Abstract
An anesthetic mixture of medetomidine (MED), midazolam (MID), and butorphanol (BUT) has been used in laboratory animals. We previously reported that this anesthetic mixture produced closely similar anesthetic effects in BALB/c and C57BL/6J strains. We also demonstrated the efficacy of atipamezole (ATI), an antagonist of MED that produced quick recovery from anesthesia in mice. Anesthetics have various anesthetic effects among animal strains. However, the differences in the effects of anesthetic mixtures in rats are unclear. In the present study, we first examined effects of the abovementioned anesthetic mixture using three different rat strains: Wistar (WST), Sprague-Dawley (SD), and Fischer 344 (F344). Second, we examined how different dosages and optimum injection timing of ATI affected recovery from anesthesia in rats. We used the anesthetic score to measure anesthetic duration and a pulse oximeter to monitor vital signs. We found no significant differences in anesthetic duration among the three different strains. However, recovery from anesthesia in the SD strain took significantly longer than in the other strains. The antagonistic effects of ATI (0.15 mg/kg and 0.75 mg/kg) were equivalent when administered at 30 min after anesthetic mixture administration. The antagonistic effects of ATI 0.75 mg/kg were stronger than those of ATI 0.15 mg/kg at 10 min after anesthetic mixture administration. This anesthetic mixture is a useful drug that can induce similar anesthetic effects in three different strains and has an antagonist, ATI, that makes rats quickly recover from anesthesia. These results may contribute to the welfare of laboratory animals.
Keywords: anesthetic mixture, antagonist, pulse oximeter, rats, strain difference
Introduction
The anesthetic mixture of medetomidine (MED), midazolam (MID), and butorphanol (BUT) has been recently introduced [13]. This anesthetic mixture was reported to produce an anesthetic duration of around 40 min in ICR mice. We reported that it produced closely similar anesthetic effects in both male and female BALB/c and C57BL/6J strains [14]. We also demonstrated that there were no significant differences in anesthetic duration among intraperitoneal (IP), subcutaneous (SC), and intravenous (IV) injection route groups in mice [15]. Several studies have shown that there are different anesthetic effects among different rat strains [1, 6]. However, the differences in the effects of the anesthetic mixture of MED, MID, and BUT in rats remain unclear. In the present study, we first examined effects of the anesthetic mixture using the Wistar (WST), Sprague-Dawley (SD), and Fischer 344 (F344) rat strains. At the 57th Annual Meeting of the Japanese Association for Laboratory Animal Science (2010, Kyoto, Japan), Pang et al. showed an anesthetic duration of about 60 min after administration of an anesthetic mixture of MED 0.15 mg, MID 2 mg, and BUT 2.5 mg/kg body weight (b.w.)/rat [19]. Therefore, we used exactly the same dosage of anesthetic mixture as Pang et al. used.
Atipamezole (ATI) is a synthetic alpha2-adrenergic receptor antagonist that can antagonize an alpha2-adrenergic receptor agonist, MED [7]. After administration of the anesthetic mixture, injection of ATI caused rapid recovery from anesthesia. We reported the efficacy of ATI with a suitable dosage and timing in mice [15]. However, neither the appropriate dosage nor the optimum injection timing of ATI after administration of the anesthetic mixture is clear in rats.
In this study, we used the anesthetic score to assess the effects of the anesthetic mixture administered to the 3 different rat strains. During the experiment, we measured vital signs just before and after administration of the anesthetic mixture because parameters such as oxygen (O2) saturation, heart rate, and respiratory rate are related to the anesthetic condition of rats under anesthesia [8]. To investigate differences among the three rat strains without administration of an anesthetic, we also measured vital signs just before and after administration of physiological saline (saline) for 90 min.
Materials and Methods
Animals and housing conditions
Animal care and experimental procedures were approved by the Animal Research Committee of Shimane University and conducted according to the Regulations for Animal Experimentation at Shimane University.
We used 8, 8, and 6 male rats of the WST, SD, and F344 strains, respectively, in the first experiment. In the second experiment, we used the same 8 male rats of the WST strain repeatedly after allowing them to rest for at least 2 days after drug administration. The rats were purchased at 5 weeks of age from a commercial supplier (Japan SLC, Inc., Shizuoka, Japan) and habituated for 2 weeks in the animal room before starting the experiment. The rats were 7 to 9 weeks of age during the experiment.
Two or 3 rats were housed per TPX cage (KN-601-T®, W270 × L440 × H187 mm, Natsume Seisakusho, Co., Ltd., Tokyo, Japan) under a strict light cycle (light on at 7:00 and off at 19:00). Autoclaved bedding (Pure Chip®, Shimizu Laboratory Supplies, Co., Ltd., Kyoto, Japan) was provided for each cage and changed twice a week.
The animal room was maintained at a constant temperature (23 ± 2°C) and humidity (55 ± 10%). The rats were given a standard diet (MF®, Oriental Yeast Co., Ltd., Tokyo, Japan) and filtered tap water by an automatic water supply system ad libitum.
Experimental procedure
The experiment was conducted during daytime (13:00–17:00). The experimental room was controlled at the same temperature and humidity as the animal room. The rats were weighed before receiving saline or the anesthetic mixture.
In the first experiment, saline or the anesthetic mixture was administered by IP injection at 0.5 ml/100 g b.w./rat. We used 8 (WST and SD) or 6 (F344) rats per strain group. After administration of the anesthetic mixture, the rats were kept on a heater pad (Heater Mat KN-475®, Natsume Seisakusho, Co., Ltd., Tokyo, Japan) maintained at approximately 38°C. When saline was administered, rats were kept in a rat holder. After administration of the anesthetic mixture, the anesthetic score for each rat was measured every 5 min until the rat was completely recovered from anesthesia. At the same time, we measured O2 saturation, heart rate, and respiratory rate using a pulse oximeter.
In the second experiment, we used 4 groups of rats. We used the same 8 male rats of the WST strain repeatedly after allowing them to rest for at least 2 days after drug administration. Drugs were administered by IP injection. Each group of 8 rats was given ATI after administration of the anesthetic mixture. The rats in group 1 were administered 0.15 mg/kg b.w. ATI at 30 min after administration of the anesthetic mixture. The rats in group 2 were administered 0.75 mg/kg b.w. ATI at 30 min after administration of the anesthetic mixture. The rats in group 3 were administered 0.15 mg/kg b.w. ATI at 10 min after administration of the anesthetic mixture. The rats in group 4 were administered 0.75 mg/kg b.w. ATI at 10 min after administration of the anesthetic mixture. After administration of the anesthetic mixture, the anesthetic score was measured every 5 min. After injection of ATI, the anesthetic score was measured every 1 min.
The rats were euthanatized by IV injection of sodium pentobarbital (80 mg/kg b.w.) (Somnopentyl®, Kyoritsu Seiyaku Corporation, Tokyo, Japan) after completion of the experiment.
Measurement of anesthetic scores
The method of measuring the anesthetic scores was previously described elsewhere [14]. The score was based on 5 reflexes. The first was a front paw reflex, the second was a hind paw reflex, the third was a tail reflex, the fourth was a corneal reflex, and the fifth was a body righting reflex. If a rat showed no reflex, it was given a score of 1. If it reacted, it was given a score of 0. The total anesthetic score was graded from 0 to 5. A rat that showed a score of 0 to 3 was not considered to be anesthetized. A rat that showed a score of 4 or 5 was considered to be anesthetized, and the duration for which at a rat showed a score of 4 or 5 was considered the anesthetic duration. The time required for the anesthetic score to return to 0 was considered the recovery time.
Measurement of O2 saturation, heart rate, and respiratory rate
A pulse oximeter (Mouse Ox Plus®, STARR Life Sciences Corp., Oakmont, PA, USA) was used to measure O2 saturation, heart rate, and respiratory rate of rats during the experiment. The day before the experiment, all hair covering both carotid arteries of each rat was removed using an electric shaver and a depilatory under inhalational isoflurane (Escain®, Mylan Seiyaku, Tokyo, Japan) anesthesia using an anesthetic instrument (KN-1071-I, Natsume Seisakusho Co., Ltd., Tokyo, Japan).
A sensor clip of the pulse oximeter was placed at the cervical parts of the rat. Then, we recorded O2 saturation, heart rate, and respiratory rate until 90 min after administration of saline. After administration of the anesthetic mixture, we recorded vital signs until the time when each rat was completely recovered from anesthesia.
Drug preparation
Saline (Otsuka Normal Saline®, Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) and drugs were purchased. The anesthetic mixture was prepared as a mix of three drugs: MED (Domitor®, Nippon Zenyaku Kogyo Co., Ltd., Tokyo, Japan), MID (Dormicum®, Astellas Pharma Inc., Tokyo, Japan), and BUT (Vetorphale®, Meiji Seika Pharma Co., Ltd., Tokyo, Japan). We mixed MED 0.15 mg, MID 2 mg, and BUT 2.5 mg/kg b.w./rat and added distilled sterile water (Otsuka Distilled Water®, Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) to adjust the mixture to an administrative volume of 0.5 ml/100 g b.w./rat. For example, 0.3 ml of Domitor, 0.8 ml of Dormicum, 1.0 ml of Vetorphale, and 7.9 ml of distilled sterile water were mixed to make 10 ml of an experimental anesthetic mixture. The anesthetic mixture was prepared on the day before the experiment and kept in a refrigerator. The mixed drug was allowed to be used up to 1 week after being mixed.
In the second experiment, we made 0.75 mg/kg b.w./rat of ATI (Antisedan®, Nippon Zenyaku Kogyo Co., Ltd., Tokyo, Japan) and adjusted it to an administrative volume of 0.5 ml/100 g b.w./rat. To make 10 ml of 0.75 mg/kg of ATI, 0.3 ml of Antisedan and 9.7 ml of distilled sterile water were mixed. To make 0.15 mg/kg of ATI, 2 ml of a solution of 0.75 mg/kg of ATI and 8 ml of distilled sterile water were mixed. The solution of ATI was also allowed to be used up to 1 week after being made.
Drug preparation was conducted at a clean bench in a sterile manner. Just before administration, the drug was warmed in an incubator set at a temperature of around 37°C.
Statistical analysis
Statistical analysis was conducted using the StatView software (Hulinks Inc., Tokyo, Japan). Graph data are presented as means ± SD until 90 min after drug administration except for a graph of the anesthetic scores. Body weight differences, anesthetic duration, and recovery time for each experimental group were analyzed using one-way analysis of variance (ANOVA). Time course differences in anesthetic score, O2 saturation, heart rate, and respiratory rate were analyzed by one-way repeated measures ANOVA. Scheffe’s test was used for post hoc analysis. A P value less than 0.05 was considered to be statistically significant.
Results
All rats used in this experiment recovered from anesthesia.
First experiment
Body weight
The body weights of the SD (n=6), WST (n=6), and F344 (n=6) strains before saline was administered were 296.6 ± 8.0, 264.4 ± 6.6, and 187.8 ± 3.7 g, respectively. Significant differences in body weights were recorded among the 3 rat strains administered saline (Table 1). The body weights of the SD (n=8), WST (n=8), and F344 (n=6) strains before the anesthetic mixture was administered were 279.4 ± 17.7, 261.2 ± 19.6, and 168.9 ± 18.6 g, respectively. The body weight of the F344 strain was significantly lighter than those of the other 2 rat strains administered the anesthetic mixture (Table 1).
Table 1. Body weight (g), anesthetic duration (min), and recovery time (min) of the SD, WST, and F344 rat strains.
| Strain | n. | Body weight (g) (mean ± SD) | Anesthetic duration (min) | Recovery time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline | n. | Anesthetic mixture | Mean ± SD | Shortest | Longest | Mean ± SD | Shortest | Longest | ||||
| SD | 6 | 296.6 ± 8.0# | 8 | 279.4 ± 17.7$ | 44.4 ± 14.0 | 25 | 65 | 101.9 ± 20.0 | 75 | 125 | ||
| WST | 6 | 264.4 ± 6.6# | 8 | 261.2 ± 19.6$ | 56.9 ± 6.5 | 45 | 65 | 86.3 ± 16.9* | 70 | 115 | ||
| F344 | 6 | 187.8 ± 3.7# | 6 | 168.9 ± 18.6 | 45.0 ± 13.8 | 30 | 65 | 72.5 ± 6.9* | 60 | 80 | ||
Data are presented as means ± SD or as the shortest and longest times for each strain. Differences between each strain were analyzed by one-way ANOVA followed by Scheffe’s test. A P value less than 0.05 was considered statistically significant. *P<0.05 compared with the SD strain. $P<0.05 compared with the F344 strain. #P<0.05 compared with the other strains.
Anesthetic duration
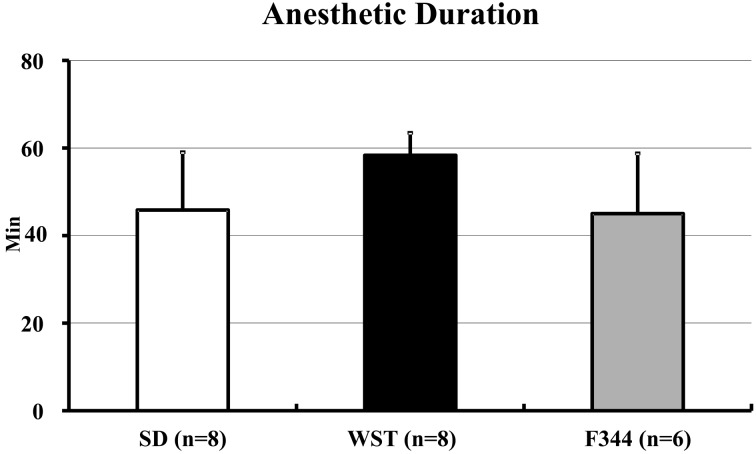
The anesthetic durations of the SD, WST, and F344 strains were 44.4 ± 14.0, 56.9 ± 6.5, and 45.0 ± 13.8 min, respectively (Fig. 1). There were no significant differences among the 3 strains. The shortest anesthetic durations of the SD, WST, and F344 strains were 25, 45, and 30 min, respectively. The longest anesthetic duration of each strain was 65 min (Table 1).
Fig. 1.
Anesthetic duration of the SD, WST, and F344 rat strains administered the anesthetic mixture. Data are presented as means ± SD. Differences between strains were analyzed by one-way ANOVA followed by Scheffe’s test. A P value less than 0.05 was considered to be statistically significant.
Recovery time
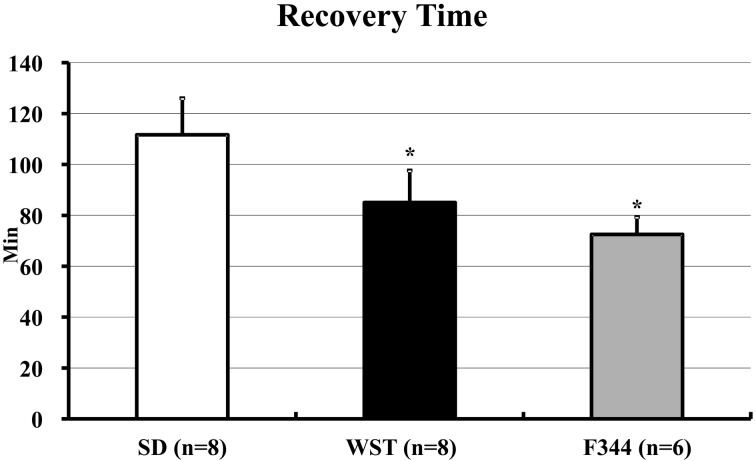
The recovery times of the SD, WST, and F344 strains were 101.9 ± 20.0, 86.3 ± 16.9, and 72.5 ± 6.9 min, respectively (Fig. 2). The recovery time of the SD strain was significantly longer than those of the other 2 strains. The shortest recovery times of the SD, WST, and F344 strains were 75, 70, and 60 min, respectively. The longest recovery times of SD, WST, and F344 strains were 125, 115, and 80 min, respectively (Table 1).
Fig. 2.
Recovery times of the SD, WST, and F344 rat strains administered the anesthetic mixture. Data are presented as means ± SD. Differences between strains were analyzed by one-way ANOVA followed by Scheffe’s test. A P value less than 0.05 was considered to be statistically significant. *P<0.05 compared with the SD strain.
Anesthetic score
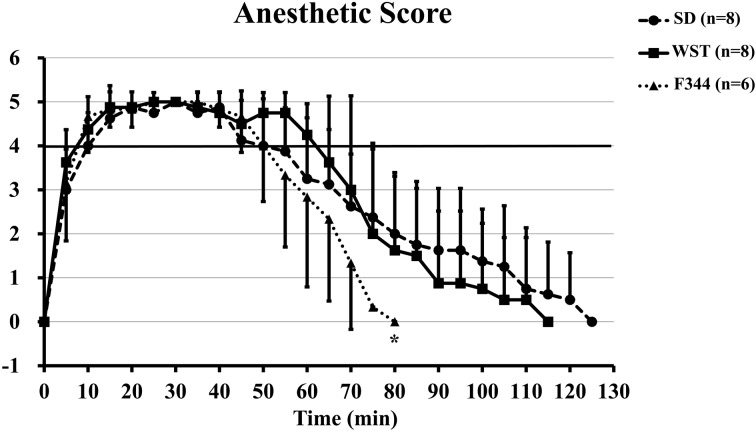
There were no significant differences in anesthetic scores among the 3 rat strains during anesthesia (anesthetic scores of 4 or 5), but 80 min after drug administration, the anesthetic score of the F344 strain was significantly lower than that of the SD strain (0.0 ± 0.0 vs. 2.0 ± 1.3) (Fig. 3).
Fig. 3.
Time courses of the anesthetic score of the SD, WST, and F344 rat strains administered the anesthetic mixture. Data are presented as means ± SD. Differences between strains were analyzed by one-way repeated measures ANOVA followed by Scheffe’s test. A P value less than 0.05 was considered to be statistically significant. *P<0.05 compared with the SD strain.
Measurement by pulse oximeter
1) O2 saturation
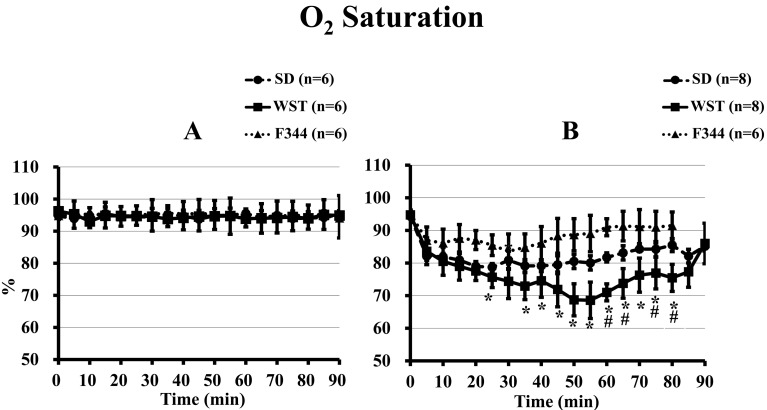
There were no significant differences in O2 saturation among the 3 rat strains before administration of saline or the anesthetic mixture (Fig. 4). After administration of saline, the 3 strains showed no significant differences in O2 saturations over the course of 90 min (Fig. 4A).
Fig. 4.
Time courses of O2 saturation of the SD, WST, and F344 rat strains administered saline (A) or the anesthetic mixture (B). Data are presented as means ± SD. Differences between strains were analyzed by one-way repeated measures ANOVA followed by Scheffe’s test. A P value less than 0.05 was considered to be statistically significant. *P<0.05 compared with the F344 strain. #P<0.05 compared with the SD strain.
From 25 to 80 min after administration of the anesthetic mixture, the O2 saturation of the WST strain was significantly lower than that of the F344 strain (excluding that at 30 min). It was also lower than that of the SD strain from 60 min to 80 min after administration (excluding that at 70 min) (Fig. 4B).
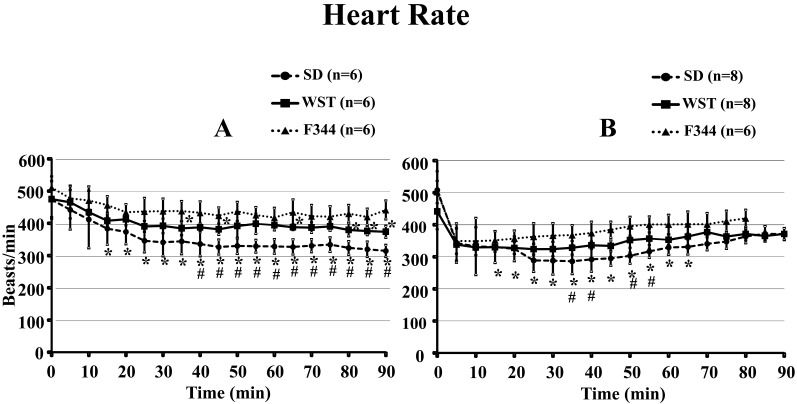
2) Heart rate
There were no significant differences in heart rate among the 3 rat strains before administration of saline or the anesthetic mixture (Fig. 5). From 15 to 90 min after administration of saline, the heart rate of the SD strain was significantly lower than that of the F344 strain, and it was lower that of the WST strain from 40 to 90 min after administration. At 35, 45, and 65 min and 80 to 90 min after administration of saline, the heart rate of the WST strain was significantly lower than that of the F344 strain (Fig. 5A).
Fig. 5.
Time courses of heart rate of the SD, WST, and F344 rat strains administered saline (A) or the anesthetic mixture (B). Data are presented as means ± SD. Differences between strains were analyzed by one-way repeated measures ANOVA followed by Scheffe’s test. A P value less than 0.05 was considered to be statistically significant. *P<0.05 compared with the F344 strain. #P<0.05 compared with the WST strain.
From 15 to 65 min after administration of the anesthetic mixture, the heart rate of the SD strain was significantly lower than that of the F344 strain, and it was also lower than that of the WST strain from 35 to 55 min after administration (excluding that at 45 min), respectively.
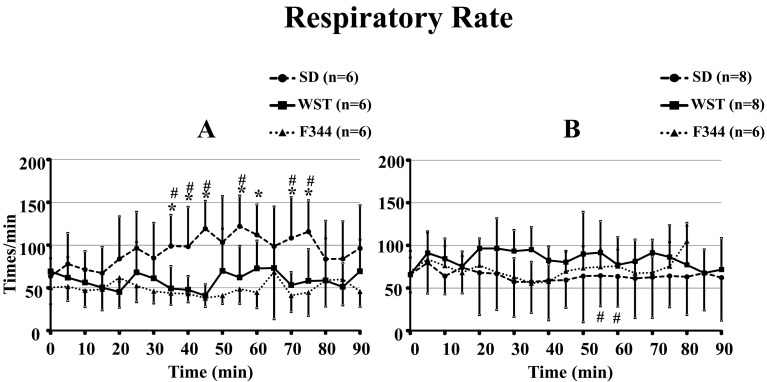
3) Respiratory rate
There were no significant differences in respiratory rate among the 3 rat strains before administration of saline or the anesthetic mixture (Fig. 6). From 35 min to 75 min after administration of saline, the respiratory rate of the SD strain was significantly higher than that of the F344 strain (excluding that at 50 and 65 min), and it was also higher than that of the WST strain from 35 to 75 min after administration (excluding those at 50, 60, and 65 min) (Fig. 6A).
Fig. 6.
Time courses of respiratory rate of the SD, WST, and F344 rat strains administered saline (A) or the anesthetic mixture (B). Data are presented as means ± SD. Differences between strains were analyzed by one-way repeated measures ANOVA followed by Scheffe’s test. A P value less than 0.05 was considered to be statistically significant. *P<0.05 compared with the F344 strain. #P<0.05 compared with the WST strain.
At 55 and 60 min after administration of the anesthetic mixture, the respiratory rate of the SD strain was significantly lower than that of the WST strain (Fig. 5B).
Second experiment
Body weight
There were no significant differences in the body weights of the 4 groups in male WST rats (Table 2).
Table 2. Body weights (g) of the 4 groups of male WST rats. Injection route, concentration of atipamezole, and injection timing after administration of the anesthetic mixture are shown in addition to body weights in this table.
| Group | n. | Body weight (g) | Anesthetic mixture | Atipamezole | Injection timing after anesthetic mixture (min) | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Route | Route | Concentration | |||||
| 1 | 8 | 270.9 ± 21.4 | IP | IP | 0.15 mg/kg | 30 | ||
| 2 | 8 | 257.0 ± 27.9 | IP | IP | 0.75 mg/kg | 30 | ||
| 3 | 8 | 267.6 ± 22.7 | IP | IP | 0.15 mg/kg | 10 | ||
| 4 | 8 | 264.0 ± 22.5 | IP | IP | 0.75 mg/kg | 10 | ||
Data for body weight are presented as means ± SD. Differences between experimental groups were analyzed by one-way ANOVA followed by Scheffe’s test. A P value less than 0.05 was considered to be statistically significant.
Recovery time
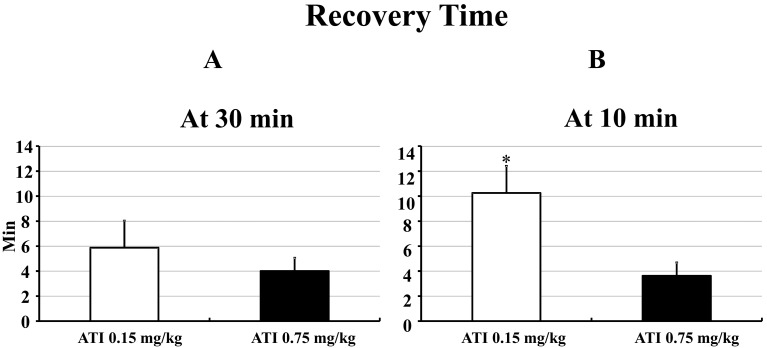
At 30 min after administration of the anesthetic mixture, the recovery times of the rats injected 0.15 mg/kg and 0.75 mg/kg of ATI were 5.9 ± 2.2 and 4.0 ± 1.1 min, respectively. There were no significant differences between the 2 dosages of ATI (Fig. 7A).
Fig. 7.
Recovery time after IP injection of ATI (0.15 mg/kg or 0.75 mg/kg) at 30 min (A) and 10 min (B) after administration of the anesthetic mixture in male WST rats. Data are presented as means ± SD. Differences between groups were analyzed by one-way ANOVA followed by Scheffe’s test. A P value less than 0.05 was considered to be statistically significant. *P<0.05 compared with AIT 0.75 mg/kg at 10 min.
At 10 min after administration of the anesthetic mixture, the recovery times of the rats injected 0.15 mg/kg and 0.75 mg/kg of ATI were 10.3 ± 3.3 and 3.6 ± 0.7 min, respectively. The recovery time for 0.15 mg/kg of ATI was significantly longer than that for 0.75 mg/kg of ATI at 10 min after administration of the anesthetic mixture (Fig. 7B).
Discussion
An anesthetic mixture of MED, MID, and BUT for laboratory mice has been recently introduced [13,14,15]. Originally, this mixture was used as anesthesia for dogs [10, 20, 24], monkeys [12, 17], and African lions in a zoo [25]. Kawai et al. reported that it produced an anesthetic duration of around 40 min in ICR mice [13]. We demonstrated that it produced closely similar anesthetic effects in both male and female BALB/c and C57BL/6J strains [14]. We also demonstrated that there were no significant differences in anesthetic duration among the 3 different (IP, IV, and SC) injection routes in mice [15].
There are only a few studies for the anesthetic mixture of MED, MID, and BUT in rats. Konno et al. reported the efficacy of this anesthetic mixture for endotracheal intubation in rats [16]. Bellini et al. evaluated the efficacy of an anesthetic mixture of MED, MID, and BUT compared with MED only or a combination of MED and BUT using rats [3]. Several studies have shown that there are different anesthetic effects among different rat strains. Siller-Matula et al. reported that WST strain rats were more sensitive to long-lasting anesthesia with isoflurane as compared with SD rats [21]. Avsaroglu et al. used 8 inbred strains to compare anesthetic responses for several anesthetics and found some strain differences [1]. However, the differences in the effects of the anesthetic mixture of MED, MID, and BUT in rats remain unclear.
In the present study, we first examined effects of the anesthetic mixture using 2 outbred strains (WST and SD) and 1 inbred F344 strain. These strains are very common and often used for rat experiments. We found that there were no significant differences in anesthetic duration after administration of the anesthetic mixture among the 3 strains, although the WST strain had a tendency for a longer anesthetic duration compared with the other 2 strains (Fig. 1). Otherwise, the recovery time for the SD strain was significantly longer than those for the other 2 strains (Fig. 2). After an anesthetic duration of around 45 min, the anesthetic score of the F344 strain quickly decreased, and the score reached 0 at 80 min. Compared with the F344 strain, the SD and WST strains needed more time (125 and 115 min, respectively) to reach an anesthetic score of 0 (Fig. 3). In this experiment, the mean body weight of the F344 strain (168.9 g) was lighter than those of the SD (279.4 g) and WST (261.2 g) strains (Table 1). The anesthetic mixture was administered according to body weight (0.5 ml/100 g b.w./rat). However, one reason for the rapid recovery from anesthesia for the F344 strain may be related to its lighter body weight as compared with the heavier SD and WST strains. Also, Avsaroglu et al. reported that the F344 strain slept a significantly shorter amount of time after administration of MED than other inbred strains [1]. Therefore, we guessed that the F344 strain would recover quickly from anesthesia as compared with the other 2 strains.
In our previous study using 2 mouse strains, we recorded no significant differences in anesthetic effects for the anesthetic mixture between male and female mice [14]. Several studies have reported sex differences in anesthesia for rats [5, 26]. However, further exploration of the sex differences for rats using the anesthetic mixture will require more experiments and research.
We measured the vital signs of 3 the strains without anesthesia because there were some differences in physiological parameters among various strains [4, 9, 22]. After administration of saline, there were no significant differences in O2 saturation among the 3 strains (Fig. 4A). After administration of saline, the heart rate of the SD strain was significantly lower than that of the other 2 strains (Fig. 5A). Furthermore, after administration of saline, the respiratory rate of the SD strain was significantly higher than that of the other 2 strains (Fig. 6A). However, the normal ranges for the heart rate and respiratory rate in several rat strains were 260–450 beats/min and 66–115 times/min, respectively [18, 22]. Therefore, in spite of statistical differences in heart rate and respiratory rate among the 3 different strains after administration of saline, it is not an important issue compared with the heart rate and respiratory rate after administration of the anesthetic mixture.
All 3 rat strains in this experiment showed lower O2 saturation after administration of the anesthetic mixture (Fig. 4B). In particular, the WST strain showed significantly lower O2 saturation than the F344 strain. O2 saturation is one of the parameters used to estimate anesthetic depth and condition under anesthesia in laboratory animals [8]. According to the changes in O2 saturation after administration of the anesthetic mixture, the WST strain seemed to be in a deeper anesthetic condition from 40 to 80 min after administration compared with the 2 other strains. However, our results showed that there were no significant differences in anesthetic duration among the 3 different strains (Fig. 1). One reason for this discrepancy may be related to our scoring method for estimation of anesthetic depth because it cannot measure an anesthetic score over 5.
After administration of the anesthetic mixture, all rat strains showed lower heart rates compared with those after administration of saline (Fig. 5B). However, we found that heart rate differences among the 3 strains were reduced and stable under anesthesia (Fig. 5).
After administration of the anesthetic mixture, the respiratory rate of the SD strain was lower than that after administration of saline (Fig. 6). However, the respiratory rates of the WST and F344 strains after administration of the anesthetic mixture were not different from those after administration of saline (Fig. 6). After administration of the anesthetic mixture, the respiratory rates of 3 different strains were almost the same and were stable during the rest of the experiment (Fig. 6B).
In our previous mice study, we showed an anesthetic duration of about 46 min after IP administration of the anesthetic mixture [15]. In the present rat experiment, we demonstrated almost the same anesthetic duration, about 49 min, after IP administration in 3 different rat strains. When we compared the cardiorespiratory influences of the anesthetic mixture between mice and rats, we found similar tendencies. Both rats and mice showed reduced O2 saturation and a reduced heart rate after administration of the anesthetic mixture. We did not find any respiratory rate changes after administration of the anesthetic mixture in either mice or rats.
ATI is a synthetic alpha2-adrenergic receptor antagonist that can antagonize an alpha2-adrenergic receptor agonist, MED [7]. After administration of the anesthetic mixture, injection of ATI caused rapid recovery from anesthesia. However, neither the appropriate dosage nor optimum injection timing of ATI after administration of the anesthetic mixture is clear in rats.
We previously reported the efficacy of ATI with a suitable dosage and timing in mice [15]. In this previous mouse experiment, we used the same dosage of ATI as MED (0.3 mg/kg) and a 5-times higher dosage of ATI (1.5 mg/kg) than MED and chose injection timings of 10 and 30 min after administration of the anesthetic mixture. Therefore, we used the same dose as MED (0.15 mg/kg) and a 5-times higher dose (0.75 mg/kg) than MED and injection timings of 10 and 30 min after administration of the anesthetic mixture in our rat experiment.
At 30 min after administration of the anesthetic mixture, injection of ATI at 0.15 mg/kg and 0.75 mg/kg resulted in almost the same rapid recovery time (Fig. 7A). On the other hand, at 10 min after administration of the anesthetic mixture, injection of ATI 0.15 mg/kg resulted in the need for more time to recover from anesthesia compared with ATI 0.75 mg/kg (Fig. 7B). Baker et al. reported that there were no significant differences in recovery times after receiving 5 mg/kg of ATI at 10 min and 40 min after administration of a combination of KET (75 mg/kg) and MED (1 mg/kg) in mice [2]. Our previous study using mice also showed no significant differences in recovery times at 10 min and 30 min after administration of the anesthetic mixture when ATI was administered at 1.5 mg/kg, a 5-times higher dosage than that of MED (0.3 mg/kg) [15]. In a study by Jang et al. using SD rats, a 5-times higher dosage of ATI than MED was injected at 70 min after administration of a combination of MED (0.2, 0.4 or 0.8 mg/kg) and KET (60 mg/kg) [11]. They reported that a 5-times higher dosage of ATI caused reversal of anesthesia within 10 min after administration of MED and KET. However, Jang et al. theorized that ATI at a dosage 5-times higher than the MED might not be adequate for satisfactory reversal of anesthesia induced by MED and KET in rats [11]. In our rat study, we also used a 5-times higher dosage of ATI (0.75 mg/kg) than MED (0.15 mg/kg) and observed quick recovery, in 5 min, from anesthesia.
When ATI at a dosage 5-times higher than the dosage of MED is used, a combination of MED and KET may result in the need for more time to recover from anesthesia compared with in the case of administration of the anesthetic mixture. At 10 min after administration of the anesthetic mixture, rats injected with ATI (0.15 mg/kg) at the same dosage as MED (0.15 mg/kg) needed more time (10.3 ± 3.3 min) to recover from anesthesia as compared with the time needed by mice (6.2 ± 3.3 min) [15].
Regarding the safety of ATI, all rats injected with ATI (0.75 mg/kg) perfectly recovered from anesthesia and showed normal values for O2 saturation, heart rate, and respiratory rate. In this experiment, we used rats repeatedly and confirmed that the parameters described above were normal before proceeding to the next injection experiment. In our previous study using mice, we injected a 5-times higher dosage of ATI (1.5 mg/kg) than MED (0.3 mg/kg) and found no significant changes in health condition [15]. Baker et al. reported that there were no mortalities and no weight loss or reduced water intake at 24 and 48 h after administration of ATI (5 mg/kg) in mice [2]. Also, Jang et al. injected ATI (4 mg/kg) in rats and reported rapid increases in respiratory rate, but there was no mention of health problems after recovering from anesthesia [11]. Therefore, we suggest that 0.75 mg/kg is a safe and proper dosage of ATI for helping rats quickly recover from anesthesia at both 10 and 30 min after administration of the anesthetic mixture.
In summary, our study indicated that an anesthetic mixture of MED, MID, and BUT produced a closely similar anesthetic duration in 3 different rat strains: SD, WST, and F344. The anesthetic mixture of MED, MID, and BUT is a useful anesthetic that can be antagonized with an antagonist such as ATI to help rats quickly recover from anesthesia. These results may contribute to the welfare of laboratory animals.
Acknowledgments
The authors wish to thank Mr. John Telloyan of Shimane University, School of Medicine, for English assistance.
References
- 1.Avsaroglu H., van der Sar A.S., van Lith H.A., van Zutphen L.F., Hellebrekers L.J.2007. Differences in response to anaesthetics and analgesics between inbred rat strains. Lab. Anim. 41: 337–344. doi: 10.1258/002367707781282811 [DOI] [PubMed] [Google Scholar]
- 2.Baker N.J., Schofield J.C., Caswell M.D., McLellan A.D.2011. Effects of early atipamezole reversal of medetomidine-ketamine anesthesia in mice. J. Am. Assoc. Lab. Anim. Sci. 50: 916–920. [PMC free article] [PubMed] [Google Scholar]
- 3.Bellini L., Banzato T., Contiero B., Zotti A.2014. Evaluation of three medetomidine-based protocols for chemical restraint and sedation for non-painful procedures in companion rats (Rattus norvegicus). Vet. J. 200: 456–458. doi: 10.1016/j.tvjl.2014.03.024 [DOI] [PubMed] [Google Scholar]
- 4.Boyd R.L., Halderman L.W., Harris J.O., Mangos J.A.1982. Strain differences in pulmonary function of laboratory rats. Lab. Anim. Sci. 32: 42–43. [PubMed] [Google Scholar]
- 5.Ciccone G.K., Holdcroft A.1999. Drugs and sex differences: a review of drugs relating to anaesthesia. Br. J. Anaesth. 82: 255–265. doi: 10.1093/bja/82.2.255 [DOI] [PubMed] [Google Scholar]
- 6.Fender C., Fujinaga M., Maze M.2000. Strain differences in the antinociceptive effect of nitrous oxide on the tail flick test in rats. Anesth. Analg. 90: 195–199. doi: 10.1097/00000539-200001000-00039 [DOI] [PubMed] [Google Scholar]
- 7.Flecknell P.A. 2009. Chapter 2, Anesthesia. p.24 In: Laboratory Animal Anaesthesia. 3rd ed. ELSEVIER, London. [Google Scholar]
- 8.Flecknell P.A. 2009. Chapter 3, Analgesic management. p.83–87 In: Laboratory Animal Anaesthesia. 3rd ed. ELSEVIER, London. [Google Scholar]
- 9.Gordon C.J., Watkinson W.P.1995. Strain differences in the laboratory rat: impact on the autonomic, behavioral, and biochemical response to cholinesterase inhibition. J. Toxicol. Environ. Health 45: 59–73. doi: 10.1080/15287399509531980 [DOI] [PubMed] [Google Scholar]
- 10.Itamoto K., Hikasa Y., Sakonjyu I., Itoh H., Kakuta T., Takase K.2000. Anaesthetic and cardiopulmonary effects of balanced anaesthesia with medetomidine-midazolam and butorphanol in dogs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 47: 411–420. doi: 10.1046/j.1439-0442.2000.00302.x [DOI] [PubMed] [Google Scholar]
- 11.Jang H.S., Choi H.S., Lee S.H., Jang K.H., Lee M.G.2009. Evaluation of the anaesthetic effects of medetomidine and ketamine in rats and their reversal with atipamezole. Vet. Anaesth. Analg. 36: 319–327. doi: 10.1111/j.1467-2995.2009.00463.x [DOI] [PubMed] [Google Scholar]
- 12.Kalema-Zikusoka G., Horne W.A., Levine J., Loomis M.R.2003. Comparison of the cardiorespiratory effects of medetomidine-butorphanol-ketamine and medetomidine-butorphanol-midazolam in patas monkeys (Erythrocebus patas). J. Zoo Wildl. Med. 34: 47–52. doi: 10.1638/1042-7260(2003)34[0047:COTCEO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 13.Kawai S., Takagi Y., Kaneko S., Kurosawa T.2011. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 60: 481–487. doi: 10.1538/expanim.60.481 [DOI] [PubMed] [Google Scholar]
- 14.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Kurosawa T.2013. Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp. Anim. 62: 173–180. doi: 10.1538/expanim.62.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirihara Y., Takechi M., Kurosaki K., Kobayashi Y., Saito Y., Takeuchi T.2015. Anesthetic effects of a three-drugs mixture—comparison of administrative routes and antagonistic effects of atipamezole in mice. Exp. Anim. 64: 39–47. doi: 10.1538/expanim.14-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konno K., Shiotani Y., Itano N., Ogawa T., Hatakeyama M., Shioya K., Kasai N.2014. Visible, safe and certain endotracheal intubation using endoscope system and inhalation anesthesia for rats. J. Vet. Med. Sci. 76: 1375–1381. doi: 10.1292/jvms.14-0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochi T., Nishiura I., Tatsumi M., Hirano Y., Yahagi K., Sakurai Y., Matsuyama-Fujiwara K., Sudo Y., Nishina N., Koyama H.2014. Anesthetic effect of a combination of medetomidine-midazolam-butorphanol in cynomolgus monkeys (Macaca fascicularis). J. Vet. Med. Sci. 76: 917–921. doi: 10.1292/jvms.13-0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohno M., Hirose H., Sugano S.1987. Alteration in heart rate and conduction time during the growing process of rats, mice and guinea pigs. (Japanese). Adv. Anim. Cardiol. 20: 24–32. [Google Scholar]
- 19.Pang H., Kawai S., Shioya K., Katahira K., Yabuuci K., Tajima M., Kurosawa T.2010. Short anesthesia with a combination of three injectable anesthetic in rat. Exp. Anim. 59: 370. [Google Scholar]
- 20.Pypendop B., Verstegen J.1999. Cardiorespiratory effects of a combination of medetomidine, midazolam, and butorphanol in dogs. Am. J. Vet. Res. 60: 1148–1154. [PubMed] [Google Scholar]
- 21.Siller-Matula J.M., Jilma B.2008. Strain differences in toxic effects of long-lasting isoflurane anaesthesia between Wistar rats and Sprague Dawley rats. Food Chem. Toxicol. 46: 3550–3552. doi: 10.1016/j.fct.2008.08.033 [DOI] [PubMed] [Google Scholar]
- 22.Tazima Y. 1972. p.12 In: Experimental Zoology Detailed Discussion. (Japanese) Asakura Publishing Co., Ltd., Tokyo. [Google Scholar]
- 23.Tazima Y. 1989. p.16–17 In: Biological Reference Data Book on Experimental Animals. (Japanese) SOFT SCIENCE, INC., Tokyo. [Google Scholar]
- 24.Verstegen J., Petcho A.1993. Medetomidine-butorphanol-midazolam for anaesthesia in dogs and its reversal by atipamezole. Vet. Rec. 132: 353–357. doi: 10.1136/vr.132.14.353 [DOI] [PubMed] [Google Scholar]
- 25.Wenger S., Buss P., Joubert J., Steenkamp J., Shikwambana P., Hatt J.M.2010. Evaluation of butorphanol, medetomidine and midazolam as a reversible narcotic combination in free-ranging African lions (Panthera leo). Vet. Anaesth. Analg. 37: 491–500. doi: 10.1111/j.1467-2995.2010.00569.x [DOI] [PubMed] [Google Scholar]
- 26.Zambricki E.A., Dalecy L.G.2004. Rat sex differences in anesthesia. Comp. Med. 54: 49–53. [PubMed] [Google Scholar]