Abstract
Background
Video-assisted thoracoscopic surgery (VATS) has been applied to resection of small and well-encapsulated thymomas. However, few data are available regarding to the application of VATS in stage III thymomas.
Methods
A novel subxiphoid and subcostal arch approach for thoracoscopic extended thymectomy was developed by us. From January 2014 to August 2015, 14 patients with stage III thymoma were treated by using this new technique in the Department of Thoracic Surgery, Tangdu hospital, Xi’an, China. These patients were retrospectively reviewed and analyzed.
Results
Among the 14 patients, 1 patient was converted to transsternal approach owning to invasion of the superior vena cava. The other 13 patients with thymomas invading the pericardium, lung tissues and left innominate vein (LIV), were successfully operated on by using this new technique. The average operation time was 120.0±32.7 min (80–170 min), the average volume of estimated blood loss was 51.5±44.8 min (10–150 mL) and the average postoperative hospital stay was 4.8±1.5 days (3-9 days). There was no perioperative death. Two patients suffered postoperative complications including one patient with atrial fibrillation (AF) and the other one with myasthenic crisis (MC). The postoperative pain score decreased dramatically from 3.8±1.0 [3–6] at 24 hours to 1.5±0.9 [0–6] at 48 hours, and finally to 0 at 3 months after surgery (P=0.000). The patients reported a higher cosmetic score of 92.6±2.7 [90–96]. There was no tumor recurrence and the five patients with myasthenia gravis had improvement and did not need any medication until follow-up.
Conclusions
Based on our limited experience, the subxiphoid and subcostal arch thoracoscopic extended thymectomy is safe and feasible for selective stage III thymoma, and might reduce the postoperative pain and provide satisfied cosmetic effect.
Keywords: Invasive thymoma, video-assisted thoracic surgery (VATS), thymectomy, subxiphoid approach
Introduction
Thymoma is the most common primary anterior mediastinal tumor in adults. Surgical resection plays critical role in the treatment of thymomas, and completeness of resection is the most important prognostic factor (1). Traditionally, transsternal total thymectomy has been considered as the standard treatment for thymomas. However, it has been fiercely challenged by video-assisted thoracoscopic surgery (VATS) thymectomy, a minimally invasive surgical procedure for thymectomy. Several reports have shown that thoracoscopic thymectomy performed by well trained surgeons might achieve equivalent clinical outcomes compared with that of open surgery in the treatment of early stage thymomas (Masaoka stage I and II) (2-9). However, the application of VATS thymectomy in locale invasive thymoma (Masaoka stage III) was controversial (10). There were few articles reported the experiences of VATS thymectomy in the treatment of stage III thymomas (11-13).
Recently, a novel subxiphoid and subcostal arch thoracoscopic extended thymectomy has been developed by us and has been successfully applied in the treatment of non-thymomas myasthenia gravis and early stage thymomas (The results has been reported in the 14th Annual Congress of Chinese Society for Thoracic and Cardiovascular Surgery, 2014; the manuscript has been submitted for publication). Here, the experiences of this novel procedure in stage III thymomas resection were summarized and analyzed.
Materials and methods
Patients
From January 2014 to August 2015, 65 patients with thymomas were treated by the subxphoid and subcostal arch thoracoscopic extended thymectomy in the Department of Thoracic Surgery, Tangdu hospital, Xi’an, China. All these patients were carefully evaluated before operation by using computed tomography (CT). The tumor location was carefully assessed to make sure there was no major invasion to the trachea, the heart, the aorta and the other big vessels. After operation, the patients were classified according to the Masaoka’s staging system (14). The patients with postoperative pathological confirmed Masaoka stage III were retrospectively reviewed and analyzed.
The informed consents were obtained from all patients. The patients were informed about the risks and potential benefits of this new approach, as well as the possibility to convert to transsternal approach according to the judgments of the surgeon during operation. All the patients were also informed that the lack of long-term data on this new procedure for the treatment of thymomas. This retrospective study was approved by the ethics committee of Tangdu Hospital.
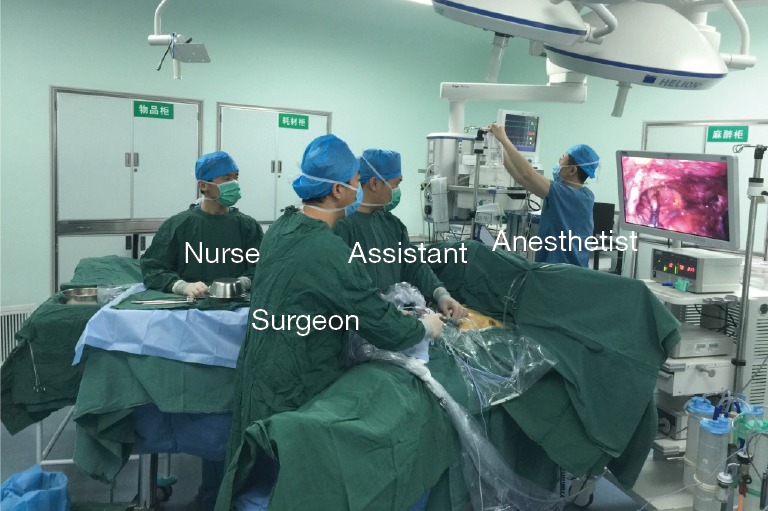
Position and anesthesia
The patient was placed in the supine position on the operating table with the legs open. The surgeon stood between the patient’s legs and the assistant was on the left or right side (Figure 1). After intravenous induction, the patient was anesthetized and intubated with a single lumen endotracheal tube.
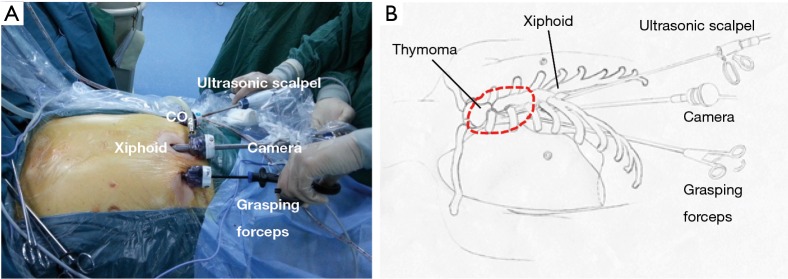
Figure 1.
The position of the patient and the operation team during the subxiphoid and subcostal arch thoracoscopic extended thymectomy.
Surgical procedure
A 3-cm incision was made below the xiphoid. The rectus abdominis muscle was separated vertically, and the retrosternal space was dissected blindly by using a finger. The bilateral extra pleural space was enlarged and two 5-mm extra pleural thoracic ports under the bilateral costal arches were created. Under the guidance of the finger, a longer thoracoscopic grasping forceps (45 cm) and an ultrasonic scalpel (45 cm) were placed. Thereafter, a 30-degree oblique, 10 mm thoracoscope was introduced into the retrosternal space. The carbon dioxide (CO2) with an 8-cm H2O positive pressure was insufflated into the anterior mediastinum, which helped to enlarge the retrosternal space and blow the fume out of the anterior mediastinum during operation (Figure 2).
Figure 2.
The incisions and the setup of the surgical instruments during the subxiphoid and subcostal arch thoracoscopic extended thymectomy. CO2, carbon dioxide.
Then, according to the “no touch, tumor last” principle (11,12), the non-tumorous parts of the thymus was always dissected firstly. In order to minimize the risk of tumor seeding, the surgeon should pay much attention to grasp the normal tissues but not touch the tumor capsule. During dissection, the thymic veins, usually branched from the innominate veins, were carefully identified and cut with the ultrasonic scalpel. After dissecting the normal parts of the thymus, the thymus with the tumor was carefully assessed. If the pericardium was invaded, the normal part of the pericardium was grasped and cut by using the ultrasonic scalpel (Figure 3). If the lung was invaded, one of the ports under the costal arches was enlarged to 1 cm, and a wedge resection of the lung was made by using a stapler (Figure 4). If the left innominate vein (LIV) was invaded, the LIV was carefully mobilized and two staplers were used to cut the involved vein (Figure 5). If the bilateral mediastinal pleura were invaded, or the patients suffered myasthenia gravis, the bilateral mediastinal pleura were resected completely. The whole thymus with the thymoma was en bloc resected, put into a plastic bag and removed through the subxiphoid port. If the patients suffered myasthenia gravis at the same time, the fat tissues near the cardiac-diaphragmatic angles, the aortopulmonary window, the phrenic nerves and the lower poles of the thyroid gland were dissected and removed.
Figure 3.
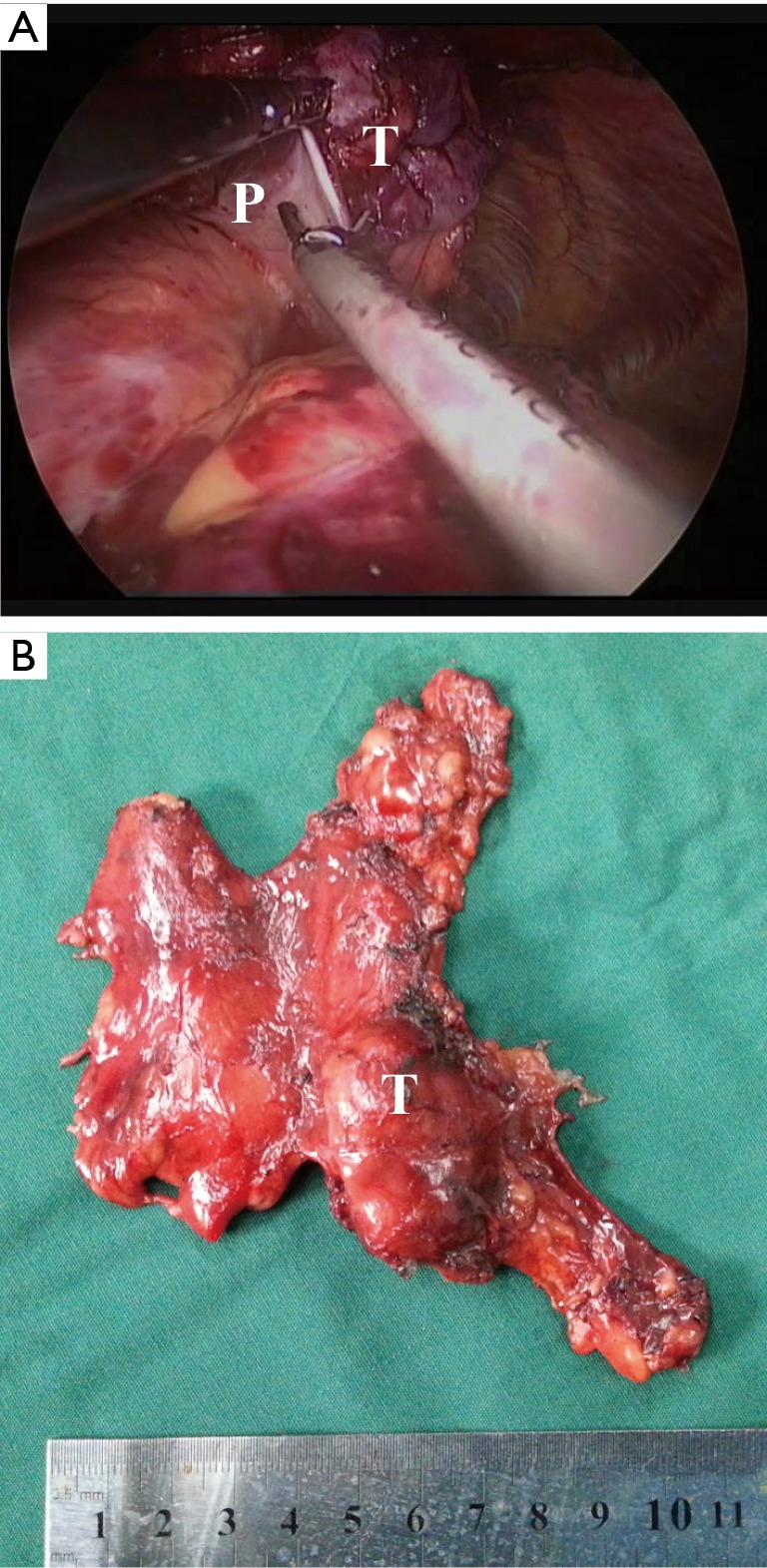
The thymoma invading the pericardium (Case No. 1): (A) the invaded pericardium could be resected by the ultrasonic scalpel; (B) the sample after operation. P, pericardium; T, tumor.
Figure 4.
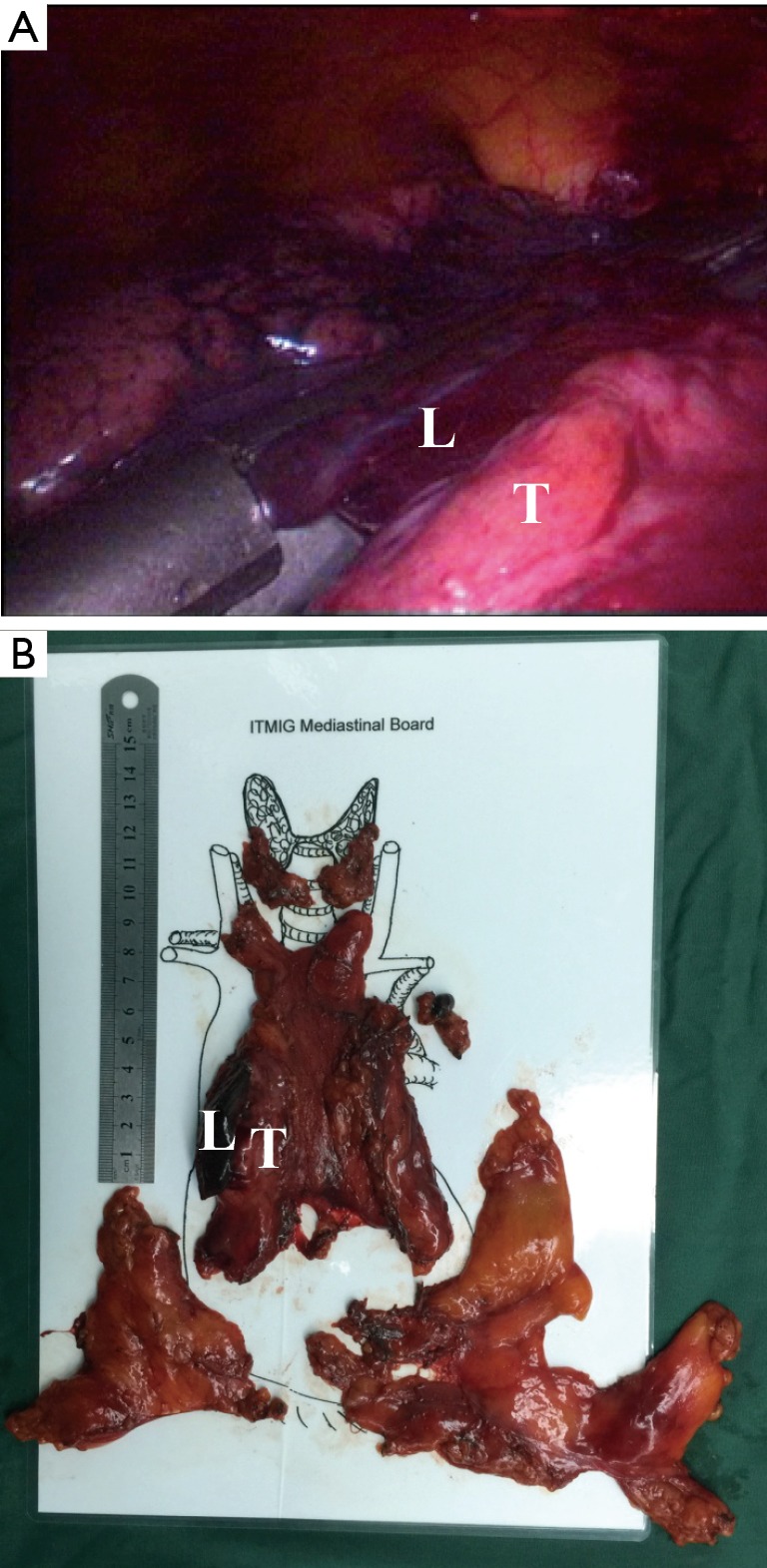
The thymoma invading the lung tissue (Case No. 10): (A) the invaded lung tissue could be resected by using an endoscopic stapler; (B) the sample after operation. L, lung tissue; T, tumor.
Figure 5.
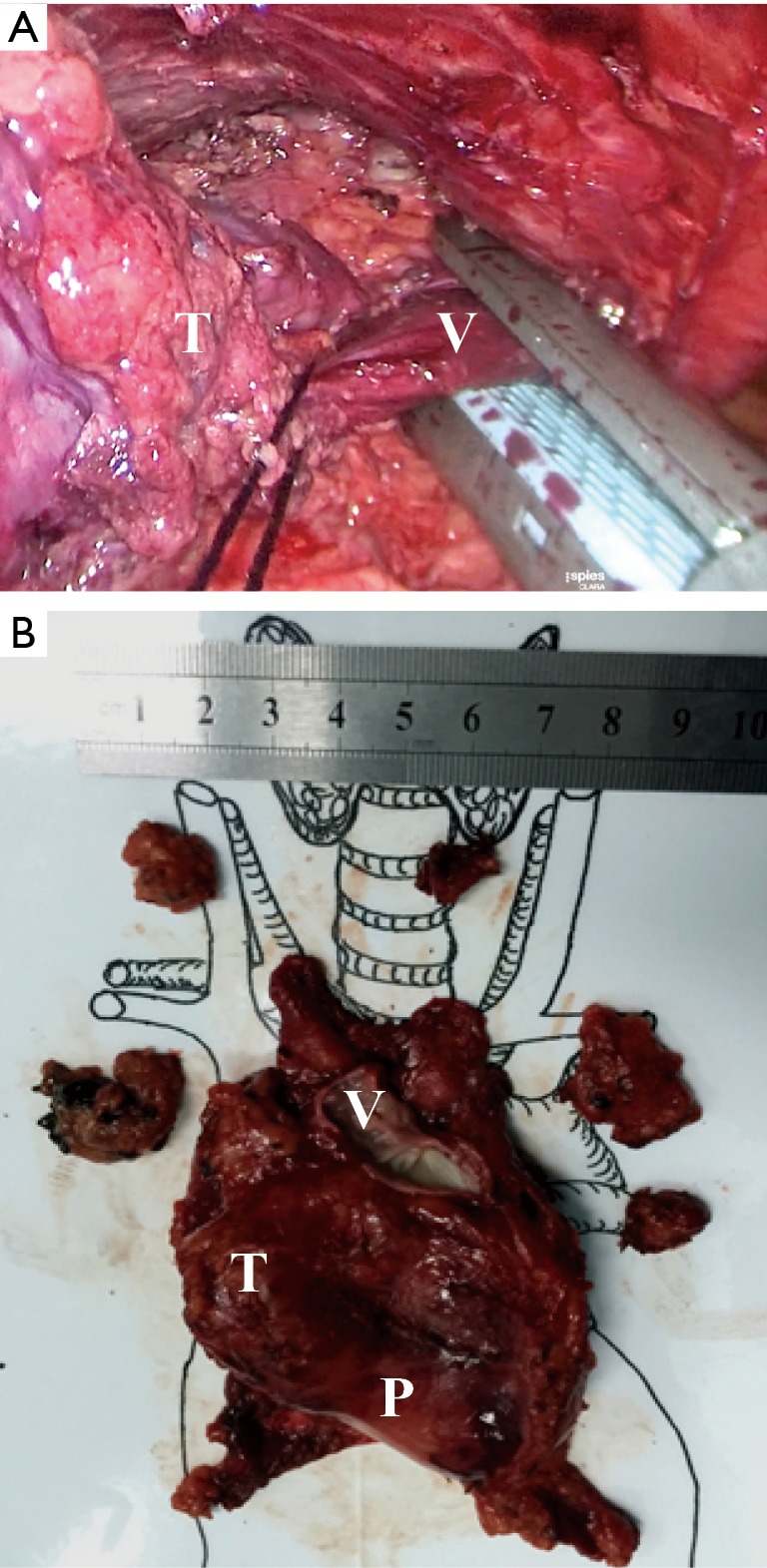
The thymoma invading the left innominate vein (LIV) and the pericardium (Case No. 13): (A) the invaded left innominated vein could be resected by using an endoscopic stapler; (B) the sample after operation. V, vessel; P, pericardium; T, tumor.
The surgical field was checked and the thoracoscope was removed. The two subcostal arch ports were sutured first. Then, a drainage tube was inserted into the anterior mediastinum through the subxiphoid incision. The air was drained out by inflating the lungs. After that, the drainage tube was removed and the incisions were sutured.
The specimens were sectioned, examined and classified according to the World Health Organization (WHO) classification by the pathologists in the Department of Pathology, Tangdu Hospital.
Postoperative care and evaluation
After operation, the oxygen saturation and electrocardiography monitoring was performed in the early period. A sitting chest X-ray was taken the day after operation.
The postoperative pain was evaluated by using the Visual Analog Pain Scale at 24, 48 hours after operation and 3 months after operation. A patient self-reported cosmetic evaluation score was used to evaluate the cosmetic effect when the patients were discharged. The cosmetic evaluation was reported by the patients themselves with a score from 0 to 100 to indicate very dissatisfied status to very satisfied status.
The patients were followed up at 3, 6, 12 months for the first year after operation and once yearly later. All the clinical data was collected. The GraphPad Prism software (Version 5.0, GraphPad Software, Inc., CA, USA) was used for the data analysis.
Results
From January 2014 to August 2015, 14 patients were finally classified as Masaoka’s stage III according to the postoperative pathologic results, and were treated by the subxphoid and subcostal arch thoracoscopic extended thymectomy at the Tangdu hospital, Xi’an, China. Among them, one patient was converted to transsternal approach owning to tumor invasion to the superior vena cava, and the angioplasty cannot be performed under the thoracoscope. The other 13 patients’ clinical characteristics were reviewed and summarized in Table 1.
Table 1. Clinical characters.
| Case | Age (years) | Sex | Tumor size (centimeters) | WHO classification | Involved organs | Postoperative complications |
|---|---|---|---|---|---|---|
| 1 | 61 | M | 3.0×2.5×2.0 | B2 | P | N |
| 2* | 56 | M | 4.0×3.5×3.0 | B1 | P | N |
| 3 | 47 | M | 7.5×6.5×4.0 | B2 | P | N |
| 4 | 54 | M | 7.0×3.0×3.0 | B2–B3 | P | N |
| 5* | 44 | M | 6.0×4.0×3.0 | B2–B3 | RUL | MC |
| 6 | 45 | F | 7.0×6.5×4.0 | B1 | P | N |
| 7 | 45 | F | 6.0×5.5×3.0 | AB | P | N |
| 8 | 69 | M | 6.0×6.0×5.0 | B2–B3 | P | AF |
| 9* | 70 | M | 6.0×5.0×5.0 | B2–B3 | P | N |
| 10* | 53 | M | 3.0×2.0×2.0 | B1–B2 | RUL | N |
| 11 | 67 | M | 5.0×4.0×3.0 | B2–B3 | LIV | N |
| 12* | 52 | F | 4.0×4.0×4.0 | B2–B3 | P | N |
| 13 | 46 | M | 5.0×4.0×3.0 | B2–B3 | P, LIV | N |
*, Co-morbidity: ocular myasthenia gravis. F, female; M, male; WHO, World Health Organization; RUL, right upper lobe; LIV, left innominate vein; P, pericardium; N, none; MC, myasthenic crisis; AF, atrial fibrillation.
According to the postoperative pathological examination, there were one patient with AB, two patients with B1, two patients with B2, one patient with B1-B2 and seven patients with B2–B3. All the patients had clear surgical margins. The average longest diameter of thymoma was 5.4±1.6 cm (range, 3.0–7.5 cm). The invaded organs included the pericardiums in nine patients, the lung tissues in two patients, the LIVs in one patients, and both the pericardium and the LIV in one patient. Of the 13 cases accepted the subxphoid and subcostal arch thoracoscopic extended thymectomy, the average operation time was 120.0±32.7 min (80–170 min), the average volume of estimated blood loss was 51.5±44.8 mL (10–150 mL) and the average postoperative hospital stay was 4.8±1.5 days (3–9 days) .
Two patients suffered postoperative complications including one patient with atrial fibrillation (AF) and the other one with myasthenic crisis (MC). All these two patients recovered well when discharged. There was no perioperative death. The postoperative pain score decreased dramatically from 3.8±1.0 [3–6] at 24 hours to 1.5±0.9 [0–6] at 48 hours, and finally to 0 at 3 months after surgery (P=0.000). The patients reported a higher cosmetic score of 92.6±2.7 [90-96]. All the 13 patients were alive and no tumor recurrence happened. All the five patients with myasthenia gravis had improvement and did not need any medication.
Discussion
Surgery has been proved to be an effective method to treat the resectable thymomas (1). Even though subtotal resection may prolong the survival of patients with later stages thymomas (Masaoka’s stage III and VI), complete resection has been shown as the most important prognostic factor (1). Recently, as a minimal invasive approach, VATS thymectomy has been prevalent in the treatment of thymomas. A number of studies have shown that the patients with thymomas treated by VATS had smaller size tumors, less blood loss, shorter hospital stay, shorter duration of chest tube drainage, but similar longer term survival, compared with those treated by sternotomy (2-9). Therefore, VATS thymectomy has been recommended as an option for the treatment of early stagy thymomas (9). Different approaches have been developed for the thoracoscopic resection of thymomas including unilateral, bilateral, uniportal and subxiphoid approaches (11,12,15-18).
However, the reports of VATS thymectomy in the treatment of stage III thymomas were limited (11-13). There were some possible reasons. Firstly, it was a technique challenge to resect an invasive tumor under thoracoscope. Secondly, the retraction of tumors during VATS may induce the tumor seeding, recurrence and metastasis. Dr. Axel Aubert reported a woman with well encapsulated thymoma was biopsied by VATS, but suffered tumor recurrence at the port sites four years later (10). But Dr. Thirugnanam Agasthian reported that 13 patients with stage III thymomas were operated on through unilateral VATS approach (11). During operation, the surgeons followed the “no touch, tumor last” principle, which mean that the non-tumorous part of the thymus should be dissected firstly, and the tumor was dissected lastly and should be not touched during dissection. The mean duration of follow-up was 4.9 years and no port sites recurrence happened. But the mean size of tumor was small (3.4 cm). Thereafter, Dr. Guofei Zhang and his colleagues reported bilateral VATS thymectomy for stage III thymomas in 13 patients (12). They followed the same oncological principle. By using the bilateral approach, the mean size of tumor increased to 6.4 cm. All the patients recovered well with no recurrence after 17.4 months follow-up. The VATS thymectomy combined with lateral thoracotomy also been reported for the resection of stage III thymomas (13). By using the approaches mentioned above, some of the thymomas invading the lung and pericardium could be safely resected by VATS. But no thymomas invaded great vessels was operated on through VATS.
Regarding to the novel subxiphoid and subcostal arch approach, we thought there were several advantages for thoracoscopic extended thymectomy in the treatment of thymomas. Firstly, the anterior mediastinum could be well exposed through this approach and three ports facilitated the operation, which avoided the bilateral incisions. The great visualization allowed the resection of invaded tissues and the mediastinal fat tissues possible. Our results have shown that the invaded lung tissues, pericardium and even the LIV could be safely resected by using this approach. The fat tissues near the cardiac-diaphragmatic angles, the aortopulmonary window, the phrenic nerves, even near the lower poles of the thyroid gland, could be easily removed. Secondly, the intercostals nerves compression or injury could be avoided by this new approach, which might reduce the postoperative pain. Our results prove that all the patients reported no pain 3 months after operation. Thirdly, no separate ventilation was needed by using this approach, which may be benefit to the patients who cannot tolerate the single lung ventilation due to the severe lung diseases or poor lung function. However, If the thymoma was bigger (>8 cm, according to our limited experiences), this big mass might influence the exposure of important structures in the anterior mediastinum. At the same time, the reconstruction of invaded big vessels was difficult even by using this approach. Therefore, the surgeon should convert to sternotomy without any hesitation in these situations. In certain situations, the application of this novel technique might be limited. For example, the retrosternal space might be difficult to open for a patient with previous operations in the chest or the anterior mediastinum; a cervical incision might be added to facilitate the dissection for a patient with a mass extended to the cervical part.
Although there were several limitations in this study including small sample size, shorter follow-up and its restrospective nature, our study provided initial experiences regarding to the thoracoscopic resection of stage III thymomas through this novel subxiphoid and subcostal arch approach. To our knowledge, this is the first report of successful thorascopic resection of thymomas invading the vessels. In future, larger studies with longer follow-up data were required to further confirm the oncological results of this new procedure.
Conclusions
The subxiphoid and subcostal arch thoracoscopic extended thymectomy is safe and feasible for the resection of selective stage III thymomas.
Acknowledgements
Funding: This study was partially supported by National Nature Science Foundation of China (No. 81001041), and Science and Technology Innovation Development Foundation of Tangdu Hospital, Fourth Military Medical University.
Footnotes
Conflicts of Interests: The authors have no conflicts of interest to declare.
References
- 1.Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. [DOI] [PubMed] [Google Scholar]
- 2.Gu ZT, Mao T, Chen WH, et al. Comparison of video-assisted thoracoscopic surgery and median sternotomy approaches for thymic tumor resections at a single institution. Surg Laparosc Endosc Percutan Tech 2015;25:47-51. [DOI] [PubMed] [Google Scholar]
- 3.Liu TJ, Lin MW, Hsieh MS, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol 2014;21:322-8. [DOI] [PubMed] [Google Scholar]
- 4.Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg 2014;45:e187-93. [DOI] [PubMed] [Google Scholar]
- 5.Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [DOI] [PubMed] [Google Scholar]
- 6.Tagawa T, Yamasaki N, Tsuchiya T, et al. Thoracoscopic versus transsternal resection for early stage thymoma: long-term outcomes. Surg Today 2014;44:2275-80. [DOI] [PubMed] [Google Scholar]
- 7.Zielinski M. Minimally invasive subxiphoid-right, videothoracoscopic technique of thymectomy for thymoma and rethymectomy. Multimed Man Cardiothorac Surg 2012;2012:mms005. [DOI] [PubMed]
- 8.Takeo S, Tsukamoto S, Kawano D, et al. Outcome of an original video-assisted thoracoscopic extended thymectomy for thymoma. Ann Thorac Surg 2011;92:2000-5. [DOI] [PubMed] [Google Scholar]
- 9.Xie A, Tjahjono R, Phan K, et al. Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann Cardiothorac Surg 2015;4:495-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aubert A, Chaffanjon P, Brichon PY. Video-assisted extended thymectomy in patients with thymoma by lifting the sternum: is it safe? Ann Thorac Surg 2004;77:1878; author reply 1878. [DOI] [PubMed]
- 11.Agasthian T. Can invasive thymomas be resected by video-assisted thoracoscopic surgery? Asian Cardiovasc Thorac Ann 2011;19:225-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang G, Li W, Chai Y, et al. Bilateral video-assisted thoracoscopic thymectomy for Masaoka stage IIIA thymomas. Thorac Cardiovasc Surg 2015;63:206-11. [DOI] [PubMed] [Google Scholar]
- 13.Hirai K, Ibi T, Bessho R, et al. Video-assisted thoracoscopic thymectomy (VAT-T) with lateral thoracotomy for stage II and III thymoma. Ann Thorac Cardiovasc Surg 2013;19:79-82. [DOI] [PubMed] [Google Scholar]
- 14.Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304-12. [DOI] [PubMed] [Google Scholar]
- 15.Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i54-i58. [DOI] [PubMed] [Google Scholar]
- 16.Wu L, Lin L, Liu M, et al. Subxiphoid uniportal thoracoscopic extended thymectomy. J Thorac Dis 2015;7:1658-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zieliński M, Rybak M, Wilkojc M, et al. Subxiphoid video-assisted thorascopic thymectomy for thymoma. Ann Cardiothorac Surg 2015;4:564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu CF, Gonzalez-Rivas D, Wen CT, et al. Single-port video-assisted thoracoscopic mediastinal tumour resection. Interact Cardiovasc Thorac Surg 2015;21:644-9. [DOI] [PubMed] [Google Scholar]