Abstract
This study, aimed the development of a methodology for rapid manufacture of orthopedic implants simultaneously with the surgical intervention, considering two potential applications in the fields of orthopedics: the manufacture of anatomically adapted implants and implants for bone loss replacement. This work innovation consists on the capitation of the in situ geometry of the implant by direct capture of the shape using an elastomeric material (polyvinylsiloxane) which allows fine detail and great accuracy of the geometry. After scanning the elastomeric specimen, the implant is obtained by machining using a CNC milling machine programmed with a dedicated CAD/CAM system. After sterilization, the implant is able to be placed on the patient. The concept was developed using low cost technology and commercially available. The system has been tested in an in vivo hip arthroplasty performed on a sheep. The time increase of surgery was 80 minutes being 40 minutes the time of implant manufacturing. The system developed has been tested and the goals defined of the study achieved enabling the rapid manufacture of an implant in a time period compatible with the surgery time.
Keywords: Prostheses and implants, Bone loss, Arthroplasty
INTRODUCTION
There are several reasons that favor the use of so–called custom implants. The use of an implant that is designed and manufactured according to the anatomy of the patient should be able to replace the functionality of the limb or joint in a manner close to the physiological1, 2, 3, allowing for its greater longevity and a more efficient transfer of the load resulting from the better adjustment to the host bone4, 5, 6.
The geometry of the implant can be defined in preoperative and intraoperative processes. The preoperative procedures are based on medical imaging techniques such as radiography (XR), magnetic resonance imaging (MRI), and computerized axial tomography (commonly referred to as CAT) to obtain the geometry of the implant. Information is collected from the patient in advance and from this information the implant is fabricated and then placed in the patient7, 8. Other methods use invasive techniques, obtaining the geometry of the implant during surgery(9).
Today, computerized tomography (CT) and magnetic resonance imaging (MRI) are the processes most commonly used to survey the shape of an anatomical model. For the CT, the patient or any other model being studied undergoes a scanning process to generate a two-dimensional image with 1 to 3 mm spacing corresponding to the cross sections of the study model. The images produced are placed in their relative positions, and by detecting the gray gradient builds a boundary contour that is used to create a three-dimensional virtual model of the implant10, 11.
The implant is manufactured by machining via computerized numerical control (CNC), using computer-aided design and computer-aided manufacturing (CAD/
CAM) to change or modify the 3D virtual model, and generate the control program of the paths taken by the machine during the manufacture of the implant.
The process of manufacturing customized implants, with a shape survey based on CT presents some critical aspects, including those relating to the recognition of the gray contrast level necessary for the correct and precise definition of the geometry of the contour. In these cases, the contrast level depends on the density of matter and not all patients have the same bone mass. Another aspect is related to the final precision of the implant, which often show deviations greater than 1 mm(12) resulting from, among other factors, the resolution of the system involved in the production of images and the spacing between 1 and 10 mm for borderline situations between images13, 14.
PROCESS AND METHODOLOGY
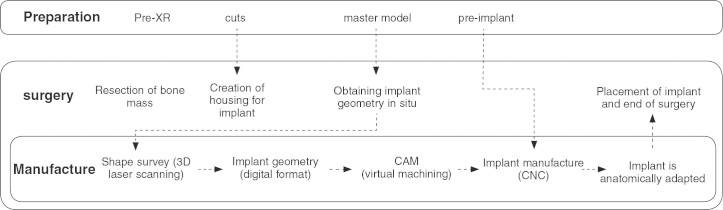
The process was aimed at developing a system for the rapid manufacture of anatomically adapted implants which allows for the faithful reproduction of the fine details of any anatomical structure, and can be used simultaneously with surgery. The approach was based on the intraoperative procedure developed by Mulier et al.(9) to obtain the geometry of the implant by direct capture during surgery of the housing structure. The methodology was developed to minimize the costs of acquiring the necessary technology and the time for implementing the solution.
To perform the surgery, there is a preliminary stage that begins with an XR, and based on this information, the surgery-specific instruments that may be necessary are designed and manufactured. A master model is also made, which will support the material used for printing in obtaining the geometry of the implant and an implant with an oversized shape so as to decrease the time of manufacture of the implant during surgery, because the geometry and final sizing of the implant will be determined during surgery and after obtaining the contour of the housing in situ.
During surgery, after establishing the housing structure of the implant, the doctor uses the previously manufactured master model and obtains the geometry of the implant in detail using the printing material. This geometry is then placed in a 3D laser scanning machine to generate a digital model of the contour of the implant.
This virtual 3D model is transferred to the CAM system that will generate the control program of the CNC machine where the implant will be made. The pre-implant obtained with the oversized shape is then subjected to machining that will determine its final geometry. After sterilization, the implant is ready to be placed in the patient. Figure 1 depicts the process of the intraoperative manufacture of implants.
Figure 1.
Procedure for the rapid manufacture of implants during surgery.
MATERIALS AND METHODS
A system for shape acquisition via 3D scanning (3D Laser Scanner, Roland LPX 250) with a resolution of 0.2 mm and a work area of Ø200mm by 400mm height was used for testing. Implants were manufactured on a computerized numerical control milling machine with four programmable axes (Roland MDX 650), and its programming was done using an automatic programming system (CAM) (PowerMill – Delcam PLC, Birmingham, United Kingdom). Experimental testing aimed at the manufacture of an anatomically adapted femoral stem that would be implanted during hip replacement. This began by obtaining the XR of the zone undergoing surgery and using it as the base for manufacturing the cutters necessary to open the femoral canal, using the master model as a support for obtaining the geometry of the femoral canal in situ and manufacturing a pre-implant with an oversized thickness in the area of the femoral stem. The previous manufacturing of the prosthesis is performed so that only the final geometry needs to be machined during surgery, thereby shortening the manufacturing time and consequently the total time of surgery.
Sheep were the animals chosen for in vivo testing, which underwent arthroplasty of a custom prosthesis. The surgeries were performed on the premises of the University of Évora (Portugal), by a team of researchers and experts in the field of veterinary medicine and orthopedic surgery. The choice of the animal for study and the site of surgery resulted from some previous studies developed between the Biomechanics Research Group, Department of Mechanical Engineering, University of Aveiro and the Department of Veterinary Medicine, University of Évora.
Following normal procedures, surgery was initiated with an incision, dislocation of the hip joint, exposing the femoral head, and cutting the neck. The geometry of the implant housing in the femoral canal was performed using a progressing set of three cutters pre-manufactured for this purpose. The third cutter allowed for the final geometry of the end of the canal to be produced identically to the geometry of the end of either the master model or the end of the prosthesis.
To obtain the geometry of the femoral canal by direct printing, the previously made master model was used, with a latex membrane in its proximal zone that served to retain the fast-setting printing material (polyvinylsiloxane – Zhermack, Elide HD+, light body fast setting). After placing the master model according to the position of fixation of the prosthesis, we proceeded to inject polyvinylsiloxane. We injected approximately 3 ml of polyvinylsiloxane, for which it was necessary to wait 3 minutes, corresponding to the period of material trapping.
The master model manufactured for the direct printing of the geometry of the implant during surgery is shown at the top of Figure 2. At the bottom of the same figure the pre-prosthesis model can be seen endowed with an overly thick proximal zone made with polymeric material to be machined to the geometry of the femoral canal during surgery.
Figure 2.

Top: the master model manufactured to receive the polyvinylsiloxane. Bottom: the pre-prosthesis prepared to be machined with the geometry of the femoral canal.
This was followed by the removal of the master model, which was placed in the 3D scanning machine and in which the geometry of the proximal area of the canal was obtained in digital format. This shape survey process took about two minutes and the data file was transferred to the CAM system.
The role of a CAM application is to calculate the trajectory paths of a cutting tool that allows it to make cuts into the material during its movements to generate the surfaces of the 3D model. In this case, the surfaces desired are the stem of the prosthesis, which were generated from the 3D model imported from the 3D scanner. The result of this step is an NC program with the control instructions for the CNC milling machine used in the manufacture of the implant.
Once the NC program is obtained, it is sent to the CNC milling machine with the pre-model of the prosthesis inside. Machining of the proximal zone of prosthesis followed, preparing it to be sterilized and then implanted in the femur, requiring approximately 20 minutes. Figure 3 shows the final configuration of the implant after machining.
Figure 3.

Final configuration of the implant after machining.
The various software applications (3D scanning system, CAM, and CNC) were integrated into one computing platform with a unique working environment that is user-friendly and able to be used by medical professionals or others without specific knowledge of computer-aided design and manufacturing technology, as can be seen in Figure 4.
Figure 4.
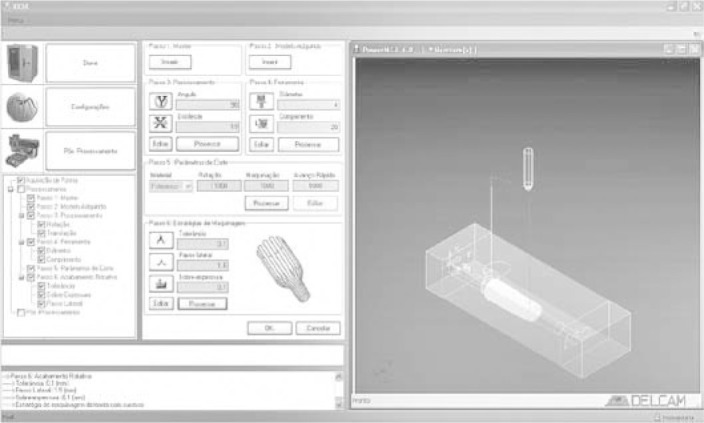
General view of the computing platform designed to allow for the greater ease of use of various technologies.
The prosthesis was implanted in the sheep after sterilization. The surgery was completed with the placement of the acetabular component and after obtaining acceptable positioning and coupling of the two implant components.
Figure 5 shows one of the stages of surgery, performed at the Veterinary Hospital of the University of Évora.
Figure 5.
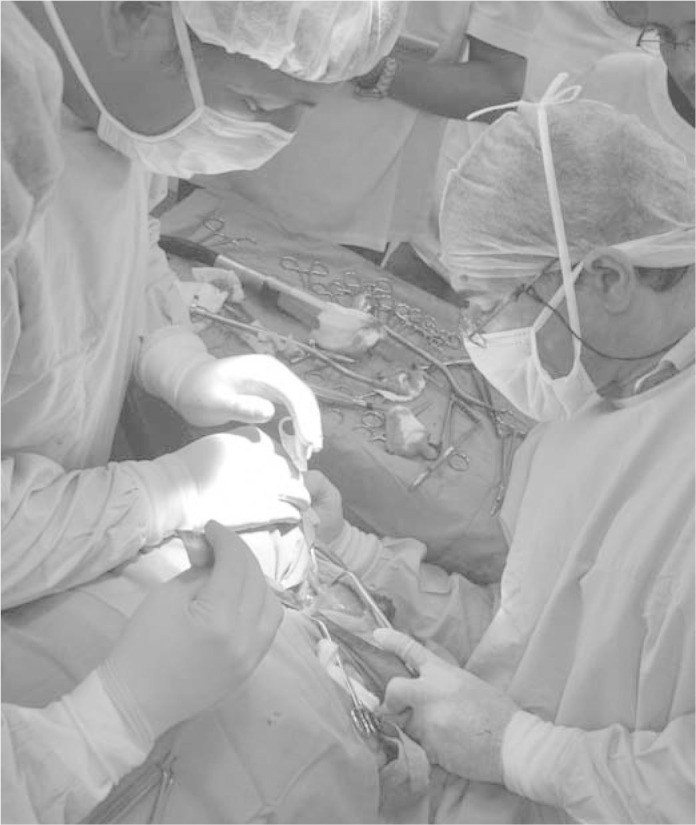
Surgical implant placement.
RESULTS
The total time of surgery was 2 hours and 50 minutes. The time required to capture the shape of the femoral canal in situ and manufacture the customized implant simultaneously with surgery has proved to be adjusted and perfectly compatible with the time during which an animal can be kept under anesthesia.
In the test performed, it should be noted that the total time required for the manufacture of the implant did not exceed 40 minutes and another 40 minutes should be added for the sterilization of the prosthesis prior to its implementation. All other times are for common stages of surgery and are identical to those of any bone graft placement.
Another aim of this study was to develop a more cost-effective solution than the alternatives currently on the market. This can be determined by the value of the initial investment of the implemented system, about 36,000, and the cost of surgery, about 400, according to the values presented in Table 1 that reflect the costs of the equipment and materials used in the surgery.
Table 1.
Initial investment and cost estimate of the surgical intervention.
| Designation | Acquisition cost (euros) | Total estimated use | Actual use | Cost of use (euros) |
|---|---|---|---|---|
| Roland MDX 650 | 20000 | 2500 h | 20 min | 2.64 |
| Roland LPX 250 | 7500 | 1000 h | 3 min | 0.38 |
| PowerMill (software) | 7500 | 800 h | 8 min | 1.25 |
| Cutting tool | 71 | 2 h | 20 min | 11.85 |
| Scanner fixation device | 200 | 10 X | 20.00 | |
| Milling machine fixation device | 200 | 10 X | 20.00 | |
| Master model | 250 | 10 X | 25.00 | |
| Prosthesis pre-model | 250 | 1 X | 250.00 | |
| Acetabular component | 20 | 1 X | 20.00 | |
| Polyvinylsiloxane | 27 | 100 ml | 3 ml | 0.80 |
| Other | 50 | 1 X | 50.00 | |
| Initial investment | 36068 | Procedure total | 401.92 | |
Only the actual costs related to the acquisition of equipment and its use are reflected in the table. The values obtained were based on a scenario of five years of use for the equipment and 200 days of work per year. For some of the manufactured devices, it was assumed that these could be reused in similar future situations. No rates of return for the initial investment costs were taken into consideration.
DISCUSSION
Considering hip replacement surgery, and despite commercial prostheses being supplied in various sizes and configurations, the anatomy of femurs varies greatly in geometry and size15, 16, 17, 18. In this sense it is very difficult to provide commercial prostheses that allow a proper fit with the surrounding bone.
In revision arthroplasty, the geometrical component of the prosthesis plays an important role. Revision prosthesis should consider the geometry of the section of the stem and that of the metaphysis, in addition to its modularity, features essential to this type of prosthesis. In fact, the modularity of the revision prosthesis is the “necessary evil” because it is necessary to provide more flexible intraoperative solutions, with changes in the geometry of the stems and independent modifications of stem length, offset, and the cervical diaphysis angle19, 20. But the modular solution also has its own disadvantages, such as the likelihood of the emergence of debris and its potential for corrosion(21).
An anatomical prosthesis allows for adaptation to the natural curvature of the femur and accommodation to possible bone defects, and allowing for the independent correction of anteversion and retroversion.
A manufactured implant that is customized can be applied in primary or revision surgery, although in revision situations the concept seems to have a higher potential22, 23. Revision arthroplasties, whether hip or of other joints, are usually complex and very costly. Thus, bone defects detected during surgery may allow for the selection of the implant, which should objectively maintain stable fixation, make the joint functional, maintain or restore the volume of bone tissue, and offer the patient quality of life, with a functional and painless joint.
The manufacture of customized and anatomically adapted implants have functional advantages compared to conventional implants with a pre-defined geometry and even in relation to custom implants obtained by preoperative processes. The manufacture of custom implants based on the information generated by computerized axial tomography or magnetic resonance imaging requires a somewhat lengthy prior preparation in addition to presenting some constraints on the generation of the geometry of bone structures and the final precision of the implant(12). Moreover, they do not allow for any kind of correction when the need for such is detected during surgery.
Thus, the development of a system for the rapid manufacture of orthopedic implants capable of faithfully reproducing the fine details of any anatomical structure that can be used intraoperatively is highly advantageous because it can allow medicine to restore not only the function but also the shape of a damaged bone structure.
The new concept of rapidly manufacturing anatomically adapted implants simultaneously with surgery was developed with the hip prosthesis, but it can be used in other pathological situations such as the repair of bone defects (such as those caused by bone loss) or the correction of anatomical bone structures.
An increase in the total time of surgery by 40 to 60 minutes still allows the total time of surgery to be compatible with the viability of the process and the use of a faster process of sterilization would represent a significant improvement.
The use of systems and technologies supported by computer such as 3D CAD, CAM, shape-acquisition systems, and manufacturing by numerical control allow us to confirm the assumption that this is the process that best guarantees the minimization of the dimensional and geometric deviations between the design and the final implant, ensuring the best conditions for repeatable processes. The results were always observed within the specifications of the manufacturer (± 0.1 mm), which is perfectly compatible with the requirements necessary to ensure good fixation of the implant(24).
The choice of equipment and commercial applications has resulted in more accessible costs and greater ease in the recruitment and training of qualified technicians, that is, the use of less expensive and easier to use equipment allowed for its use in unusual situations. With regard to the cost of the equipment and materials used in surgery, it can be concluded that although they present high values, they are not unaffordable and are lower than the values reported for alternative methods.
CONCLUSIONS
The system developed for the rapid manufacture of custom orthopedic implants allowed for developments in order to produce a proper fit between the implant and the bone, improving postoperative discomfort and potentially improving success rates in the medium and long-term. It is expected that the final costs of this solution may be further reduced with continued technological development.
The use of processes for directly capturing the geometry of the implant and manufacturing simultaneously with surgery proved to be able to faithfully reproduce the fine details of the anatomical structure, thus fulfilling the objective of improving the dimensional quality and the geometry of the desired model, ensuring better functional performance.
Testing revealed that the system was able to meet the proposed objectives and be implemented over a period of time that is perfectly compatible with the time of surgery.
The supporting technology, namely the computer applications that were used, also proved adequate, a fact demonstrated by the average time required for information processing and the reliability of results produced in the manufacture of the implants.
The editor chose to publish in the Portuguese language as it was written in its original form.
REFERENCES
- 1.Bargar WL. Shape the implant to the patient. A rationale for the use of custom-fit cementless total hip implants. Clin Orthop Relat Res. 1989;(249):249–258. [PubMed] [Google Scholar]
- 2.McCarthy JC, Bono JV, O'Donnell PJ. Custom and modular components in primary total hip replacement. Clin Orthop Relat Res. 1997;(344):344–371. [PubMed] [Google Scholar]
- 3.Stulberg SD, Stulberg BN, Wixson RL. The rationale, design characteristics, and preliminary results of a primary custom total hip prosthesis. Clin Orthop Relat Res. 1989;(249):249–296. [PubMed] [Google Scholar]
- 4.Decking R, Puhl W, Simon U, Claes LE. Changes in strain distribution of loaded proximal femora caused by different types of cementless femoral stems. Clin Biomech (Bristol, Avon) 2006;21(5):495–501. doi: 10.1016/j.clinbiomech.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Gillies RM, Morberg PH, Bruce WJ, Turnbull A, Walsh WR. The influence of design parameters on cortical strain distribution of a cementless titanium femoral stem. Med Eng Phys. 2002;24(2):109–114. doi: 10.1016/s1350-4533(01)00124-2. [DOI] [PubMed] [Google Scholar]
- 6.Viceconti M, Brusi G, Pancanti A, Cristofolini L. Primary stability of an anatomical cementless hip stem: A statistical analysis. J Biomech. 2006;39(7):1169–1179. doi: 10.1016/j.jbiomech.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Robertson DD, Walker PS, Granholm JW, Nelson PC, Weiss PJ, Fishman EK. Design of custom hip stem prostheses using three-dimensional CT modeling. J Comput Assist Tomogr. 1987;11(5):804–809. doi: 10.1097/00004728-198709000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Winder J, Cooke RS, Gray J, Fannin T, Fegan T. Medical rapid prototyping and 3D CT in the manufacture of custom made cranial titanium plates. J Med Eng Technol. 1999;23(1):26–28. doi: 10.1080/030919099294401. [DOI] [PubMed] [Google Scholar]
- 9.Mulier JC, Mulier M, Brady LP, Steenhoudt H, Cauwe Y, Goossens M, Elloy M. A new system to produce intraoperatively custom femoral prosthesis from measurements taken during the surgical procedure. Clin Orthop Relat Res. 1989;(249):249–252. [PubMed] [Google Scholar]
- 10.Boissonat JD. Shape reconstruction from planar crosssections. Comput Vis Graph Image Process. 1988;44:1–29. [Google Scholar]
- 11.Fuchs H, Kedem ZM, Uselton SP. Optimal surface reconstruction from planar contours. Commun ACM. 1977;20(10)::693–:702. [Google Scholar]
- 12.Rubin PJ, Leyvraz PF, Aubaniac JM, Argenson JN, Estève P, de Roguin B. The morphology of the proximal femur. A three-dimensional radiographic analysis. J Bone Joint Surg Br. 1992;74(1):28–32. doi: 10.1302/0301-620X.74B1.1732260. [DOI] [PubMed] [Google Scholar]
- 13.Laine HJ, Kontola K, Lehto MU, Pitkänen M, Jarske P, Lindholm TS. Image processing for femoral endosteal anatomy detection: description and testing of a computed tomography based program. Phys Med Biol. 1997;42(4):673–689. doi: 10.1088/0031-9155/42/4/005. [DOI] [PubMed] [Google Scholar]
- 14.Liu JG, Li DS, Ma WH, Zhou ZP, Xu XX. Computer assisted reconstruction of three-dimensional canal model of femur and design for custom-made stem. Chin Med J (Engl) 2004;117(8):1265–1267. [PubMed] [Google Scholar]
- 15.Averill RG, Pachtman N, Jaffe W E. Basic dimensional analysis of normal human proximal femora. In: Proceedings of the 8th Annual Northeast Bioengineering Conference, Cambridge, 1980; MA, p. 352.
- 16.Dai KR, An KM, Hein T, Nakahjima I, Chao E. Geometric and biomechanical analysis of the human femur. Orthop Trans. 1985;10:99. [Google Scholar]
- 17.Huang HK, Suarez FR. Evaluation of cross-sectional geometry and mass density distributions of humans and laboratory animals using computerized tomography. J Biomech. 1983;16(10):821–832. doi: 10.1016/0021-9290(83)90006-4. [DOI] [PubMed] [Google Scholar]
- 18.Morscher EW. Cementless total hip arthroplasty. Clin Orthop Relat Res. 1983;(181):76–91. [PubMed] [Google Scholar]
- 19.Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G. Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model. Health Technol Assess. 1998;2(6):1–133. [PubMed] [Google Scholar]
- 20.Bono JVS. Rom modular total hip replacement. Oper Techn Orthop. 2001;11(4):279–287. [Google Scholar]
- 21.Bobyn JD, Tanzer M, Krygier JJ, Dujovne AR, Brooks CE. Concerns with modularity in total hip arthroplasty. Clin Orthop Relat Res. 1994;(298):27–36. [PubMed] [Google Scholar]
- 22.Toni A, Ciaroni D, Sudanese A, Femino F, Marraro MD, Bueno Lozano AL. Incidence of intraoperative femoral fracture. Straight-stemmed versus anatomic cementless total hip arthroplasty. Acta Orthop Belg. 1994;60(1):43–54. [PubMed] [Google Scholar]
- 23.Walker PS, Culligan SG, Hua J, Muirhead-Allwood K, Bentley G. Stability and bone preservation in custom designed revision hip stems. Clin Orthop Relat Res. 2000;(373):373–374. doi: 10.1097/00003086-200004000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Relvas C. Concepção e estudo de próteses de anca anatomicamente adaptadas por obtenção in situ da geometria do canal femoral [tese]. Universidade de Aveiro; 2007.