Abstract
Valproic acid (VPA) is a widely used antiepileptic drug, which has recently been reported to modulate the neuronal differentiation of adipose tissue-derived stem cells (ASCs) in humans and dogs. However, controversy exists as to whether VPA really acts as an inducer of neuronal differentiation of ASCs. The present study aimed to elucidate the effect of VPA in neuronal differentiation of rat ASCs. One or three days of pretreatment with VPA (2 mM) followed by neuronal induction enhanced the ratio of immature neuron marker βIII-tubulin-positive cells in a time-dependent manner, where the majority of cells also had a positive signal for neurofilament medium polypeptide (NEFM), a mature neuron marker. RT-PCR analysis revealed increases in the mRNA expression of microtubule-associated protein 2 (MAP2) and NEFM mature neuron markers, even without neuronal induction. Three-days pretreatment of VPA increased acetylation of histone H3 of ASCs as revealed by immunofluorescence staining. Chromatin immunoprecipitation assay also showed that the status of histone acetylation at H3K9 correlated with the gene expression of TUBB3 in ASCs by VPA. These results indicate that VPA significantly promotes the differentiation of rat ASCs into neuron-like cells through acetylation of histone H3, which suggests that VPA may serve as a useful tool for producing transplantable cells for future applications in clinical treatments.
Keywords: adipose tissue-derived stem cells, histone deacetylase inhibitor, neuronal differentiation, neuron marker, rats
Introduction
Adipose tissue-derived stem cells (ASCs) are mesenchymal stem cells that are isolated from the stromal vascular fraction of adipose tissues [3, 14, 19]. In a similar way to bone marrow-derived mesenchymal stem cells (BMSCs), ASCs can differentiate into not only mesenchymal lineage cells [17, 18], but also into neurogenic lineage cells [16, 18]. However, unlike BMSCs, ASCs can easily be obtained in large quantities and with minimum risk from invasive surgery [5]. Therefore, it is reasonable to conclude that ASCs will be the preferred adult stem cells for future clinical applications [14].
Valproic acid (VPA), well known as an antiepileptic and anticonvulsant drug, is an inhibitor of class I histone deacetylase (HDACs) [2, 15]. The acetylation of histone N-terminal tails is thought not only to alter the interaction between histone and DNA, but also to bind acetylated histone to bromodomain proteins and transcription activators [8], thus inducing the expression of genes on the loci [4, 6, 7, 10]. We have previously shown that VPA induces neuronal differentiation of canine ASCs [11], a result that suggests a safer and more direct method for preparing the cell source for future applications. However, Lee et al. [12] showed that the pretreatment of VPA diminished efficiency of neuronal differentiation of human ASCs. Thus there is debate over the role of VPA as an inducer of neuronal differentiation of ASCs. To address this, we examined the effect of VPA on neuronal differentiation of ASCs isolated from adipose tissue in rats in the present study. The results indicate that VPA induced differentiation of rat ASCs into neuron-like cells through acetylation of histone H3.
Materials and Methods
Isolation and culture of rat ASCs
ASCs were isolated as previously described [11]. Briefly, 10 to 12-week-old male Wistar rats (Charles River Japan, Yokohama, Japan) were euthanatized by decapitation under light anesthesia. Subcutaneous adipose tissue was excised from the inguinal regions and extensively washed with a washing buffer of sterile phosphate-buffered saline (PBS) containing penicillin (100 U/ml) and streptomycin (100 μg/ml) to remove contaminating blood cells and local anesthetics. The tissue was minced into small pieces and then incubated in washing buffer with added 0.05% collagenase type 1A (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 1 h with vigorous shaking. The top lipid layer was removed, and the remaining liquid portion was centrifuged at 200 × g for 10 min. The pellet was resuspended in a growth medium of Dulbecco’s modified Eagle’s medium (DMEM, Nissui, Tokyo, Japan) supplemented with 10% newborn bovine serum (NBS, Invitrogen, Carlsbad, CA, USA) and antibiotics above described, and then spread in 100-mm collagen type 1-coated dishes (Iwaki, Tokyo, Japan) at a density of 1 × 106 cells per dish. Cells were maintained in growth medium at 37°C and 5% CO2. After 24 h, the unattached cells were removed by rinsing with washing buffer. ASCs were identified by confirming multilineage potentials to adipogenic, osteogenic and neurogenic cells (data not shown). Twice-passaged ASCs were used in the present study. All animal experiments in the present study were carried out according to the guidelines of the Committee for Animal Experimentation at Azabu University.
In vitro neuronal differentiation assay
In vitro assay of neuronal differentiation was carried out as previously described [1] with a minor modification. Briefly, ASCs were seeded on non-coating glass coverslips in 35-mm dishes at a density of 1 × 104 cells per dish. ASCs were incubated for one or three days in growth medium containing 2 mM of VPA. After pretreatment with and without VPA, vehicle dimethylsulfoxide (DMSO), the medium was changed to a neuronal induction medium (NIM) of DMEM supplemented with 100 μM dibutyryl cyclic adenosine monophosphate (dbcAMP, Wako Pure Chemical Ind., Osaka, Japan) and 125 μM isobutylmethylxanthine (IBMX, Wako Pure Chemical Ind.) for 2 h [1, 11]. Control for NIM was NIM minus dbcAMP and IBMX. After pretreatment of VPA followed by NIM, the cells were fixed in PBS containing 3.7% formaldehyde for 15 min at room temperature. Similarly incubated ASCs on non-coating 35-mm dishes were removed by a cell-scraper and the removed-cells were immersed in ISOGEN (Nippon Gene, Tokyo, Japan) and stored at –80°C until further analysis. Neuronal differentiation was determined by immunofluorescence staining for βIII-tubulin and neurofilament medium polypeptide (NEFM) and by RT-PCR analysis for expression of genes, βIII-tubulin (TUBB3), NEFM, microtubule-associated protein 2 (MAP2), glial fibrillary acidic protein (GFAP) and housekeeping gene hypoxanthine-guanine phosphoribosyl-transferase (HPRT).
Immunofluorescence staining
Immunocytochemical analyses for βIII-tubulin, NEFM and acetylation of histone H3 were performed. Formaldehyde-fixed ASCs were rinsed with PBS three times. The cells were then permeated with 0.2% Triton X-100 in PBS for 10 min at room temperature, and then incubated with, an anti-βIII-tubulin antibody (1:200; Abcam, ab74978, Cambridge, UK), an anti-NEFM antibody (1:400; Proteus BioSciences, Inc., 40-1259, Ramona, CA, USA) and an anti-acetylated histone H3 (K9) antibody (1:100; Novus Biologicals, NB21-1081SS, Littleton, CO, USA) for 1 h at room temperature. After being washed with PBS, the cells were incubated with secondary antibody (Cy3-conjugated goat anti-rabbit IgG, 1:1600 or FITC-conjugated goat anti-mouse IgG, 1:200, Jackson ImmunoResearch, West Grove, PA) for 30 min at room temperature. The cells were then rinsed with PBS and counterstained with 4’, 6-diamidino-2-phenylindole (DAPI) for nuclear staining before fluorescence microscopic observation. The percentage of βIII-tubulin-positive cells was determined based on the total number of counting cells. At least 300 cells were evaluated per culture.
Reverse transcription-PCR
Total RNA was extracted using ISOGEN and reverse-transcribed for single-strand cDNA, using oligo (dT) primer and Superscript III reverse transcriptase (Invitrogen) according to the manufacture’s instruction. PCR was performed using Taq DNA polymerase (KAPA Biosystems, Woburn, MA, USA) and specific primers, and each cycle consisted of the following steps: denaturation at 98°C for 10 s, annealing at 57°C to 65°C for 30 s, and elongation at 72°C for 30 s (Table 1). Reaction products were electrophoresed on a 2.0% agarose gel and visualized with ethidium bromide.
Table 1. Primer sequences used in RT-PCR.
| Gene | Primer sequence (5’-3’) | Anealing temperature | Product length (bp) | |
|---|---|---|---|---|
| TUBB3 | Forward | 5’-GGCCTCCTCTCACAAGTATGT-3’ | 58°C | 167 |
| Reverse | 5’-CGCCCTCTGTATAGTGC-3’ | |||
| NEFM | Forward | 5’-AGGCTGAGTCCCCAGTGAAA-3’ | 58°C | 220 |
| Reverse | 5’-TCCACCTCCCCATTGATAGC-3’ | |||
| MAP2 | Forward | 5’-ACCTTCCTCCATCCTCCCTC-3’ | 57°C | 151 |
| Reverse | 5’-AGTAGGTGTTGAGGTGCCGC-3’ | |||
| GFAP | Forward | 5’-ACATCGAGATCGCCACCTAC-3’ | 58°C | 228 |
| Reverse | 5’-GCACACCTCACATCACATCC-3’ | |||
| HPRT | Forward | 5’-AATGTCTGTTGCTGCGTC-3’ | 55°C | 92 |
| Reverse | 5’-TGTCTGTCTACAAGGGAAG-3’ |
Effect of VPA on the acetylation of histone H3
To confirm the effect of VPA on the acetylation of histone H3, ASCs were incubated for three days using a similar method to that described above, in growth medium containing 2 mM VPA or valpromide (VPM), an analogue of VPA without HDAC inhibitory activity. After treatment with these reagents, the cells were fixed with formaldehyde in a similar method to that described above. The effect of VPA on the acetylation of histone H3 was examined by immunofluorescence staining for acetylated-histone H3.
Chromatin Immunoprecipitation assay
Tissue samples were treated with chromatin immunoprecipitation (ChIP) reagents (Nippon Gene), following the manufacturer’s instructions with minor modifications. Cells were harvested and mixed with formaldehyde at a final concentration of 1.0% for 5 min at room temperature to cross-link protein to DNA. Cells were then suspended in lysis buffer (Protease Inhibitors, Pierce, Rockford, IL, USA) and incubated on ice for 10 min. DNA cross-linked with protein was sonicated into fragments of 200–1,000 bp by an Ultrasonic Homogenizer (Taitec, Koshigaya, Japan). Samples were subsequently incubated at 4°C with Dynabeads (Invitrogen) to obtain the soluble chromatin fraction. Dynabeads were previously incubated with 4 μg rabbit anti-histone H3 (acetyl K9) antibody (Novus Biologicals, Littleton) or ChIP rabbit IgG control antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 16 h overnight at 4°C. The chromatin-antibody complexes were eluted with ChIP direct elution buffer. Protein-DNA cross-links were reversed with 5 M NaCl for 12 h incubation at 65°C. Proteinase K treatment and phenol-chloroform extraction were carried out, and the DNA was then precipitated in ethanol, and used as a template for PCR. PCR amplification was carried out with primers specific for promoter region of TUBB3. Primer sequences were shown as follows, forward; 5’- ACTCCATACCCCCTCTTTGC-3’, reverse; 5’-AGTCCATGGCTCCACAAAAG-3’. Input DNA and DNA immunoprecipitated by anti-IgG served as positive and negative control, respectively.
Statistical analysis
Results are expressed as means ± standard error. Multiple comparisons were performed with the Turkey-Kramer test after one-way analysis of variance (ANOVA). A P value of less than 0.05 was considered statistically significant.
Results
VPA promotes neuronal differentiation in a time-dependent manner
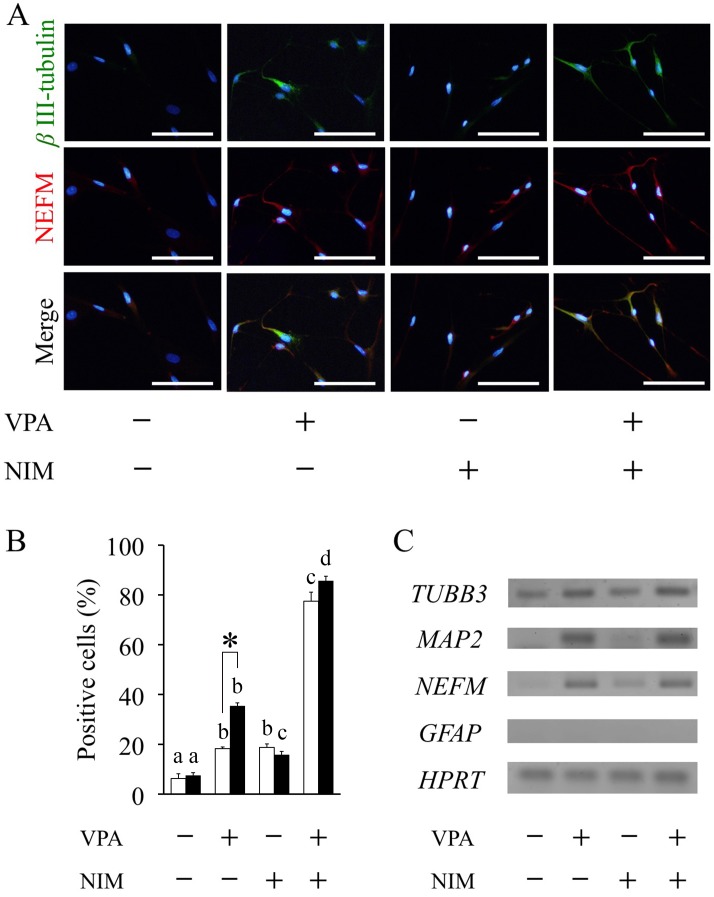
Using immunocytochemistry and RT-PCR analysis, neuronal differentiation was determined in ASCs with and without VPA pretreatment for 1 or 3 days, followed by treatment with and without NIM for 2 h. Regardless of the duration of VPA treatment, differentiated ASCs showed neuron-like morphology with several branching neurite-like cell processes, in which most of the cells had positive signals of an immature neuron marker βIII-tubulin and a mature neuron marker NEFM. Three-days treatment with VPA significantly enhanced the positive signal of βIII-tubulin and NEFM with and even without NIM treatment. The majority of βIII-tubulin positive cells also expressed NEFM, and both immunofluorescence intensities were reduced without VPA pretreatment (Fig. 1A). Neuronal induction significantly increased the ratio of βIII-tubulin-positive cells from 15.7 ± 1.4% without VPA to 85.6 ± 1.9% with three-days treatment of VPA (Fig. 1B). Three-days treatment of VPA also significantly increased the ratio of βIII-tubulin-positive cells from 35.4 ± 1.2% without NIM to 85.6 ± 1.9% with NIM. Without NIM groups, VPA significantly increased the ratio of βIII-tubulin-positive cells in a time-dependent manner (Fig. 1B).
Fig. 1.
Valproic acid promotes neuronal differentiation. Adipose tissue-derived stem cells (ASCs) were pretreated with valproic acid (VPA) for 1 or 3 days followed by neuronal induction medium (NIM) for 2 h. (A) Neuronal differentiation was assessed by immunocytochemistry using anti-βIII-tubulin antibody (green) and anti-NEFM antibody (Red) after fixing at the end of incubation in 3-days VPA treatment group. Nuclei were counterstained with DAPI (blue). Scale bar, 100 μm. (B) The percentage of βIII-tubulin-positive cells was determined based on the total number of counting cells in 1 or 3 days VPA treatment. At least 300 cells were evaluated per culture. Data are the means ± S.E. of 4 independent experiments. Open and solid bars represent 1 or 3 days VPA treatment, respectively. a, b, c, d: bars with different letters at the top differ significantly among same duration group, asterisk indicates significantly different compared to one-day treatment group, P<0.05. (C) RT-PCR analysis of neuronal markers TUBB3, MAP2 and NEFM, the glial marker GFAP and house keeping gene HPRT was performed using total RNA extracted from ASCs after 3-days VPA treatment followed by 2 h of neuronal induction.
mRNA expressions of immature neuron marker TUBB3 were observed in all groups with and without pretreatment of VPA, but mRNA expression of the glial cell marker GFAP was not detected in any groups (Fig. 1C). Furthermore, three-days treatment of VPA markedly elevated not only the mRNA expression levels of immature neuron marker TUBB3, but also mature neuron markers MAP2 and NEFM in ASCs with and without NIM. One-day treatment of VPA also induced the expression of these mature neuron markers (data not shown). In addition, NIM treatment without VPA did not significantly enhance the expressions of these neuron markers when compared to those observed in the groups with VPA.
VPA induces acetylation of histone H3
To confirm the effect of VPA on acetylation of histone H3, we examined the acetylated histone H3 by immunofluorescence staining. Minimal acetylation of histone H3 were observed in the control ASCs. Three-days pretreatment of VPA gave morphological changes of the ASCs such as flattened and expanded shapes, and increased their acetylation of histone H3 (Fig. 2A). In contrast, VPM, an analogue of VPA without HDAC inhibitory activity, did not cause any morphological changes or significant changes in acetylation-positive signal of histone H3 in ASCs.
Fig. 2.
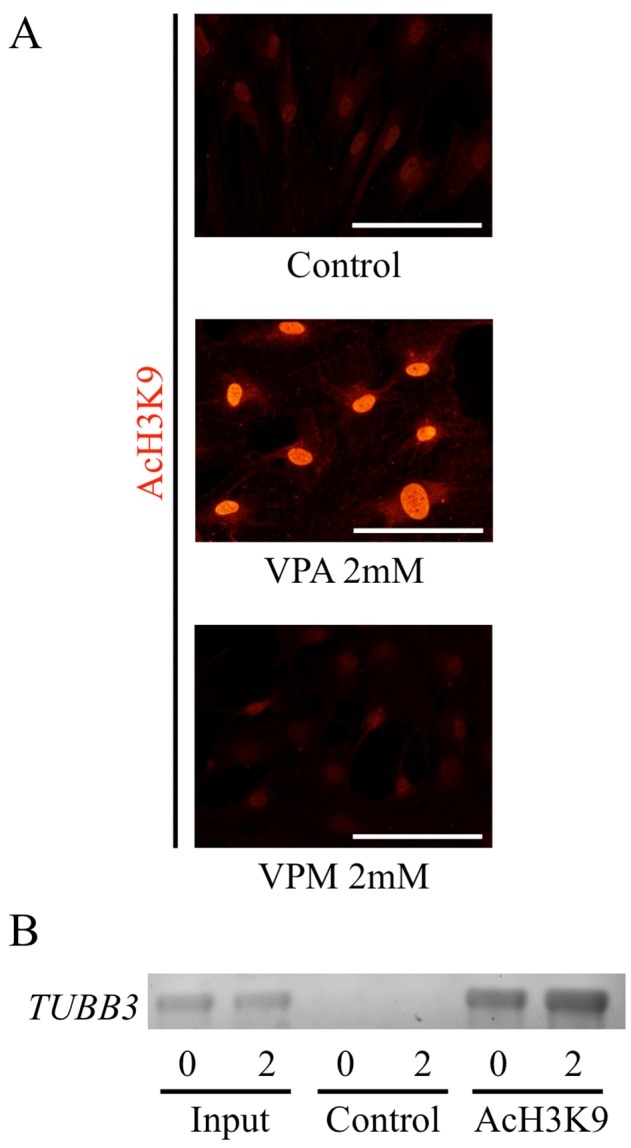
Effects of valproic acid on histone H3 acetylation. (A) Adipose tissue-derived stem cells (ASCs) from rats were incubated with valproic acid (VPA) or valpromide (VPM), an analogue of VPA without histone deacetylase inhibitory activity, for 3 days. Control group was treated with solvent, DMSO. ASCs were immunostained with an anti-acetylated histone H3 (K9) antibody (Red). Acetylated histone H3 (K9) antibody was visualized with Cy3-conjugated goat anti-rabbit IgG. Scale bar, 100 μm. (B) VPA increased acetylation levels at the proximal region of TUBB3. The state of the histone tails of ASCs pretreated with VPA for 3 days was analyzed by ChIP assay using antibody to acetylated lysine 9 on histone H3 (anti-AcH3K9). Typical electrophoresis of PCR analysis of TUBB3 was shown.
VPA increases H3K9 acetylation levels at the proximal promoter region of TUBB3
The state of the histone tails of ASCs pretreated with VPA for three days was analyzed by ChIP assay using antibody to acetylated lysine 9 on histone H3 (anti-AcH3K9). The acetylated state of histone H3 tails associated with the proximal region of TUBB3 increased in ASCs pretreated by VPA compared to those in the ASCs without VPA (Fig. 2B), indicating that the increase of histone acetylation of H3K9 correlated with the expression of the TUBB3 gene of ASCs pretreated with VPA.
Discussion
In this study we demonstrated that VPA promoted the differentiation of approximately 86% of ASCs into βIII-tubulin-positive neuron-like cells after three-days treatment followed by neuronal induction. The differentiated cells showed neuron-like morphology with branching neurite-like cell protrusions, and were significantly immunostained to an immature neuron marker βIII-tubulin, and a mature neuron marker NEFM. Pretreatment with VPA followed by neuronal induction also significantly enhanced mRNA expressions of TUBB3, MAP2 and NEFM, compared to the groups without VPA. We also demonstrated that VPA induced differentiation of ASCs into neuron-like cells in a time-dependent manner. The present study is in agreement with our previous study demonstrating that VPA promotes neuronal differentiation of canine ASCs [11], and further indicates that VPA not only promotes neuronal differentiation but can also promote more mature neuron-like cells. The present data also show that VPA enhances expression of neuron markers, whereas no effect was observed for glial marker. This suggests that VPA induces the differentiation of ASCs into neuron-like cells by neuronal induction, although further confirmation by means of a detailed study is required.
In the present study, we used a well-known neuronal differentiation medium including IBMX and dbcAMP. We showed that VPA increased the neuronal morphological changes and neuron markers expression even without neuronal induction treatment, thus indicating that VPA can induce neuronal differentiation in the absence of neuronal induction. We also demonstrated that VPA increased the acetylation of histone H3K9 on the proximal region of TUBB3 in rat ASCs, leading to the neuronal differentiation. Present results suggest that the promotional effect of VPA on neuronal differentiation occurs through inducing the acetylation of histone H3.
The investigation demonstrated that VPA flattened and expanded the shape of rat ASCs and markedly induced their acetylation of histone H3. These observations are in accordance with the findings of Lee et al. [12] and Kurihara et al. [11]. Lee et al. [12], however, showed that VPA diminished the efficiency of neuronal differentiation of human ASCs. The study showed that one-day pretreatment of VPA (1, 10 mM) followed by neuronal induction decreased neurite formation and expression of βIII-tubulin in human ASCs [12]. On the other hand, the study of Kurihara et al. [11] showed that VPA induced neuronal differentiation of canine ASCs with three-days pretreatment of VPA (4 mM) followed by neuronal induction [11]. Differing treatment regimens of VPA were implemented in these studies and this can partially explain the different effect on neuronal differentiation of ASCs. In the present study, we examined the time-course effects of VPA on the neuronal differentiation of ASCs by immunocytochemistry and RT-PCR analysis. This investigation clearly demonstrated that VPA induced differentiation of ASCs into neuron-like cells, not only as a result of three-days treatment, but also from a one-day course of treatment. Together with the previous study [11], the present results demonstrate that VPA is highly effective in neuronal differentiation of ASCs at least for rats and dogs, when VPA treatment is applied over one and three days. Taken together with the present study, an appropriate treatment of VPA promotes the differentiation of rat ASCs into neuron-like cells, although species difference should be examined in future.
It is well known that the differential potential of neural stem cells (NSCs) into neurons depends on the stages occurring during brain development; early stage NSCs prefer to differentiate into neurons, whereas late stage NSCs into astrocytes [13]. VPA has also been shown to promote the differentiation of adult hippocampal neural progenitors into neurons, but inhibited their glial differentiation in adult neural progenitor cells [6, 9]. These reports offer another explanation that the different effect of VPA on neuronal differentiation depends on the stages of ASCs similarly observed in NSCs during brain development.
In conclusion, the results have shown that, through the acetylation of histone H3, VPA significantly promotes the differentiation of rat ASCs into neuron-like cells, with expression of mature neuron markers. The findings suggest that VPA may prove to be a useful tool for producing transplantable cells for future applications in clinical treatments.
Acknowledgments
This work was supported in part by the Science Research Promotion Fund of The Promotion and Mutual Aid Corporation for Private Schools of Japan. This work was also supported in part by the MEXT Program for the Strategic Research Foundation at Private Universities, 2011–2015.
References
- 1.Deng W., Obrocka M., Fischer I., Prockop D.J.2001. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem. Biophys. Res. Commun. 282: 148–152. doi: 10.1006/bbrc.2001.4570 [DOI] [PubMed] [Google Scholar]
- 2.Göttlicher M., Minucci S., Zhu P., Krämer O.H., Schimpf A., Giavara S., Sleeman J.P., Lo Coco F., Nervi C., Pelicci P.G., Heinzel T.2001. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20: 6969–6978. doi: 10.1093/emboj/20.24.6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gronthos S., Franklin D.M., Leddy H.A., Robey P.G., Storms R.W., Gimble J.M.2001. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 189: 54–63. doi: 10.1002/jcp.1138 [DOI] [PubMed] [Google Scholar]
- 4.Grunstein M.1997. Histone acetylation in chromatin structure and transcription. Nature 389: 349–352. doi: 10.1038/38664 [DOI] [PubMed] [Google Scholar]
- 5.Housman T.S., Lawrence N., Mellen B.G., George M.N., Filippo J.S., Cerveny K.A., DeMarco M., Feldman S.R., Fleischer A.B.2002. The safety of liposuction: results of a national survey. Dermatol. Surg. 28: 971–978. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh J., Nakashima K., Kuwabara T., Mejia E., Gage F.H.2004. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA 101: 16659–16664. doi: 10.1073/pnas.0407643101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenuwein T., Allis C.D.2001. Translating the histone code. Science 293: 1074–1080. doi: 10.1126/science.1063127 [DOI] [PubMed] [Google Scholar]
- 8.Josling G.A., Selvarajah S.A., Petter M., Duffy M.F.2012. The role of bromodomain proteins in regulating gene expression. Genes Basel 3: 320–343. doi: 10.3390/genes3020320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung G.A., Yoon J.Y., Moon B.S., Yang D.H., Kim H.Y., Lee S.H., Bryja V., Arenas E., Choi K.Y.2008. Valproic acid induces differentiation and inhibition of proliferation in neural progenitor cells via the beta-catenin-Ras-ERK-p21Cip/WAF1 pathway. BMC Cell Biol. 9: 66. doi: 10.1186/1471-2121-9-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo M.H., Allis C.D.1998. Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays 20: 615–626. doi: [DOI] [PubMed] [Google Scholar]
- 11.Kurihara Y., Suzuki T., Sakaue M., Murayama O., Miyazaki Y., Onuki A., Aoki T., Saito M., Fujii Y., Hisasue M., Tanaka K., Takizawa T.2014. Valproic acid, a histone deacetylase inhibitor, decreases proliferation of and induces specific neurogenic differentiation of canine adipose tissue-derived stem cells. J. Vet. Med. Sci. 76: 15–23. doi: 10.1292/jvms.13-0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S., Park J.R., Seo M.S., Roh K.H., Park S.B., Hwang J.W., Sun B., Seo K., Lee Y.S., Kang S.K., Jung J.W., Kang K.S.2009. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 42: 711–720. doi: 10.1111/j.1365-2184.2009.00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namihira M., Nakashima K., Taga T.2004. Developmental stage dependent regulation of DNA methylation and chromatin modification in a immature astrocyte specific gene promoter. FEBS Lett. 572: 184–188. doi: 10.1016/j.febslet.2004.07.029 [DOI] [PubMed] [Google Scholar]
- 14.Ning H., Lin G., Fandel T., Banie L., Lue T.F., Lin C.S.2008. Insulin growth factor signaling mediates neuron-like differentiation of adipose-tissue-derived stem cells. Differentiation 76: 488–494. doi: 10.1111/j.1432-0436.2007.00240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phiel C.J., Zhang F., Huang E.Y., Guenther M.G., Lazar M.A., Klein P.S.2001. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276: 36734–36741. doi: 10.1074/jbc.M101287200 [DOI] [PubMed] [Google Scholar]
- 16.Safford K.M., Hicok K.C., Safford S.D., Halvorsen Y.D., Wilkison W.O., Gimble J.M., Rice H.E.2002. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 294: 371–379. doi: 10.1016/S0006-291X(02)00469-2 [DOI] [PubMed] [Google Scholar]
- 17.Wu P., Sato K., Yukawa S., Hikasa Y., Kagota K.2001. Differentiation of stromal-vascular cells isolated from canine adipose tissues in primary culture. J. Vet. Med. Sci. 63: 17–23. doi: 10.1292/jvms.63.17 [DOI] [PubMed] [Google Scholar]
- 18.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., Alfonso Z.C., Fraser J.K., Benhaim P., Hedrick M.H.2002. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13: 4279–4295. doi: 10.1091/mbc.E02-02-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H.2001. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7: 211–228. doi: 10.1089/107632701300062859 [DOI] [PubMed] [Google Scholar]