Abstract
Background
Single-port video-assisted thoracoscopic surgery (VATS) has attracted much attention recently; however, it is still very challenging to perform especially on more technically demanding sublobar anatomic resection procedures such as segmentectomy. Therefore we conducted a retrospective study on the perioperative results of single-port segmentectomy using a propensity-matched method for comparison with multi-port segmentectomy in patients with primary lung cancer.
Methods
For procedures of anatomic segmentectomy performed between May 2006 and March 2014, we retrieved data on patients’ demographic information, medical history, cancer information, and postoperative outcomes from our surgical database of thoracoscopic lung cancer surgery. Outcome variables included the number of lymph nodes retrieved during the surgery, the amount of blood loss, the duration of hospitalization, the length of the wound, the operation duration in minutes, and incidence and types of complication. The t-test and Chi-squared test were used to compare demographic and clinical variables between single- and multi-port approaches.
Results
A total of 98 consecutive patients who underwent VATS segmentectomy for lung cancer treatment were identified in our database: 52 (53.1%) underwent a single-port segmentectomy and 46 (46.9%) had a multi-port segmentectomy. After propensity score matching, the differences in patients’ age, pulmonary function tests, tumor size, and operating surgeons were no longer significant between the two sample groups. The length of the wound was the only surgical outcome for which single-port segmentectomy had a significantly better outcome than multi-port segmentectomy (P value <0.001).
Conclusions
This study showed that single-port VATS segmentectomy yielded comparable surgical outcomes to multi-port segmentectomy despite technique difficulties and smaller wound in our setting.
Keywords: Single-port, video-assisted thoracoscopic surgery (VATS), segmentectomy
Introduction
Thoracoscopic surgery for lung cancer was first described in the early 1990s, and it is now accepted as a technically feasible (even standard) option for many kinds of lung surgery after two decades of development. The many recognized advantages of the procedure include decreased postoperative pain, reduced impairment of pulmonary function, shorter duration of chest tube insertion, and consequently shorter hospital stays (1). Single-port video-assisted thoracoscopic surgery (VATS) was first described by Rocco et al., who reported wedge resection of the lung with a single-port approach in 2004 (2). In addition, Gonzalez et al. reported performing lobectomy and segmentectomy through a single incision (3). Recently, single-port VATS has become an increasingly popular approach for managing thoracic diseases. This popularity can be attributed to the continuous innovations in endoscopic systems, energy devices, and surgical instruments as well as, most importantly, the obligation and desire of surgeons to reduce surgical trauma and ameliorate patients’ discomfort (4).
Not only the technique of VATS has been evolving towards less invasiveness, but a lesser extent of surgical resection also has been suggested over the years. In 1995, a milestone study by the Lung Cancer Study Group (LCSG) concluded that lobectomy was the standard procedure for lung cancer treatment because of the higher rate of local recurrence following segmentectomy (5). However, the landscape of thoracic oncology has changed remarkably in subsequent decades and new developments have led to an era of minimally invasive thoracoscopic approaches, which include segmentectomy for carefully selected patients. Recent articles pointed out that there was no significant difference in disease-free survival following lobectomy and segmentectomy among stage IA lung cancer patients (6,7). Studies also suggest that segmentectomy has comparable oncologic outcomes with lobectomy for early non-small cell lung cancer (8). Literatures often recommend thoracoscopic segmentectomy over thoracoscopic lobectomy on account of results demonstrating reduced postoperative complications and hospital stay, equivalent oncologic results, rate of recurrence, and survival in selected lung cancer patients (9).
We started using the thoracoscopic approach for lobectomy and segmentectomy with radical lymph node dissection to treat lung cancer patients in 2005, and we adapted to using the two-port thoracoscopic approach in 2007. In November 2010, we omitted the thoracoscopic port and began using a single-port approach for lung cancer surgery to simplify the surgical wound and reduce postoperative discomfort. The positive feedback from patients’ clinical outcomes constantly encourages us to modify existing procedures and invent new ones to solve technical problems. We have also developed several simple and effective methods to facilitate the dissection of mediastinal lymph nodes (10). With the single-port approach, there is no need for additional grasping of lung tissues; furthermore, instrument fencing can be avoided. The anterior-to-posterior order of dissection described by Pham et al. (11), which we adopted in 2007, was particularly helpful when we were developing our method of single-port surgery.
Single-port VATS is now as widely used as multi-port VATS in lung cancer segmentectomy in our hospital. However, there remains a lack of comparative information on the postoperative outcomes between these two techniques. Hence, we conducted a retrospective cohort analysis to compare single- and multi-port segmentectomies using a propensity score matching method to verify the clinical application of single-port thoracoscopic surgery in patients who received segmentectomy.
Materials and methods
Surgical technique
Our previous study had described the details of single-port segmentectomy surgical techniques (12). In brief, the surgery was performed under general anesthesia with a single lung ventilation in the lateral decubitus position. Both the surgeon and the assistant stood at the anterior side of the patient. A single incision of approximately 4 cm was made in the fifth or sixth intercostal space along the anterior axillary line and a wound protector (Alexis wound protector/retractor, Applied Medical Technology Inc., Brecksville, OH, USA) was routinely used without rib spreading. All procedures were performed with thoracoscopic assistance, in which a 10-mm, 30-degree thoracoscopic video camera and several thoracoscopic instruments were simultaneously inserted into a single incision. The surgical field was visualized primarily on the screen via the thoracoscopic view. The majority of the dissection was performed with endoscopic hook electrocautery and energy devices such as a Harmonic scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). The pulmonary vessels and bronchi were sectioned with the use of endoscopic staplers or vascular clips (Hemo-lock vascular clips, Weck Closure Systems, Research Triangle Park, NC, USA). Energy devices were used to aid lymphadenectomy (systemic lymph node sampling: 2R, 4R, 7, 8, and 9 for right-sided cancers; 4L, 5, 6, 7, 8, and 9 for left-sided cancers). After the divisions of segmental vessels and bronchi, the parenchymal excision was completed either by staplers or electrocautery along the inflated-deflated zone. At the end of surgery, a protective specimen bag was always used to retrieve the specimen and a single chest drain (14 Fr Pigtail, 16 or 20 Fr chest tube) was placed at the edge of the incision.
Data sources and patient selection
We retrieved data from our prospective database, which was established in 2000 at the Department of Thoracic Surgery, Koo-Foundation Sun Yat-Sen Cancer Center, Taipei, Taiwan. The study was approved by the institutional review board of the hospital.
For each individual, demographic information (age and gender), medical history (chronic obstructive pulmonary disease, diabetic mellitus, and tuberculosis), and cancer information (stage, year of intervention, location, histologic type of cancer, TNM classification, and FEV1/FVC ratio) were collected prospectively. Histological typing was established according to the World Health Organization classification. TNM stage was determined according to the American Joint Committee on Cancer staging system, 7th Edition.
Outcome variables included the number of lymph nodes retrieved during the surgery, the amount of blood loss, the duration of hospitalization, the length of the wound, the operation duration in minutes, and incidence and types of complication.
Most data were collected at the time of diagnosis; however, some data (e.g., cancer information and hospital outcomes) were collected during the course of surgery.
In total, 107 adult patients who had a segmentectomy from May 2006 to March 2014 were identified. We excluded patients with a subxiphoid port placement and patients not diagnosed with lung cancer. Nine patients were excluded. For the remaining 98 patients included in the study, 52 (53.1%) underwent single-port surgery and 46 (46.9%) had multi-port surgery (Figure 1). The multi-port surgery group included the two-port approach and three-port approach procedures.
Figure 1.
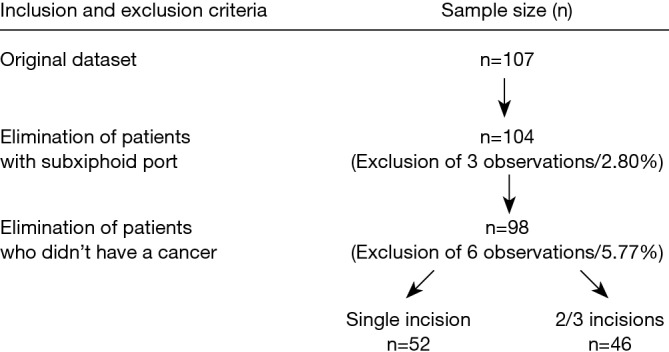
Flow chart showing patient selection process.
Statistical analysis
To control for potential selection bias, we used a propensity score matching method. The cohort of patients who had single-port surgery was matched with patients who had multi-port surgery using the nearest neighbor-matching algorithm with a “greedy” heuristic. Matching occurred if the difference in the logits of the propensity scores was less than 0.2 times the standard deviation of the scores. To generate the propensity score, we applied a 1:1 ratio and used the following covariates in the logistic regression: age, tumor size, FEV1/FVC ratio, and the identifier of the surgeon. A total of 29 pairs of patients were selected after the propensity score matching.
Differences between the baseline characteristics of patients and parameters after propensity score matching were tested using t-test for continuous variables and Chi-squared test for categorical data. We also compared outcome parameters at baseline and after propensity score matching between patients who underwent single-port surgery and those who received multi-port. Finally, we cross-tabulated the demographic variables and clinical variables with the outcome variables in the single-port group to look for potential predictors of the surgical outcomes.
P values less than 0.05 were considered statistically significant. All statistical analyses were conducted using SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Among the single-port group (Table 1), 34 traditional segmentectomies were performed, including trisegmentectomy, lingulectomy, common basal segmentectomy, and superior segmentectomy of lower lobe; the other 18 atypical segmentectomies included apicoposterior segmentectomy of left upper lobe, right apical segmentectomy, posterior segmentectomy of right upper lobe, apical segmentectomy of right upper lobe, right segment 8+9 bisegmentectomy right segment 7+8 bisegmentectomy, and right segment 9+10 bisegmentectomy.
Table 1. Type of surgical procedure among the single-port approach group (n=52).
| Type of surgical procedure | No. of patients |
|---|---|
| Traditional segmentectomies (n=34) | |
| Trisegmentectomy | 17 |
| Lingulectomy | 7 |
| Superior segmentectomy of lower lobe | 8 |
| Common basal segmentectomy | 2 |
| Atypical segmentectomies (n=18) | |
| Apicoposterior segmentectomy of left upper lobe | 4 |
| Right apical segmentectomy | 2 |
| Posterior segmentectomy of right upper lobe | 5 |
| Apical segmentectomy of right upper lobe | 2 |
| Right segment 8+9 bi-segmentectomy | 1 |
| Right segment 7+8 bi-segmentectomy | 2 |
| Right segment 9+10 bisegmentectomy | 2 |
The clinical and demographic characteristics of the patients before and after the propensity matching are presented in Table 2. All variables included in the logistic regression model for the propensity score matching (i.e., age, FEV1/FVC ratio, tumor size, and surgeon) were initially statistically significant and became non-significant after matching. Gender and pathological stage group were significantly different between patients that underwent the single-port surgery and those that underwent multi-port surgery. Even though they were not specifically matched in the process, these two factors were not significantly different after the propensity score matching.
Table 2. Clinical characteristics of segmentectomy patients before and after propensity-score matching.
| Characteristics | All patients (n=98) |
Propensity-matched patients (n=58) |
|||||
|---|---|---|---|---|---|---|---|
| Single-port (n=52) | Multi-port (n=46) | P value | Single-port (n=29) | Multi-port (n=29) | P value | ||
| Sex | |||||||
| Female | 43 (82.69) | 26 (56.52) | 0.005* | 25 (86.21) | 18 (62.07) | 0.070 | |
| Male | 9 (17.31) | 20 (43.48) | 4 (13.79) | 11 (37.93) | |||
| Age, y# | 59.00±11.63 | 66.8±9.95 | <0.001* | 61.72±12.24 | 67.24±9.57 | 0.061 | |
| FEV1/FVC, L# | 80.15±7.42 | 71.93±8.75 | <0.001* | 77.14±7.79 | 74.39±6.23 | 0.222 | |
| Tumor size, cm# | 2.15±1.03 | 2.92±1.87 | 0.016* | 2.24±0.96 | 2.38±1.11 | 0.623 | |
| Surgeon# | |||||||
| A | 45 (86.54) | 30 (65.22) | 0.013* | 22 (75.86) | 21 (72.41) | 0.764 | |
| B | 7 (13.46) | 16 (34.78) | 7 (24.14) | 8 (27.59) | |||
| Pathologic stage (AJCC 7th) | |||||||
| 1 | 38 (80.85) | 22 (66.67) | 0.486 | 21 (80.77) | 16 (72.73) | 0.854 | |
| 2 | 6 (12.77) | 7 (21.21) | 4 (15.38) | 5 (22.73) | |||
| 3 | 2 (4.26) | 3 (9.09) | 1 (3.85) | 1 (4.55) | |||
| 4 | 1 (2.13) | 1 (3.03) | 0 (0) | 0 (0) | |||
*, statistically significant; #, represented as matched variables.
Table 3 lists the details of the outcome variables in each cohort analyzed before and after propensity score matching. After propensity score matching, the number of lymph nodes retrieved, blood loss, length of hospital stay, operation time, and complication rate did not differ significantly between the groups. Only the length of the wound remained significantly different after matching; specifically, the average length of wounds was 3.71 and 4.36 cm in the single- and multi-port groups, respectively.
Table 3. Surgical outcomes among single-port and multi-port patients.
| Outcome variables | All patients (n=98) |
Propensity-matched patients (n=58) |
|||||
|---|---|---|---|---|---|---|---|
| Single-port (n=52) | Multi-port (n=46) | P value | Single-port (n=29) | Multi-port (n=29) | P value | ||
| No. of lymph nodes retrieved | 19.20±10.73 | 17.70±10.50 | 0.489 | 19.46±10.96 | 18.79±11.97 | 0.826 | |
| Blood loss, mL | 63.27±78.38 | 60.22±50.44 | 0.817 | 66.38±93.43 | 55.52±49.40 | 0.583 | |
| Hospital stay, day | 5.77±1.98 | 6.93±2.17 | 0.007* | 6.17±2.28 | 6.66±2.38 | 0.434 | |
| Length of wound, cm | 3.62±0.74 | 4.58±1.02 | <0.001* | 3.71±0.74 | 4.36±0.61 | <0.001* | |
| Operation time, min | 3.31±0.97 | 3.46±0.93 | 0.425 | 3.48±1.00 | 3.26±0.83 | 0.358 | |
| Complication rate, % | 44 (89.80) | 37 (80.43) | 0.198 | 22 (84.62) | 23 (79.31) | 0.733 | |
*, statistically significant.
Table 4 describes the cross tabulation of some demographic and clinical variables with the outcome variables for the single-port group (29 patients). In this cohort, females lost an average of 73.40 mL of blood during the intervention, which contrasted with an average of 22.50 mL blood loss among male patients (P value =0.028). We also noted that patients who had diabetes mellitus before surgery had significantly longer wounds than patients without diabetes (average length of 4.00 vs. 3.69 cm; P value=0.041). The other associations tested between outcomes and clinical variables were not statistically significant.
Table 4. Comparison of clinical variables and outcome variables for the single-incision group after propensity-score matching (n=29).
| Characteristics | Outcome variables (mean ± σ) |
Total, n (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymph node retrieved, no. | P value | Blood loss, mL | P value | Hospital stay, day | P value | Length of wound, cm | P value | Operation time, min | P value | Complication | P value | ||
| Sex | 0.843 | 0.028* | 0.874 | 0.229 | 0.410 | 0.580 | |||||||
| Female | 19.29±11.22 | 73.40±98.83 | 6.20±2.36 | 3.64±0.74 | 3.42±1.05 | 0.14±0.35 | 25 (86.21) | ||||||
| Male | 20.50±10.66 | 22.50±18.93 | 6.00±2.00 | 4.12±0.63 | 3.87±0.63 | 0.25±0.50 | 4 (13.79) | ||||||
| Age, y | 0.096 | 0.315 | 0.286 | 0.469 | 0.952 | 0.930 | |||||||
| <65 | 21.84±10.33 | 49.00±41.66 | 5.75±1.41 | 3.77±0.82 | 3.47±1.01 | 0.15±0.37 | 20 (68.97) | ||||||
| ≥65 | 14.44±11.10 | 105.00±154.90 | 7.11±3.48 | 3.56±0.53 | 3.50±1.06 | 0.17±0.41 | 9 (31.03) | ||||||
| Stage I cancer | 0.233 | 0.212 | 0.277 | 0.711 | 0.657 | 0.376 | |||||||
| Yes | 17.13±8.34 | 84.69±121.40 | 5.75±2.11 | 3.66±0.47 | 3.41±1.17 | 0.21±0.43 | 16 (55.17) | ||||||
| No | 22.15±13.21 | 43.85±30.97 | 6.69±2.46 | 3.77±0.99 | 3.58±0.79 | 0.08±0.29 | 13 (44.83) | ||||||
| Antecedent of DM | 0.605 | 0.354 | 0.674 | 0.041* | 0.741 | 0.171 | |||||||
| Yes | 15.50±13.43 | 50.00±0.00 | 5.50±2.12 | 4.00±0.00 | 3.25±1.06 | 0.50±0.71 | 2 (6.90) | ||||||
| No | 19.77±11.01 | 67.59±96.85 | 6.22±2.33 | 3.69±0.76 | 3.50±1.02 | 0.12±0.34 | 27 (93.10) | ||||||
| Antecedent of TB* | |||||||||||||
| Yes | – | – | – | – | – | – | 0 (0.00) | ||||||
| No | – | – | – | – | – | – | 29 (100.00) | ||||||
Standard deviations are reported in parentheses. *, the data was not applicable for analysis due to no patient with antecedent TB infection.
Discussion
In our sample, patients receiving single-port segmentectomy, as compared to those receiving multi-port segmentectomy, were more likely to be female, younger, with better lung functioning, having smaller tumor, and in stage I. Using propensity score matching, we were able to control for most of the confounding factors. The differences in gender, age, FEV1/FVC ratio, tumor size, operating surgeon, and pathological stage were not statistically significant after matching between the two groups in our dataset.
This study has shown that single-port thoracic segmentectomy can yield comparable surgical outcomes to multi-port segmentectomy in most of the tested parameters. The length of the wound was the only surgical outcome for which single-port segmentectomy had a better outcome than multi-port segmentectomy. There is a growing body of literature that compares the oncologic efficacy of thoracoscopic segmentectomy with that of lobectomy (6,13-16). These studies propose technical modifications for anatomic resection, and present the feasibility of single-port segmentectomy. However, comparisons of postoperative outcomes between multi-port and single-port segmentectomy have rarely been reported. This study helps fill the gap of information.
We started using single-port VATS segmentectomy in December 2010 for the removal of a centrally located carcinoid tumor. We then used it for lung cancers with tumors of less than 2 cm in diameter and for elderly patients with compromised cardio-pulmonary function. Our preliminary results of single port VATS, including 14 lobectomies and five segmentectomies, were performed successfully without the need for conversion to conventional open surgery (17). We also reported that 233 lung cancer patients underwent thoracoscopic lobectomy or segmentectomy via a single-port or multi-port technique without surgical mortality, and showed that these two techniques produced comparable lengths of hospitalization and postoperative complication rates. Furthermore, patients in single-port approach group had shorter operative times, more lymph nodes removed, and less intraoperative blood loss (18). In recent years, our team has addressed single-port thoracoscopic surgery in several published articles (10,12,17,19,20).
After gaining experience of the procedure and advancing the technique and instrument, we introduced precise resection of the so-called subsegment, or combination of subsegments, according to the approach of Illustrated Anatomical Segmentectomy for Lung Cancer for deep-seated small lung nodules proposed by Hiroaki and Morihito (21). Cases of complicated subsegmental resection have increased in the last 2 years and may have required more operative time and possibly longer hospital stays due to prolonged air leaks. The effect of different single-port approach procedures has been analyzed (not shown here), but no significant differences between simple and complicated segmentectomy were identified regarding demographic parameters or postoperative outcomes. It showed that, after years of experience and instrumental refinement, single-port VATS has been a routine procedure and treatment of choice for dealing with general thoracic malignancies in our hospital.
Segmentectomy was once regarded as an inadequate procedure for lung cancer treatment and was reserved only for poorly functioning elderly patients. Thanks to the development of low-dose chest CT scans, early detection of lung cancer has become more common in recent years. Segmentectomy could be the treatment of choice for this group of patients who have a low chance of lymph node metastases, and it was advocated to cope with the rapidly increasing numbers of small early lung cancer identified by CT screening programs. For small lung malignancies, both the single-port thoracoscopic approach and segmentectomy can minimize injury and provide benefits such as reducing wound trauma, preserving lung parenchymal function, and improving respiratory recovery, which can lead to shorter lengths of hospitalization and early return to work.
This study shows that single-port thoracoscopic segmentectomy with radical lymph node dissection can be performed safely and feasibly with perioperative outcomes that are comparable to conventional VATS segmentectomy as well as improved surgical wound trauma. In further research, the oncologic validity of segmentectal resection in relation to lung cancer should be investigated, possibly with prospective study conducted for scientific comparison.
Acknowledgements
None.
Footnotes
Conflicts of Interests: The authors have no conflicts of interest to declare.
References
- 1.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [DOI] [PubMed] [Google Scholar]
- 2.Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg 2004;77:726-8. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [DOI] [PubMed] [Google Scholar]
- 4.Ng CS, Rocco G, Wong RH, et al. Uniportal and single-incision video-assisted thoracic surgery: the state of the art. Interact Cardiovasc Thorac Surg 2014;19:661-6. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [DOI] [PubMed] [Google Scholar]
- 6.Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Li M, Yin R, et al. Comparison of the oncologic outcomes of anatomic segmentectomy and lobectomy for early-stage non-small cell lung cancer. Ann Thorac Surg 2015;99:728-37. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Sun Y, Wang R, et al. Meta-analysis of lobectomy, segmentectomy, and wedge resection for stage I non-small cell lung cancer. J Surg Oncol 2015;111:334-40. [DOI] [PubMed] [Google Scholar]
- 9.Yang CF, D'Amico TA. Thoracoscopic segmentectomy for lung cancer. Ann Thorac Surg 2012;94:668-81. [DOI] [PubMed] [Google Scholar]
- 10.Liu CC, Shih CS, Pennarun N, et al. Transition from a multiport technique to a single-port technique for lung cancer surgery: is lymph node dissection inferior using the single-port technique?†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i64-i72. [DOI] [PubMed] [Google Scholar]
- 11.Pham D, Balderson S, D'Amico TA.Technique of Thoracoscopic Segmentectomy. Oper Tech Thorac Cardiovasc Surg 2008;13:188-203. [DOI] [PubMed] [Google Scholar]
- 12.Wang BY, Tu CC, Liu CY, et al. Single-incision thoracoscopic lobectomy and segmentectomy with radical lymph node dissection. Ann Thorac Surg 2013;96:977-82. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Ma W, Li Y, et al. Comparative study of the anatomic segmentectomy versus lobectomy for clinical stage IA peripheral lung cancer by video assistant thoracoscopic surgery. J Cancer Res Ther 2013;9 Suppl 2:S106-9. [DOI] [PubMed] [Google Scholar]
- 14.Deng B, Cassivi SD, de Andrade M, et al. Clinical outcomes and changes in lung function after segmentectomy versus lobectomy for lung cancer cases. J Thorac Cardiovasc Surg 2014;148:1186-1192.e3. [DOI] [PMC free article] [PubMed]
- 15.Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren M, Meng Q, Zhou W, et al. Comparison of short-term effect of thoracoscopic segmentectomy and thoracoscopic lobectomy for the solitary pulmonary nodule and early-stage lung cancer. Onco Targets Ther 2014;7:1343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang BY, Liu CC, Shih CS. Short-term results of thoracoscopic lobectomy and segmentectomy for lung cancer in koo foundation sun yat-sen cancer center. J Thorac Dis 2010;2:64-70. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang BY, Liu CY, Hsu PK, et al. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: a propensity-matched analysis. Ann Surg 2015;261:793-9. [DOI] [PubMed] [Google Scholar]
- 19.Hsu PK, Lin WC, Chang YC, et al. Multiinstitutional analysis of single-port video-assisted thoracoscopic anatomical resection for primary lung cancer. Ann Thorac Surg 2015;99:1739-44. [DOI] [PubMed] [Google Scholar]
- 20.Liu CY, Cheng CT, Wang BY, et al. Number of Retrieved Lymph Nodes and Postoperative Pain in Single-incision and Multiple-incision Thoracoscopic Surgery. Ann Surg 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Nomori H, Okada M. Illustrated Anatomical Segmentectomy for Lung Cancer. Japan: Springer Japan, 2012. [Google Scholar]


