Abstract
Short-chain cyanoacrylates (SCCA), such as ethyl-2-cyanoacrylate (KrazyGlue, Aron Alpha, Columbus, OH) are commonly used as commercial fast-acting glues. Although once used in clinical medicine as skin adhesives, these products caused tissue toxicity and thus their use in live tissue was discontinued. SCCA were replaced by longer-chain versions (LCCA), such as butyl-cyanoacrylate (Vetbond, 3M, St Paul, Minnesota), which were found to be less toxic than the short-chain formulations. Some researchers prefer to use SCCA due to the belief that they create a stronger bond than do the longer-chain counterparts. In survival surgeries, we compared the bone thickness, bone necrosis, fibrosis, inflammation, and bone regeneration in the calvaria of control (naïve), surgery-only, SCCA-treated, and LCCA-treated mice (n = 20 per group). At 1 and 14 d after surgery, all mice except those treated with SCCA showed statistically similar bone measurements to those of the naive control group. The SCCA group had significantly less bone regeneration than did all other groups. These results suggest that the application of SCCA causes bone damage resulting in the loss of bone regeneration. This finding may assist investigators in choosing a tissue glue for their studies and may support the IACUC in advocating the use of pharmaceutical-grade tissue glues.
Abbreviations: LCCA, long-chain cyanoacrylates; SCCA, short-chain cyanoacrylates
Cyanoacrylates have been used as tissue adhesives since their synthesis in 1949.6 Synthetic cyanoacrylate adhesives belong in the family of liquid monomers formed by alkyl esters of 2-cyanoacrylic acid. The basic formula of the cyanoacrylate adhesive (alkyl-2-cyanoacrylate) has been manipulated to form different cyanoacrylate adhesives with different properties.29 Several 2-cyanoacrylate esters have been synthesized by changing the length of the alkyl chain attached.42 The first cyanoacrylates were short-chained, poorly manufactured, and toxic to animals at pharmacologic doses.24 Short-chain cyanoacrylates (SCCA), such as methyl-2-cyanoacrylate and ethyl-2-cyanoacrylate (KrazyGlue [Aron Alpha, Columbus, OH]), continue to be used as fast-acting adhesives.5 Although appropriated as tissue glues soon after their discovery, these early SCCA caused tissue toxicity and thus were discontinued in the clinical arena.4 Research showed that changing the type of alcohol in the compound to one with a longer molecular chain reduced tissue toxicity. Over time, nontoxic, longer-chain cyanoacrylates (LCCA), such as butyl-cyanoacrylate (Vetbond [3M, St Paul, Minnesota]) and octylcyanoacrylate, were manufactured, leading to their use once again in clinical medicine33 (Figure 1).
Figure 1.
Trade names for different types of cyanoacrylates.
Many researchers contend that SCCA is superior to LCCA in regard to the strength and tenacity of the bond when used to create cranial windows and as an application prior to an overlay of acrylic for cranial implants. However, SCCA is not pharmaceutical-grade, as mandated by the USDA in Policy 32 and the Guide for the Care and Use of Laboratory Animals,22 and therefore can only be used after specific review and approval by an institution's IACUC. The determination for substitution is generally based on scientific necessity or compound availability.
In this study, the effects of applying an SCCA product to mice calvaria were compared with those of an LCCA glue. Specifically, we evaluated bone regeneration, osteocyte numbers, inflammation, and bone remodeling at 2 time points after application. We hypothesized that mice calvaria treated with the SCCA product would show signs of toxicity, compared with skulls treated with the LCCA glue.
Materials and Methods
Animals.
We obtained 80 female CD1 (weight, 20 to 24 g; age: 35 d, n = 70; 49 d, n = 10) from Charles River Laboratories (Wilmington, MA). All mice were housed in an SPF, AAALAC-accredited facility, where sentinel mice were tested quarterly and remained negative for mouse parvovirus, minute virus of mice, mouse norovirus, mouse hepatitis virus, Sendai virus, lymphocytic choriomeningitis virus, polyomavirus, K virus, pneumonia virus of mice, mouse adenovirus, Rotavirus, Mouse encephalomyelitis virus, reovirus, ectromelia virus, Mycoplasma pulmonis, and Helicobacter spp. as well as endo- and ectoparasites. After experimental manipulation, mice were singly housed in polycarbonate cages (Lab Products, Seaford, DE) on corncob bedding (BedO'Cobs, The Andersons, Maumee, OH) and were given enrichment nesting material (Cotton squares, Ancare, Bellmore, NY). Mice had unrestricted access to rodent diet (PMI Nutrition International, Richmond, IN) and water. The animal room was environmentally controlled: temperature, 68 to 79 °F (20.0 to 26.1 °C); relative humidity, 30% to 70%; and a 12:12-h light:dark cycle. The IACUC of the University of California–Los Angeles approved the use of animals in this study.
Experimental groups.
The 80 mice were divided into experimental groups as follows. The first group of 20 mice (10 each at 35 and 49 d of age) had no experimental manipulation and were used as baseline controls. These mice were euthanized on arrival at the facility. A group of 20 mice (35 d old) were surgery-only controls and underwent the surgical approach to expose the skull, but no bonding agent was applied. Of these mice, 10 each were euthanized at 1 and 14 d after surgery. Another group of 35-d-old mice (n = 20) underwent cranial bone exposure followed by the application of an LCCA tissue glue (Vetbond); 10 each of these mice were euthanized at 1 and 14 d after surgery. The remaining 20 mice (age, 35 d) underwent cranial bone exposure followed by the application of an SCCA product (KrazyGlue); 10 each of these mice were euthanized at 1 and 14 d after surgery.
Anesthesia and analgesia.
Anesthesia was induced by placing mice in an anesthesia chamber containing 5% isoflurane and then maintained with 2% isoflurane by mask. Oxygen was at a flow rate of 0.5L/min. Each mouse was weighed and received buprenorphine (0.1mg/kg SC) at the time of induction. The skull was shaved, followed by 3 alternating scrubs of povidone–iodine scrub and alcohol, and then transferred aseptically to a surgery table, where isoflurane was maintained at 2%. Each surgery was performed under sterile conditions with sterile drapes, instruments, and gloves.
Surgery.
All experimental mice were age-matched. A 1-cm, full-thickness incision was made on the midline of the cranium by using a no. 15 surgical blade, and the underlying skull was exposed by gentle dissection with a cotton swab. For the surgery-only controls, the skin was closed with wound clips after dissection with cotton swabs. For experimental mice, a 25-gauge needle was attached to the tube of each bonding agent and used to apply 1 drop to the skull, in the same area for each mouse. Both products spread to the limit of the skin, thus covering the entire surgical area. Once the product had dried, the skin was closed over the skull by using wound clips. The toenails of all 4 feet were trimmed to reduce postsurgical trauma, and the mice were then allowed to recover from anesthesia. The same surgeon performed all surgeries.
At either 1 or 14 d after the application of the bonding agents, mice were euthanized by CO2 inhalation, and their heads (rostral to the occiput) were collected, fixed in 10% formalin for 24 h, and then decalcified by using 10% hydrochloric acid overnight. The entire skull was sectioned from the nose to the occipital crest in approximately 1/8-in. increments. All tissues were processed, embedded in paraffin, and sectioned to a thickness of 5 μm by using a microtome. The section used to measure bone thickness corresponded to the cross section that included the optic chiasm. For each mouse, a 40× digital image was collected approximately 0.3 cm right or left from the midline suture, wherever the tissue damage was the greatest. In addition, the surgery-only and unmanipulated control mice were euthanized and their tissues processed as described.
One researcher, blinded to the identity of the subject, chose 3 representative areas of bone on each 40× digital image and measured the bone thickness in nanometers; the average of these 3 measurements was recorded. Another blinded researcher, given the same set of instructions, measured 3 representative areas on each of 10 random samples, acting as a control for the first researcher.
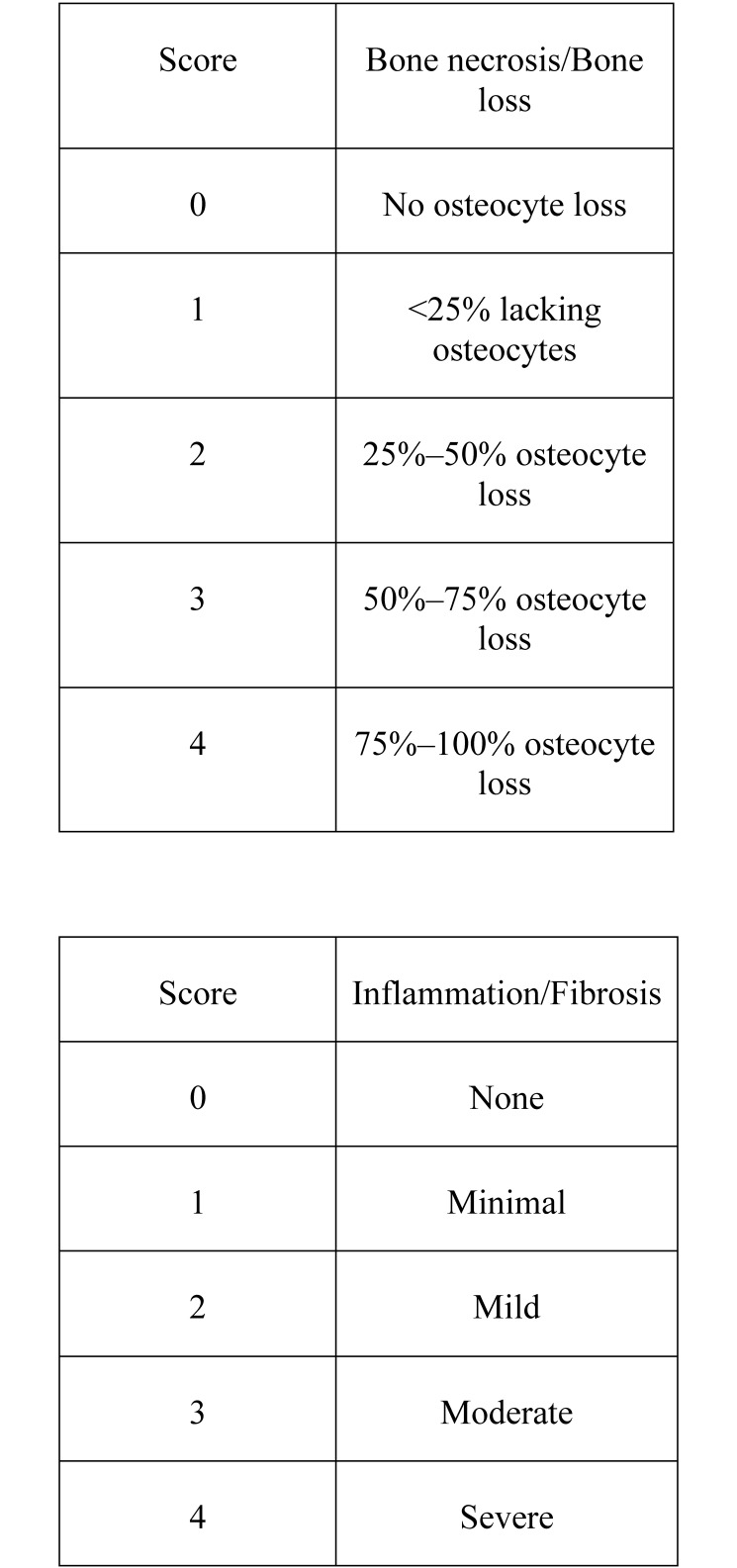
In addition, each section was scored for bone necrosis, bone remodeling, inflammation, and fibrosis. Bone necrosis and bone remodeling both were scored a scale of 0 to 4 (Figure 2).
Figure 2.
Histologic scoring scale.
Statistical methods.
The JMP statistical program (SAS Institute, Cary, NC) was used to analyze the results. ANOVA was performed on the mean measurements from the 4 groups of mice on each of the 2 test days. Histologic scores were analyzed by using nonparametric comparisons for each pair according to the Wilcoxon method. A P value less than 0.05 was considered significant.
Results
Interobserver reliability correlation for the bone measurements was R2 = 0.99, indicating excellent correlation. To establish a baseline for bone regeneration, we compared the calvarial thickness of 35- and 49-d-old unmanipulated mice. The average thickness increased from 189,010 nm in 35-d-old mice to 251,053 nm in 49-d-old mice.
On day 1 after surgery, bone measurements were similar between untreated control and LCCA-treated mice. Bone was thinner (P < 0.032) in mice treated with SCCA or surgery only than in the untreated control and LCCA-treated groups.
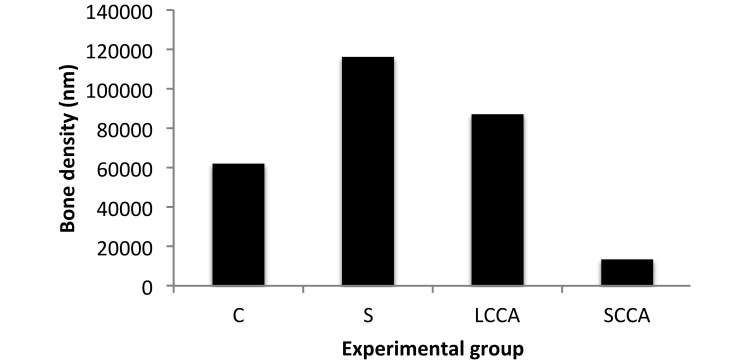
At 14 d after surgery, bone measurements were similar between the control, surgery-only, and LCCA-treated mice, with marked bone regeneration evident. In comparison, the SCCA-treated animals showed significant (P < 0.05) lack of bone regeneration. In addition, the surgery-only group showed more (P < 0.05) bone regeneration than did the unmanipulated control mice. Only histologic scores regarding lack of bone regeneration differed between any groups, with significant (P < 0.05) lack of regeneration at 14 d after surgery in the SCCA-treated mice compared with the LCCA-treated group (Figure 3).
Figure 3.
Change in bone density between days 1 and 14. C, control (naïve) mice; S, surgery-only mice; LCCA, mice treated with long-chain cyanoacrylate tissue glue; SCCA, mice treated with short-chain cyanoacrylate product.
Discussion
Multiple human and veterinary versions of tissue glue are available, and studies have shown that their efficacy and outcomes are similar to those of sutures.30,34,35 In addition to their use in skin closure, cyanoacrylate tissue adhesives have been used for bronchoplueral fistula repair, endoscopic treatment of ulcers, high-risk intestinal anastomoses, middle-ear surgery, and inguinal hernia repair.10 These products have also been used as an adjuvant in laboratory animal medicine for cranial implants1,7 and cranial windows, where cover glasses are glued onto the skulls of mice21.
In rabbits, ethyl-2-cyanoacryalte produced severe histotoxicity compared with the minimal toxic effects of 2-butyl-cyanoacrylate; specifically, ethyl-2-cyanoacrylate induced a pronounced acute inflammatory response with a persistent foreign-body giant-cell reaction.38 Degradation of short side chains such as methyl and ethyl-2 incited a strong acute inflammatory response followed by a chronic foreign-body giant-cell reaction.26 The SCCA are thought to degrade rapidly, thereby releasing large amounts of toxic byproducts such as cyanoacetate and formaldehyde,40 whereas the slower degradation of LCCA is considered to create less of an inflammatory effect.3,28,39 At 15 d after application in rabbits, ethyl-cyanoacrylate induced an inflammatory reaction around the adhesive that was to biodegradation and the release of cyanoacetate and formaldehyde during curing.31 Furthermore, ethyl-cyanoacrylate on rabbit skull elicited an intense inflammatory reaction within 10 d.12
Investigations with LCCA such as butyl-cyanoacrylate have yielded varied results. In a histologic study, bone growth was not impaired by using butyl-2-cyanoacylate,14 similar to another study in which isobutyl-2-cyanoacrylate (in limited amounts) did not harm bone and cartilage or retard adjacent fracture healing.16 When the effects of butyl-cyanoacrylate on rabbit zygomatic bone was studied, however, there was evidence of mild inflammation, with inflammation regression at day 14 after application.8 Another study concluded that although the long-chain alkyl group cyanoacrylates caused a mild tissue reaction, that reaction contributed to acting as a barrier to callus formation.11 A similar study found that the limitations to isobutyl-2-cyanoacrylate included impermeability to osteogenic cells, decreased tensile strength over time, and slow biodegradability.15 In contrast, other researchers found that butyl-2-cyanoacrylate did not result in inflammatory response in tissues adjacent to its placement, including the scalp, bone, and brain,32 and further hypothesized that the degenerating glue provided a scaffold for new bone formation, stimulating an inflammatory response that might provide growth factors, particularly bone morphogenetic proteins.32
Researchers sometimes challenge veterinarians and IACUC to provide proof that the use of nonpharmaceutical-grade compounds is not safe for the animals. In this study, we compared a commonly requested SCCA substitute for tissue glue with a veterinary-approved LCCA product. We measured bone thickness at 2 time points and examined several histologic parameters, including inflammation, osteocyte necrosis, bone loss, and fibrosis.
We established a baseline of bone growth by comparing the bone thickness of 35-d-old mice with that of 49-d-old animals. By comparing the other experimental groups with the control, we were able to assess whether the manipulations we performed had any effect on bone thickness or growth. At 1 d after surgery, calvarial bone was thinner (P < 0.032) in the SCCA-treated mice than in the surgery-only, control, or LCCA-treated groups. At both 1 and 14 d after surgery, the surgery-only group showed a significant increase in bone thickness compared with all other groups, including the unmanipulated control group, suggesting that surgery alone stimulates bone regeneration.
At 14 d after surgery, bone regeneration was similar between the LCCA-treated and control mice. The SCCA group was the only group that showed a significant lack of bone growth, suggesting that the SCCA product inhibited regeneration of bone whereas the LCCA glue and surgical manipulation did not. However, the surgery-only mice showed greater bone regeneration than did the unmanipulated and LCCA-treated mice. If we assume that the bone regeneration seen in the surgery-only mice is the normal bone reaction to surgery, then it becomes apparent that both the LCAA glue and SCCA glue interfered with regeneration. However, although both products might have dampened the body's natural reaction, the SCCA glue clearly had the greater effect on bone regeneration (Figure 3).
Comparing the groups histologically in regard to bone loss, inflammation, fibrosis, and bone necrosis revealed significant changes only in the bone loss category, specifically between the SCCA and LCCA groups at 14 d after treatment. This result implies that the SCCA product was significantly more damaging than was the LCCA tissue glue.
The reasons for the lack of bone regeneration in SCCA-treated mice likely are varied and multifactorial. The periosteum contains the cellular layer that is osteogenic,36 and the cotton-tipped applicator used to clean the muscle layers of the skull might have disturbed the integrity of the bone periosteum.1 In light of the delicate balance between osteoblastic and osteoclastic activity in the regeneration of bone and given that the periosteum is required for the regeneration process, the cyanoacetate and formaldehyde produced as byproducts by the SCCA used31 might have destroyed osteoblasts or retarded their activation. Compared with LCCA, SCCA are known to produce more and stronger toxic byproducts, which in turn interfere with healing.11,40 The decreased bone measurement of SCCA-treated mice compared with the unmanipulated control mice at 1 d after surgery was surprising and suggests that the SCCA product caused sufficient damage during just the first 24 h after application to cause significant bone loss.
Another possible explanation for the lack of regeneration in the SCCA-treated mice might be related to the heat that the compound generates. Studies have shown that osteoblast-like cells are susceptible to heat-induced cellular death.9,25 Cyanoacrylates generate heat when they transform from the monomeric to polymeric form.40 Large quantities of cyanoacrylates are associated with cumulative heat dissipated during polymeration.38 The rate at which heat can dissipate is dependent on the monomer. One study showed that methyl-2-cyanoacyrlate, an SCCA, yielded a net rise of 4 °C, whereas butyl-2-cyanoacrulate showed a 1.5 °C rise in temperature.19 Another study documented a temperature increase of 19.1 °C per microliter of methyl-cyanoacrylate compared with 8.6 °C per microliter of isobutyl-acrylate.27 Clinically, there have been occasional cases where humans have been burned by application of the 2-cyanoacrylates, including the SCCA product tested,13,23,37 as well as reports of mild discomfort due to the heat of polymerization.41 We did not measure the temperatures associated with the 2 products we tested, so we cannot say whether temperature played an important role in the results. This area, however, merits further investigation.
Angiogenesis also plays an important role in bone repair,20 and the inhibition of angiogenic growth factors can dramatically alter this process.17 One group found that the 2-ethyl-cyanoacryate affected new bone formation by delaying the formation of osseous trabeculae until after the adhesive was phagocytized.12 Either individually or in combination, the heat, toxic byproducts, or the physical presence of the SCCA product we used might have interrupted angiogenesis, resulting in delayed bone repair. In addition, the physical presence of the glue might have altered the healing process.
One study documented signs of bone healing at 64 d after cyanoacrylate administration,15 and another group found evidence of bone remodeling at 120 d after treatment with 2-ethyl-cyanoacrylate.31 Because a wound typically is considered to be healed after 10 to 14 d, we chose 14 d as the end point for the current study. Perhaps the calvaria treated with the SCCA compound might have regenerated after a longer period; however, this evaluation was beyond the scope of this study.
Cyanoacrylates were adopted for medical use soon after their discovery as strong adhesives. However, the use of cyanoacrylates in humans was discontinued after unacceptable results regarding bonding strength, tissue toxicity, and possible carcinogenesis emerged.18 The Food and Drug Administration ultimately banned methylcyanoacrylates for human use.18 In comparison, LCCA have fewer toxic effects, resulting in their approval for human and animal use in the clinical realm. Our current study showed that, unlike the LCCA glue evaluated, the SCCA product inhibits bone regeneration. Although the exact mechanism underlying the inhibition of bone regeneration is unknown, investigators and IACUC should consider this effect when choosing a bonding agent for work on live animal tissues.
Acknowledgments
Special thanks to Lisa Williams, Luis Papa, and Aulani Navarro-King and to all of the dedicated DLAM staff members.
References
- 1.Agterberg MJ, Spoelstra EN, van der Wijst S, Brakkee JH, Wiegant VM, Hamelink R, Brouns K, Westerink BH, Remie R. 2010. Evaluation of temperature rise and bonding strength in cements used for permanent head attachments in rats and mice. Lab Anim 44:264–270. [DOI] [PubMed] [Google Scholar]
- 2.Animal Welfare Act as Amended. 2008. 7 USC §2131–2159. [Google Scholar]
- 3.Cascarini L, Kumar A. 2007. Case of the month. Honey I glued the kids—tissue adhesives are not the same as “superglue”. Emerg Med J 24:228–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou SN. 1977. Use of cyanoacrylate. J Neurosurg 46:266. [DOI] [PubMed] [Google Scholar]
- 5.Clarke TF. 2011. Cyanoacrylate glue burn in a child—lessons to be learned. J Plast Reconstr Aesthet Surg 64:e170–e173. [DOI] [PubMed] [Google Scholar]
- 6.Coover HN, Joiner FB, Sheere NH. 1959. Chemistry and performance of cyanoacrylate adhesive. J Soc Plast Surg Eng 15:5–6. [Google Scholar]
- 7.Criado A, Barford J, Parker F, Bate S, Whelan G. 2003. Use of cyanoacrylate gel as a substitute for dental cement in intracerebroventricular cannulations in rats. ContempTop Lab Anim Med 42:13–16. [PubMed] [Google Scholar]
- 8.Dadas B, Alkan S, Cifci M, Basak T. 2007. Treatment of tripod fracture of zygomatic bone by N-2-butyl cyanoacrylate glue fixation, and its effects on the tissues. Eur Arch Otorhinolaryngol 264:539–544. [DOI] [PubMed] [Google Scholar]
- 9.Dolan EB, Haugh MG, Tallon D, Casey C, McNamara LM. 2012. Heat-shock-induced cellular responses to temperature elevations occurring during orthopaedic cutting. J R Soc Interface 9:3503–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edlich RF, Gubler K, Stevens HS, Wallis AG, Clark JJ, Dahlstrom JJ, Rhoads SK, Long WB., 3rd 2010. Scientific basis for the selection of surgical staples and tissue adhesives for closure of skin wounds. J Environ Pathol Toxicol Oncol 29:327–337. [DOI] [PubMed] [Google Scholar]
- 11.Ekelund A, Nilsson OS. 1991. Tissue adhesives inhibit experimental new bone formation. Int Orthop 15:331–334. [DOI] [PubMed] [Google Scholar]
- 12.Esteves JC, Borrasca AG, Aranega AM, Garcia Junior IR, Filho OM. 2011. Histomorphometric analysis of the repair process of autogenous bone grafts fixed at rat calvaria with cyanoacrylate. J Appl Oral Sci 19:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez Arenas ME, Ruiz Moreno O, Marcuello Melendo B, Ferrer Novella E, Torron Fernandez-Blanco C, Honrubia F. 2002. Orcular cyanoacrylate injury. Arch Soc Esp Oftalmol 77:47–50.[Article in Spanish]. [PubMed] [Google Scholar]
- 14.Gosain AK, Plastic Surgery Educational Foundation DATA Committee. 2002. The current status of tissues glue: I. For bone fixation. Plast Reconstr Surg 109:2581–2583. [DOI] [PubMed] [Google Scholar]
- 15.Hampel NL, Pijanowski GJ, Johnson RG. 1986. Effects of isobutyl-2-cyanoacrylate one bone healing. Am J Vet Res 47:1605–1610. [PubMed] [Google Scholar]
- 16.Harper MC, Ralston M. 1983. Isobutyl-2-cyanoacrylate as an osseous adhesive in the repair of osteochondral fractures. J Biomed Mater Res A 17:167–177. [DOI] [PubMed] [Google Scholar]
- 17.Hausman MR, Schaffler MB, Majeska RJ. 2001. Prevention of fracture healing in rats by an inhibitor of angiogensis. Bone 29:560–564. [DOI] [PubMed] [Google Scholar]
- 18.Heiss C, Kraus R, Schluckebier D, Stiller A-C, Wenisch S, Schnettler R. 2006. Bone adhesives in trauma and orthopedic surgery. Eur J Trauma 32:141–148. [Google Scholar]
- 19.Hida T, Sheta SM, Proia AD, McCuen BW., 2nd 1988. Retinal toxicity of cyanoacrylate tissue adhesive in the rabbit. Retina 8:148–153. [DOI] [PubMed] [Google Scholar]
- 20.Holstein JH, Becker SC, Fiedler M, Garcia P, Histing T, Klein M, Laschke MW, Corsten M, Pohlemann T, Menger MD. 2011. Intravital microscopic studies of angiogensis during bone defect healing in mice calvaria. Injury 42:765–771. [DOI] [PubMed] [Google Scholar]
- 21.Holtmaat A, de Paola V, Wilbrecht L, Trachtenerg JT, Svoboda K, Portera-Cailliau C. 2012. Imaging neocortical neurons through a chronic cranial window. Cold Spring Harb Protoc 2012:694 –701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th edition. Washington (DC): National Academic Press. [Google Scholar]
- 23.Jamnadas-Khoda B, Khan MA, Thomas GP, Ghosh SJ. 2011. Histoacryl glue: a burning issue. Burns 37:e1–e3. [DOI] [PubMed] [Google Scholar]
- 24.Leonard F. 1968. The N-alkaalphacyanoacrylate tissue adhesives. Ann N Y Acad Sci 146:203–213. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Chien S, Branemark P. 1999. Heat shock-induced necrosis and apoptosis in osteoblasts. J Orthop Res 17:891–899. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Heisterkiamp CA., 3rd 1969. Long-term study of aerosol cyanoacrylate tissue adhesive spray: carcinogenicity and other untowrad effects. Am Surg 35:825–827. [PubMed] [Google Scholar]
- 27.Morikawa K. 1990. Biochemical study on the application of α-cyanoacrylate instant adhesives in dentistry. Shikwa Gakuho 90:201–224.[Article in Japanese]. [PubMed] [Google Scholar]
- 28.Pani KC, Gladieux G, Brandes G, Kulkarni R, Lenoard F. 1968. The degrdation of n-butylalphacyanoacrylate tissue adhesive.II. Surgery 63:481. [PubMed] [Google Scholar]
- 29.Quinn J. 2005. Tissue adhesives in clinical medicine. Palo Alto (CA): BC Decker. [Google Scholar]
- 30.Quinn JV, Drezwiecki A, Li MM, Stiell IG, Sutcliff T, Elmslie TJ, Wood WE. 1993. A randomized, controlled trial comparing a tissue adhesive with suturing in the repair of pediatric facial lacerations. Ann Emerg Med 22:1130–1135. [DOI] [PubMed] [Google Scholar]
- 31.Saska S, Hochuli-Vieira E, Minarelli-Gaspar AM, Gabrielli MF, Capela MV, Gabreilli MA. 2009. Fixation of autogenous bone grafts with ethyl-cyanoacrylate glue or titanium screws in the valvaria of rabbits. Int J Oral Maxillofac Surg 38:180–186. [DOI] [PubMed] [Google Scholar]
- 32.Shermak MA, Wong L, Inoue N, Crain BJ, Im MJ, Chao EY, Manson P. 1998. Fixation of the craniofacial skeleton with butyl-2-cyanoacrylate and healing. Plast Reconstr Surg 102:309–318. [DOI] [PubMed] [Google Scholar]
- 33.Singer AJ, Quinn JV, Hollander JE. 2008. The cyanoacrylate topical skin adhesives. Am J Emerg Med 26:490–496. [DOI] [PubMed] [Google Scholar]
- 34.Singer AJ, Quinn JV, Clark RE, Hollander JE, TraumaSeal Study Group. 2002. Closure of lacerations and incisions with octylcyanoacrylate: a multi-center randomized clinical trial. Surgery 131:270–276. [DOI] [PubMed] [Google Scholar]
- 35.Souza SC, Oliveira WL, Soares D, Briglia CH, Athanazio PR, Cerqueira MD, Guimaraes PH, Cerreiro MC. 2007. Comparative study of suture and cyanoacrylates in the skin closure of rats. Acta Cir Bras 22:309–316. [DOI] [PubMed] [Google Scholar]
- 36.Stedman TL. 2006. Stedman's medical dictionary, 28th ed. Philadelphia (PA): Lippincott, Williams, and Wilkins. [Google Scholar]
- 37.Takeru N, Keisuki I, Hiroyuki K, Takuya F, Arita K, Toshinori M. 2003. Burn caused by a cyanoacrylate adhesive agent: a case report. Jpn J Burn Injury 29:49–53. [Google Scholar]
- 38.Toriumi DM, Raslan W, Friendman M, Tard E. 1990. Histotoxicity of cyanoacrylate tissue adhesives. Arch Otolaryngol Head Neck Surg 116:546–550. [DOI] [PubMed] [Google Scholar]
- 39.Tseng YC, Tabata M, Hyon SH, Ikada Y. 1990. In vitro toxicity test of 2-cyanoacrylate polymers by cell culture method. J Biomed Mater Res 24:1355–1367. [DOI] [PubMed] [Google Scholar]
- 40.Vinters HV, Galil KA, Lundie MJ, Kaufmann JCE. 1985. The histotoxicity of cyanoacrylates. A selective review. Neuroradiology 27:279–291. [DOI] [PubMed] [Google Scholar]
- 41.Watson DP. 1989. Use of cyanoacrylate tissue adhesive for closing facial lacerations in children. BMJ 299:1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber SC, Chapman MW. 1984. Adhesives in orthopaedic surgery. A review of the literature and in vitro bonding strengths of bone-bonding agents. Clin Orthop Relat Res 191:249–261. [PubMed] [Google Scholar]