Abstract
Sex steroid hormones are major determinants of bone morphology and quality and are responsible for sexually dimorphic skeletal traits. Hypogonadism results in suboptimal skeletal development and may lead to an increased risk of bone fracture later in life. The etiology of delayed puberty and/or hypothalamic amenorrhea is poorly understood, and experimental animal models addressing this issue are predominantly based upon short-term experimental induction of hormonal suppression via gonadotropin releasing hormone antagonists (GnRH-a). This acute change in hormone profile does not necessarily emulate the natural progression of hypogonadic bone disorders. We propose a novel animal model with which to explore the effects of chronic hypogonadism on bone quality, the naked mole-rat (NMR; Heterocephalus glaber). This mouse-size rodent may remain reproductively suppressed throughout its life, if it remains as a subordinate within the eusocial mole-rat colony. NMRs live in large colonies with a single dominant breeding female. She, primarily by using aggressive social contact, naturally suppresses the hypothalamic gonadotropic axis of subordinate NMRs and thereby their reproductive expression. However should an NMR be separated from the dominant breeder, within less than a week reproductive hormones may become elevated and the animal attains breeding status. We questioned if sexual suppression of subordinates impact upon the development and maintenance of the femora, and lead to a sexually indistinct monomorphic skeleton. Femora were obtained from male and female NMRs that were either non-breeders (subordinate) or breeders at the time of sacrifice. Diaphyseal cross-sectional morphology, metaphyseal trabecular micro-architecture and tissue mineral density of the femur was measured using MicroComputed tomography and diaphyseal mechanical properties were assessed by four-point bending tests to failure.
Subordinates were sexually monomorphic and showed no significant differences in body weight or femoral bone structure and quality between male and females. Femora of subordinate females differed significantly from that of breeding animals, whereas in males, the divergent trend among breeders and non-breeders did not reach statistical significance. Subordinate NMRs, naturally suppressed from entering puberty, may prove to be a useful model to tease apart the relationship between bone morphology and hypogonadism and evaluate skeletal development during pubertal maturation.
Keywords: Naked mole-rats, bone strength, bone quality, aging, hypogonadic, delayed puberty
Introduction
Sexual dimorphism in bone traits is attributed to the differential action of androgens and estrogen. Current studies show that the actions of androgens at puberty are mediated by estrogen [1, 2], and that differences in hormone levels among males and females results in pronounced sexual dimorphism in most bone traits. Indeed, subperiosteal expansion during the adolescent growth spurt is greater in males resulting in a larger bone diameter [3]. Current studies show that the actions of androgens at puberty are mediated by estrogen [1, 2]. Chronic estrogen deficiency during growth and maturity has negative effects on the skeleton of both sexes. Young female athletes commonly show a delay in the onset of puberty, delayed menarche, and increased fracture risk despite the bone-strengthening effects generally associated with high levels of activity [4, 5]. Even in female athletes that have completed puberty, exercise induced amenorrhea, associated with low body fat, is a common occurrence, and, like delayed menarche, leads to suboptimal skeletal development, and a concomitant decrease in bone strength, bone mass, and an increase in the incidence of bone fragility [4, 5]. The etiology of delayed puberty and/or hypothalamic amenorrhea is however currently unknown.
Male endurance athletes also have very low levels of body fat, and exhibit concomitant low levels of sex steroid hormones commensurate with hypogonadism. These athletes often present in later life with bone-related problems that are exacerbated if excessive training occurred during adolescence and development [6]. The effects of hypogonadism in males are equivocal [7, 8] and to a large extent depend upon the age at which hypogonadism occurred. A delay in puberty may result in slower rates of peak bone growth with a result that these males may fail to reach their genetic target height and have shorter stature than expected [8]. There are, however, also many reports that a delay in male pubertal onset results in tall stature as a result of persistent linear growth due to a delay in epiphyseal closure with concomitant delayed bone age, and increased risk for osteopenia/osteoporosis later in life [2, 9, 10]. Indeed, chronic hypogonadism during puberty may lead to a reduction in BMD and an increase in osteoporosis, osteopenia, eunuchoid skeletal traits and delayed epiphyseal closure in both males and females [10, 11].
Hypogonadism in both sexes impacts upon bone quality, resulting in impaired bone strength with reduced bone mass and changes in trabecular architecture and bone area [12-14]. Suboptimal skeletal development during puberty affects long-term bone strength, leading to an increase in fracture risk later in life [5, 14-16]. The effects of hypogonadism on both human and rodent (mice and rat) skeletal tissue have been extensively characterized in females and to a lesser degree in males. Hypogonadism in animal models is generally experimentally induced over a relatively short duration via gonadotropin releasing hormone (GnRH) antagonists (GnRH-a). While these studies have yielded considerable information in this regard, in particular about cortical bone remodeling through the use of double labeling, the short-term nature of these studies as well as the extended duration of bone remodeling and turnover, limits these studies in what they can ell us about “catch-up” growth or chronic hypogonadism and begs the question of whether or not there is a natural animal model for long-term hypogonadic effects on bone. One such novel animal model may be the Bathyergid rodent, the naked mole-rat (NMR; Heterocephalus glaber, Ruppell 1842).
NMRs are eusocial, exceptionally long-living (~30 years), mouse-size (~35 g) mammals that live in large subterranean colonies of up to 300 individuals in north east Africa [17, 18]. They have a strict social organization that culminates in the presence of a single breeding female in a colony [18]. She suppresses reproductive hormone levels and sexual maturation in both non-breeding males and females [17]. Any female, older than six months of age, within the colony is capable of becoming a breeder. Should the dominant female die or be removed from the colony, the remaining females removed from the stimuli for reproductive suppression will fight to the death to establish dominance and become reproductive [17, 19]. Similarly any female, older than 6 months, isolated from her natal colony, and either housed on her own or with a mate, may show reproductive hormone cycling, although not all females necessarily recover this capacity to breed after prolonged suppression. When a female becomes a breeder she exhibits an estrogen-dependent “pubescent” growth surge [20], and may increase her head to tail length 1.4-fold [19]. Breeding males do not show an obvious hormone-dependent growth surge, but nevertheless show markedly different steroid hormone profiles to those of their non-breeding siblings [21]. The dominant female will breed with her original mate for life, and also will select 1-3 of her sons as additional sires.
Non-breeding subordinate females are anovulatory due to the inactivity of GnRH and the low hormone levels of the hypothalamic pituitary gonadal axis (HPGA) [22, 23] while subordinate males have lower levels of plasma luteinizing hormone (LH) and testosterone and abnormal sperm development when compared to their breeding counterpart [24]. NMRs also have naturally low levels of thyroxine, insulin like growth factor (IGF) and vitamin D [25] and attenuated levels of these hormones also may impact upon the onset of puberty and skeletal maturation [26].
Suppression of reproductive hormones in NMRs can delay their puberty for life [20] and they remain monomorphic in overall body size, ano-genital distance, expression of a wide range of behaviors, brain and spinal cord structure and function [27, 28]. NMRs may show elevated sex steroid levels within 7 days of isolation from the dominant female. Activation of growth processes following “restoration of puberty” may take considerably longer, and growth changes occur over at least three and often as long as seven or eight pregnancy cycles. Growth surges that accompany the changes in breeding status of the dominant female appear to mainly target lumbar vertebral bone rather than limb bones [20]. This differential growth of the vertebrae with changes in reproductive status seems to be similar to that observed during delayed puberty in humans where they also have shorter stature attributed to decreased truncal growth rather than limb growth [29, 30] and may reflect differential sex steroid influence on axial and appendicular growth [29].
We questioned whether sexual suppression and the resulting low steroid hormones [31, 32] impact upon development, maintenance and mechanical properties of the femora, and if so, whether these sexually suppressed rodents showed signs of sexual dimorphism in their skeletal properties. We propose that suppression of puberty gives rise to a monomorphic skeleton in the NMR. Furthermore, because HPGA hormones remain low throughout the entire life span of subordinate male and female NMRs, and the relative ease of removing them from this suppression by simply isolating individuals from the dominant female, we propose the NMR as a new animal model to study the effects of chronic hormonal suppression on the mechanical and structural properties of the skeletal system.
Materials and methods
Animals study and design
Femora were obtained from both male (2-5 yrs, n = 7; 6-10 yrs, n = 3; 11-15 yrs, n = 4) and females (2-5 yrs, n = 9; 6-10 yrs, n = 4; 11-15 yrs, n = 7) young to middle aged NMRs, that were non breeders (subordinate) at the time of sacrifice. These age cohorts were chosen because we wanted to use only animals that were no longer growing (i.e. at least 2 years-old) and that would approximate humans at 7-10%, 25-30% and 45-50% of maximum lifespan and ought to represent peak bone quality as well as the periods in which noticeable changes in human bone are evident. These were compared with data from a small cohort of young to middle-aged NMRs breeders (male 2-7 yrs, n=4 and female 2-8 yrs, n=6). Although we did not measure hormone levels, since our colony has been extensively characterized in behavioral and physiological studies and obvious genitalia and skin color differences are evident amongst breeders and non-breeders, we were able to easily recognize breeders from non-breeders. All NMRs were born in captivity and maintained in colonies at The City College of New York (CCNY) and at the University of Texas Health Science Center at San Antonio in simulated, multi-chambered burrow systems under constant climatic conditions that were designed to approximate their native habitat (30°C; 75% RH). NMRs were fed fruit and vegetables ad libitum and supplemented with a high protein and vitamin enriched cereal (Pronutro, South Africa). All animal handling procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at both CUNY and UTHSCSA.
Physical bone traits
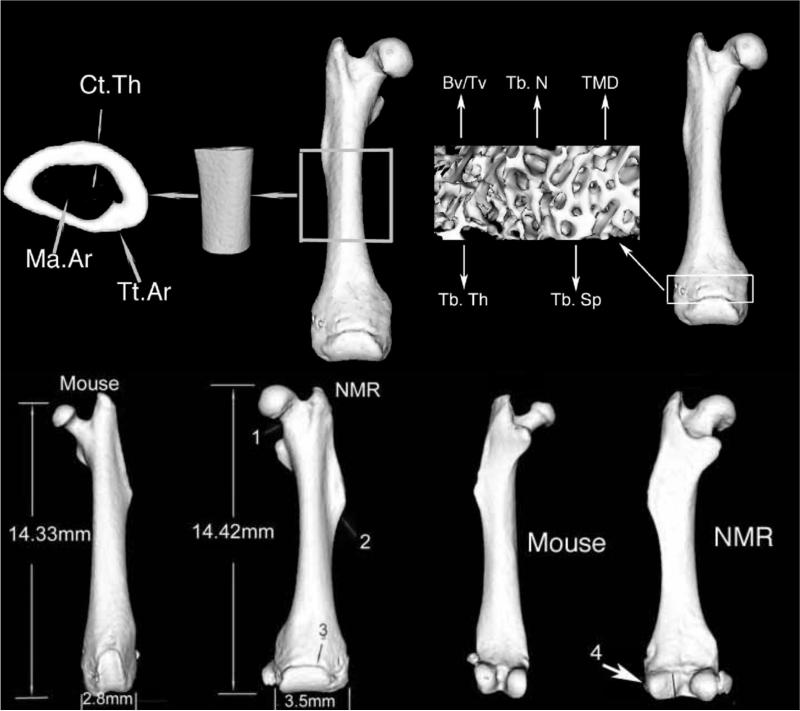
Diaphyseal cross-sectional morphology, metaphyseal trabecular micro-architecture and tissue mineral density (TMD) of the femur were measured using an Explore Locus SP Pre-Clinical Specimen MicroComputed Tomography system (GE Healthcare, London, Ontario, Canada). Three-dimensional images of the entire femur were reconstructed at 8.7 μm voxel size. The mid-diaphysis analysis region was standardized by selecting a 3mm region that was located immediately distal to the third trochanter while the trabecular bone analysis region was standardized by selecting a 1mm region that was located immediately under the distal growth plate (Fig. 1).
Fig 1.
3D rendered tomographic image of a naked mole-rat (NMR) femur showing the regions of analysis and representative mid-diaphyseal cross section and metaphyseal trabecular bone. The region through the mid shaft of the femur is used to measure total area (Tt.Ar.); marrow area (Ma.Ar) and cortical thickness (Ct.Th). Metaphyseal trabecular bone measurements included trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular spacing (Tb.Sp) and tissue mineral density (TMD), in addition the ratio of trabecular bone volume (Tv) to total bone volume (Bv) was calculated. This tomographic image of the NMR is shown next to that of a 16 week-old male C57BL/6J mouse. Note that the NMR has a wide femoral neck (1), a third trochanter that is enlarged and distally shifted (2), and a wide epiphysis (3). In addition, it has a lateral condyle that has a small articulating surface relative to the medial condyle (4).
Femoral mid-diaphyseal and trabecular metaphyseal images were individually analyzed using a standard thresholding algorithm to segment bone and non-bone voxels [33]. A custom analysis program (The Math-works, Inc., Natick, MA USA) was developed to quantify morphologic traits describing the amount of tissue (cortical area [Ct. Ar]; marrow area [Ma.Ar]; total area [Tt.Ar]; cortical thickness [Ct.Th] and the spatial distribution of tissue (moment of inertia [J.]). Moment of inertia is a measure of the proximity of the tissue to the geometric centroid of the cross section. The amount and distribution of tissue are both necessary to properly relate diaphyseal morphology to mechanical function, because bones having the same cross-sectional area but different moments of inertia will exhibit different mechanical behaviors under bending and torsional loads. Total bone area was defined as the sum of the cortical and marrow areas. The relative cortical area [RCA] was determined from the ratio of Ct. Ar/Tt. Ar and provided a measure of the proportion of the total area that was occupied by bone. These traits were quantified for each cross-section and the values were averaged over the volume of interest.
A custom analysis program (The Math-works, Inc., Natick, MA USA) was also developed to reduce noise in the raw trabecular volumes by using a median filter. Trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.N), trabecular separation (Tb.Sp) were assessed using MicroView advanced Bone analysis (v. 1.23; GE Healthcare).
Tissue mineral density (TMD) is the average mineral value of the bone voxels only and was expressed in hydroxyapatite (HA) density equivalents. TMD was calculated by converting the gray-scale output of bone voxels in Hounsfield units (HU) to mineral values (mg/cc of HA) through the use of a calibration phantom containing air, water and HA (SB3:Gamex RMI, Middleton, WI, USA). TMD in cortical and trabecular bone was defined as the average bone voxel HU value divided by the average value of the HA phantom multiplied by 1130 mg/cc (HA physical density). The same calibration phantom was included in all the scans to account for the variation in X-ray attenuation inherent to independent scan sessions.
Mechanical Properties
Femora from a subset of the males (6-15 yrs, n = 9) and females (6-15 yrs, n = 13) mentioned above were loaded to failure in four-point bending at 0.05 mm/sec using a servohydraulic materials test system (Instron Corp.; Canton, MA, USA) to assess whole-bone mechanical properties. Load deflection curves were analyzed for stiffness (the slope of the initial portion of the curve), maximum load (Max Load), post-yield deflection (PYD), and work-to-failure (Work). PYD, which is a measure of ductility, was defined as the deflection at failure minus the deflection at yield. Yield was defined as a 10% reduction of stiffness relative to the initial (tangent) stiffness. Work, which is a measure of toughness, was defined as the area under the load deflection curve. Femora were tested at room temperature and kept moist with phosphate buffered saline during all tests.
Analysis
Sexual dimorphism in cortical and trabecular bone was determined by comparing the slopes and intercepts of the Tt.Ar, Ma.Ar, Ct.Ar, Ct.Th, stiffness, PYD, max load, work, trabecular BV/TV, Tb.Th, Tb.Sp and TMD versus age and bodyweight curves using ANCOVA to measure the effects of gender or body size, and two way ANOVA to measure the effects of breeding status, gender and age on bone traits followed by Bonferonni post-tests (GraphPad Prism; San Diego, CA, USA). Statistically significant differences between groups are defined as p values less than 0.05 adjusted for multiple comparisons and data are presented as means and standard deviation.
Results
Qualitative characterization of naked mole-rat bone
The epiphysis of the NMR femur was 3.5mm wide and it flared medially and laterally whereas the patellar articulating cartilage surface extended across 75% of the width of the epiphysis (Fig. 1 B). NMR femora accommodated a thick and wide patella that measured 2.9 mm ± 0.189 in length and 1.54 mm ± 0.136 in width. The femoral head of NMRs was spherical in shape, whereas the femoral neck was short (1.11 mm ± 0.02 in length) with an even distribution of tissue throughout. No differences in cross-sectional area were observed between the anchorage of the femoral neck to the femoral head and the anchorage of the shaft to the femoral neck (Fig. 1 B). There were no gender differences in bone length, femoral head thickness or femoral neck dimensions among subordinate NMRs.
Cortical bone
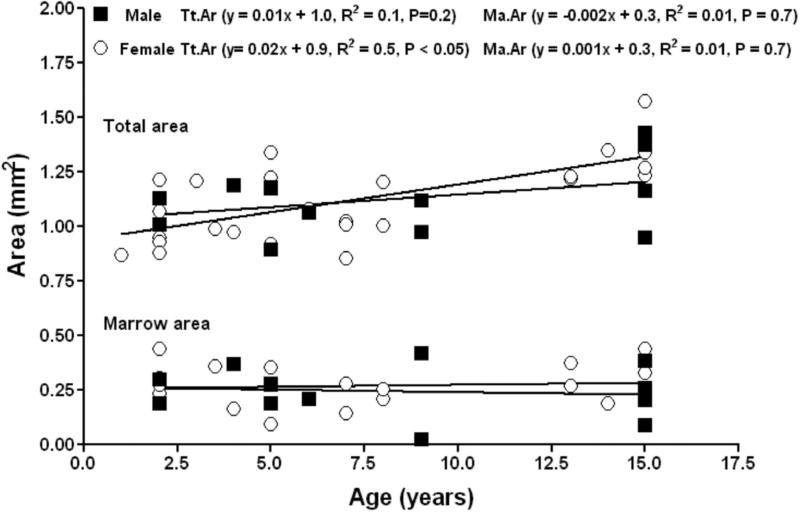
Two way ANOVA showed that the majority of femoral bone traits differ with age but not sex (Table. 1). Subordinate male and female NMRs showed no differences in subperiosteal expansion and endocortical apposition with size or age. Total area was observed to increase in females with age, but not in males, but no differences in the slope or intercept of the total area versus age regressions were found between males and females (Fig. 2). Marrow area was observed not to change with age in males and females. No sex differences were found in the slope or y-intercept of the age versus marrow area regressions. Thus, with age both males and females showed similar subperiosteal expansion and no marrow infilling.
Table 1.
Sexual monomorphism in body weight and femoral traits; femoral length, tissue mineral density (TMD) and mid diaphysis morphology: total area (Tt.Ar), marrow area (Ma.Ar) relative cortical area (RCA), polar moment of inertia (Jo), cortical thickness (Ct.Th), cortical area (Ct.Ar), robustness and stiffness (N) measured in 3 age cohorts of subordinate males. Two-way ANOVA analysis showed significant age related changes but no significant sex differences in any of these variables. Post-hoc tests showed significant differences in certain traits shown in bold between age cohorts but not between the sexes.
| 2-5 years | 6-10 years | 11-15 years | Two way ANOVA (P) | |||||
|---|---|---|---|---|---|---|---|---|
| Male (n = 7) | Female (n = 9) | Male (n = 3) | Female (n = 4) | Male (n = 4) | Female (n = 7) | Sex | Age | |
| Weight (g) | 35.5 ± 5.8 | 28.7 ± 10.2 | 41.2 ± 5.4 | 30.0 ± 4.7 | 45.8 ± 17.3 | 46.6 ± 7.7* | 0.18 | 0.02 |
| F. Length (mm) | 13.7 ± 0.5 | 13.3 ± 1.3 | 14.0 ± 0.9 | 14.0 ± 0.5 | 15.0 ± 0.5 | 15.0 ± 0.7* | 0.74 | <0.00 |
| Tt.Ar (mm2) | 1.09 ± 0.11 | 1.03 ± 0.15 | 1.05 ± 0.07 | 1.02 ± 0.14 | 1.22 ± 0.21 | 1.31 ± 0.12*† | 0.96 | <0.00 |
| Ct.Ar (mm2) | 0.82 ± 0.10 | 0.75 ± 0.11 | 0.76 ± 0.08 | 0.79 ± 0.13 | 0.98 ± 0.10 | 1.00 ± 0.16* | 0.98 | <0.00 |
| Ct.Th (mm) | 0.25 ± 0.02 | 0.24 ± 0.03 | 0.23 ± 0.04 | 0.25 ± 0.03 | 0.29 ± 0.01 | 0.27 ± 0.04 | 0.98 | 0.03 |
| Ma.Ar (mm2) | 0.26 ± 0.06 | 0.27 ± 0.10 | 0.21 ± 0.19 | 0.22 ± 0.05 | 0.23 ± 0.12 | 0.29 ± 0.08 | 0.64 | 0.46 |
| Jo (mm4) | 0.21 ± 0.04 | 0.16 ± 0.06 | 0.19 ± 0.01 | 0.18 ± 0.05 | 0.28 ± 0.08 | 0.31 ± 0.07* | 0.67 | <0.00 |
| RCA (Ct.Ar/Tt.Ar) | 0.75 ± 0.05 | 0.73 ± 0.08 | 0.72 ± 0.11 | 0.78 ± 0.05 | 0.80 ± 0.07 | 0.76 ± 0.07 | 0.96 | 0.40 |
| Robustness (TtAr/F.Le) | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.001 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.92 | 0.09 |
| TMD (mg/cc) | 1370 ± 56 | 1370 ± 63 | 1415 ± 40 | 1371 ± 20 | 1423 ± 20 | 1386 ± 37 | 0.17 | 0.18 |
| Stiffness (N) | N/A | N/A | 110 ± 7 | 119 ± 25 | 151.6 ± 34 | 165 ± 41 | 0.09 | 0.72 |
Difference relative to 2-5 year data (within sex) Bonferroni's test P < 0.05
Difference relative to 6-10 year data (within sex) Bonferroni's test P < 0.05
Figure 2.
Femoral mid-diaphysis total area and marrow area in subordinate male (closed squares) and female (open circles) naked mole-rats are phenotypically similar.
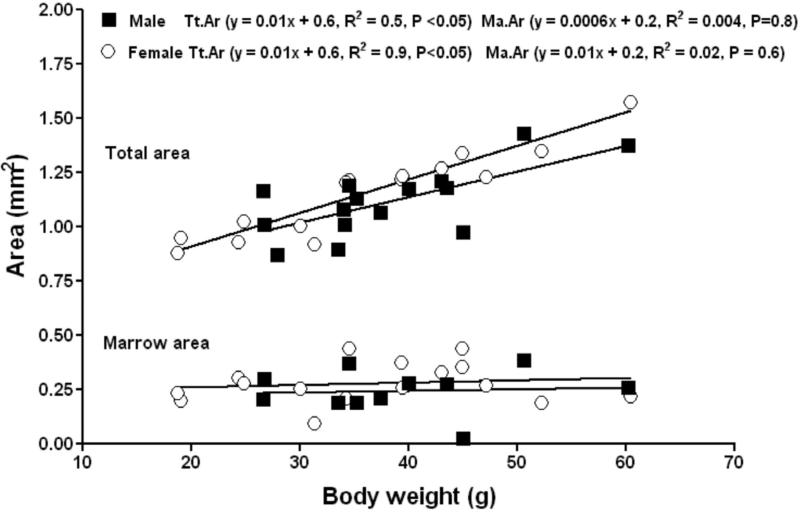
No obvious sex differences in cortical bone were evident among subordinate NMRs. Body weight was shown to be a strong determinant of total area, cortical area and cortical thickness in femora from both male and female subordinate NMRs. Total area increased significantly with body weight for both males and females. No sex differences were found in the slope or intercept of the total area versus body weight regressions of males and females (Fig. 3). Marrow area did not correlate with body weight, and no differences in the slope or intercept of the marrow area versus body weight regressions (Fig. 3).
Figure 3.
Femoral mid-diaphysis in subordinate male (closed squares) and female (open circles) naked mole-rats functionally adapts to body weight by increasing the total area with no marrow infilling observed in either gender.
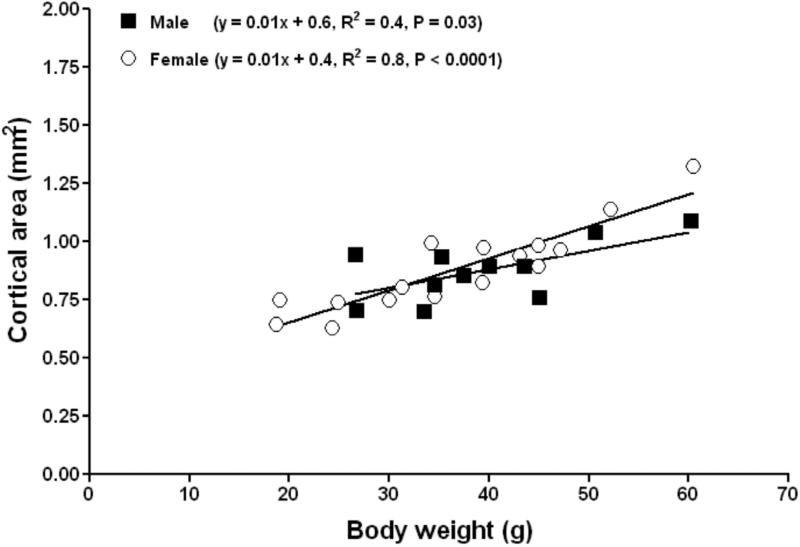
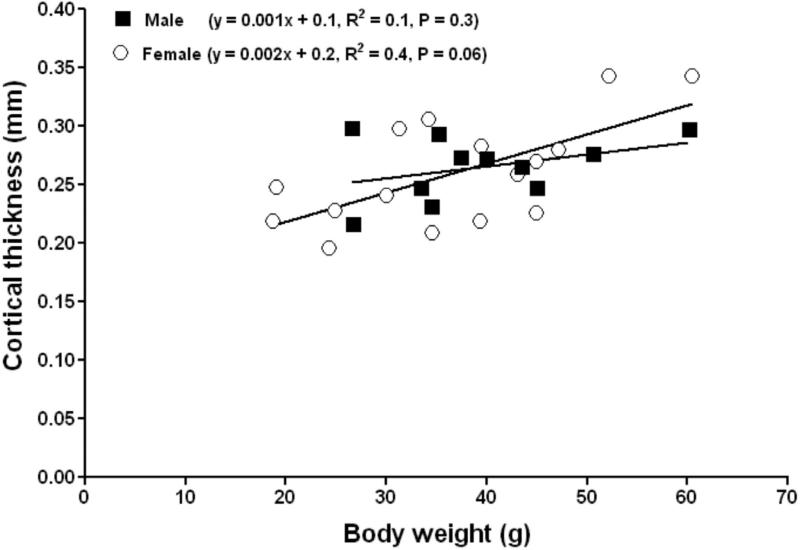
Cortical area increased significantly with body weight in both males and females (Fig. 4). No differences in the slopes or intercepts of the linear relationship between cortical area versus body weight were evident between males and females (P = 0.1, ANCOVA). Cortical thickness increased significantly with body weight in females (Figure 5). No differences in the slopes or intercepts of the linear relationship between cortical thickness versus body weight were evident between males and females (P = 0.2, ANCOVA). The femora of both subordinate male and female NMRs appear to adapt to increasing weight through subperiosteal expansion with no endocortical infilling or resorption occurring in the endosteal surface. This yielded femora with greater diameter, cortical area and cortical thickness in larger individuals regardless of sex.
Figure 4.
Femoral mid-diaphys in subordinate male (closed squares) and female (open circles) naked mole-rats adapts to increasing body weight by increasing cortical area.
Figure 5.
Femoral mid-diaphys cortical thickness in subordinate female (open circles) naked mole-rats adapts to increasing body weight by increasing cortical thickness.
Trabecular bone
Body weight was shown to be also a strong determinant of trabecular BV/TV, and trabecular number. No gender differences were observed between slopes or intercepts of the BV/TV versus body weight, similarly gender differences were absent in the relationship between the trabecular number versus body weight (Table 2). In both genders, trabecular bone adapts to increasing body weight by increasing both BV/TV and trabecular number (Tables 2 and 3). Age-related effects were however evident in trabecular BV/TV and trabecular thickness. BV/TV and trabecular thickness increased in females while trabecular spacing was observed to decline in females. No differences among genders were observed in either the slope or the intercept for the relationship between trabecular BV/TV versus age, trabecular thickness versus age and trabecular spacing versus age (Tables 2 and 3).
Table 2.
Regression analysis of metaphyseal trabecular bone BV/TV, trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp) and trabecular number (Tb.N) in subordinate male (n = 14) and female (n = 20) naked mole-rats. Strong correlations between BV/TV and BW, BV/TV and age, and Tb.Th and age are evident in females. ANCOVA analysis revealed no differences between the sexes.
| Trabecular bone | Regression analysis | ANCOVA analysis | ||||
|---|---|---|---|---|---|---|
| Equation | R2 | P | F | P | F | |
| BV/TV vs BW (M) | y = 0.006x - 0.02 | 0.42 | 0.08 | 4.4 | 0.74 | 0.1 |
| BV/TV vs BW (F) | y = 0.005x + 0.06 | 0.40 | <0.00 | 10.9 | 0.74 | 0.1 |
| BV/TV vs Age (M) | y = 0.014x + 0.14 | 0.24 | 0.08 | 3.6 | 0.24 | 1.4 |
| BV/TV vs Age (F) | y = 0.002x + 0.16 | 0.42 | <0.00 | 1.5 | 0.24 | 1.4 |
| Tb.Th vs age (M) | y = 3.19x + 101.0 | 0.24 | 0.17 | 2.2 | 0.35 | 0.9 |
| Tb.Th vs age (F) | y = 6.319x + 75.8 | 0.37 | 0.02 | 2.2 | 0.35 | 0.9 |
| Tb.Sp vs age (M) | y = 4.972x + 1356 | 0.27 | 0.06 | 4.2 | 1.00 | −1.0 |
| Tb.Sp vs age (F) | y = 1.739x + 1362 | 0.04 | 0.39 | 0.7 | 1.00 | −1.0 |
| Tb.N vs BW (M) | y = −0.017 + 6.1 | 0.01 | 0.77 | 0.1 | 0.1 | 3.4 |
| Tb.N vs BW (F) | y = 0.092 + 2.21 | 0.50 | <0.00 | 12.6 | 0.1 | 3.4 |
M = male, F = female
Table 3.
Mid diaphyseal morphology of subordinates differed in several traits from those of breeding naked mole-rats. Total area (Tt.Ar), marrow area (Ma.Ar), relative cortical area (RCA), tissue mineral density (TMD), polar moment of inertia (Jo), cortical thickness (Ct.Th), cortical area (Ct.Ar), and metaphyseal trabecular bone architecture: Trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular spacing (Tb.Sp), and tissue mineral density (TMD) measured in subordinate and breeding male and female naked mole-rats.
| 2-8 years | 2-8 years | 2-7 years | 2-7 years | Two way ANOVA (P) | ||
|---|---|---|---|---|---|---|
| Female (n = 13) | Breeding Female (n = 6) | Male (n = 8) | Breeding Male (n = 4) | Sex | Breeders | |
| Weight (g) | 29.1 ± 8.4 | 38.5 ± 8.0 | 35.8 ± 5.1 | 37.0 ± 5.2 | 0.43 | 0.07 |
| F. Length (mm) | 13.5 ±1.1 | 14.5 ± 1.2 | 13.7 ± 0.5 | 14.9 ± 0.4 | 0.60 | 0.01 |
| Tt.Ar (mm2) | 1.02 ± 0.14 | 1.19 ± 0.17 | 1.09 ± 0.10 | 1.13 ± 0.10 | 0.98 | 0.07 |
| Ct.Ar (mm2) | 0.77 ± 0.11 | 0.84 ± 0.07 | 0.82 ± 0.09 | 0.86 ± 0.07 | 0.80 | 0.80 |
| Ma.Ar (mm2) | 0.25 ± 0.09 | 0.34 ± 0.21 | 0.25 ± 0.06 | 0.27 ± 0.17 | 0.60 | 0.40 |
| Ct.Th (mm) | 0.24 ± 0.03 | 0.25 ± 0.05 | 0.25 ± 0.03 | 0.27 ± 0.06 | 0.30 | 0.30 |
| Jo (mm4) | 0.17 ± 0.06 | 0.23 ± 0.04 | 0.21 ± 0.04 | 0.21 ± 0.02 | 0.60 | 0.10 |
| RCA (Ct.Ar/Tt.Ar) | 0.75 ± 0.07 | 0.72 ± 12.70 | 0.76 ± 5.18 | 0.77 ± 0.13 | 0.75 | 0.54 |
| Trabecular bone BV/TV | 0.16 ± 0.05 | 0.22 ± 0.03 | 0.20 ± 0.07 | 0.27 ± 0.10 | 0.10 | 0.01 |
| Tb.Th (mm) | 0.03 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.20 | 0.42 |
| Tb.Sp (mm) | 0.22 ± 0.14 | 0.12 ± 0.02 | 0.16 ± 0.06 | 0.13 ± 0.05 | 0.53 | 0.14 |
| Tb.N | 4.40 ± 1.33 | 6.43 ± 1.08 | 5.61 ± 2.37 | 6.30 ± 2.42 | 0.40 | 0.04 |
| Cortical bone TMD (mg/cc) | 1370 ± 50 | 1377 ± 40 | 1381 ± 60 | 1405 ± 60 | 0.50 | 0.30 |
| Trabecular bone TMD (mg/cc) | 797 ± 106 | 890 ± 55 | 871 ± 110 | 902 ± 137 | 0.36 | 0.10 |
Mechanical properties of bone
No gender differences were observed between the slopes and intercepts of the stiffness versus age and stiffness versus body weight curves (Table 4). However, in females the relationship between stiffness and body weight reached statistical significance, and because the older females were also the heavier females this relationship with age was also maintained. Both male and female NMRs exhibit similar resistance to bending and this trait, in females but not in males, is correlated with body weight.
Table 4.
Regression analysis of cortical stiffness versus age and body weight in subordinate male (n = 14) and female (n = 20) naked mole-rats. Strong correlations were observed in females with both age and body weight; however, ANCOVA analysis revealed no differences between the sexes.
| Cortical bone | Regression analysis | ANCOVA analysis | ||||
|---|---|---|---|---|---|---|
| Equation | R2 | P | F | P | F | |
| Stiffness vs age (M) | y = 3.19x + 101.0 | 0.24 | 0.17 | 2.2 | 0.35 | 0.9 |
| Stiffness vs age (F) | y = 6.319x + 75.8 | 0.37 | 0.02 | 2.2 | 0.35 | 0.9 |
| Stiffness vs BW (M) | y = 1.666x + 1.10 | 0.31 | 0.19 | 2.3 | 0.09 | 3.2 |
| Stiffness vs BW (F) | y = 3.732x - 5.8 | 0.77 | <0.00 | 35.2 | 0.09 | 3.2 |
M = male, F = female
Material composition
Tissue mineral density (TMD) did not change with age in male and female cortical bone (Table 5). However, trabecular bone TMD increased in both males and females with age and in females with body weight (Table 5). No sex difference in the slope or intercept of the TMD versus age and TMD versus BW regressions were found among males and females for either cortical or trabecular bone (Table 5).
Table 5.
Regression analysis of cortical and trabecular bone tissue mineral density (TMD) versus age and body weight (BW) in subordinate male (n = 14) and female (n = 20) subordinate naked mole-rats. ANCOVA analysis showed no differences between the sexes.
| Regression analysis | ANCOVA analysis | |||||
|---|---|---|---|---|---|---|
| Equation | R2 | P | F | P | F | |
| TMD vs age Ct. (M) | y = 4.972x + 1356 | 0.27 | 0.06 | 4.2 | 0.30 | 1.0 |
| TMD vs age Ct. (F) | y = 1.739x + 1362 | 0.04 | 0.39 | 0.8 | 0.30 | 1.0 |
| TMD vs BW Ct. (M) | y = 1.973x + 1313 | 0.15 | 0.23 | 1.6 | 0.30 | 2.9 |
| TMD vs BW Ct. (F) | y = 0.758x + 0.135 | 0.04 | 0.45 | 0.6 | 0.50 | 0.5 |
| TMD vs Age Tb. (M) | y = 11.69x + 852.2 | 0.38 | 0.04 | 5.5 | 0.45 | 0.6 |
| TMD vs Age Tb. (F) | y = 18.56x + 712.4 | 0.38 | 0.01 | 8.1 | 0.45 | 0.6 |
| TMD vs BW Tb. (M) | y = 4.640x + 757.6 | 0.22 | 0.13 | 2.6 | 0.47 | 0.5 |
| TMD vs BW Tb. (F) | y = 7.975x + 584.6 | 0.35 | 0.01 | 7.1 | 0.47 | 0.5 |
Cortical bone (Ct.), Trabecular bone (Tb)
M = male, F = female
Collectively these data reveal that among subordinates no sex differences in morphology, material composition and mechanical properties are evident in both trabecular or cortical bone and clearly subordinates show a monomorphic hind limb skeleton, even when analyzed relative to age and BW.
Quantitative analysis between subordinate and breeding naked mole-rats
Significant differences between young breeders and age matched subordinates were evident in females, but not males. In males, although similar trends towards larger bone in breeders were evident, differences that could be attributed to reproductive status did not reach statistical significance.
The mid-diaphysis of young (2-8 year) breeding females had a 27% bigger total area, a 25% larger marrow area, a 34% greater cortical thickness and a 23% increase in cortical area (Table 3). Breeding females also had significantly longer femora, greater trabecular bone BV/TV and increased trabecular number when compared with those measurements in smaller-sized subordinates (Table 3). Morpholological differences in data from our breeding and subordinate male cohorts did not reach significance however a similar trend to that observed in females was evident whereby breeding males had a 9% larger total area, and16% higher trabecular bone BV/TV (Table 3).
Data for femora of breeding females were not significantly different from those of breeding males.
Discussion
In this study we used MicroComputed Tomography to characterize the NMR bone phenotype, and to assess if reproductively-suppressed hypogonadic subordinate NMRs show a monomorphic femoral bone profile, and whether or not this differs from the bone phenotype of their breeding counterparts. Briefly, femoral bone morphology of both subordinates and breeders showed no sexual dimorphism, although there were some significant differences between female breeders and subordinates. Despite reproductive suppression and concomitant low sex steroid hormone profiles [24, 34], and unlike other models of delayed puberty and hypogonadism, NMRs maintain bone structure and strength for at least 50% of their exceptional 30 year lifespan (Fig. 2, Tables 1 and 4).
Characterization of the naked mole-rat hind limb
The NMR hind limb has not been previously characterized and differs from that of common laboratory rodents. NMR hind limb morphology may reflect adaptive responses to the subterranean milieu that these rodents have inhabited since the early Miocene [35]. Given their life in self-excavated burrows, NMRs have evolved a fusiform cylindrical shape with a concomitant reduction in limb length relative to the axial skeleton. Although NMRs use their procumbent incisors as chisels for burrow excavation, the hind limb provides the power for locomotion, braces the NMR while digging and assists in dirt removal [36] by kicking the dirt out behind them using backward thrusts. The fore limbs and hind limbs of the NMR are nearly equal in length. The femora of NMRs, like that of most subterranean rodents, are thickened (Fig. 1) presumably to reduce torsion and bending stresses. Cross sectional morphology of the femora scale with body mass (Fig. 3), as is common in other hystricognath rodents [37].
NMRs live permanently in the dark and naturally have very low levels of the principal circulating metabolite of the photo-hormone vitamin D, 25-hydroxy vitamin D (25(OH)D). These levels are below the detection limit for routinely used assays [38]. They nevertheless have detectable, albeit very low levels of the active hormone, 1,25 dihydroxy vitamin D (1,25(OH)2D). Given their well-mineralized bones (Tables 1, 3 and 5) that are sustained with age for at least half of their 30 year lifespan, these low levels of 1,25(OH)2D may be adequate to maintain bone mineral quality. Neither oral supplementation with vitamin D, nor controlled exposure to sunlight significantly altered bone mineral density or calcium balance [25, 39, 40], as such it is possible that these strictly subterranean rodents may have very low requirements for 1,25(OH)2D or have evolved vitamin D independent bone metabolism.
Hind limbs of subordinate naked mole-rats are sexually monomorphic
Femur length of both male and females is similar and scales with body size (Table 1). Cross sectional areas of the bones of subordinate females (Fig. 2) more closely follow a male pattern of bone development where you see continued subperiosteal expansion with age. This bone profile and in particular the subperiosteal expansion, interestingly, is independent of testosterone but is highly correlated with body weight. Sexual monomorphism in subordinates of this species has been documented for body size, ano-genital distance, expression of behavior, perineal muscles and perineal motoneurons and the brain and spinal cord [23, 28, 41-43] and these traits are significantly different from those of breeding individuals, where regardless of gender, they too show sexual monomorphism. Here we show that femoral morphology, trabecular micro-architecture and strength also follow a pronounced monomorphic phenotype (Tables 1, 2, and 5).
Subordinate male and female NMRs show pronounced physiological differences during reproductive suppression: Females are anovulatory, maintaining low levels of luteinizing hormone, progesterone and estrogen while males show impaired fertility manifested by poorer quality sperm and low sperm counts. They have extremely small testes with fewer Leydig cells and 5-fold lower levels of testosterone than observed in breeding males [24, 31, 32].
Comparative differences among femora of breeders and subordinates
Any individual within the colony is capable of changing its status from a subordinate to a breeder once removed from the dominant female. When females become breeders they exhibit a growth surge in the lumbar vertebral bone that continues at least over the first 6 pregnancies, however femoral length does not change significantly [19, 44]. Although, our sample size for breeding animals is small, marked differences in femoral traits among breeding females versus non-breeders were evident (Table 3). Breeding females showed a more robust bone with a significantly larger diameter, trabecular bone volume and trabecular number. Even though, cortical thickness was not observed to be significantly larger, considerable variability was observed in this and other traits. This may reflect the large variability among the various individual bone measurements and our comparatively small sample size of 4 male and 6 female breeders in our experimental cohorts. Further, this may reflect that breeders may have been killed at different stages of their reproductive cycle and that we did not control for those animals that were successfully raising pups, litter size or prior reproductive history. An alternate explanation may be that the differences in femoral bone features among breeding females and subordinate females simply reflect the fact that breeding females tended to be larger animals; whereas body size in males was similar amongst breeders and non-breeders. The exact effects of puberty on bone in NMRs need to be further analyzed. We believe that the timing of reproductive onset, the number of pregnancies the female has had, and her success in raising her pups, as well as the stage of the reproductive cycle when the breeding female died, all contribute the variability in these traits. This may reflect the use of cortical bone as mineral reservoirs for fetal bone mineralization and for lactation. Indeed, in some early pregnant animals we observed that the bone marrow cavity was almost completely occluded, while in late pregnancy and during lactation, we noted extensive evidence of endocortical bone resorption.
The greater cross sectional area seen in breeding females (Table 3) when compared with subordinates was not expected since breeding females have higher levels of estrogen and this hormone has been shown to inhibit subperiosteal expansion in rats and humans [45-47]. It is possible that changes in bone traits with breeding status, rather than reflecting sex steroid actions, are due to adaptive changes associated with greater body weight. Breeding females more than double their mass during pregnancies and even post lactation are among the biggest animals within the colony [48]. Because most bone traits of male and female subordinates correlate closely with body size, we expect this may also hold in breeding females.
Compared to the subordinate males our comparatively small (n=4) cohort of breeding males did not show significant morphological or tissue quality differences compared to the non-breeding counterparts. This was unexpected since breeders in this species have five-fold higher testosterone levels than non-breeders [24]. Elevated testosterone levels have been linked to subperiosteal expansion [45, 47]. It is possible that femoral bone development is complete before animals become breeders and does not change substantially thereafter. Given that both breeding males and subordinate males have a similar body size (Table 3), the lack of a significant difference in these variables may also suggest that body size rather than steroid hormone level, is a key determinant of the bone phenotype in these animals.
Bones of breeding females were similar to those of breeding males. Lack of sexual dimorphism even when sex steroid hormones are elevated is intriguing. This may reflect our small sample size and the large inherent variability in bone traits, or it may suggest that femoral bone development is complete before the animals become breeders. Although animals can breed from as young as 6 months of age, most animals only become breeders when older than 2 years of age. While vertebral bone changes accompany changes in breeding status, femoral length and bone quality, quantity and strength are unchanged, suggesting that these are only modestly influenced by changes in hormone status after the animals achieved adult stature. Further studies are needed exploring the relationship between age, and breeding status and changes in skeletal traits at multiple anatomic sites to state with confidence that bones of breeders are sexually monomorphic. If this were indeed the case, our data would support neural morphological studies in this species which report that NMR breeders lack many of the sex differences in the brain and spinal cord commonly found in other rodents [27, 49]. Indeed their studies found no evidence of sexual dimorphism; rather breeders, regardless of sex, differed substantially from the subordinates by having more neurons in the ventromedial nucleus of the hypothalamus (VMH), larger overall volumes of specific brain regions and pronounced differences in perineal muscles and motor neurons [49, 50].
Delayed puberty
Puberty is induced by changes in the gonadotropin axis and a concomitant surge in the sex steroids. Theoretically, NMRs can suppress this surge for their entire life. Interestingly, a fifteen-year delay in puberty did not affect the strength of their femora, total mineral density, or cause a decrease in cortical thickness (Tables 1 and 5 and Fig. 5). Quite the opposite, cortical thickness was observed to increase over this period. Humans on the other hand attain peak bone quality by 30 years of age [51] and by 50% of their maximum lifespan (61 years) may have lost a considerable amount of bone tissue. Prolonged and sustained good bone health in NMRs is at variance with age-related changes in bones of mice and humans [52-55] and demands indepth analyses as to the mechanisms facilitating sustained bone quality over more than 50% of their extraordinary lifespan [56] .
In humans, a delay in menarchy has been associated with lower BMD, higher incidence of stress fractures, greater cortical area, lower BV/TV, and thinner cortical shell [12, 13, 57, 58]. In mice and rats, a delay in pubertal onset has also been shown to reduce trabecular number, increase trabecular separation, and reduce femoral length and strength [5, 53, 59-61]. Subordinate NMRs circumvent the observed detrimental effects of hypogonadism on the skeletal system that are evident in humans, rats and mice. Rather, they appear to maintain bone morphology, strength and trabecular micro-architecture. Understanding the mechanisms by which hypogonadic NMRs protect their bone may identify pivotal points of intervention that could mimic the prolonged good bone health found in this naturally long-lived species.
Current animal models to study delayed puberty have several drawbacks. Ovariectomy models often manifest with high GnRH, since its inhibition by sex steroids and inhibin is reduced, whereas GnRH-antagonists affect not only the entire hypothalamic-pituitary-gonadal axis, but also have a tendency to increase body weight, serum IGF-1 levels, and complete suppression of the onset of puberty is dose dependent [5, 62, 63]. NMRs on the other hand, are naturally suppressed from entering puberty and do not show differences in other hypothalamic pituitary hormones or serum IGF-1 levels, a clear advantage to other models. Further, “puberty” can be initiated within 7 days once isolated from the dominant female [21] and levels of the sex steroid hormones in these recently isolated NMRs reach concentrations comparable to those of their long-term breeding counterparts [24, 64]. This activation of gonadal activity and fertility is extremely rapid, especially when considering that non-breeders may have been reproductively suppressed for many years, and even 24 year old females are capable of exhibiting rapid changes from reproductive suppression to ovarian cycling. Further studies in which hormone status may be experimentally manipulated in young individuals both prior to and after attainment of adult mass may shed more light as to whether or not bone morphology and mechanical properties are sustained by sex steroid hormone independent mechanisms. Elucidating the mechanisms facilitating sustained bone structure and strength in hypogonadic subordinates may yield pivotal insights into potential mechanisms and therapies for sustaining bone in delayed puberty and exercise-induced hypogonadism.
Conclusion
Subordinate NMRs are sexually monomorphic with no significant differences in femoral bone structure and quality among males and females. Similarly sexual dimorphism was not evident among breeding males and females; however as breeders showed considerable variability in bone histomorphometry and our sample size was small we cannot conclude with confidence that breeding males and females are also sexually monomorphic. Significant differences exist between the dominant (reproductive) and subordinate (non-reproductive) female bone morphological and functional properties. Despite reproductive suppression and concomitant low sex steroid hormone profiles [17, 32], unlike other models of delayed puberty and hypogonadism, NMRs maintain a robust bone structure and strength for at least 50% of their unusually long lifespan. The challenge ahead is to understand how this species is able to maintain bone quality and structure independent of reproductive status. Elucidating these mechanisms may enable therapies aimed at protecting bone health independent of reproductive hormone status and this may have extensive collateral health benefits.
Acknowledgements
Financial support from National Institutes of Health (RB AG 022981 and KJ AR 44927) is gratefully acknowledged. Blazej Andziak, Adriana Biney, Yael Edrey, Joy Kang, Yael Kramer and Ting Yang assisted in the collection of most of these samples and the Animal Care Staff at CCNY and UTHSCSA are thanked for their assistance in caring for these animals. Hayden Courtland, Steven Tommassini, Damien Laudier, Philip Nasser and Vallerie Williams at MSSM provided technical support in the various bone measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have nothing to disclose.
References
- 1.Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–60. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zirilli L, Rochira V, Diazzi C, Caffagni G, Carani C. Human models of aromatase deficiency. J Steroid Biochem Mol Biol. 2008;109:212–8. doi: 10.1016/j.jsbmb.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 3.Garn S. The earlier gain and the later loss of cortical bone. Charles C Thomas; Springfield, IL, USA: 1970. [Google Scholar]
- 4.Golden NH, Carlson JL. The pathophysiology of amenorrhea in the adolescent. Ann N Y Acad Sci. 2008;1135:163–78. doi: 10.1196/annals.1429.014. [DOI] [PubMed] [Google Scholar]
- 5.Yingling VR, Taylor G. Delayed pubertal development by hypothalamic suppression causes an increase in periosteal modeling but a reduction in bone strength in growing female rats. Bone. 2008;42:1137–43. doi: 10.1016/j.bone.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennell KL, Brukner PD, Malcolm SA. Effect of altered reproductive function and lowered testosterone levels on bone density in male endurance athletes. Br J Sports Med. 1996;30:205–8. doi: 10.1136/bjsm.30.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cools BL, Rooman R, Op De Beeck L, Du Caju MV. Boys with a simple delayed puberty reach their target height. Horm Res. 2008;70:209–14. doi: 10.1159/000137663. [DOI] [PubMed] [Google Scholar]
- 8.Ambler GR. Androgen therapy for delayed male puberty. Curr Opin Endocrinol Diabetes Obes. 2009;16:232–9. doi: 10.1097/med.0b013e32832b20a8. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein JS, Neer RM, Biller BM, Crawford JD, Klibanski A. Osteopenia in men with a history of delayed puberty. N Engl J Med. 1992;326:600–4. doi: 10.1056/NEJM199202273260904. [DOI] [PubMed] [Google Scholar]
- 10.Rochira V, Balestrieri A, Madeo B, Zirilli L, Granata AR, Carani C. Osteoporosis and male age-related hypogonadism: role of sex steroids on bone (patho)physiology. Eur J Endocrinol. 2006;154:175–85. doi: 10.1530/eje.1.02088. [DOI] [PubMed] [Google Scholar]
- 11.Kohrt WM, Van Pelt RE, Gozansky WS. Effects of estrogen replacement on metabolic factors that influence physical performance in female hypogonadism. J Endocrinol Invest. 2003;26:902–10. doi: 10.1007/BF03345242. [DOI] [PubMed] [Google Scholar]
- 12.Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Deleterious effect of late menarche on distal tibia microstructure in healthy 20-year-old and premenopausal middle-aged women. J Bone Miner Res. 2009;24:144–52. doi: 10.1359/jbmr.080815. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal DI, Mayo-Smith W, Hayes CW, Khurana JS, Biller BM, Neer RM, Klibanski A. Age and bone mass in premenopausal women. J Bone Miner Res. 1989;4:533–8. doi: 10.1002/jbmr.5650040412. [DOI] [PubMed] [Google Scholar]
- 14.Johnell O, Gullberg B, Kanis JA, Allander E, Elffors L, Dequeker J, Dilsen G, Gennari C, Lopes Vaz A, Lyritis G, et al. Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. J Bone Miner Res. 1995;10:1802–15. doi: 10.1002/jbmr.5650101125. [DOI] [PubMed] [Google Scholar]
- 15.Park KH, Lee SJ, Kim JY, Kim JY, Bai SW, Kim JW. A concomitant decrease in cortical and trabecular bone mass in isolated hypogonadotropic hypogonadism and gonadal dysgenesis. Yonsei Med J. 1999;40:444–9. doi: 10.3349/ymj.1999.40.5.444. [DOI] [PubMed] [Google Scholar]
- 16.Silman AJ. Risk factors for Colles' fracture in men and women: results from the European Prospective Osteoporosis Study. Osteoporos Int. 2003;14:213–8. doi: 10.1007/s00198-002-1364-1. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis JU. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–3. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- 18.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–77. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 19.O'Riain MJ, Jarvis JU, Alexander R, Buffenstein R, Peeters C. Morphological castes in a vertebrate. Proc Natl Acad Sci U S A. 2000;97:13194–7. doi: 10.1073/pnas.97.24.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dengler-Crish CM, Catania KC. Phenotypic plasticity in female naked mole-rats after removal from reproductive suppression. J Exp Biol. 2007;210:4351–8. doi: 10.1242/jeb.009399. [DOI] [PubMed] [Google Scholar]
- 21.Faulkes CG, Bennett NC. Family values: group dynamics and social control of reproduction in African mole-rats. Trends Ecol Evol. 2001;16:184–190. doi: 10.1016/s0169-5347(01)02116-4. [DOI] [PubMed] [Google Scholar]
- 22.Faulkes CG, Abbott DH, Jarvis JU, Sherriff FE. LH responses of female naked mole-rats, Heterocephalus glaber, to single and multiple doses of exogenous GnRH. J Reprod Fertil. 1990;89:317–23. doi: 10.1530/jrf.0.0890317. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis JU. Sherman PW, Jarvis JU, Alexander RD, editors. Reproduction in naked mole-rats. The Biology of the Naked Mole-Rat New Jersey. 1991. pp. 384–425.
- 24.Clarke FM, Faulkes CG. Hormonal and behavioural correlates of male dominance and reproductive status in captive colonies of the naked mole-rat, Heterocephalus glaber. Proc Biol Sci. 1998;265:1391–9. doi: 10.1098/rspb.1998.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buffenstein R, Sergeev IN, Pettifor JM. Vitamin D hydroxylases and their regulation in a naturally vitamin D-deficient subterranean mammal, the naked mole rat (Heterocephalus glaber). J Endocrinol. 1993;138:59–64. doi: 10.1677/joe.0.1380059. [DOI] [PubMed] [Google Scholar]
- 26.Flor-Cisneros A, Roemmich JN, Rogol AD, Baron J. Bone age and onset of puberty in normal boys. Mol Cell Endocrinol. 2006;254-255:202–6. doi: 10.1016/j.mce.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes MM, Rosen GJ, Jordan CL, de Vries GJ, Goldman BD, Forger NG. Social control of brain morphology in a eusocial mammal. Proc Natl Acad Sci U S A. 2007;104:10548–52. doi: 10.1073/pnas.0610344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes MM, Goldman BD, Forger NG. Social status and sex independently influence androgen receptor expression in the eusocial naked mole-rat brain. Horm Behav. 2008;54:278–85. doi: 10.1016/j.yhbeh.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E. The differing tempo of growth in bone size, mass, and density in girls is region-specific. J Clin Invest. 1999;104:795–804. doi: 10.1172/JCI7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeman E. Invited Review: Pathogenesis of osteoporosis. J Appl Physiol. 2003;95:2142–51. doi: 10.1152/japplphysiol.00564.2003. [DOI] [PubMed] [Google Scholar]
- 31.Faulkes CG, Abbott DH, Jarvis JU. Social suppression of reproduction in male naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1991;91:593–604. doi: 10.1530/jrf.0.0910593. [DOI] [PubMed] [Google Scholar]
- 32.Faulkes CG, Abbott DH, Jarvis JU. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1990;88:559–68. doi: 10.1530/jrf.0.0880559. [DOI] [PubMed] [Google Scholar]
- 33.Otsu N. A threshold selection mothod from gray-level histograms. IEEE Transactions on Systems, Man and Cybernetics. 1979;9:62–66. [Google Scholar]
- 34.Faulkes CG, Bennett NC. African Mole-rats Ecology and Eusociality. Press Syndicate of the University of Cambridge; Cambridge: 2000. [Google Scholar]
- 35.Buffenstein R. Ecophysiological responses to an underground habitat. In: E. Lacey E, Patton J, Cameron G, editors. The Biology of Subterranean Rodents. Chicago Chicago University Press; 2000. pp. 62–110. [Google Scholar]
- 36.Stein BR. Morphology of subterranean rodents. In: Lacey E, Patton J, Cameron G, editors. The Biology of Subterranean Rodents. Chicago University Press; Chicago: 2000. pp. 19–91. [Google Scholar]
- 37.Biknevicius AR. Biomechanical scaling of limb bones and differential limb use in the caviomorph rodents. J Mammal. 1993;74:95–107. [Google Scholar]
- 38.Buffenstein R, Jarvis JU, Opperman LA, Cavaleros M, Ross FP, Pettifor JM. Subterranean mole-rats naturally have an impoverished calciol status, yet synthesize calciol metabolites and calbindins. Eur J Endocrinol. 1994;130:402–409. doi: 10.1530/eje.0.1300402. [DOI] [PubMed] [Google Scholar]
- 39.Pitcher T, Pettifor JM, Buffenstein R. The effect of dietary calcium content and oral vitamin-D-3 supplementation on mineral homeostasis in a subterranean mole-rat Cryptomys damarensis. Bone and Mineral. 1994;27:145–157. doi: 10.1016/s0169-6009(08)80216-4. [DOI] [PubMed] [Google Scholar]
- 40.Pitcher T, Sergeev IN, Buffenstein R. Vitamin-D Metabolism in the damara mole-rat is altered by exposure to sunlight yet mineral metabolism is unaffected. J Endocrinol. 1994;143:367–374. doi: 10.1677/joe.0.1430367. [DOI] [PubMed] [Google Scholar]
- 41.Lacey EA, Alexander RD, Braude SH, Sherman PW, Jarvis JU. An ethogram for the naked mole-rat: nonvocal behaviors. In: Sherman PW, Jarvis JU, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; New Jersey: 1991. pp. 209–242. [Google Scholar]
- 42.Lacey EA, Sherman PW. Social organization of naked mole-rat colonies: Evidence for divisions of labor. In: Sherman PW, Jarvis JU, Alexander RD, editors. The Biology of the Naked Mole-Rat Princeton. Princeton University Press; New Jersey: 1991. pp. 275–336. [Google Scholar]
- 43.Peroulakis ME, Goldman B, Forger NG. Perineal muscles and motoneurons are sexually monomorphic in the naked mole-rat (Heterocephalus glaber). J Neurobiol. 2002;51:33–42. doi: 10.1002/neu.10039. [DOI] [PubMed] [Google Scholar]
- 44.Henry EC, Dengler-Crish CM, Catania KC. Growing out of a caste-reproduction and the making of the queen mole-rat. J Exp Biol. 2007;210:261–8. doi: 10.1242/jeb.02631. [DOI] [PubMed] [Google Scholar]
- 45.Seeman E. Estrogen, androgen, and the pathogenesis of bone fragility in women and men. Curr Osteoporos Rep. 2004;2:90–6. doi: 10.1007/s11914-004-0016-0. [DOI] [PubMed] [Google Scholar]
- 46.Turner RT, Vandersteenhoven JJ, Bell NH. The effects of ovariectomy and 17 beta-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res. 1987;2:115–22. doi: 10.1002/jbmr.5650020206. [DOI] [PubMed] [Google Scholar]
- 47.Zhang XZ, Kalu DN, Erbas B, Hopper JL, Seeman E. The effects of gonadectomy on bone size, mass, and volumetric density in growing rats are gender-, site-, and growth hormone-specific. J Bone Miner Res. 1999;14:802–9. doi: 10.1359/jbmr.1999.14.5.802. [DOI] [PubMed] [Google Scholar]
- 48.Urison NT, Buffenstein R. Metabolic and body-temperature changes during pregnancy and lactation in the naked mole-rat (Heterocephalus-glaber). Physiol Zool. 1995;68:402–420. [Google Scholar]
- 49.Seney M, Goldman BD, Forger NG. Breeding status affects motoneuron number and muscle size in naked mole-rats: recruitment of perineal motoneurons? J Neurobiol. 2006;66:1354–64. doi: 10.1002/neu.20314. [DOI] [PubMed] [Google Scholar]
- 50.Holmes MM, Goldman BD, Goldman SL, Seney ML, Forger NG. Neuroendocrinology and sexual differentiation in eusocial mammals. Front Neuroendocrinol. 2009 doi: 10.1016/j.yfrne.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniels ED, Pettifor JM, Schnitzler CM, Moodley GP, Zachen D. Differences in mineral homeostasis, volumetric bone mass and femoral neck axis length in black and white South African women. Osteoporos Int. 1997;7:105–12. doi: 10.1007/BF01623684. [DOI] [PubMed] [Google Scholar]
- 52.Schnitzler CM, Pettifor JM, Mesquita JM, Bird MD, Schnaid E, Smyth AE. Histomorphometry of iliac crest bone in 346 normal black and white South African adults. Bone Miner. 1990;10:183–99. doi: 10.1016/0169-6009(90)90261-d. [DOI] [PubMed] [Google Scholar]
- 53.Price C, Herman BC, Lufkin T, Goldman HM, Jepsen KJ. Genetic variation in bone growth patterns defines adult mouse bone fragility. J Bone Miner Res. 2005;20:1983–91. doi: 10.1359/JBMR.050707. [DOI] [PubMed] [Google Scholar]
- 54.Ramanadham S, Yarasheski KE, Silva MJ, Wohltmann M, Novack DV, Christiansen B, Tu X, Zhang S, Lei X, Turk J. Age-related changes in bone morphology are accelerated in group VIA phospholipase A2 (iPLA2beta)-null mice. Am J Pathol. 2008;172:868–81. doi: 10.2353/ajpath.2008.070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22:1197–207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 56.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol [B] 2008;178:439–45. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 57.Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab. 2002;87:3162–8. doi: 10.1210/jcem.87.7.8637. [DOI] [PubMed] [Google Scholar]
- 58.Ito M, Yamada M, Hayashi K, Ohki M, Uetani M, Nakamura T. Relation of early menarche to high bone mineral density. Calcif Tissue Int. 1995;57:11–4. doi: 10.1007/BF00298989. [DOI] [PubMed] [Google Scholar]
- 59.Yingling VR, Xiang Y, Raphan T, Schaffler MB, Koser K, Malique R. The effect of a short-term delay of puberty on trabecular bone mass and structure in female rats: a texture-based and histomorphometric analysis. Bone. 2007;40:419–24. doi: 10.1016/j.bone.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krewson TD, Supelak PJ, Hill AE, Singer JB, Lander ES, Nadeau JH, Palmert MR. Chromosomes 6 and 13 harbor genes that regulate pubertal timing in mouse chromosome substitution strains. Endocrinology. 2004;145:4447–51. doi: 10.1210/en.2004-0543. [DOI] [PubMed] [Google Scholar]
- 61.Jepsen KJ, Akkus OJ, Majeska RJ, Nadeau JH. Hierarchical relationship between bone traits and mechanical properties in inbred mice. Mamm Genome. 2003;14:97–104. doi: 10.1007/s00335-002-3045-y. [DOI] [PubMed] [Google Scholar]
- 62.Yingling VR, Khaneja A. Short-term delay of puberty causes a transient reduction in bone strength in growing female rats. Bone. 2006;38:67–73. doi: 10.1016/j.bone.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roth C, Leonhardt S, Seidel C, Luft H, Wuttke W, Jarry H. Comparative analysis of different puberty inhibiting mechanisms of two GnRH agonists and the GnRH antagonist cetrorelix using a female rat model. Pediatr Res. 2000;48:468–74. doi: 10.1203/00006450-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Clarke FM, Faulkes CG. Dominance and queen succession in captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc Biol Sci. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]