Abstract
Background
Anastomotic leak following esophagectomy is associated with significant morbidity and mortality. As hospital length of stay decreases, the timely diagnosis of leak becomes more important. We evaluated CT esophagram, white blood count (WBC), and drain amylase levels in the early detection of anastomotic leak.
Methods
The diagnostic performance of CT esophagram, drain amylase >800 IU/L, and WBC >12,000/µL within the first 10 days after surgery in predicting leak at any time after esophagectomy was calculated.
Results
Anastomotic leak occurred in 13 patients (13%). CT esophagram performed within 10 days of surgery diagnosed six of these leaks with a sensitivity of 0.54. Elevation in drain amylase level within 10 days of surgery diagnosed anastomotic leak with a sensitivity of 0.38. When the CT esophagram and drain amylase were combined, the sensitivity rose to 0.69 with a specificity of 0.98. WBC elevation had a sensitivity of 0.92, with a specificity of 0.34. Among 30 patients with normal drain amylase and a normal WBC, one developed an anastomotic leak.
Conclusions
Drain amylase adds to the sensitivity of CT esophagram in the early detection of anastomotic leak. Selected patients with normal drain amylase levels and normal WBC may be able to safely forgo CT esophagram.
Keywords: Esophagectomy, anastomotic leak, amylase
Introduction
Esophageal resection is associated with major operative morbidity with perioperative mortality in the National Inpatient Sample (1) of 11%. High-volume centers have demonstrated improvement in perioperative morbidity and mortality with protocoled systems (2,3), which include team-based approaches that quickly identify and manage postoperative complications (4).
Anastomotic leak following esophageal resection occurs in up to 30% of patients (4) and remains one of the most significant postoperative complications (5), correlating with shortened cancer-specific survival (6-8).
Among patients who experience an anastomotic leak, the diagnosis is made a median of 7 days after surgery (9-11). Traditionally, length of stay after esophagectomy has been long enough [median of 15 days in several studies (12,13)] that most anastomotic leaks would have manifested themselves before hospital discharge.
With the advent of minimally invasive esophagectomy and enhanced recovery after surgery protocols, the early detection of anastomotic leak following esophagectomy is imperative. Ideally, the methods used to predict leaks would be inexpensive, safe, and easily replicable.
Contrast swallow examination has traditionally been used to evaluate for anastomotic leak but is limited by poor sensitivity (14-18). CT esophagram has been shown to be more sensitive (15,19,20), with reported sensitivity from 88% to 100%.
In this study, we retrospectively analyzed our patients who underwent minimally invasive transthoracic esophagectomy. Our protocol-defined postoperative care includes CT esophagram and the routine measurement of daily drain amylase levels and white blood count (WBC). Here, we compare the utility of these measures in the early detection of anastomotic leak following esophagectomy.
Methods
This study was a retrospective review of patients from November 2009 through April 2014. Approval was obtained from the Carolinas Medical Center Institutional Review Board (Charlotte, NC, USA). Demographics drain amylase values, serum WBC, CT esophagram, postoperative complications, and mortality is collected. Of the 106 patients who underwent transthoracic esophagectomy at Carolinas Medical Center during the study period, 6 were excluded due to incomplete data.
Operative technique
All patients underwent some form of minimally invasive transthoracic esophagectomy using techniques previously described (21). Laparoscopic mobilization of the stomach was performed using 3 ports with a hand port placed for retraction. The gastric conduit was formed using a cutting linear stapler to fashion a 5-6 cm tube. The thoracic esophagus was mobilized thoracoscopically using 5 to 6 thoracic ports. The esophagogastric anastomosis was formed using a 25 or 21 mm EEA circular stapler (Covidien, New Haven CT, USA). A 28-French Blake drain was positioned in the right pleural space and a 19-French round Blake drain was placed within the chest alongside the gastric conduit, passed through the hiatus, and brought out through the abdominal wall.
Postoperative care
All patients were admitted to the ICU in the immediate postoperative period. Daily drain amylase levels were collected from the intrathoracic 19-French round Blake drain. Patients were initiated on low-volume jejunostomy tube feeds on the first postoperative day, followed by slow advancement of enteral feedings. CT esophagram was performed routinely on postoperative days 7, 8, or 9 for evaluation of anastomotic leak prior to initiating oral intake. If an anastomotic leak was suspected clinically, CT esophagram was performed earlier. If an anastomotic leak was suspected and CT esophagram was negative, the study was repeated at weekly intervals as long as patients remained in the hospital. Drains were removed prior to discharge if the CT esophagram was negative and drain amylase levels were normal. As a result, the majority of drains were removed by postoperative day 10.
Because the intention of the study was to examine the role of drain amylase in the early (prior to hospital discharge) detection of leaks, the performance of CT esophagram, drain amylase, and elevated WBC in the first 10 days after surgery were evaluated.
Statistical analysis
Anastomotic leak was defined by contrast extravasation on postoperative CT esophagram or presence of empyema on chest CT. CT findings were used as the standard for defining a leak, so the sensitivity and specificity in diagnosing a leak at any time after surgery was 1.00 (100%). Because the purpose of the study was to examine the early detection of leaks, the sensitivity and specificity of each test were calculated as the ability of that test within the first 10 days after surgery to predict whether or not the patient would experience a leak at any time after surgery.
Drain amylase levels were defined as elevated for any value >800 IU/L. Threshold amylase level was determined by visual inspection of the data. Four patients had elevations of drain amylase within the first 3 postoperative days, which then normalized; these were assumed to be due to intraoperative spill of gastric contents. For the purpose of analysis, drain amylase levels were included from the sixth and subsequent days after surgery.
WBC count was defined as elevated if greater than 12,000/µL on any day in the early postoperative period from days 0-10. Leaks were divided into early leaks (detected by CT esophagram on or before postoperative day 10) and late leaks (detected after postoperative day 10). Sensitivity, specificity, positive predictive value, and negative predictive value were calculated for drain amylase levels greater than 800 IU/L, elevated WBC greater than 12,000/µL, and CT esophagram findings for determination of anastomotic leak at any time after surgery (including early and late leaks). Statistical analysis was performed using R Statistics version 3.02 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study captured 100 patients who underwent esophagectomy. All procedures were performed by a surgical team comprised of a GI surgical oncologist and thoracic surgeon. The majority were treated with some form of minimally-invasive esophagectomy and an anastomosis with a circular EEA stapler; 8 patients with size 21 mm and 92 with size 25 mm. Intrathoracic anastomoses were performed in 98 cases, and transhiatal anastomoses were performed in 2 with benign disease. Ninety three patients underwent hand-assisted laparoscopic mobilization of the stomach and creation of the gastric conduit with thoracoscopic resection and reconstruction. Six patients underwent a laparoscopic abdominal phase and thoracotomy for resection and reconstruction. Of those patients who underwent thoracotomy, two patients underwent planned thoracotomy that had a history of prior thoracotomy; four underwent a thoracotomy converted from thoracoscopy. One patient underwent laparotomy converted from laparoscopy and thoracoscopic reconstruction. Median operative time was 503 min (range, 325-791 min).
Patient characteristics are listed in Table 1. Average patient age was 63 years (range, 33-81 years), 80% of the patients were male, 88 patients were White, 11 were African-American, and 1 was Asian. Indications for surgery included adenocarcinoma (85 patients), squamous cell carcinoma (7 patients), neuroendocrine tumor (3 patients), and benign disease (5 patients, which included 3 esophageal strictures and 2 leiomyomata of the esophagus). Neoadjuvant chemotherapy along with radiation therapy was administered in 66 patients, and neoadjuvant chemotherapy alone in 1. Adjuvant therapy was given postoperatively in 12 patients, 1 of whom received radiation only, 7 of whom received chemotherapy only, and 4 of whom received chemotherapy and radiation therapy.
Table 1. Patient characteristics (n=100).
| Characteristics | N [%] |
|---|---|
| Age | |
| Median | 63 |
| Range | 33-81 |
| Gender | |
| Male | 80 [80] |
| Female | 20 [20] |
| Race | |
| White | 88 [88] |
| Black | 11 [11] |
| Asian | 1 [1] |
| Histology | |
| Adenocarcinoma | 85 [85] |
| Squamous cell | 7 [7] |
| Neuroendocrine | 3 [3] |
| Benign | 5 [5] |
| Clinical T classification | |
| T1a | 9 [10] |
| T1b | 9 [10] |
| T2 | 12 [13] |
| T3 | 65 [67] |
| Clinical N classification | |
| N0 | 55 [59] |
| N1 | 37 [39] |
| N2 | 2 [2] |
| Clinical M classification | |
| M0 | 94 [99] |
| M1 | 1 [1] |
| Pathologic T classification | |
| T0 | 12 [13] |
| T1a | 15 [16] |
| T1b | 14 [15] |
| T2 | 12 [14] |
| T3 | 13 [14] |
| Pathologic N classification | |
| N0 | 49 [52] |
| N1 | 40 [43] |
| N2 | 1 [1] |
| N3 | 4 [4] |
| Pathologic M classification | |
| M0 | 93 [98] |
| M1 | 2 [2] |
| Neoadjuvant therapy | |
| None | 32 [32] |
| Chemotherapy | 1 [1] |
| Chemo/XPT | 67 [67] |
| Adjuvant therapy | |
| None | 89 [89] |
| Radiation | 1 [1] |
| Chemotherapy | 6 [6] |
| Chemo/XPT | 4 [4] |
| R0 resection | 93 [98] |
| Lymph node harvest | [median, 12] |
In the 95 patients who underwent surgery for malignancy, 91 R0 resections and 4 R1 resections were performed. Median lymph node harvest for patients with cancer was 12 nodes (range, 2-45 nodes), median hospital length of stay was 12 days (range, 7-86 days), and 30-day mortality was zero. There were two patient deaths within 60 days (2%) due to strokes and one additional death within 90 days (3%) due to prolonged respiratory failure and subsequent withdrawal of support. Table 2 lists postoperative complications and mortality. The most frequently occurring postoperative complication was pneumonia, which occurred in 26 patients (26%).
Table 2. Postoperative complications and mortality (n=100).
| Complications | N [%] |
|---|---|
| Pneumonia | 26 [26] |
| ARDS | 13 [13] |
| Pulmonary embolism | 3 [3] |
| Postoperative ventilator dependence | 24 [24] |
| Respiratory failure requiring tracheostomy | 17 [17] |
| Atrial arrhythmia | 23 [23] |
| Myocardial infarction | 3 [3] |
| Acute renal failure | 3 [3] |
| Central neurological event-CVA | 4 [4] |
| Chyle leak | |
| Medical management | 1 [1] |
| Surgical management | 1 [1] |
| Unexpected admission to ICU | 6 [6] |
| Anastomotic leak | 13 [13] |
| Medical management | 7 [7] |
| Endoscopic stent | 6 [6] |
| Thirty-day mortality | 0 [0] |
| Sixty-day mortality | 2 [2] |
| Ninety-day mortality | 3 [3] |
Anastomotic leak occurred in 13 patients (13%). Details are found in Table 3. Early leaks (diagnosis by CT esophagram in the first 10 postoperative days) occurred in seven patients. Among these seven early leaks, drain amylase was elevated in three and normal in four. Figure 1 shows the time course of a typical patient with an anastomotic leak diagnosed by elevation in drain amylase. The sensitivity of CT esophagram performed in the first 10 postoperative days in predicting whether a patient would have a leak at any time after surgery (early leaks + late leaks) was 0.54 (Table 4). By definition, the sensitivity and specificity of CT esophagram performed at any time was 100% (1.00), as CT scan provided the diagnostic criteria for a leak for the purposes of the study.
Table 3. Patients with anastomotic leak (n=13) and false-positive drain amylase (n=1).
| Patient ID | CT esophagram |
Drain amylase |
|||||
|---|---|---|---|---|---|---|---|
| Day | Findings | Day | Value (IU/L) | Results | |||
| Early leaks (diagnosed by CT esophagram within 10 days of surgery) | |||||||
| 21 | 6 | Leak on CTE | 6 | 37,481 | True positive | ||
| 1 | 8 | Leak on CTE | 8 | 54,410 | True positive | ||
| 37 | 7 | Leak on CTE | 7 | 55,432 | True positive | ||
| 2 | 9 | Leak on CTE | 9 | 63.0 | False negative | ||
| 84 | 7 | Leak on CTE | 7 | 20 | False negative | ||
| 76 | 8 | Leak on CTE | 8 | 28 | False negative | ||
| 13 | 7 | Leak on CTE | 5 | 14.0 | False negative | ||
| Late leaks (diagnosed by CT esophagram more than 10 days after surgery) | |||||||
| 6 | 7 | No Leak on CTE | 1 | 90.0 | False negative | ||
| 14 | Leak on CTE | 14 | 171,689 | False negative | |||
| 67 | 7 | No leak on CTE | 7 | 15 | True positive | ||
| 15 | No leak on CTE | 10 | 37,997 | True positive | |||
| 23 | Leak on CTE | 16 | 75,041 | True positive | |||
| 38 | 10 | No leak on CTE | 9 | 7,700 | True positive | ||
| 12 | No Leak on CTE | 10 | 21,664 | True positive | |||
| 23 | No Leak on CTE | 30 | 19,062 | True positive | |||
| 32 | No Leak on CTE | 32 | 15,767 | True positive | |||
| 46 | Leak on CTE | 33 | 23,949 | True positive | |||
| 18 | 7 | No leak on CTE | 7 | 52 | False negative | ||
| 13 | Leak on CTE | 8 | Drain removed | False negative | |||
| 33 | 7 | No leak on CTE | 7 | 21.0 | False negative | ||
| 11 | Leak on CTE | 8 | Drain removed | False negative | |||
| 12 | 7 | No leak on CTE | 7 | 14.0 | False negative | ||
| 15 | Leak on CTE | 8 | Drain removed | False negative | |||
| False positive drain amylase | |||||||
| 61 | 7 | No leak on CTE | 6 | 20 | False positive | ||
| 13 | No leak on CTE | 10; 11; 12; 13 | 1,820; 2,464; 947; 394 | False positive | |||
Figure 1.

A 68-year-old woman (patient #21) with T1aN0M0 adenocarcinoma of the gastroesophageal junction treated with minimally invasive esophagectomy. Significant elevation of drain amylase (>800 IU/L) was noted on postoperative day 6. CT esophagram on postoperative day 6 showed evidence of a leak. She was treated with intraluminal stent. Note that drain amylase level appears on a semi-logarithmic scale.
Table 4. Postoperative leak diagnostic tests: sensitivity, specificity, positive and negative predictive values.
| Test used for diagnosis of leak | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| CT esophagram± | 0.54 | 1.00 | 1.00 | 0.94 |
| Drain amylase >800 IU/L* | 0.38 | 0.99 | 0.83 | 0.91 |
| CT esophagram± or drain amylase >800 IU/L* | 0.69 | 0.98 | 0.82 | 0.96 |
| WBC count >12,000/µL∞ | 0.92 | 0.34 | 0.17 | 0.97 |
±, CT esophagram performed in postoperative days 1-10; *, maximum drain amylase value any time in postoperative days 6-10; ∞, WBC count, elevation >12,000/µL any time in postoperative days 1-10. WBC, white blood count.
Late leaks (diagnosed by detection of a leak on CT esophagram after the first 10 postoperative days) occurred in six patients. Three of these late leaks had normal drain amylase levels (patients #18, #33 and #12). One patient (patient #6) had a simultaneous diagnosis of leak by CT esophagram and elevated drain amylase on postoperative day 14. Significantly, two patients with late leaks (patients #67 and #38) had elevations in drain amylase significantly earlier than their diagnosis by CT esophagram. Both had elevation of drain amylase within the first 10 postoperative days, and had diagnosis by CT esophagram on postoperative days 23 and 46, respectively.
The combination of CT esophagram and elevated drain amylase within the first 10 days after surgery in predicting whether or not a patient would experience a leak was 0.69, with a negative predictive value of 0.96. The increase in the sensitivity of the combination of CT esophagram and elevated drain amylase was due to the two patients who were detected within the first 10 days by elevated drain amylase, but were not detected by CT esophagram until postoperative days 23 and 46.
Among the 87 patients without leaks on CT esophagram, 1 had elevated drain amylase levels of 1,820 IU/L on postoperative day 10 and 2,464 IU/L on postoperative day 11, as depicted in Figure 2. CT esophagrams on postoperative days 7 and 13 showed no evidence of leak, and the patient showed no clinical signs of a leak.
Figure 2.
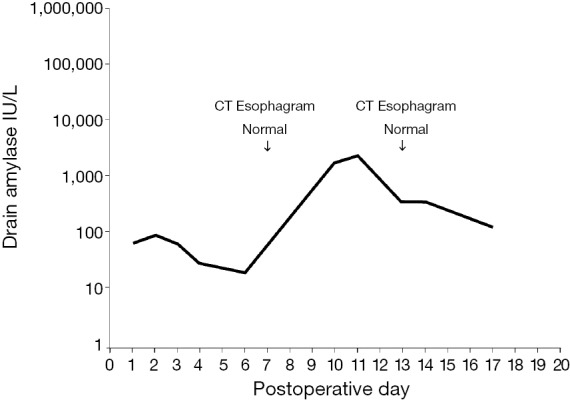
A 58-year-old woman (patient #61) with adenocarcinoma of the gastroesophageal junction treated with preoperative carboplatin, paclitaxel, and radiation therapy followed by minimally invasive esophagectomy. Transient elevation in drain amylase levels peaked at 2,464 IU/L on postoperative day 11 and subsequently normalized. CT esophagrams performed postoperative day 7 and postoperative day 13 were normal. Patient was discharged postoperative day 17 without clinical signs of anastomotic leak. She was treated with NPO status and jejunostomy feedings until her drain amylase declined. Note that drain amylase level appears on a semi-logarithmic scale.
Seventy patients had elevation of WBC greater than 12,000/µL, including 12 of 13 with leaks and 58 of the 87 without leaks. Elevation in WBC greater than 12,000/µL in the first 10 postoperative days was highly sensitive (0.92) for leak but was of low specificity (0.34). Among 30 patients with a WBC count less than 12,000/µL, only one patient experienced a leak.
Discussion
The significant morbidity and mortality associated with esophagectomy has prompted changes in operative techniques and postoperative patient care and surveillance (22,23). As a result, minimally-invasive esophagectomy has in many centers replaced open esophagectomy due to the significant reduction in postoperative morbidity and hospital length of stay (24,25).
An obstacle to early discharge is the timeframe of the detection of anastomotic leaks: in our study, the median time of diagnosis of a leak by CT esophagram was 9 days. With progressive decrease in length of stay after esophagectomy, a need exists for the inexpensive, safe, and early detection of anastomotic leaks to avoid the untoward consequences of an anastomotic leak which does not become evident until after discharge.
The routine care of postoperative esophagectomy patients has historically included evaluation for detection of anastomotic leak (26,27). Contrast fluoroscopic swallow (“Barium Swallow”) is limited by poor sensitivity, while CT esophagram is relatively expensive (15,18,20). The search for safer, less expensive, and more effective measures for detection of anastomotic leak has led some surgeons to routinely follow drain amylase levels after esophagectomy with cervical anastomosis (28,29). Should an anastomotic leak occur, amylase from gastrointestinal secretions would collect in the drainage catheters, indicating presence of a leak.
The problematic clinical situations are late leaks. Two possibilities exist: (I) lake leaks could be clinically occult leaks which do not become manifest until late in the postoperative period; or (II) late leaks may simply occur late in the postoperative period (and would therefore defy detection in the early postoperative period). One possible example of the latter is patient #33, who had an uneventful postoperative course with normal drain amylase levels, a normal CT esophagram at postoperative day 7, and normal WBC during his hospitalization. The patient’s drain was discontinued prior to discharge on postoperative day 8. He returned on postoperative day 11 with a leak that was treated with intraluminal stent placement. One possibility is that increased oral intake upon discharge may have precipitated the leak during the early postoperative phase of healing (30). Nonetheless, there is likely a subset of patients in whom more sensitive testing would facilitate the earlier diagnosis of anastomotic leak.
The first purpose of our study was to determine whether serum WBC or drain amylase levels would improve the early detection (prior to postoperative day 10) of anastomotic leak over CT esophagram alone. Drain amylase was elevated in six patients, of whom five were confirmed to have anastomotic leaks. The sixth patient had elevation of drain amylase to 1,820 IU/L on postoperative day 10, then 2,464 on postoperative day 11, after which time the drain amylase levels declined. CT esophagram was negative and the patient developed no clinical signs of a leak.
We found that drain amylase improved the ability of CT esophagram to predict which patients would leak at any time after their surgery, as two patients with elevations in drain amylase within the first 10 days were found to have leaks that were not detected by CT esophagram until much later.
The second purpose of our study was to determine whether we could identify a subset of patients with a low enough risk of leak that they could forgo CT esophagram, with the goal of reducing costs and radiation exposure.
All patients with anastomotic leak had an elevation of the WBC greater than 12,000/µL. At the same time, WBC elevation was found in a majority of patients at some point during the early postoperative period, making the elevation of WBC sensitive but of limited specificity. Among 30 patients (out of 100 in our study cohort) with normal WBC, only 1 developed an anastomotic leak, which was patient #33 described above.
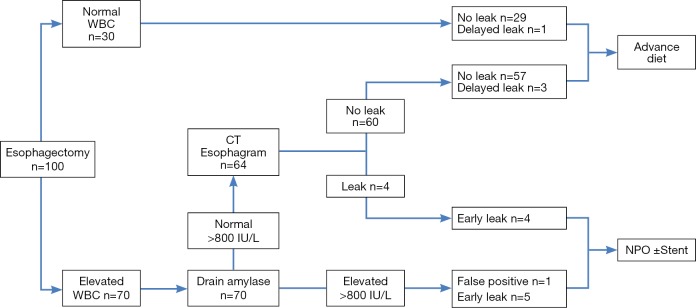
A proposed pathway for detection of anastomotic leaks is shown in Figure 3, which also shows the outcomes of our study cohort. Patients with normal WBC <12,000 µL (30% of patients) have a low risk of anastomotic leak (3%) and can be treated without the need for CT esophagram. Patients with elevated drain amylase after postoperative day 6 (represented by 6% of patients in our series) are considered to have a leak and are treated conservatively (NPO with jejunostomy feedings) until the drain amylase has normalized and CT esophagram confirms healing of the leak. In our cohort, this group included three patients diagnosed simultaneously by CT esophagram and drain amylase, two patients whose diagnosis was made earlier by drain amylase than by CT esophagram and one false positive. Patients with elevated WBC and normal drain amylase (64% of patients) would undergo CT esophagram, of whom seven (11% of this group) would be positive for leak. Four of the seven leaks in this group would be detected within the first 10 days by CT esophagram, and three would be expected to be late leaks and diagnosed subsequently. Using this pathway, initial CT esophagram could be safely eliminated in 36% of patients.
Figure 3.
Proposed diagnostic pathway for postoperative evaluation for anastomotic leak and outcomes after esophagectomy, based upon the study population. Elevated WBC is defined as exceeding 12,000/µL and elevated drain amylase as exceeding 800 IU/L. WBC, white blood count.
Conclusions
Drain amylase is a simple and inexpensive test that appears to have utility for the early detection of anastomotic leak after transthoracic esophagectomy. In the patient with normal drain amylase levels and normal WBC, CT esophagram is unlikely to detect a leak and can therefore be avoided, sparing both cost and radiation exposure.
Acknowledgements
The editorial assistance of Jennifer Barnes is gratefully acknowledged.
Footnotes
Conflicts of Interest: A portion of this work was presented as a poster presentation at the Society of Surgical Oncology 2013 and ASCO GI 2013.
References
- 1.Rodgers M, Jobe BA, O'Rourke RW, et al. Case volume as a predictor of inpatient mortality after esophagectomy. Arch Surg Sep 2007;142:829-39. [DOI] [PubMed] [Google Scholar]
- 2.Dikken JL, Dassen AE, Lemmens VE, et al. Effect of hospital volume on postoperative mortality and survival after oesophageal and gastric cancer surgery in the Netherlands between 1989 and 2009. Eur J Cancer 2012;48:1004-13. [DOI] [PubMed] [Google Scholar]
- 3.Low DE, Kunz S, Schembre D, et al. Esophagectomy--it’s not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg 2007;11:1395-402; discussion 1402. [DOI] [PubMed] [Google Scholar]
- 4.Khithani A, Jay J, Galanopoulos C, et al. Zero leaks with minimally invasive esophagectomy: a team-based approach. JSLS 2009;13:542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutegård M, Lagergren P, Rouvelas I, et al. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol 2012;19:99-103. [DOI] [PubMed] [Google Scholar]
- 6.Lagarde SM, de Boer JD, ten Kate FJ, et al. Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg 2008;247:71-6. [DOI] [PubMed] [Google Scholar]
- 7.Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 2009;250:798-807. [DOI] [PubMed] [Google Scholar]
- 8.Sierzega M, Kolodziejczyk P, Kulig J. Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. Br J Surg 2010;97:1035-42. [DOI] [PubMed] [Google Scholar]
- 9.Cooke DT, Lin GC, Lau CL, et al. Analysis of cervical esophagogastric anastomotic leaks after transhiatal esophagectomy: risk factors, presentation, and detection. Ann Thorac Surg 2009;88:177-84; discussion 184-5. [DOI] [PubMed] [Google Scholar]
- 10.Findlay JM, Tilson RC, Harikrishnan A, et al. Attempted validation of the NUn score and inflammatory markers as predictors of esophageal anastomotic leak and major complications. Dis Esophagus 2015;28:626-33. [DOI] [PubMed] [Google Scholar]
- 11.Cools-Lartigue J, Andalib A, Abo-Alsaud A, et al. Routine Contrast Esophagram has Minimal Impact on the Postoperative Management of Patients Undergoing Esophagectomy for Esophageal Cancer. Ann Surg Oncol 2014;21:2573-9. [DOI] [PubMed] [Google Scholar]
- 12.Doty JR, Salazar JD, Forastiere AA, et al. Postesophagectomy morbidity, mortality, and length of hospital stay after preoperative chemoradiation therapy. Ann Thorac Surg 2002;74:227-31; discussion 231. [DOI] [PubMed] [Google Scholar]
- 13.Papenfuss WA, Kukar M, Attwood K, et al. Transhiatal versus transthoracic esophagectomy for esophageal cancer: A 2005-2011 NSQIP comparison of modern multicenter results. J Surg Oncol 2014;110:298-301. [DOI] [PubMed] [Google Scholar]
- 14.Doerfer J, Meyer T, Klein P, et al. The importance of radiological controls of anastomoses after upper gastrointestinal tract surgery-a retrospective cohort study. Patient Saf Surg 2010;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauss C, Mal F, Perniceni T, et al. Computed tomography versus water-soluble contrast swallow in the detection of intrathoracic anastomotic leak complicating esophagogastrectomy (Ivor Lewis): a prospective study in 97 patients. Ann Surg 2010;251:647-51. [DOI] [PubMed] [Google Scholar]
- 16.Honing J, Pultrum BB, van der Jagt EJ, et al. Routine or on demand radiological contrast examination in the diagnosis of anastomotic leakage after esophagectomy. J Surg Oncol 2009;100:699-702. [DOI] [PubMed] [Google Scholar]
- 17.Boone J, Rinkes IB, van Leeuwen M, et al. Diagnostic value of routine aqueous contrast swallow examination after oesophagectomy for detecting leakage of the cervical oesophagogastric anastomosis. ANZ J Surg 2008;78:784-90. [DOI] [PubMed] [Google Scholar]
- 18.Hogan BA, Winter DC, Broe D, et al. Prospective trial comparing contrast swallow, computed tomography and endoscopy to identify anastomotic leak following oesophagogastric surgery. Surg Endosc 2008;22:767-71. [DOI] [PubMed] [Google Scholar]
- 19.Lantos JE, Levine MS, Rubesin SE, et al. Comparison between esophagography and chest computed tomography for evaluation of leaks after esophagectomy and gastric pull-through. J Thorac Imaging 2013;28:121-8. [DOI] [PubMed] [Google Scholar]
- 20.Upponi S, Ganeshan A, D'Costa H, et al. Radiological detection of post-oesophagectomy anastomotic leak - a comparison between multidetector CT and fluoroscopy. Br J Radiol 2008;81:545-8. [DOI] [PubMed] [Google Scholar]
- 21.Hanna EM, Norton HJ, Reames MK, et al. Minimally invasive esophagectomy in the community hospital setting. Surg Oncol Clin N Am 2011;20:521-30, ix. [DOI] [PubMed] [Google Scholar]
- 22.Hamouda AH, Forshaw MJ, Tsigritis K, et al. Perioperative outcomes after transition from conventional to minimally invasive Ivor-Lewis esophagectomy in a specialized center. Surg Endosc 2010;24:865-9. [DOI] [PubMed] [Google Scholar]
- 23.Munitiz V, Martinez-de-Haro LF, Ortiz A, et al. Effectiveness of a written clinical pathway for enhanced recovery after transthoracic (Ivor Lewis) oesophagectomy. Br J Surg 2010;97:714-8. [DOI] [PubMed] [Google Scholar]
- 24.Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [DOI] [PubMed] [Google Scholar]
- 25.Levy RM, Trivedi D, Luketich JD. Minimally invasive esophagectomy. Surg Clin North Am 2012;92:1265-85. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen NT, Rudersdorf PD, Smith BR, et al. Management of gastrointestinal leaks after minimally invasive esophagectomy: conventional treatments vs. endoscopic stenting. J Gastrointest Surg 2011;15:1952-60. [DOI] [PubMed] [Google Scholar]
- 27.Price TN, Nichols FC, Harmsen WS, et al. A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg 2013;95:1154-60; discussion 1160-1. [DOI] [PubMed] [Google Scholar]
- 28.Machens A, Busch C, Bause H, et al. Gastric tonometry and drain amylase analysis in the detection of cervical oesophagogastric leakage. Br J Surg 1996;83:1614-5. [DOI] [PubMed] [Google Scholar]
- 29.Pines G, Klein Y, Melzer E, et al. One hundred transhiatal esophagectomies: a single-institution experience. Isr Med Assoc J 2011;13:428-33. [PubMed] [Google Scholar]
- 30.Bolton JS, Conway WC, Abbas AE. Planned delay of oral intake after esophagectomy reduces the cervical anastomotic leak rate and hospital length of stay. J Gastrointest Surg 2014;18:304-9. [DOI] [PubMed] [Google Scholar]