Abstract
The main objective of this study was to compare the physiological changes (withdrawal and corneal reflexes, respiratory and cardiac frequency, blood oxygen saturation, and rectal temperature) following intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg) to 3-, 6-, 12- and 18-month-old male Sprague Dawley rats (n=6/age group). Plasma pharmacokinetics, liver metabolism, and blood biochemistry were examined for a limited number of animals to better explain anesthetic drug effects. Selected organs were collected for histopathology. The results for the withdrawal and corneal reflexes suggest a shorter duration and decreased depth of anesthesia with aging. Significant cardiac and respiratory depression, as well as decreased blood oxygen saturation, occurred in all age groups however, cardiac frequency was the most affected parameter with aging, since the 6-, 12-, and 18-month-old animals did not recuperate to normal values during recovery from anesthesia. Pharmacokinetic parameters (T1/2 and AUC) increased and drug clearance decreased with aging, which strongly suggests that drug exposure is associated with the physiological results. The findings for liver S9 fractions of 18-month-old rats compared with the other age groups suggest that following a normal ketamine anesthetic dose (80 mg/kg), drug metabolism is impaired, leading to a significant increase of drug exposure. In conclusion, age and related factors have a substantial effect on ketamine and xylazine availability, which is reflected by significant changes in pharmacokinetics and liver metabolism of these drugs, and this translates into shorter and less effective anesthesia with increasing age.
Keywords: anesthesia, ketamine, liver metabolism, pharmacokinetics, xylazine
Introduction
The combination of ketamine and xylazine (KX) is commonly used for anesthesia in rodents [10, 33]. Ketamine is an antagonist of the NMDA glutamate receptor [6, 31], and it can also bind to opioids and GABAergic receptors [18]. Ketamine is known for its analgesic properties and its induction of a dissociative anesthesia [9, 10]. Xylazine is an α2-adrenergic agonist that causes sedation, analgesia, and muscular relaxation [6, 10, 12, 31]. KX combinations have proven to be nonirritating and suitable for intraperitoneal injections in laboratory rats [13, 44]. The sedative and muscle relaxant properties of xylazine are beneficial because they reduce the side effects of ketamine, such as tremors and muscular rigidity [37]. KX combinations induce bradycardia, hypercapnia, and acidosis [35, 47]. Other adverse effects, such as respiratory depression, may be additive [40]. In rodents, the KX combination causes mainly hypotension and hypoventilation [6]. KX anesthesia varies greatly between individuals, and some rodents do not achieve an adequate surgical anesthesia, which is reflected by the high ranges of anesthetic doses of these drugs that are reported in the literature [6, 37].
In a previous publication [50], we showed that the pharmacokinetics of ketamine (125 mg/kg) and xylazine (10 mg/kg) differed greatly between young (3 months) and old (>2 yrs of age) Sprague Dawley rats. The half-lives of both ketamine and xylazine in old rats were significantly increased, which could be explained in part by a decrease in function of liver enzymes with aging. However, no pharmacodynamic parameters were evaluated during anesthesia in that study, and only a limited number of organs were collected for histopathology, which limits the rationalization of these findings. No study has evaluated the pharmacodynamics and pharmacokinetic changes associated with this increased anesthesia effect with aging.
The main objective of this study was therefore to compare the pharmacodymanic (withdrawal and corneal reflexes) changes associated with the administration of 80 mg/kg of ketamine and 10 mg/kg of xylazine in rats from 3 to 18 months of age, as well as other associated anesthetic changes (cardiac and respiratory frequency, blood oxygen saturation, and rectal temperature), to better understand if the anesthesia changes with aging are progressive or change significantly after a given age. A reduced dose of ketamine was chosen due to the severe toxicity seen in 12-month-old rats in our previous study [11]. Pharmacokinetics and blood biochemistry were performed in a reduced number of animals to explain changes associated with physiological measures that occur during anesthesia. Ketamine is metabolized primarily by liver CYP3A [28, 37] to its active metabolite norketamine [29]. Alterations in liver metabolism (e.g., hepatic clearance) with aging could explain the higher area under the curve (AUC) observed in the aging rats. The aim of the in vitro study was to assess the influence of aging on CYP3A metabolism of ketamine using liver S9 fractions from 3-, 6-, 12-, and 18-month-old rats.
Materials and Methods
Subjects
Twenty-four male SPF Sprague Dawley (Crl:CD (SD)) rats from Charles River (St-Constant, QC, Canada) were used for this study. Two- and 3-month-old rats (n=6/age group) were purchased and kept until they were, respectively, 3 and 6 months old. Twelve 8-month-old retired breeder rats were purchased and kept until they were at 12 and 18 months of age (n=6/age group). At the time of experimentation, the 3-, 6-, 12-, and 18-month-old rats weighed, respectively, 484.0 ± 18.0 g, 732.1 ± 50.4 g, 790.0 ± 65.1 g, and 998.0 ± 74.7 g. All rats were housed in a standard laboratory animal environment under a 12:12-h light cycle in a controlled environment with a temperature of 21 ± 2°C, humidity of 50 ± 20%, and fresh filtered air with 15 changes/hour. The rats had ad libitum access to food (2018 Teklad Global 18% Protein Rodent Diet, Harlan Teklad, Bartonville, IL, USA) and reverse osmosis water. The rats were housed individually in ventilated cages (Green Line IVC Sealsafe Plus, Tecniplast Chester, PA, USA) changed once a week. They were housed on corncob bedding (7097 Corncob, Harlan Teklad, Bartonville, IL, USA) and had a high temperature polycarbonate rat retreat (Bio-Serv, Flemington, NJ, USA) and one Nylabone (Bio-Serv, Flemington, NJ, USA) for environmental enrichment. The Institutional Animal Care and Use Committee of the Ste-Justine Hospital Research Center approved the protocol prior to animal use in agreement with the guidelines of the Canadian Council on Animal Care [3].
Treatments
The study was performed in two phases. Rats were first examined for physiological changes and reflexes following KX administration. The KX pharmacokinetics were then evaluated in 3 animals of each age group after a one week washout period. It was considered that this interval was sufficient, since repeated administration of ketamine will only affect drug efficacy when rats receive moderate doses (40 mg/kg) of ketamine for 10 consecutive days [51]. The other 3 animals of each group were used to evaluate in vitro liver metabolism. For both study phases, animals received 80 mg/kg of ketamine (Ketalean, Bimeda-MT, Cambridge, ON, Canada) and 10 mg/kg of xylazine (Xylamax, Bimeda-MTC, Cambridge, ON, Canada) intraperitoneally and non-medicated ophthalmic gel to maintain a good lubrication of the cornea following KX induction. Animals remained on a circulating hot water blanket (Heat Therapy Pump, Kent Scientific, Harrington, CT, USA) during anesthesia up to recovery.
Evaluation of reflexes and physiological changes
Following the intraperitoneal KX injection, different parameters were monitored at chosen time points (5, 15, 30, 45, 60, 90, and 120 min). The corneal reflex was evaluated by softly pressing on the cornea with a cotton tip, and the withdrawal reflex was assessed by pressing the interdigital hind paw skin with hemostatic forceps. A small animal oximeter (CANL-425V, Med Associates, St-Alban, VT, USA) was used to monitor cardiac frequency and blood oxygen saturation (SaO2) by taping the probe onto the right hind paw. Respiratory frequency was taken over 1 min by direct observation, and rectal temperature (Thermalert TH-8, Physitemp, Clifton, NJ, USA) was taken with a rectal probe. The recovery time was the time at which the first voluntary movement occurred following KX injection.
Blood sampling for biochemistry and pharmacokinetics
During anesthesia, jugular vein blood collections (0.2 ml/time point) were rapidly collected for the pharmacokinetic study. When necessary (when the withdrawal reflex was present; after 45, 60, and 90 min for the 3-, 6-, and 12–18-month-old rats, respectively), individual blood collections were performed under isoflurane anesthesia (0.5 ml/min oxygen) using a face mask (total collection time: less than 1 min). Blood was collected in 1 ml microtainer K3-EDTA tubes (Becton, Dickenson and Co., Franklin Lakes, NJ, USA), preserved on ice, and centrifuged within 30 min. Plasma was then collected and kept at −80°C until HPLC-MS/MS analysis. At the last blood collection of pharmacokinetics study, rats were euthanized with CO2. Using the same 3 animals for the pharmacokinetic study, intracardiac blood samples (1 ml) were the collected at the end of the study in serum tubes (Becton, Dickenson and Co., Franklin Lakes, NJ, USA) for biochemistry and refrigerated until processed (within 24 h) at the diagnostic service of the Faculty of Veterinary Medicine of the University of Montreal by automatic evaluation with a Synchron CX5 Clinical System (Beckman Coulter, Fullerton, CA, USA). Since all rats were under the influence of KX anesthesia, the values obtained in each groups should vary with age. The selected biochemical parameters included glucose, blood urea nitrogen, creatinine, alanine aminotransferase, alkaline phosphatase, total protein, albumin, and globulins. Experimental values were compared with normal ranges taken from published findings [4, 39].
Histological preparations
Immediately following euthanasia, the kidneys, liver, heart, and lungs of each animal were collected and preserved in a formalin buffered solution (10%) prior to histological preparations (paraffin embedding; hematoxylin-eosin staining). Specimens were sent to the pathology department of the Faculty of Veterinary Medicine of the University of Montreal for processing. All slides were evaluated by a board certified veterinary pathologist (Dr Pierre Hélie DMV, DACVP).
Bioanalytical methods and pharmacokinetics and statistical analyses
A high-performance liquid chromatography-tandem mass spectrometer (HPLC-MS/MS) that has previously been described [20] was used for the analysis of ketamine and xylazine plasmatic concentrations. All pharmacokinetic parameters were calculated using WinNonLin 5.2 (Pharsight Corporation, Mountain View, CA, USA) and noncompartmental methods [36]. The elimination rate constant (kel) was calculated using a minimum of three measured plasma concentrations, and a terminal elimination half-life (T1/2) was calculated using 0.693/kel. The area under the curve from time 0 to the last measurable concentration (AUC0-t) was calculated using the linear trapezoidal rule, and with the last measured plasma concentration, the area under the curve extrapolated to infinity (AUC0-∞) was calculated using the equation AUC0-t + Clast/kel. Relative clearance (CL/F) was calculated by dividing the administered drug dosage by the AUC0-∞.
Drug metabolism in liver S9 fractions
Liver S9 fractions from 3-, 6-, 12-, and 18-month-old male Sprague Dawley rats were prepared, and Michaelis-Menten parameters were determined for primary metabolic pathways. Midazolam was used to verify and validate CYP3A activity in liver S9 fractions prepared from tissues obtained from animals of different age groups [15, 27, 41].
Ketamine, d4-ketamine, norketamine, d4-norketamine, midazolam, d4-midazolam, α-hydroxymidazolam, and d4-α-hydroxymidazolam were obtained in solution from Cerilliant (Round Rock, TX, USA). Other chemicals, including acetonitrile, formic acid, methanol, sodium phosphate dibasic, and sodium phosphate monobasic were purchased from Fisher Scientific (Ottawa, ON, Canada). Commercial rat liver S9 fractions and NADPH regeneration solutions were obtained from Corning Gentest (Tewksbury, MA, USA).
For each age group, three livers were pooled and homogenized in a 50 mM TRIS-HCl buffer, pH 7.4, containing 150 mM KCl and 2 mM EDTA at a ratio of 1:4 (w:v). The homogenates were centrifuged at 9,000 g for 20 min. The total amount of protein in each supernatant was determined using the standard Coomassie protein assay (Bradford). Supernatant aliquots were kept at −80°C until usage. No significant differences in total amount of proteins were noted between age groups. The incubations were performed as previously described [20, 38] and were performed minimally in triplicate. They were performed at various concentrations ranging from 1 to 100 µM of ketamine or midazolam in 0.5 mg/ml of S9 fraction proteins diluted in 100 mM phosphate buffer (pH 7.4). Liver S9 enzyme suspensions (total volume of 100 µl) were fortified with 5 µl of NADPH-regenerating solution A (Cat. No. 451200, Corning Inc., Corning, NY, USA) and 1 µl of solution B (Cat. No. 451200, Corning Inc., Corning, NY, USA) and preincubated at 37°C for 5 min prior to the addition of ketamine or midazolam. For the determination of Km and Vmax, the concentration of norketamine or α-hydroxymidazolam was determined after 10 min incubation to calculate the initial rate of formation (i.e., Vi). Fifty microliters of samples were taken and mixed with 250 µl of the deuterated internal standard solution (1 µM d4-norketamine or d4-α-hydroxymidazolam in acetonitrile) in a 1.5 ml centrifuge tube. Samples were centrifuged at 12,000 g for 10 min, and 200 µl of the supernatant was transferred into an injection vial for HPLC-MS/SRM analysis.
The concentrations of norketamine and α-hydroxymidazolam were determined using an HPLC-MS/SRM assay derived from our previous publication [38]. Metabolites and corresponding deuterium-labeled molecule analogues were analyzed in full-scan MS/MS using a Thermo Scientific linear ion trap mass spectrometer (Thermo LTQ-XL) and the quantification was based on specific post-processing selected reaction monitoring (SRM) extracted ion chromatograms. The SRM transitions were set to m/z 242.1 → 179.0, 246.1 → 183.0, 342.1 → 297.0, and 346.1 → 301.0 for norketamine, d4-norketamine, α-hydroxymidazolam, and d4-α-hydroxymidazolam, respectively. The analytical range used was from 0.05 µM to 50 µM.
Michaelis-Menten equation [25] analyses were performed with GraphPad PRISM (6.0f) software (GraphPad Sofware Inc., La Jolla, CA, USA) using the non-linear curve-fitting module with an estimation of the goodness of fit.
The initial velocity (vi) was determined using Equation 1.
 (1) (1)
|
The initial rate (vi) was calculated based on the concentration of norketamine or α-hydroxymidazolam measured after a 10-min incubation of rat liver S9 enzyme suspensions in ketamine or midazolam. Additionally, the enzyme-mediated clearance (CLuint) that would occur without physiological limitations including protein binding or hepatic blood flow was determined using Equation (2).
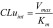 (2) (2)
|
Statistics
For the physiological results (heart rate, respiratory frequency, oxygen saturation, and rectal temperature), statistical analyses were performed for each age group using an analysis of variance (ANOVA) linear model with repeated measures and a post hoc Dunnett’s test. All analyses were performed with SAS (version 9.3, SAS Institute Inc., Cary, NC, USA) to evaluate the effect of time for rectal temperature, heart rate, respiratory frequency, and oxygen saturation results. An ANOVA linear model with repeated measures was used to compare the age effect. A priori contrasts were done with an adjustment of alpha by Bonferroni sequential correction to compare means of each group. All results were expressed as the mean ± SEM (excepted for pharmacokinetic and biochemistry results, which were expressed with the standard deviation (SD)), and differences were considered significant at P<0.05.
For the in vitro liver S9 fraction study, the statistical differences were assessed with a one-way ANOVA and a Tukey’s multiple comparisons test using GraphPad PRISM (version 6.0f); P<0.05 was considered significant.
Results
Reflexes and physiological changes
The withdrawal reflex (WR) (Fig. 1) showed a clear difference in anesthesia duration between the different age groups following the administration of KX. The WR was absent in all 3- and 6-month-old animals at 15 and 30 min, whereas it was absent at 30 min only in all the 12-month-old animals. In 18-month-old animals, only 83% lost the reflex at 15 to 45 min. The WR was present again at 45, 60, 120, and 90 min in all the 3-, 6-, 12-, and 18-month-old rats, respectively. The first voluntary movements were noted at 66.7 ± 7.3, 88.8 ± 29.7, 122.2 ± 16.5, and 104.3 ± 17.5 min in the 3-, 6-, 12-, and 18-month-old rats, respectively. The corneal reflex results following KX anesthesia are shown in Fig. 2. The reflex was absent in all groups at 15 and 30 min, except for two 3-month-old rats. The 3-month-old rats lost their corneal reflex very rapidly, since it was already absent following KX administrations. The corneal reflex was present again in 40–60% of rats in all groups at tentatively selected time points, with the evaluations ending when all animals had a positive corneal reflex.
Fig. 1.
Percent of Sprague Dawley rats (n=6/group; 3, 6, 12, and 18 months old) showing a positive withdrawal reflex when evaluated at selected time points (5, 15, 30, 45, 60, 90, and 120 min), following the intraperitoneal administration of 80 mg/kg of ketamine and 10 mg/kg xylazine.
Fig. 2.
Percent of Sprague Dawley rats (n=6/age group; 3, 6, 12, and 18 months old) showing a positive corneal reflex evaluated at tentatively selected time points (5, 15, 30, 45, 60, 90, and 120 min), with evaluation ending when all animals had a positive corneal reflex following the intraperitoneal administration of 80 mg/kg of ketamine and 10 mg/kg xylazine.
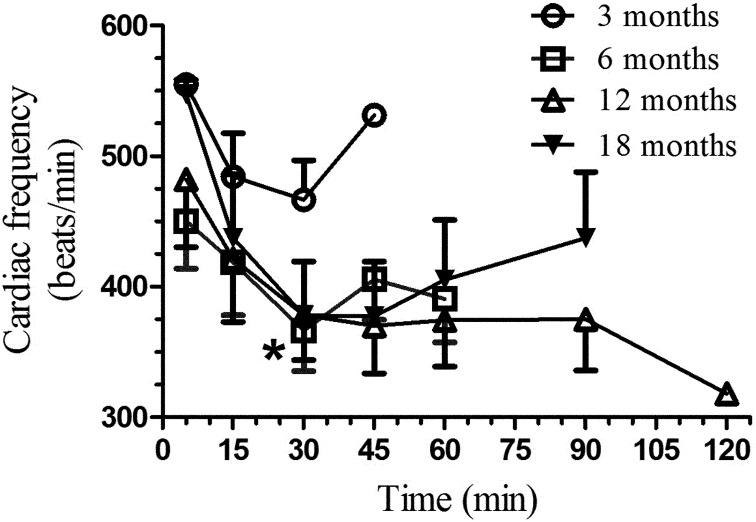
Cardiac frequency (Fig. 3) varied significantly with time in the 3- (F4,20=2.71, P=0.05), 6- (F4,25=3.12, P<0.05), 12- (F6,30=7.13, P<0.01), and 18-month-old rats (F5,25=4.61, P<0.01). Significant age group differences are shown in Fig. 3. Respiratory frequency (Fig. 4) varied significantly with time in the 3- (F4,20=6.99, P=0.002), 6- (F4,20=7.21, P<0.001), 12- (F6,30=4.78, P<0.002), and 18-month-old rats (F5,25=6.45, P<0.01). No significant age group differences occurred (Fig. 4). Blood oxygen saturation (Fig. 5) did not vary significantly for 6-month-old rats but varied significantly in the 3- (F4,20=3.71, P=0.03), 12- (F6,30=10.12, P<0.001), and 18-month-old rats (F5,25=7.07, P<0.0005). Since these comparisons were done with the results for the 5-min time point result, this suggests that blood oxygen saturation returned to near normal values (>90%) at the end of anesthesia, except in the 6-month-old rats. Significant age group differences are shown in Fig. 5.
Fig. 3.
Mean (± SE) cardiac frequency (beat/min) in Sprague Dawley rats (n=6/age group; 3, 6, 12, and 18 months old) following an intraperitoneal administration of 80 mg/kg of ketamine and 10 mg/kg xylazine. The heart rate was evaluated with a pulse oximeter tentatively at selected time points (5, 15, 30, 45, 60, 90, and 120 min), with evaluation ending when all animals had a positive withdrawal reflex. A significant difference between the 3-month-old group and the older groups was seen only at the 30 min time point. * P<0.01 (post hoc Dunnett’s test).
Fig. 4.
Mean (± SE) respiratory frequency (breaths/min) in Sprague Dawley rats (n=6/age group; 3, 6, 12, and 18 months old) following an intraperitoneal administration of 80 mg/kg of ketamine and 10 mg/kg xylazine. The respiratory frequency was measured by direct observation at 5, 15, 30, 45, 60, 90, and 120 min, and measurement ended when all animals had a positive withdrawal reflex. No significant difference between groups.
Fig. 5.
Mean (± SE) blood oxygen saturation (%) in Sprague Dawley rats (n=6/age group; 3, 6, 12, and 18 months old) following an intraperitoneal administration of 80 mg/kg of ketamine and 10 mg/kg xylazine. The SaO2 was evaluated with a pulse oximeter at tentatively selected time points (5, 15, 30, 45, 60, 90, and 120 min), with evaluation ending when all animals had a positive withdrawal reflex. A significant difference (post hoc Dunnett’s test*) between groups was seen only at 5 min between 3- (P<0.002) and 6-month-old (P<0.02) animals compared with 12-month-old animals.
Rectal temperature (Fig. 6) did not vary with time in the 3- (F4,20=1.79, P<0.17), 6- (F4,20=2.30, P<0.09), and 12-month-old rats (F6,30=1.32, P<0.28). However, rectal temperature was significantly affected in the 18-month-old rats (F5,25=5.57, P<0.0005).
Fig. 6.
Mean (± SE) rectal temperature (°C) in Sprague Dawley rats (n=6/age group; 3, 6, 12, and 18 months old) following an intraperitoneal administration of 80 mg/kg of ketamine and 10 mg/kg xylazine. The rectal temperature was evaluated at tentatively selected time points (5, 15, 30, 45, 60, 90, and 120 min), with evaluation ending when all animals had a positive withdrawal reflex. A significant difference between 6-month-old animals and other age groups was seen at 30 and 45 min (P<0.01; post hoc Dunnett’s test)
Pharmacokinetics
Mean pharmacokinetic parameters (± SD) are presented in Table 1, and mean concentration-time profiles are presented in Fig. 7.> Drug exposure increased for both ketamine and xylazine with aging, as shown by the AUC0-t and AUC0-∞. Compared with the 3-months-old rats, ketamine exposure (AUC0-t) increased by factors of 1.98, 2.94, and 4.15 in the 6-, 12-, and 18-month-old rats, respectively, whereas xylazine exposure increased by factors of 1.33, 1.85 and 2.78. The terminal elimination rate constant (kel) decreased, and the terminal elimination half-lives (T1/2) increased for both ketamine and xylazine with increasing age. These parameters were not calculated for 18-month-old rats, since the correlation of the data measured from the last three data points was less than 90%. The relative clearance of both drugs decreased with aging. When compared with the 3-month-old rats, relative clearance was decrease by 58, 74, and 77% for ketamine and by 30, 52, and 67% for xylazine in the 6-, 12-, and 18-month-old rats, respectively.
Table 1. Mean (SD) plasmatic pharmacokinetic parameters following a single intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) administered to male Sprague Dawley rats (n=3/group) of different ages.
| Pharmacokinetic parameters (units) | Age (months) | ||||
|---|---|---|---|---|---|
| 3 | 6 | 12 | 18 | ||
| Ketamine | |||||
| AUC0-t (µg.h/ml) | 2.02 (0.03) | 4.01 (0.28)4 | 5.88 (0.13)4,3 | 8.39 (0.44)4,4 | |
| AUC0-∞ (µg.h/ml) | 3.24 (0.16) | 7.77 (0.71) 1 | 12.75 (2.25)4,2 | 13.94 (1.22)4,2 | |
| kel (h-1) | 0.14 (0.01) | 0.08 (0.02)2 | 0.07 (0.02)2,ns | ND | |
| T1/2 (h) | 5.61 (0.28) | 8.41 (1.44) ns | 10.29 (2.90)2,ns | ND | |
| CL/F (ml·min-1) | 24.76 (1.24) | 10.35 (0.95)4 | 6.41 (1.15)4,1 | 5.77 (0.50)4,ns | |
| Xylazine | |||||
| AUC0-t (µg.h/ml) | 2.67 (0.19) | 3.54 (0.17)2 | 4.94 (0.35)4,3 | 7.42 (0.22)4,3 | |
| AUC0-∞ (µg.h/ml) | 2.83 (0.26) | 4.00 (0.11)2 | 5.87 (0.01)4,4 | 8.56 (0.38)4,4 | |
| kel (h-1) | 0.34 (0.04) | 0.26 (0.02)1 | 0.22 (0.05)1,ns | ND | |
| T1/2 (h) | 2.09 (0.29) | 2.71 (0.26) ns | 3.25 (0.73) ns, ns | ND | |
| CL/F (ml·min-1) | 3.55 (0.32) | 2.50 (0.07)3 | 1.70 (0.01)4,2 | 1.17 (0.05) 4,1 | |
AUC0-∞, area under curve extrapolated to infinity; AUC0-t, area under curve from time zero to the last measured concentration; kel, terminal elimination rate constant; T1/2, terminal elimination half-life; CL/F, relative clearance rate; ND, not determined. Post hoc Tukey statistics reported for 6-, 12-, and 18-month-old rats when compared with 3-month-old animals (first value) as well as for 6-12 and 12-18 groups (second value): 1P<0.05; 2P<0.01; 3P<0.001; 4P<0.0001. ns, non-significant.
Fig. 7.
Mean (± SD) concentration-time profiles of ketamine (top) and xylazine (bottom) in male Sprague Dawley rats (n=3/group; 3, 6, 12, and 18 months old) following a single intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg).
Histopathology
No lesions were observed in selected tissues from the animals independently of the age group.
Blood biochemistry
Mean biochemical parameters (± SD) are presented in Table 2. All parameters were within normal limits except for an increase with age above the higher limit values for BUN and ALT. ALT fell within normal limits if we consider the post-anesthesia concentrations [49]. Glucose concentrations increased with aging and were slightly above normal in 12-month-old rats and very high in 18-month-old rats. Creatinine concentrations also increased with aging but stayed within normal limits. Total proteins were within normal limits in all groups, however, albumin decreased with aging. In another study, it was reported that a decrease in total proteins occurred with aging and post KX administration [49].
Table 2. Mean (SD) biochemistry parameters following a single intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) administered to male Sprague Dawley rats (n=3/group) of different ages.
| Biochemistry parameters (units) | Normal values | Age (months) | |||
|---|---|---|---|---|---|
| 3 | 6 | 12 | 18 | ||
| Glucose (mmol/l) | 4.4–16.7 | 9.3 (1.3) | 12.9 (3.2) | 17.6 (6.0) | 28.8 (9.0) |
| BUN (mmol/l) | 3.9–8.2 | 9.6 (1.1) | 9.9 (1.7) | 11.8 (1.8) | 13.0 (2.3) |
| Creatinine (mmol/l) | 35–123 | 40.0 (3.5) | 45.3 (5.8) | 58.7 (8.3) | 65.7 (16.0) |
| ALT (U/l) | 28–40 | 55.7 (9.2) | 48.7 (9.0) | 56.0 (15.9) | 62.0 (7.2) |
| ALP (U/l) | 150–220 | 215.3 (53.4) | 138.0 (28.2) | 142.3 (23.7) | 164.7 (35.3) |
| Total proteins (g/l) | 58–66 | 64.6 (0.6) | 61.1 (1.0) | 56.1 (6.1) | 59.4 (7.2) |
| Albumin (g/l) | 33–46 | 32.8 (0.3) | 31.5 (1.8) | 28.3 (2.6) | 28.3 (3.9) |
| Globulins (g/l) | 17–30 | 31.8 (0.6) | 29.6 (1.8) | 27.8 (3.6) | 31.1 (1.3) |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; BUN, blood urea nitrogen.
Drug metabolism in liver S9 fractions
The results were consistent with the kinetics following a Michaelis-Menten enzymatic reaction for all rat liver S9 fractions from all age groups (Fig. 8), and the data were compatible with those of commercial rat liver S9 fractions (data not shown). The derived data are presented in Table 3. Midazolam is a well-characterized substrate of CYP3A and the primary biotransformation product is α-hydroxymidazolam. The observed Km values were not significantly different when comparing age groups (Table 3). This suggests that the enzyme-substrate complex structure was not significantly different between midazolam and CYP3A with age. However, the derived Vmax suggested a rapid saturation of the CYP3A enzyme active sites in liver S9 fractions of 18-month-old rats, thus affecting significantly the intrinsic clearance (CLuint) of the drug. Interestingly, the derived Km value for ketamine significantly changed in 18-month-old rats. This observation is very distinctive compared with the rat liver CYP3A-mediated metabolism of midazolam.
Fig. 8.
Determination of Michaelis constant (Km) and maximum velocity (Vmax) using nonlinear regression fitting in S9 liver fractions from 3-, 6-, 12-, and 18-month-old rats. Each point represents the mean (± SD) of experiments in triplicate. Significant differences in the initial rate of formation (Vi) were observed starting at the 5 µM substrate concentration for liver S9 fractions of 18-month-old rats. * P<0.05; ** P<0.01; **** P<0.0001.
Table 3. Kinetic parameters associated with the formation α-hydroxymidazolam and norketamine in liver S9 fractions from aging rats.
| α-Hydroxymidazolam | Vmax nmol min–1 mg–1 |
Km µM (nmol ml–1) |
CLuint ml min–1 |
|---|---|---|---|
| 3 month liver S9 fractions | 0.247 (± 0.017) | 4.32 (± 0.26) | 0.057 |
| 6 month liver S9 fractions | 0.264 (± 0.021) | 6.22 (± 0.99) | 0.042 |
| 12 month liver S9 fractions | 0.216 (± 0.020) | 4.18 (± 0.05) | 0.052 |
| 18 month liver S9 fractions | 0.040 (± 0.003)1 | 3.76 (± 1.45)3 | 0.011 |
| Norketamine | |||
| 3 month liver S9 fractions | 2.39 (± 0.23) | 15.99 (± 4.28) | 0.150 |
| 6 month liver S9 fractions | 2.61 (± 0.18) | 18.38 (± 3.85) | 0.142 |
| 12 month liver S9 fractions | 2.07 (± 0.07) | 19.04 (± 0.94) | 0.109 |
| 18 month liver S9 fractions | 0.68 (0.02)2 | 2.65 (± 0.49)4 | 0.256 |
1,2P<0.001; 3P>0.05; 4P<0.05.
Discussion
In this study, ketamine (80 mg/kg) and xylazine (10 mg/kg) caused a short duration anesthesia in all 3- and 6-month-old rats; however, it was increased in the 12-month-old rats and even more so in 18-month-old rats. The withdrawal reflex and the first voluntary movement following KX administrations occurred progressively later with aging. These results suggest a clear effect on anesthesia depth and duration with aging and related factors. Regarding the physiological changes seen with KX anesthesia, cardiac frequency significantly decreased in 6-, 12-, and 18-month-old animals when compared with the 3-month-old rats. Cardiac frequency remained depressed in older rats at the end of anesthesia. Respiratory frequency significantly decreased in all groups but returned to near normal values during recovery from anesthesia. The decreased blood oxygen saturation is most probably associated with the decreased cardiac and respiratory frequencies. These results suggest a strong effect of KX administration that was not importantly affected by aging, except for cardiac frequency. Apart from the 18-month-old rats, rectal temperature did not show a significant difference with aging following KX administration. Our previous results [11, 50] showed an increase in the depth and duration of KX anesthesia with increasing age, although much higher doses (125 mg/kg) were administered to the animals; however, cardiac and respiratory frequency as well as blood oxygen saturation were similarly affected at both 80 and 125 mg/kg with increasing age.
KX injectable anesthesia is mostly used in laboratory animals [10, 33]. Ketamine produces short unconsciousness and has analgesic properties. Xylazine is an analgesic medication used to minimize side effects of the use of ketamine such as muscle stiffness [21, 37, 45]. The analgesic and sedative properties of this drug combination leads to an increased depth of anesthesia associated with respiratory depression, bradycardia, hypercapnia, and acidosis [35, 39, 47]. Our results confirm these cardiac and respiratory effects but show that the cardiac depression is more pronounced with aging, with a long recovery of respiratory frequency for the animals over 3 months of age. However, blood oxygen saturation is significantly decreased in younger animals in the first 5 min following KX administration.
The pharmacokinetics results confirm results obtained for the withdrawal and corneal reflexes; that is, a longer anesthesia duration was correlated with greater drug exposure. For both ketamine and xylazine, drug exposure (AUC) increases, and the clearance decreases, with aging. The significant increase in the AUC values of both ketamine and xylazine suggests that toxicity occurs in aged animals if higher drug concentrations are used [11]. The half-lives of ketamine and xylazine are, respectively, 2 and 1 h in 8–12 week-old rats [49]. In our previous study, we found that the half-lifes of ketamine and xylazine were, respectively, 8.5 and 13 h in aged rats (>2 years old) [50]. Therefore, the pharmacokinetics are affected as rats age, and this could be due to many factors. A decrease in albumin plasmatic concentrations with aging would increase the free fraction of drugs in plasma and therefore increase anesthesia depth. Many other factors can affect the pharmacokinetics of drugs, such as sex, nutrition, environmental conditions, and diseases [17, 42, 45, 50]. Many changes associated with aging could affect the metabolism of drugs, such as chronic subclinical inflammation, obesity (e.g., storage of the lipid-soluble drugs in fat tissues), and diminished exercise [25]. We evaluated S9 liver metabolism, as it is one of the important organs responsible for drug metabolism, the others being the kidney and the brain.
Ketamine is metabolized by the liver [24] into active metabolites, mainly norketamine, an NDMA receptor antagonist [16], and hydroxynorketamine, a nicotinic acetylcholine receptor antagonist [43]. Norketamine induces anesthesia, whereas hydroxynorketamine is not an anesthetic [22] but possesses antidepressive properties [42]. From our findings, we see that formation of norketamine results in a very rapid saturation in the liver S9 fractions of 18-month-old rats, suggesting that following a commonly administered anesthetic dose of ketamine (80 mg/kg), drug metabolism is impaired, leading to a significant increase of drug exposure (AUC) and a decrease of elimination. These results are in accordance with our recent in vivo investigation [9, 50]. Our results suggest that norketamine is mainly produced in animals of 3–12 months of age and would contribute much less to the anesthesia with aging. Further studies should measure the plasma concentration of norketamine to confirm this hypothesis. Xylazine is metabolized into multiple metabolites, and up to 70% is eliminated in the urine [30]. Rat cytochrome P450 enzymes, mainly CYP3A, are involved in the metabolism of ketamine and xylazine [46], and qualitative changes in liver metabolism with aging could explain the high blood concentration of both drugs as the rat ages [34]. CYP2B6 plays a non-negligible role in the metabolism of ketamine; however, when looking at the concentration of individual CYPs, CYP 3A is predominantly involved in the metabolism of these anesthetic drugs [32, 54]. A previous study demonstrated alterations of hepatic clearance of a CYP3A substrate, rate of absorption, and hepatic blood flow following anesthesia [51], and altogether liver metabolism appears to be one of the mechanisms explaining alterations in anesthetic drug effects. Although the liver is often assumed to be the main organ for drugs metabolism, Edwards et al. showed that ketamine metabolism may also occur in the kidney and to a lesser extent in the lung and gut [8]. Also, ketamine is a racemate of equal concentrations of (R)- and (S)-enantiomers, and the (R)-enantiomer is much more potent than the (S)-enantiomer. This also holds true for the enantiomers of norketamine [7]. These findings were obtained when norketamine was evaluated as an NMDA receptor antagonist in cortical and spinal cord preparations [7]. Our findings do not suggest a clear correlation between anesthesia depth and duration with liver metabolism, and this may be due in part to the metabolism in other organs, the distribution of different enantiomers in different organs, and the differences in permeability of the blood-brain barrier that occurs with aging and associated factors.
Km is an indicator of the affinity that an enzyme has for a particular substrate, hence the thermodynamic stability of the enzyme-substrate complex. The stability of the enzyme-substrate complex is closely related to the enzyme structure. It plays a central role in defining the energetically favored binding cluster of the substrate in the active enzyme site [20]. As shown in silico, the structure of the binding cluster may lead to different metabolites or affect the rate of formation [46]. Figure 8 shows results consistent with the kinetics following a Michaelis-Menten enzymatic reaction for all rat liver S9 fractions from all age groups; these data were compatible with those of commercial rat liver S9 fractions (data not shown). The derived data are presented in Table 3. Midazolam is a well-characterized substrate of CYP3A, and its primary biotransformation product is α-hydroxymidazolam. As illustrated in Table 3 and Fig. 8, the observed Km values were not significantly different when comparing age groups. This is interesting because it suggests that the enzyme-substrate complex structure was not significantly different between midazolam and CYP3A (i.e., CYP3A1 and CYP3A2) with age. However, the derived Vmax suggests a rapid saturation of the CYP3A enzyme active sites in liver S9 fractions of 18-month old rats, significantly affecting the intrinsic clearance (CLuint) of the drug. Interestingly, the derived Km value for ketamine changed significantly in geriatric rats. This observation is very distinctive compared with the rat liver CYP3A-mediated metabolism of midazolam. The Km value is directly related to the thermodynamic stability of the binding cluster of ketamine in the active site of CYP3A. The data indicates a significant decrease of Km in liver S9 fractions of 18-month-old rats and suggest that ketamine may have stronger interactions with CYP3A active site residues, leading to a more thermodynamically stable enzyme-substrate complex. However, it may also suggest that the enzyme binds the substrate more tightly and consequently requires more energy to form the activated transition state complex (EX‡), a necessary intermediary in formation of the enzyme-product complex (EP), as shown in Equation 3.
 (3) (3)
|
Free energy difference associated with the formation of the enzyme-substrate complex (ΔGbinding) can have a significant impact on the observed Km, but an increase of the ΔG‡ (difference in free energy between EX‡ and ES) can also significantly decrease the rate constant kcat (i.e., turnover number), which represents the number of substrate molecules each enzyme site can convert to a metabolite per unit of time. The rate constant kcat can be related to Vmax using the Equation 4.
 (4)
(4)
|
As illustrated in Fig. 8 and Table 3, derived Vmax values shows significant differences with age, specifically when comparing with results obtain from liver S9 fractions of 18-month-old rats. A decreased Vmax suggests a rapid saturation of the CYP3A enzyme active sites, similar to what was observed with the reference CYP3A substrate midazolam. These results are therefore compatible with the formation of a more stable enzyme-substrate complex (ES) but a less favorable transition state complex (EX‡), leading to an increase of ΔG‡ and thus a decrease of kcat and Vmax. Conformational change of the CYP3A active site with age can potentially explain these results, and protein misfolding is characteristic of several age-related problems. The interaction of the active site residues is substrate dependent, and the results appear to suggest that an energetically favored binding cluster of ketamine in the active site of CYP3A is observed with age, but interestingly, this effect was not observed for midazolam. The formation of a more stable enzyme-substrate complex (ES) may have severe consequences on drug-drug interactions, a major issue in geriatric populations. The observed CLuint diminishes with aging, which is associated with the midazolam CYP3A-catalyzed reaction, but increases for ketamine (Table 3). The calculation of CLuint assumes that the concentration of the enzyme catalytic sites remain constant. This assumption cannot be made if conformational changes of the CYP3A active site occurs with aging. Consequently, CLuint cannot be compared between age groups for the ketamine CYP3A-mediated reaction.
No gross pathologies were found, and blood biochemistry parameters were near normal considering the effects of the KX anesthesia. With high BUN and normal creatinine concentrations, the results may suggest the beginning of a subclinical renal disease; however, no histopathological findings were noted in older animals. High ALT and ALP may be the side effects of a long-term high fat diet [23, 52], however, these parameters are also increased with KX anesthesia. Increased glycemia could be caused by xylazine, which is known to induce hyperglycemia [26]. Ketamine and xylazine are molecules that bind to albumin. A lower albumin plasmatic concentration with aging will increase the unbound fraction of ketamine and xylazine in the blood and could explain in part the longer recovery from anesthesia awakening in older rats [5]. KX anesthesia associated with hypoalbuminemia could also reflect the drug effect on hepatic metabolism even without lesions [14, 24]. In the present study, blood biochemistry and histopathology offered little explanation for the physiological changes seen during KX anesthesia.
Ketamine and xylazine are widely distributed in tissues because they are lipophilic drugs [43]. Since rats show an increase in fat deposits in organs with aging and also get less exercise, these fats deposits could act as a form of storage and slow release of ketamine and xylazine. These drugs should also penetrate readily well perfused organs such as the brain; however, both ketamine and xylazine are known to decrease brain perfusion [21]. With aging, there is a disruption of the blood-brain barrier [28, 48], making it less permeable to molecules, which is caused by activation of drug efflux pumps [19]. All these characteristics explain the decrease in anesthesia depth and duration as well as the longer time to recovery seen in aging animals [50, 53]. Further studies evaluating the availability of ketamine and xylazine in brain tissue should be conducted to verify this hypothesis. However, increasing the xylazine concentrations would be detrimental in aging animals, since it causes pulmonary edema and effusion at high blood concentrations [1, 2, 11]. Reversing this effect with an α2-antagonist (e.g., yohimbine) following recovery is an option that needs to be evaluated.
Physiological differences between the experimental groups suggest that KX injectable anesthesia is a poor anesthetic choice for aging rats. Age and the related factors have a substantial effect on ketamine and xylazine availability by changing the pharmacokinetics of these drugs, which translated into shorter and less effective anesthesia in aging rats.
Acknowledgments
The authors would like to thank Dr. Guy Beauchamp, a statistician in the Faculty of Veterinary Medicine, University of Montreal, for the statistical analyses. This study was funded by the CALAM/CALAS 2014 research fund (Pascal Vachon) as well as the Fond du Centenaire de la Faculté de Médecine Vétérinaire (Marie-Chantal Giroux and Raphaël Santamaria) and the Fond de Recherche pour la Médecine des Animaux de Laboratoire (Pascal Vachon). This project was also funded by the National Sciences and Engineering Research Council of Canada (Francis Beaudry; Discovery Grant no. 386637–2010). The HPLC-MS/MS analyses were performed on instruments funded by the National Sciences and Engineering Research Council of Canada (Francis Beaudry; Research Tools and Instruments Grants no. 439748–2013).
References
- 1.Amouzadeh H.R., Sangiah S., Qualls C.W., Jr1989. Effects of some hepatic microsomal enzyme inducers and inhibitors on xylazine-ketamine anesthesia. Vet. Hum. Toxicol. 31: 532–534. [PubMed] [Google Scholar]
- 2.Amouzadeh H.R., Qualls C.W., Jr, Wyckoff J.H., 3rd, Dzata G.K., Sangiah S., Mauromoustakos A., Stein L.E.1993. Biochemical and morphological alterations in xylazine-induced pulmonary edema. Toxicol. Pathol. 21: 562–571. doi: 10.1177/019262339302100607 [DOI] [PubMed] [Google Scholar]
- 3.Canadian Council on Animal Care. 1993. Guide to the care and use of experimental animals, Vol. 1, 2nd ed. Ottawa (Canada): Canadian Council on Animal Care. [Google Scholar]
- 4.Charles River [Internet]: Clinical Laboratory parameters for CRL: CD(SD) rats. 2006, Available at http://www.criver.com /files/pdfs/rm/cd/ rm_rm_rc_clinical_parameters_cd_rat_06.aspx.
- 5.Dayton P.G., Stiller R.L., Cook D.R., Perel J.M.1983. The binding of ketamine to plasma proteins: emphasis on human plasma. Eur. J. Clin. Pharmacol. 24: 825–831. doi: 10.1007/BF00607095 [DOI] [PubMed] [Google Scholar]
- 6.Dittmar M.S., Fehm N.P., Vatankhah B., Horn M.2004. Ketamine/xylazine anesthesia for radiologic imaging of neurologically impaired rats: dose response, respiratory depression, and management of complications. Comp. Med. 54: 652–655. [PubMed] [Google Scholar]
- 7.Ebert B., Mikkelsen S., Thorkildsen C., Borgbjerg F.M.1997. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur. J. Pharmacol. 333: 99–104. doi: 10.1016/S0014-2999(97)01116-3 [DOI] [PubMed] [Google Scholar]
- 8.Edwards S.R., Mather L.E.2001. Tissue uptake of ketamine and norketamine enantiomers in the rat: indirect evidence for extrahepatic metabolic inversion. Life Sci. 69: 2051–2066. doi: 10.1016/S0024-3205(01)01287-5 [DOI] [PubMed] [Google Scholar]
- 9.Fitzgibbon E.J., Hall P., Schroder C., Seely J., Viola R.2002. Low dose ketamine as an analgesic adjuvant in difficult pain syndromes: a strategy for conversion from parenteral to oral ketamine. J. Pain Symptom Manage. 23: 165–170. doi: 10.1016/S0885-3924(01)00393-1 [DOI] [PubMed] [Google Scholar]
- 10.Flecknell P.A.1996. Laboratory animal anesthesia. Academic Press, London. [Google Scholar]
- 11.Giroux M.C., Hélie P., Burns P., Vachon P.2015. Anesthetic and pathological changes following high doses of ketamine and xylazine in Sprague Dawley rats. Exp. Anim. 64: 253–260. doi: 10.1538/expanim.14-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene S.A., Thurmon J.C.1988. Xylazine—a review of its pharmacology and use in veterinary medicine. J. Vet. Pharmacol. Ther. 11: 295–313. doi: 10.1111/j.1365-2885.1988.tb00189.x [DOI] [PubMed] [Google Scholar]
- 13.Hajighahramani S., Vesa N.2007. Evaluation of several drug combinations for intraperitoneal anaesthesia in adult male rats. Iranian J. Vet. Res. (University of Shiraz), 8 Ser. No. 19. [Google Scholar]
- 14.Hijazi Y., Boulieu R.2002. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab. Dispos. 30: 853–858. doi: 10.1124/dmd.30.7.853 [DOI] [PubMed] [Google Scholar]
- 15.Halama B., Hohmann N., Burhenne J., Weiss J., Mikus G., Haefeli W.E.2013. A nanogram dose of the CYP3A probe substrate midazolam to evaluate drug interactions. Clin. Pharmacol. Ther. 93: 564–571. doi: 10.1038/clpt.2013.27 [DOI] [PubMed] [Google Scholar]
- 16.Holtman J.R., Jr, Crooks P.A., Johnson-Hardy J.K., Hojomat M., Kleven M., Wala E.P.2008. Effects of norketamine enantiomers in rodent models of persistent pain. Pharmacol. Biochem. Behav. 90: 676–685. doi: 10.1016/j.pbb.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 17.Jenkins A.J.2007. Toxicokinetics and factors affecting pharmacokinetic parameters, p. 21–24, In: Karch SB, editor. Pharmacokinetics and Pharmacodynamics of Abused Drugs. CRC Press, London. [Google Scholar]
- 18.Knobloch M., Portier C.J., Levionnois O.L., Theurillat R., Thormann W., Spadavecchia C., Mevissen M.2006. Antinociceptive effects, metabolism and disposition of ketamine in ponies under target-controlled drug infusion. Toxicol. Appl. Pharmacol. 216: 373–386. doi: 10.1016/j.taap.2006.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krinke G.J., Eisenbrandt D.L.1994. Non-neoplastic changes in the brain, p. 3–19, In: Mohr U, Dungworth DL, Capen CC, editors. Pathobiology of the Aging Rat, Vol. 2., ILSI Press, Washington D.C. [Google Scholar]
- 20.Lavoie D.S., Pailleux F., Vachon P., Beaudry F.2013. Characterization of xylazine metabolism in rat liver microsomes using liquid chromatography-hybrid triple quadrupole-linear ion trap-mass spectrometry. Biomed. Chromatogr. 27: 882–888. doi: 10.1002/bmc.2875 [DOI] [PubMed] [Google Scholar]
- 21.Lei H., Grinberg O., Nwaigwe C.I., Hou H.G., Williams H., Swartz H.M., Dunn J.F.2001. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res. 913: 174–179. doi: 10.1016/S0006-8993(01)02786-X [DOI] [PubMed] [Google Scholar]
- 22.Leung L.Y., Baillie T.A.1986. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J. Med. Chem. 29: 2396–2399. doi: 10.1021/jm00161a043 [DOI] [PubMed] [Google Scholar]
- 23.Madsen N.B., Tuba J.1952. On the source of the alkaline phosphatase in rat serum. J. Biol. Chem. 195: 741–750. [PubMed] [Google Scholar]
- 24.Meyer R.E., Fish R.E.2008. Pharmacology of injectable anesthetics, sedatives, and tranquilizers, p. 27–82, In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and Analgesia in Laboratory Animals. Academic Press, San Diego. [Google Scholar]
- 25.Mézière A., Paillaud E., Plaud B.2013. [Anesthesia in the elderly]. Presse Med. 42: 197–201. [DOI] [PubMed] [Google Scholar]
- 26.Michaelis L., Menten M.L.1913. Die Kinetik der Invertinwirkung. Biochem. Z. 49: 334–336. [Google Scholar]
- 27.Mooiman K.D., Maas-Bakker R.F., Rosing H., Beijnen J.H., Schellens J.H., Meijerman I.2013. Development and validation of a LC-MS/MS method for the in vitro analysis of 1-hydroxymidazolam in human liver microsomes: application for determining CYP3A4 inhibition in complex matrix mixtures. Biomed. Chromatogr. 27: 1107–1116. doi: 10.1002/bmc.2913 [DOI] [PubMed] [Google Scholar]
- 28.Mooradian A.D.1988. Effect of aging on the blood-brain barrier. Neurobiol. Aging 9: 31–39. doi: 10.1016/S0197-4580(88)80013-7 [DOI] [PubMed] [Google Scholar]
- 29.Mössner L.D., Schmitz A., Theurillat R., Thormann W., Mevissen M.2011. Inhibition of cytochrome P450 enzymes involved in ketamine metabolism by use of liver microsomes and specific cytochrome P450 enzymes from horses, dogs, and humans. Am. J. Vet. Res. 72: 1505–1513. doi: 10.2460/ajvr.72.11.1505 [DOI] [PubMed] [Google Scholar]
- 30.Park Choo H.Y., Choi S.O.1991. The metabolism of xylazine in rats. Arch. Pharm. Res. 14: 346–351. doi: 10.1007/BF02876882 [DOI] [Google Scholar]
- 31.Plumb D.C.2008. Plumb’s Veterinary Drug Handbook, p. 935–938, Wiley-Blackwell, Hoboken. [Google Scholar]
- 32.Portmann S., Kwan H.Y., Theurillat R., Schmitz A., Mevissen M., Thormann W.2010. Enantioselective capillary electrophoresis for identification and characterization of human cytochrome P450 enzymes which metabolize ketamine and norketamine in vitro. J. Chromatogr. A 1217: 7942–7948. doi: 10.1016/j.chroma.2010.06.028 [DOI] [PubMed] [Google Scholar]
- 33.Richardson C.A., Flecknell P.A.2005. Anaesthesia and post-operative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern. Lab. Anim. 33: 119–127. [DOI] [PubMed] [Google Scholar]
- 34.Rikans L.E., Notley B.A.1982. Age-related changes in hepatic microsomal drug metabolism are substrate selective. J. Pharmacol. Exp. Ther. 220: 574–578. [PubMed] [Google Scholar]
- 35.Rodrigues S.F., de Oliveira M.A., Martins J.O., Sannomiya P., de Cássia Tostes R., Nigro D., Carvalho M.H., Fortes Z.B.2006. Differential effects of chloral hydrate- and ketamine/xylazine-induced anesthesia by the s.c. route. Life Sci. 79: 1630–1637. doi: 10.1016/j.lfs.2006.05.019 [DOI] [PubMed] [Google Scholar]
- 36.Rowland M., Towzer T.N.1995. Clinical pharmacokinetics: concepts and application, p. 367–389, Lippincott, Williams and Wilkins, Philadelphia. [Google Scholar]
- 37.Saha J.K., Xia J., Grondin J.M., Engle S.K., Jakubowski J.A.2005. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp. Biol. Med. (Maywood) 230: 777–784. [DOI] [PubMed] [Google Scholar]
- 38.Santamaria R., Pailleux F., Beaudry F.2014. In vitro ketamine CYP3A-mediated metabolism study using mammalian liver S9 fractions, cDNA expressed enzymes and liquid chromatography tandem mass spectrometry. Biomed. Chromatogr. 28: 1660–1669. doi: 10.1002/bmc.3199 [DOI] [PubMed] [Google Scholar]
- 39.Sharp P.E., La Regina M.C.1998. Important biological features, p. 14–16, In: Suckow MA, editor. The laboratory rat, CRC Press, London. [Google Scholar]
- 40.Schwenke D.O., Cragg P.A.2004. Comparison of the depressive effects of four anesthetic regimens on ventilatory and cardiovascular variables in the guinea pig. Comp. Med. 54: 77–85. [PubMed] [Google Scholar]
- 41.Shimizu M., Uno T., Tamura H.O., Kanazawa H., Murakami I., Sugawara K., Tateishi T.2007. A developed determination of midazolam and 1′-hydroxymidazolam in plasma by liquid chromatography-mass spectrometry: application of human pharmacokinetic study for measurement of CYP3A activity. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 847: 275–281. doi: 10.1016/j.jchromb.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 42.Singh N.S., Zarate C.A., Jr, Moaddel R., Bernier M., Wainer I.W.2014. What is hydroxynorketamine and what can it bring to neurotherapeutics? Expert Rev. Neurother. 14: 1239–1242. doi: 10.1586/14737175.2014.971760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song G., Wu H., Yoshino K., Zamboni W.C.2012. Factors affecting the pharmacokinetics and pharmacodynamics of liposomal drugs. J. Liposome Res. 22: 177–192. doi: 10.3109/08982104.2012.655285 [DOI] [PubMed] [Google Scholar]
- 44.Struck M.B., Andrutis K.A., Ramirez H.E., Battles A.H.2011. Effect of a short-term fast on ketamine-xylazine anesthesia in rats. J. Am. Assoc. Lab. Anim. Sci. 50: 344–348. [PMC free article] [PubMed] [Google Scholar]
- 45.Sumitra M., Manikandan P., Rao K.V., Nayeem M., Manohar B.M., Puvanakrishnan R.2004. Cardiorespiratory effects of diazepam-ketamine, xylazine-ketamine and thiopentone anesthesia in male Wistar rats—a comparative analysis. Life Sci. 75: 1887–1896. doi: 10.1016/j.lfs.2004.05.009 [DOI] [PubMed] [Google Scholar]
- 46.Sun H., Scott D.O.2010. Structure-based drug metabolism predictions for drug design. Chem. Biol. Drug Des. 75: 3–17. doi: 10.1111/j.1747-0285.2009.00899.x [DOI] [PubMed] [Google Scholar]
- 47.Swindle M.M., Vogler G.A., Fulton L.K., Marini R.P., Popilskis S.2002. Preanesthesia, anesthesia, analgesia and euthanasia, p. 956–960, In: Franklin ML, Quimby FW, Fox JG, Anderson LC, editors. Laboratory Animal Medicine. Elsevier Science, Amsterdam. [Google Scholar]
- 48.Tucsek Z., Toth P., Sosnowska D., Gautam T., Mitschelen M., Koller A., Szalai G., Sonntag W.E., Ungvari Z., Csiszar A.2014. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 69: 1212–1226. doi: 10.1093/gerona/glt177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veilleux-Lemieux D., Beaudry F., Hélie P., Vachon P.2012. Effects of endotoxemia on the pharmacodynamics and pharmacokinetics of ketamine and xylazine anesthesia in Sprague-Dawley rats. Vet. Med. Res. Rep. 3: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veilleux-Lemieux D., Castel A., Carrier D., Beaudry F., Vachon P.2013. Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats. J. Am. Assoc. Lab. Anim. Sci. 52: 567–570. [PMC free article] [PubMed] [Google Scholar]
- 51.Uhing M.R., Beno D.W., Jiyamapa-Serna V.A., Chen Y., Galinsky R.E., Hall S.D., Kimura R.E.2004. The effect of anesthesia and surgery on CYP3A activity in rats. Drug Metab. Dispos. 32: 1325–1330. doi: 10.1124/dmd.104.000927 [DOI] [PubMed] [Google Scholar]
- 52.Uthandi A., Ramasamy K. Hepatoprotective activity of sesame meal on high fat fed Wistar rats. Int J. Pharma. Sci. Res. 2: 205–211.
- 53.Waterman A.E., Livingston A.1978. Effects of age and sex on ketamine anaesthesia in the rat. Br. J. Anaesth. 50: 885–889. doi: 10.1093/bja/50.9.885 [DOI] [PubMed] [Google Scholar]
- 54.Zanger U.M., Klein K.2013. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance. Front. Genet. 4: 24. doi: 10.3389/fgene.2013.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]