiano G. 2007. Investigation on virucidal activity of chlorine dioxide. experimental data on feline calicivirus, HAV, and Coxsackie B5. J Prev Med Hyg 48:91–95. [PubMed] [Google Scholar]