Abstract
Background
Neoadjuvant chemoradiation (NCRT) has become standard in the treatment of locally advanced esophageal adenocarcinoma (EAC) with survival correlated to degree of pathologic response. The phosphatidyl inositol 3 kinase (PI3K)/protein kinase B (AKT)/mTOR pathway plays an important role in tumorgenesis and resistance. We sought to elucidate the role of this pathway in patients with EAC who received NCRT.
Methods
After IRB approval, a prospective trial was initiated in which patients with EAC underwent endoscopic biopsies of normal and tumor tissue prior to instituting NCRT. Patients then proceeded to esophagectomy. The pre-treatment tissues underwent gene expression profiling. SAM method was used to analyze expression of AKT within normal and tumor tissue. Expression was then correlated to degree of pathologic response.
Results
One-hundred patients were consented for the study, of which 67 met final eligibility. Nineteen patient’s tumors ultimately underwent gene expression profiling via microarray. The differential expression of all AKT isoforms in tumor tissue was markedly overexpressed compared to normal tissue (P=6×10−5). There were 3 patients designated as pNR, 6 as pPR, and 10 as pCR. Partial and non-responders had higher expressions of AKT compared to pCR with the non-responders consistently illustrated the highest expression of AKT (P=0.02). There was a significant correlation between individual isoforms of AKT-1, AKT-2, and AKT-3 and degree of pathologic response (P=0.002, 0.04, and 0.04 respectively).
Conclusions
AKT is overexpressed in patients with AC of the esophagus. Moreover, pathologic response to NCRT may be correlated with degree of AKT expression. Additional data is needed to clarify this relationship to potentially add targeted therapies to the neoadjuvant regimen.
Keywords: AKT expression, esophageal cancer, neoadjuvant therapy, esophagectomy, postoperative outcomes
Introduction
It is estimated that in the United States there were 17,990 new cases of esophageal cancer with approximately 15,210 deaths of disease in 2013 (1). Worldwide esophageal cancer is the 5th leading cause of cancer death in males and the 7th in female population combining for 400,000 deaths (2). The 5-year survival for all stages is low and estimated to be 16%; however, for localized disease in the esophagus the survival may reach 37% (3,4). While surgical resection has remained the mainstay of treatment for esophageal cancer, outcomes of patients treated with surgery alone have been dismal (5-7).
The role of neoadjuvant chemotherapy with or without the addition of radiation for the treatment of localized esophageal cancer has been investigated in an attempt to improve oncologic outcomes (8-17). While there have been some which demonstrated increased rates of complete pathologic response along with improved overall survival (OS) (18), these results have not been generally reproducible; possibly related to heterogenic patient populations and limited power to demonstrate differences in survival (17,19,20). Neoadjuvant therapy with chemoradiation (NCRT) has the potential to significantly downstage esophageal cancers and thus increase complete resection (R0) rates even in the setting of locally advanced disease (4,21-25). However, despite excellent OS in patients with pCR, the benefit of NCRT in patients whose tumors show pathologic non response remains unclear. Given the potential morbidity, delay in surgical resection, and costs associated with NCRT, it is critical to identify subpopulations of patients who may or may not benefit from such treatment. Conversely identifying those who would have a complete pathologic response may eliminate the necessity for an operation altogether.
The phosphatidyl inositol 3 kinase (PI3K)/protein kinase B (AKT)/mTOR signaling pathway plays an important role in regulating tumor cellular apoptosis, protein translation, and survival (26). PI3K is activated by receptor tyrosine kinases, and activation of these tyrosine kinases leads to allosteric joining to the cellular membrane and subsequent tyrosine phosphorylation of the regulatory subunit of PI3K. PI3K converts phosphatidyl inositol 2 phosphate (PIP2) to phosphatidyl inositol 3 phosphate (PIP3) (26,27). AKT is activated by phosphorylation at Thr308 by PIP3 and at Ser473 by mammalian target of rapamycin (mTOR) as a part of the mTOR complex (mTORC) (27). The phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a well-described negative regulator of the PI3K/AKT signaling pathway, which functions as a tumor suppressor gene by induction of G1 phase cell cycle arrest through decreasing the levels of cyclin D1 (28). Patients with high expression of activated (phosphorylated) AKT are reported to be resistant to radiation therapy (29). The purpose of this study was to examine the differential expression of AKT in patients with esophageal cancer and to correlate this expression with response to neoadjuvant chemotherapy and radiation.
Methods
Patient selection and specimen collection
After IRB approval, a prospective trial was initiated in which patients with locally advanced esophageal adenocarcinoma (EAC) requiring NCRT were consented for endoscopic biopsies of normal and tumor tissue prior to instituting therapy. All patients were staged with seemingly operable (non-metastatic) esophageal AC via endoscopic ultrasound. All patients had greater than stage II disease (T2N0 or greater according to AJCC standards), which required NCRT. Endoscopic biopsy specimens were obtained from the 19 patients with esophageal AC prior to the initiation of neoadjuvant therapy. In each patient, approximately five biopsy specimens were taken from the tumor and five from the surrounding non-cancerous epithelium. Specimens were frozen immediately at −70 °C for RNA extraction. Extracts of the RNA obtained from each tumor specimen were maintained separately for each patient, while the RNA extracted from the ‘normal’ esophageal mucosa was pooled for each patient in order to serve as a reference probe (control) for comparison with the cancer.
Neoadjuvant therapy and pathologic response
Patients underwent neoadjuvant chemotherapy with infusional 5-FU and two courses of cisplatin (on day 1 and 28) concomitantly with external beam radiation to a total dose of 50.4 Gy. Patients were treated for a period of 6 weeks, and then underwent re-staging. Patients deemed non-metastatic and medically fit for surgery then underwent esophagectomy. Based upon pathologic review of the resected specimen, patients were categorized into one of three groups: pathologic complete response (pCR) was defined as no residual tumor, partial pathologic response (pPR) as a 50% reduction in tumor size or nodal down-staging, and non-response (pNR) as no difference between pre-operative and post-operative stage based upon endoscopic ultrasound. Board certified pathologists with specialization in gastrointestinal malignancies reviewed all specimens and determined the final pathologic response.
Gene expression
The tissues underwent gene expression profiling using the Affymetrix 133 plus 2.0 Gene chip. Significance analysis of microarrays (SAM) method was used to analyze significant differentially expression of AKT within normal and tumor tissue. Differential expression of AKT, and individual isoforms AKT-1, AKT-2, and AKT-3 were investigated. Normal tissue expression was compared to tumor tissue expression of AKT and individual isoforms. Correlation of gene expression of AKT and pathologic response was also performed. Pathologic complete responders were compared to those with less than a complete response (pPR and pNR).
Statistical analysis
Tumor and pooled normal tissue samples were matched for each patient. A mean difference of expression was calculated. The SAM (15) was used to analyze gene expression data. SAM has been widely used for microarray data analysis with adjustment for multiple simultaneously testing. SAM can be used for a variety of statistical tests, such as paired t-test, two-sample t-test, ANOVA, and survival analysis. In this study, we used the two-sample t-test setting in identifying differentially expressed genes.
Results
Demographics
One hundred patients were consented for participation in this study, of which 67 met final eligibility criteria. Nineteen patients with adenocarcinoma had tissue specimens that were analyzed via microarray (5 tumors and 5 normal tissues) for each patient. Patient characteristics are reported in Table 1. Seventeen males (89.5%) and 2 (10.5%) females comprised the patient population with a mean age of 66.3±9.7 years. There were 3 (15.8%) non-responders, 6 (31.6%) partial responders, and 10 (52.6%) complete responders. T-stage was known in twelve (63.2%) patients and were T2 or higher. N-stage was also known in 12 patients (63.2%). In the patients with known nodal stage, 11 (92%) were N1, and 1 (7%) was N0. Seven (36.8%) did not undergo endoscopic ultrasound but all had what was deemed locally advanced tumors due to size or clinical symptoms. Five of these patients had a complete pathologic response, and 2 had significant response to therapy but had residual disease and were deemed as partial responders.
Table 1. Patient characteristics.
| Variables | Data (n, %) |
|---|---|
| Sex | |
| Male | 17 (89.5) |
| Female | 2 (10.5) |
| Age | 66.3±9.7 |
| Clinical T stage | |
| T2 | 1 (5.3) |
| T3 | 9 (47.4) |
| T4 | 2 (10.5) |
| Unknown | 7 (36.8) |
| Clinical N stage | |
| N0 | 1 (5.3) |
| N1 | 11 (57.9) |
| Unknown | 7 (36.8) |
| Response to NCRT | |
| Non | 3 (15.8) |
| Partial | 6 (31.6) |
| Complete | 10 (52.6) |
NCRT, neoadjuvant chemoradiation.
AKT expression in tumor vs. normal tissue
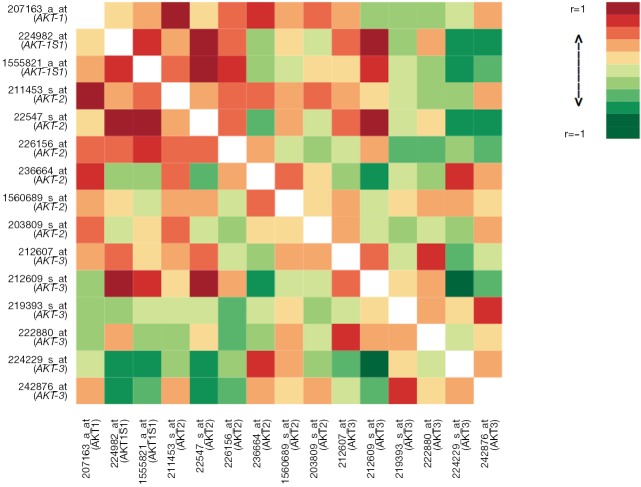
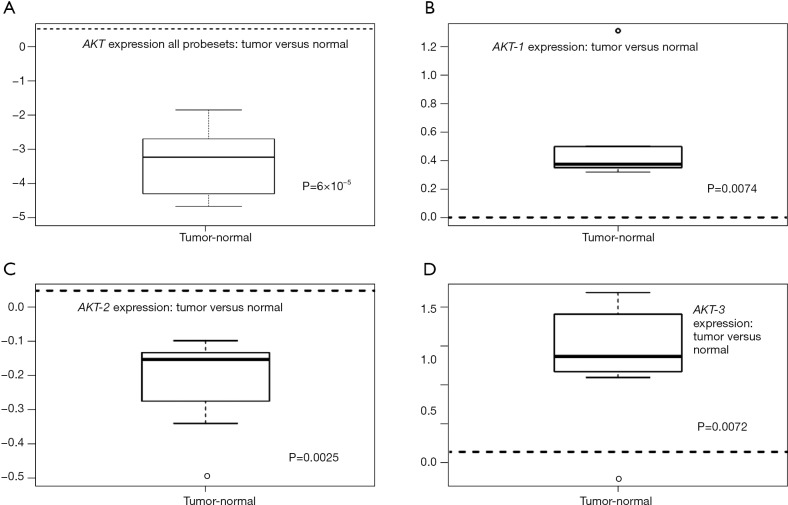
Differential gene expression analysis was used to compare overall AKT and individual isoform expression of AKT-1, AKT-2, and AKT-3 in tumor and normal tissues. Figure 1 illustrates the heat map of all AKT probesets. Significant clustering of gene expression for AKT in tumor tissues was demonstrated (P=3×10−4). Differential expression of all isoforms for AKT revealed marked overexpression in tumor tissues compared to their normal tissue counterparts (Figure 2A, P=6×10−5). As illustrated in Figure 2B-D, individual analysis of AKT-1, AKT-2, and AKT-3 also revealed overexpression in tumor tissue compared to their normal tissue cohorts. There was significant overexpression of AKT-1, with a mean difference of 0.53 between tumor and normal tissues (P=0.0074). The mean differences in expression of AKT-2 between tumor and normal tissues were 0.27 (P=0.0025). Differential expression analysis also found significant differences in AKT-3 expression between the two tissue types of 1.07 (P=0.0072).
Figure 1.
Heat map of all AKT probesets (P=3×10−4).
Figure 2.
(A) AKT expression in normal vs. tumor tissues for all isoforms. AKT is overexpressed in tumor tissues (mean diff. =−3.84; P=6×10−5); (B) AKT-1 expression (mean diff. =0.53; P=0.0074); (C) AKT-2 expression (mean diff. =−0.27; P=0.0025); (D) AKT-3 expression (mean diff. =1.07; P=0.0072).
Pathologic response and AKT expression
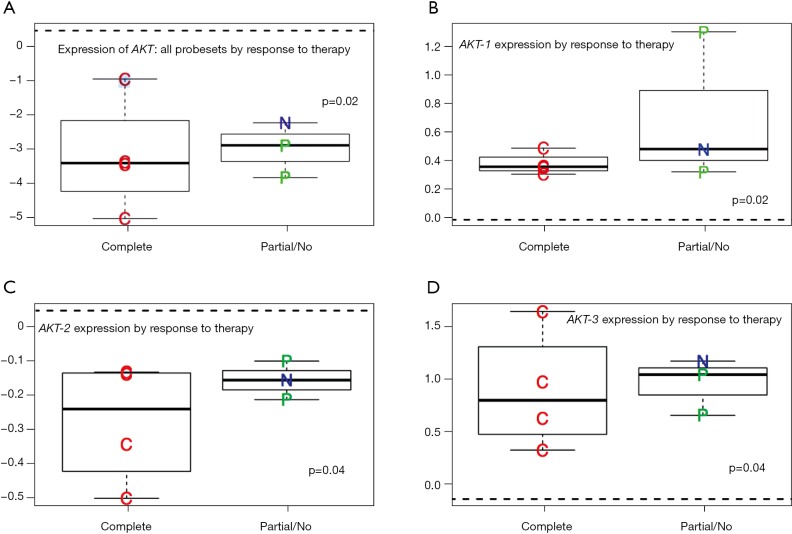
We identified a significant correlation between AKT expression and degree of pathologic response. Partial and non-responders had higher expressions of AKT compared to complete responders, with the non-responders consistently illustrating the highest expression of AKT Figure 3A. The mean difference of expression was 3.35 (P=0.02) for pCR compared to pPR/pNR. Over expression of AKT-1, AKT-2, and AKT-3 was also shown to be a predictor of pathologic response to therapy (Figure 3B-D). AKT-1 demonstrated a mean difference of expression of 0.39, P=0.002; AKT-2 demonstrated a mean difference of expression of 0.32, P=0.04, and AKT-3 demonstrated a mean difference of expression of 1.01, P=0.04.
Figure 3.
(A) AKT all isoforms expression correlated to pathologic response to NCRT (mean diff. =−3.35 for pCR vs. pPR/pNR P=0.02); (B) correlation between AKT-1 expression and pathologic response to NCRT (mean diff. =0.39; P=0.002); (C) correlation between AKT-2 expression and pathologic response to NCRT (mean diff. =0.32, P=0.04); (D) correlation between AKT-3 expression and pathologic response to NCRT (mean diff. =0.58, P=0.04). NCRT, neoadjuvant chemoradiation; pCR, pathologic complete response; pPR, partial pathologic response; pNR, pathologic non-response.
Interestingly, the expression of AKT in the tumor tissues exhibiting a pCR more closely mimicked expression of AKT in normal tissue. AKT-1 demonstrated a minimal mean difference of expression of 0.3 (P=0.1) and AKT-2 0.15 (P=0.09). Whereas differential expression of AKT-3 in pCR tumor tissues remained significantly overexpressed with a mean difference of expression of 0.7 (P=0.002) compared to normal tissues.
Discussion
We have illustrated that patients with EAC have significant overexpression of AKT in their tumor tissues compared to the normal tissue expression of AKT. Additionally, individual AKT isoforms exhibited overexpression of AKT-1 (P=0.0074), AKT-2 (P=0.0025), and AKT-3 (P=0.0072) in the tumor tissues. The correlation of the degree of expression between pathologic responses to NCRT demonstrated a linear relationship between the higher expression of AKT and decreasing degree of pathologic response. Partial and non-responders had higher expressions of AKT compared to pCR with the non-responders consistently illustrating the highest expression of AKT. Individual probesets were also found to correlate with degree of pathologic response. We demonstrated a significant overexpression of AKT-1 AKT-2, and AKT-3 in partial/non-responders versus complete responders.
The overexpression of AKT in tumor tissues and the positive correlation between AKT expression and decreasing pathological response to chemoradiation indicate the importance of the PI3K/AKT/mTOR pathway in the progression of esophageal AC and its impact on the prognosis of patients. We previously reported our series of 347 patients who underwent esophagectomy with curative intent in an attempt to assess the impact of pathologic response to neoadjuvant chemoradiation (NCRT) on OS (30). Patients with a pCR to NCRT were found to have a significantly higher 5-year disease-free survival (DFS) and OS compared to patients with partial response (pPR) and no response (pNR) (52% and 52%, respectively, compared to 36% and 38% in pPR and 22% and 19% in pNR, P<0.0001). This was consistent with other studies that have shown dramatic survival benefits in patients with complete or pPRs to NCRT (31-33).
The PI3K/AKT/mTOR pathway regulates a number of cell processes involved in tumor progression, including proliferation, invasion, and apoptosis (34). This signaling pathway is activated in malignancies and plays an important role in the development of resistance to chemotherapeutic agents (35). A significant correlation between treatment outcome in various malignancies and AKT expression has been identified by Bussink et al. (34). Rapamycin was initially considered as a promising modality for blocking mTOR phosphorylation in several cancer types; however, cancer patients with high expression AKT may have little response to mTORC1 inhibitors (36).
The PI3K/AKT/mTOR pathway also plays a significant role in the progression of a number of other malignancies (37). Chung et al. observed activated AKT expression in 84.2% of extrahe-patic cholangiocarcinoma cases. They found significant increases in p-AKT and p-mTOR expressions from normal biliary epithelial cells to infiltrating malignant neoplastic epithelial cells. Moreover there was a clear correlation between survival of patients with extra-hepatic cholangiocarcinoma and specific relative expression level of active AKT and mTOR (37).
Over-expression of AKT has also been found to be associated with the development of resistance to radiation therapy, through mechanisms including intrinsic radioresistance, tumor-cell proliferation, and hypoxia (29,34). A review on the activation of the PI3-K/AKT pathway and the implications for radioresistance mechanisms by Bussink et al. has demonstrated that pAKT expression is an independent prognostic factor for survival in patients with head and neck cancers (34). David et al. in their series of 61 patients with non-small cell lung cancer found a statistically significant decrease in survival between patients with pAKT over-expression and patients with weak expression (38). Le Page et al. investigated the correlation between the expression and localization of AKT-1, AKT-2, AKT-3, phospho-AKT proteins and the clinicopathological parameters in 63 prostate cancer specimens. They found that more than 60% of tumor tissues overexpressed AKT-1, AKT-2 or AKT-3 and that AKT-1 expression was associated with a higher risk of PSA recurrence and shorter PSA recurrence interval.
Resistance to radiation therapy is a potential explanation reason for the low survival rates and high incidence of local recurrence for esophageal cancer (39). The 5-year survival for patients with esophageal cancer is estimated at 10-20% (39). Therefore is important to identify strategies for reducing chemo and radioresistance and thereby improve the effectiveness of neoadjuvant therapy. Inhibition of the PI3K/AKT/mTOR can augment the effectiveness of radiation by blocking cellular defense mechanisms induced by radiation (34). Hildebrandt et al. found that expression of AKT1 and AKT2 were associated with an increased risk of recurrence in patients with esophageal cancer (adenocarcinoma or squamous). In addition, AKT2 was associated with a poor response to chemoradiotherapy treatment (35). In our data, we corroborated this finding that AKT-2 was associated with poor response to NCRT. However, we also demonstrated that other isoforms were also predictive of response. Since AKT plays a significant role in anti-apoptotic pathways, agents that block AKT activation could play an important role in the development of chemotherapeutic treatments that improve outcomes for patients with esophageal AC.
Le Page and colleagues evaluated the radiosensitizing effect of a COX-2 inhibitor, NS398, and its mechanism in radioresistant esophageal cancer cells. They found that NS398 blocks AKT activation and induces apoptosis in Eca109R50Gy cells and therefore increased radiosensitivity in these cell types (39,40). While the present study did not correlate survival and AKT expression, patient’s exhibiting less than a complete response to NCRT had the highest expressions of AKT. Given the poor prognosis of patient’s with a non response, it is reasonable to assume that AKT over expression may impact survival in these patients indirectly by inhibiting their potential for maximal response to therapy.
Conclusions
AKT over expression has been shown to correlate to outcomes in various malignances. In our analysis of patients with EAC, we found that AKT is overexpressed in tumor tissues compared to normal tissue AKT expression. Additionally, all isoforms of AKT exhibited over expression. Moreover, pathologic response to NCRT correlated with degree of AKT expression. Our results support the need for developing targeted agents that will decrease chemotherapy and radiation resistance and thereby increase efficacy by regulating AKT expression through the PI3K/AKT/mTOR pathway.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute, 2011. [Google Scholar]
- 4.Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 2010;17:1159-67. [DOI] [PubMed] [Google Scholar]
- 5.Earlam R, Cunha-Melo JR. Oesophageal squamous cell carcinoma: I. A critical review of surgery. Br J Surg 1980;67:381-90. [DOI] [PubMed] [Google Scholar]
- 6.Müller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1990;77:845-57. [DOI] [PubMed] [Google Scholar]
- 7.Wu PC, Posner MC, Wu PC, et al. The role of surgery in the management of oesophageal cancer. Lancet Oncol 2003;4:481-8. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds JV, Muldoon C, Hollywood D, et al. Long-term outcomes following neoadjuvant chemoradiotherapy for esophageal cancer. Ann Surg 2007;245:707-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [DOI] [PubMed] [Google Scholar]
- 10.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7. [DOI] [PubMed] [Google Scholar]
- 11.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [DOI] [PubMed] [Google Scholar]
- 12.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. [DOI] [PubMed] [Google Scholar]
- 13.Jin J, Liao Z, Zhang Z, et al. Induction chemotherapy improved outcomes of patients with resectable esophageal cancer who received chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2004;60:427-36. [DOI] [PubMed] [Google Scholar]
- 14.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979-84. [DOI] [PubMed] [Google Scholar]
- 15.Naughton P, Walsh TN, Naughton P, et al. Pre-operative chemo-radiotherapy improves 3-year survival in people with resectable oesophageal cancer. Cancer Treat Rev 2004;30:141-4. [DOI] [PubMed] [Google Scholar]
- 16.Pennathur A, Luketich JD, Landreneau RJ, et al. Long-term results of a phase II trial of neoadjuvant chemotherapy followed by esophagectomy for locally advanced esophageal neoplasm. Ann Thorac Surg 2008;85:1930-6; discussion 6-7. [DOI] [PubMed]
- 17.Walsh TN, Grennell M, Mansoor S, et al. Neoadjuvant treatment of advanced stage esophageal adenocarcinoma increases survival. Dis Esophagus 2002;15:121-4. [DOI] [PubMed] [Google Scholar]
- 18.Rohatgi P, Swisher SG, Correa AM, et al. Characterization of pathologic complete response after preoperative chemoradiotherapy in carcinoma of the esophagus and outcome after pathologic complete response. Cancer 2005;104:2365-72. [DOI] [PubMed] [Google Scholar]
- 19.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [DOI] [PubMed] [Google Scholar]
- 20.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda K, Ishida K, Sato N, et al. Chemoradiotherapy followed by surgery for thoracic esophageal cancer potentially or actually involving adjacent organs. Dis Esophagus 2001;14:197-201. [DOI] [PubMed] [Google Scholar]
- 22.Forshaw MJ, Gossage JA, Chrystal K, et al. Neoadjuvant chemotherapy for locally advanced carcinoma of the lower oesophagus and oesophago-gastric junction. Eur J Surg Oncol 2006;32:1114-8. [DOI] [PubMed] [Google Scholar]
- 23.Fréchette E, Buck DA, Kaplan BJ, et al. Esophageal cancer: outcomes of surgery, neoadjuvant chemotherapy, and three-dimension conformal radiotherapy. J Surg Oncol 2004;87:68-74. [DOI] [PubMed] [Google Scholar]
- 24.Cordin J, Lehmann K, Schneider PM. Clinical staging of adenocarcinoma of the esophagogastric junction. Recent Results Cancer Res 2010;182:73-83. [DOI] [PubMed] [Google Scholar]
- 25.Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [DOI] [PubMed] [Google Scholar]
- 26.Vivanco I, Sawyers C. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2002;2:489-501. [DOI] [PubMed] [Google Scholar]
- 27.Crowell JA, Steele VE, Fay JR. Targeting the AKT protein kinase for cancer chemoprevention. Mol Cancer Ther 2007;6:2139-48. [DOI] [PubMed] [Google Scholar]
- 28.Radu A, Neubauer V, Akagi T, et al. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol Cell Biol 2003;23:6139-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta AK, McKenna WG, Weber CN, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res 2002;8:885-92. [PubMed] [Google Scholar]
- 30.Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 2010;17:1159-67. [DOI] [PubMed] [Google Scholar]
- 31.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. [DOI] [PubMed] [Google Scholar]
- 32.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [DOI] [PubMed] [Google Scholar]
- 33.Darnton SJ, Archer VR, Stocken DD, et al. Preoperative mitomycin, ifosfamide, and cisplatin followed by esophagectomy in squamous cell carcinoma of the esophagus: pathologic complete response induced by chemotherapy leads to long-term survival. J Clin Oncol 2003;21:4009-15. [DOI] [PubMed] [Google Scholar]
- 34.Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol 2008;9:288-96. [DOI] [PubMed] [Google Scholar]
- 35.Hildebrandt MA, Yang H, Hung MC, et al. Genetic variations in the PI3K/PTEN/AKT/mTOR pathway are associated with clinical outcomes in esophageal cancer patients treated with chemoradiotherapy. J Clin Oncol 2009;27:857-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006;66:1500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung JY, Hong SM, Choi BY, et al. The Expression of Phospho-AKT, Phospho-mTOR, and PTEN in Extrahepatic Cholangiocarcinoma. Clin Cancer Res 2009;15:660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.David O, Jett J, LeBeau H, et al. Phospho-Akt Overexpression in Non–Small Cell Lung Cancer Confers Significant Stage-Independent Survival Disadvantage. Clin Cancer Res 2004;10:6865-71. [DOI] [PubMed] [Google Scholar]
- 39.Che SM, Zhang XZ, Hou L, et al. Cyclooxygenase-2 inhibitor NS398 enhances radiosensitivity of radioresistant esophageal cancer cells by inhibiting AKT activation and inducing apoptosis. Cancer Invest 2010;28:679-88. [DOI] [PubMed] [Google Scholar]
- 40.Le Page C, Koumakpayi IH, Alam-Fahmy M, et al. Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br J Cancer 2006;94:1906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]