Abstract
Background
The right hepatic artery (RHA) is the most common hepatic artery (CHA) variation. This variation may be problematic in pancreaticoduodenectomy (PD). We aimed to evaluate the impact of the RHA on postoperative and oncological outcomes.
Methods
The PubMed database was systematically searched for comparative studies reporting management of the RHA during PD for the years 1950-2014.
Results
A total of 2,278 patients were analyzed, of whom 440 (19%) had a RHA. The most CHA variation was a replaced RHA. The conservative approach was the most frequently adopted (87%) and only 8% of patients had a sacrifice without reconstruction of the RHA. Postoperative mortality and overall morbidity were similar between patients with and without RHA. Despite the preservation of the RHA in most cases, the rates of microscopic positive margin were also comparable between two groups with no impact of RHA on survival rates.
Conclusions
Postoperative and oncological outcomes seemed unaffected by the RHA in PD. Prospective studies are needed to evaluate its oncological impact.
Keywords: Pancreatoduodenectomy (PD), right hepatic artery (RHA), hepatic artery variation, aberrant artery
Introduction
The most common hepatic artery (CHA) variation is the right hepatic artery (RHA) which originates from the superior mesenteric artery (SMA). This variation is frequently observed (1,2) and may be problematic in pancreaticoduodenectomy (PD) (3) due to its course near the vascular margin, especially the SMA margin. In some cases, a pancreatic head carcinoma can invade the RHA and require its resection with or without reconstruction. The aim of this resection is to decrease the rates of microscopic margin involvement (R1). However, any intraoperative damage of the RHA can lead to bile duct or liver ischaemia, entailing a risk of anastomotic leakage at the site of the pancreaticojejunostomy, liver abscesses and patient death. Indeed, most of the blood supply to the remnant bile ducts is derived from the replaced or accessory vessel following ligation of the gastroduodenal artery (GDA) during PD. In this particular situation, the challenge is to achieve a curative resection without compromising the biliary vascularization.
On the other hand, not all variations of the RHA are likely to affect the course of PD. For instance, resection without reconstruction of an accessory RHA (i.e., in contrast with replaced RHA) may be safe (4). Moreover, in some cases, the periampullary tumor is distant from the RHA allowing adopting a more conservative approach. As a whole, preoperative identification of a hepatic artery variation and its relationship with the tumor is mandatory to avoid intraoperative vascular injury and subsequent complications after PD (5,6).
This literature review was carried out in order to evaluate the impact of the RHA on the postoperative and oncological outcomes, considering the different types of RHA and their respective intraoperative management. For this purpose, only comparative studies reporting on PD in patients with versus without RHA were considered.
Methods and materials
The PubMed database was systematically searched for comparative studies reporting management of the RHA during PD for the years 1950 to 2014. The following keywords were used: “pancreatoduodenectomy”, “right hepatic artery”, “hepatic artery variation”, “aberrant artery”. Articles were considered for inclusion when they reported the management of the RHA in patients undergoing PD for malignant or benign periampullary conditions. Studies were included provided that they compared patients according to the presence or not of a RHA and gave enough details on the postoperative and/or oncological outcomes after PD.
Exclusion criteria
Were excluded from this review studies, not focusing on the surgical management of the RHA (other vascular abnormalities, other types of pancreatic resection), studies written in languages other than English, case reports, reviews, guidelines, letters to the editor, and abstracts without available full text, studies with no comparison between groups.
Endpoints studied and definitions
Our aim was to compare postoperative and oncological outcomes between patients with RHA (RHA group) and patients without RHA (no RHA group).
The primary endpoints were the intraoperative management of the RHA, postoperative morbi-mortality, oncological status and outcome. The secondary endpoints were the intraoperative outcomes [duration of surgery (min), and blood loss (mL)], the incidence of postoperative pancreatic fistula (POPF), delayed gastric emptying (DGE), hemorrhage, and length of hospital stay (LOS).
Quantitative data were expressed as mean ± standard deviation, and categorical data as frequencies and percentages.
In order to classify the type of hepatic artery variations, we chose the International Classification of Michels, commonly used (7). Michels described ten anatomic variations of hepatic artery observed while he was conducting 200 autopsies. The second most widely-accepted classification was that of Hiatt et al. (8), based on 1,000 angiographic analyses. For studies using no classification, we posteriori classified the hepatic artery variations according to the Michels classification provided that precise anatomical description of the variation had been made by the authors. We focused on the most common variations of the RHA and those potentially impacting PD outcome, namely types III, IV, VI and IX of Michels classification or the corresponding Hiatt types, as summarized in Table 1. Type IX (replaced CHA) represents patients with CHA arising from SMA and crossing the pancreatic head. These patients were included because the replaced CHA is assimilated to the RHA for their travel near periampullary tumors.
Table 1. Classification of hepatic artery variations according to Michels and Hiatt.
| Description | Michels type | Hiatt type |
|---|---|---|
| Normal anatomy | I | I |
| Replaced RHA from SMA | III | III |
| Replaced RHA and LHA | IV | IV |
| Accessory RHA | VI | III |
| CHA from SMA | IX | V |
RHA, right hepatic artery; SMA, superior mesenteric artery; LHA, left hepatic artery; CHA, common hepatic artery.
Results
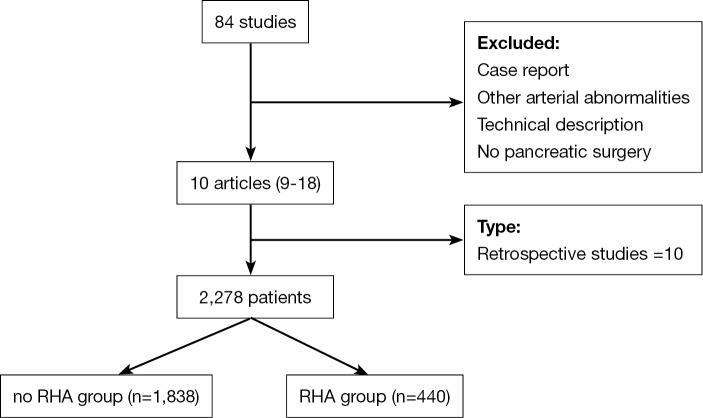
A search of the literature identified a total of 84 articles. Seventy-four articles were excluded because they were not meeting inclusion criteria. The flowchart of the study is given in Figure 1. Ten studies were finally found to be suitable for this review (9-18). All studies had been published in 2009 or later and were retrospective. Nine studies were monocentric and only one was multicentric. A total of 2,278 patients were analyzed, of whom 440 (19%) had a RHA (Table 2). The most CHA variation was a replaced RHA arising from the SMA (Michels III) that was reported in 285 patients (65.2%). This has been reported in 38% to 94% of patients with RHA variation. Replaced RHA and LHA (Michels IV), accessory RHA (Michels VI) and CHA arising from SMA (Michels IX) were rare (Table 3). Seven studies only included patients with malignant tumors (85% of patients) (10,12,14-18) and three (10% of patients) (9,11,13) with benign tumors or conditions. The periampullary carcinoma was the most frequent tumor in patients. The following malignant tumors were reported by authors: pancreatic head carcinoma (eight articles) (9,11,13-18), distal common bile duct carcinoma (five articles) (10,11,13,16,17), duodenal carcinoma (two articles) (11,17), ampullary carcinoma (11,13,16,17). In all studies, there was no difference between RHA and noRHA groups regarding the indications for PD.
Figure 1.
Table 2. Summarized of selected series.
| Author | Year | Design | Patient, n | RHA, n [%] | Surgery |
|---|---|---|---|---|---|
| Stauffer et al. (9) | 2009 | Retrospective | 191 | 31 [16] | PD |
| Lee et al. (10) | 2009 | Retrospective | 103 | 15 [15] | PD |
| Eschuis et al. (11) | 2010 | Retrospective | 758 | 143 [19] | PD |
| Perwaiz et al. (12) | 2010 | Retrospective | 200 | 39 [19] | PD |
| Jah et al. (13) | 2009 | Retrospective | 135 | 28 [20] | PD |
| Turrini et al. (14) | 2010 | Retrospective | 78 | 47 [60] | PD |
| Kim et al. (15) | 2013 | Retrospective | 289 | 40 [13] | PD |
| Sulpice et al. (16) | 2013 | Retrospective | 84 | 29 [34] | PD |
| Rammohan et al. (17) | 2014 | Retrospective | 260 | 43 [16] | PD |
| Okada et al. (18) | 2014 | Retrospective | 180 | 25 [14] | PD |
| Total | – | – | 2,278 | 440 | – |
RHA, right hepatic artery; PD, pancreaticoduodenectomy.
Table 3. Indications of PD in selected articles and types of aberrant RHA according to Michels classification.
| Author | Year | Indications of PD, n [%] |
Type of aberrant RHA, n [%] |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Malignant tumors |
Benign tumors |
Michels III | Michels IV | Michels VI | Michels IX | Others | |||||
| RHA | No RHA | RHA | No RHA | ||||||||
| Stauffer et al. (9) | 2009 | 28 [90] | – | 3 [10] | – | 23 [74] | 0 | 6 [20] | 1 [3] | 1 [3] | |
| Lee et al. (10) | 2009 | 15 [100] | 88 [100] | 0 | 0 | 12 [80] | 0 | 0 | 0 | 3 [20] | |
| Eschuis et al. (11) | 2010 | 119 [83] | 508 [83] | 19 [13] | 96 [16] | 60 [42] | 4 [3] | 22 [15] | 7 [5] | 50 [35] | |
| Perwaiz et al. (12) | 2010 | 39 [100] | 147 [100] | 0 | 0 | 29 [74] | 9 [23] | 0 | 1 [3] | 0 | |
| Jah et al. (13) | 2009 | 26 [93] | 2 [7] | 95 [89] | 12 [11] | 25 [89] | 0 | 0 | 3 [11] | 0 | |
| Turrini et al. (14) | 2010 | 47 [100] | 3 [100] | 0 | 0 | 44 [94] | 0 | 2 [4] | 1 [2] | 0 | |
| Kim et al. (15) | 2013 | 37 [100] | 212 [100] | 0 | 0 | 28 [76] | 2 [5] | 3 [9] | 2 [5] | 2 [5] | |
| Sulpice et al. (16) | 2013 | 29 [100] | 55 [100] | 0 | 0 | 11 [38] | 18 [62] | 0 | 0 | 0 | |
| Rammohan et al. (17) | 2014 | 43 [100] | 182 [100] | 0 | 0 | 31 [72] | 0 | 10 [24] | 1 [2] | 1 [2] | |
| Okada et al. (18) | 2014 | 25 [100] | 155 [100] | 0 | 0 | 22 [88] | 0 | 0 | 2 [8] | 1 [4] | |
| Total | 408 | 1,352 | 117 | 108 | 285 | 33 | 43 | 18 | 58 | ||
PD, pancreaticoduodenectomy; RHA, right hepatic artery.
Management of the RHA and intraoperative outcome
Of 440 patients with a RHA, the conservative approach was the most frequently adopted as used in 346 (87%) of patients undergoing PD (Table 4). In all cases, the RHA was reported crossing away from the tumor without any abutment or invasion of the arterial wall. Among the remaining patients, 31 (8%) had a sacrifice without reconstruction of the RHA for oncological or technical indications. Among these cases, there were 6 accessory RHA, 4 small caliber RHA, while no anatomical description was given in the 21 remaining patients with sacrificed RHA. In four series (11,15,17), the RHA was sacrificed after a clamping test with checking of normal arterial perfusion in the right hemiliver using intraoperative Doppler ultrasonography.
Table 4. Intraoperative management of the RHA in selected articles.
| Author | Dissection + preservation, n [%] | Sacrificed, n [%] | Resection + reconstruction or anastomosis, n [%] | Reconstruction, n [%] | Preoperative embolization, n [%] |
|---|---|---|---|---|---|
| Stauffer et al. (9) | 24 [77] | 0 | 7 [23] | 0 | 0 |
| Lee et al. (10) | 15 [100] | 0 | 0 | 0 | 0 |
| Eschuis et al. (11) | 130 [91] | 10 [7] | 3 [2] | 0 | 0 |
| Perwaiz et al. (12) | − | − | − | − | − |
| Jah et al. (13) | 25 [89] | 2 [7] | 1 [4] | 0 | 0 |
| Turrini et al. (14) | 44 [94] | 1 [2] | 2 [4] | 0 | 0 |
| Kim et al. (15) | 32 [86] | 3 [8] | 1 [3] | 1 [3] | 0 |
| Sulpice et al. (16) | 23 [79] | 7 [14] | 2 [7] | 0 | 0 |
| Rammohan et al. (17) | 34 [79] | 8 [19] | 1 [2] | 0 | 0 |
| Okada et al. (18) | 19 [76] | 0 | 0 | 0 | 6 [24] |
| Total | 346 [87] | 31 [8] | 17 [4] | 1 [0] | 6 [1] |
RHA, right hepatic artery.
The RHA was transected and reconstructed by primary anastomosis or by using polytetrafluoroethylene prosthesis or vein graft in the 17 remaining patients. In these series, the RHA was transected and reconstructed by anastomosing the divided RHA to the GDA stump (9,13,15). Okada et al. (18) described preoperative coil embolization for six patients with RHA encasement, with no cases of hepatic infarction or liver abscess, allowing secondarily PD combined with RHA resection. Postembolization CT demonstrated the development of a collateral system arising from the left branch and revascularizing the right liver lobe.
Regarding the intraoperative outcome, the patients with RHA had in general a prolonged operative time and increased blood losses: the mean duration of surgery ranged from 299 to 480 minutes in the RHA group compared to 300 to 439 minutes in no RHA group, and the mean blood losses from 390 to 1,400 mL compared to 360 to 1,200 mL, respectively (Table 5). With a few exceptions, no significant difference was reported in these series.
Table 5. Details of intra and postoperative outcomes of patients undergoing PD.
| Author | Mortality, n [%] |
Morbidity, n [%] |
Blood loss (mL)* |
Hemorrhage, n [%] |
Fistula, n [%] |
DGE, n [%] |
Length of hospital stay** |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RHA | No RHA | P | RHA | No RHA | P | RHA | No RHA | P | RHA | No RHA | P | RHA | No RHA | P | RHA | No RHA | P | RHA | No RHA | P | |||||||
| Stauffer et al. (9) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||||||
| Lee et al. (10) | 0 | 0 | – | 3 [20] | 10 [11] | ns | – | – | – | – | – | – | 0 | 4 | – | 1 | 0 | – | 0 | 1 | – | ||||||
| Eschuis et al. (11) | 2 [1] | 13 [2] | 0.58 | 80 [56] | 303 [49] | 0.15 | 299 | 300 | 0.96 | 1,100 | 1,050 | 0.88 | 11 [8] | 44 [7] | 0.82 | 18 [12] | 87 [14] | 0.63 | 48 [33] | 193 [31] | 0.61 | ||||||
| Perwaiz et al. (12) | 1 [2] | 3 [2] | 1 | 16 [30] | 43 [30] | 1 | 414±37 | 370±38 | <0.001 | 450±54 | 415±61 | <0.001 | 1 [2] | 2 [2] | 1 | 5 [13] | 12 [8] | 0.77 | 3 [8] | 11 [7] | 0.76 | ||||||
| Jah et al. (13) | 0 | 2 [2] | ns | 9 [32] | 31 [29] | ns | 400 | 400 | 0.58 | 1,400 | 1,200 | 0.27 | – | – | – | 5 [19] | 16 [15] | 0.43 | 4 [14] | 15 [14] | 0.47 | ||||||
| Turrini et al. (14) | 1 [2] | 1 [3] | ns | 17 [36] | 11 [35] | ns | 361 | 310 | ns | 573 | 697 | ns | – | – | – | – | – | – | – | – | – | ||||||
| Sulpice et al. (16) | 0 | 1 [0.5] | 1 | 9 [24] | 88 [42] | 0.04 | 479±85 | 439±128 | 0.05 | 950 | 650 | 0.5 | 1 [3] | 16 [7] | 0.5 | 1 [3] | 13 [6] | 0.7 | 4 [11] | 16 [7] | 0.5 | ||||||
| Ram et al. (17) | 3 [10] | 5 [9] | 0.44 | 14 [48] | 32 [58] | 0.52 | 334±84 | 341±106 | 0.98 | – | – | – | 4 [8] | 9 [16] | 1 | 3 [10] | 9 [16] | 0.53 | 10 [25] | 24 [46] | 0.56 | ||||||
| Okada et al. (18) | 1 [2] | 3 2] | ns | 26 [60] | 111 [61] | ns | 480±44 | 420±45 | <0.05 | 390±45 | 360±52 | ns | 1 [2] | 4 [2] | ns | 2 [5] | 9 [5] | ns | 23 [5] | 98 [5] | ns | ||||||
*, mean; **, median. PD, pancreaticoduodenectomy; RHA, right hepatic artery; DGE, delayed gastric emptying; ns, not significant.
Postoperative outcome
Overall, 9 of 10 studies provided details on intra- and/or postoperative outcomes of patients after PD in both RHA and no RHA groups. Postoperative mortality was defined as death occurring during the same hospital stay or within 30 days after surgery in four articles (12,13,16,18), not specified in five articles (10,11,14,15,17) and not studies in one of them (9). The postoperative morbidity rate included all complications following surgery until discharge. These complications were classified according to the Clavien-Dindo classification (19) in only two studies (15,18). The POPF, DGE, intra- and extra-abdominal bleeding was defined according to the International Study Group of Pancreatic Surgery (ISGPS) criteria (20-22) in only four series (11,15,16,18). In the other series, the definition of postoperative complications was not specified.
No statistical difference in the overall morbidity was observed in most series (Table 5), with the exception of Kim et al who reported an unexpected higher rate of complications in no RHA group (41.5% vs. 24.3% in RHA group, P=0.04) (15). Postoperative death occurred in 0-10% in RHA group compared to 0-9% in no RHA group, with no statistical difference between groups.
The incidence of POPF, postoperative hemorrhage, and DGE was respectively 15%, 9% and 39% in the RHA group and 10%, 6% and 22% in the no RHA group although the difference did not reach statistical significance. Kim et al. specifically compared short-term outcomes after PD in patients with ligated or damaged RHA versus patients with no RHA (15). There were no differences between groups in terms of surgical complications, hospital mortality, re-laparotomy rate or LOS.
Oncological outcome
The impact of the RHA on oncological outcomes was evaluated in eight series (Table 6). The presence of the RHA did not seem to influence the oncologic quality resection as indicated by the resection margins. Twenty four percent of patients (n=65) with RHA had positive margins (R1) compared to fourteen percent (n=152) of patients in no RHA group. Survival was investigated in six series. In overall survival, no differences were reported between RHA and noRHA groups.
Table 6. Microscopic positive margin (R1) in RHA and no RHA groups.
| Author | R1 |
||
|---|---|---|---|
| RHA, n (%) | No RHA, n (%) | P | |
| Stauffer et al. (9) | – | – | – |
| Lee et al. (10) | 2 [20] | 22 [25] | 1 |
| Eschuis et al. (11) | – | – | – |
| Perwaiz et al. (12) | 2 [5] | 11 [7.5] | ns |
| Jah et al. (13) | 12 [40.8] | 53 [42.6] | ns |
| Turrini et al. (14) | 8 [17] | 5 [16] | ns |
| Kim et al. (15) | 4 [11] | 34 [16] | 1 |
| Sulpice et al. (16) | 4 [14] | 6 [11] | ns |
| Rammohan et al. (17) | 3 [7] | 13 [7] | ns |
| Okada et al. (18) | 30 [19] | 8 [32] | ns |
RHA, right hepatic artery; ns, not significant.
Discussion
In this review, we aimed to overview all studies reporting the management of the RHA in PD in order to evaluate the impact of this variation on postoperative and oncological outcomes. PD remains a complex procedure that is associated with high morbidity and mortality rates, except when it is performed in expert centers (23). The presence of an aberrant hepatic artery may expose to a risk of injury of the hepatic arterial supply and subsequently to severe hepatic and/or biliary ischemia. Additionally, the close dissection of the RHA during PD increases the risk of pseudoaneurysm, especially in the presence of pancreaticojejunostomy leakage, while its preservation exposes to high rate of microscopic positive margin. Although most studies have not found any difference in postoperative and oncological outcomes, the current review shows that the real impact of the RHA remains to be determined.
As shown in this literature review, it is only recently that surgeons were interested in this issue, with no study published before 2009 despite high incidence of RHA (nearly one fifth of patients in this review). In parallel, over the same period, some authors first demonstrated that the standardization of histological study resulted in a significant increase in R1 resection rates, in particular at the site of the vascular (portal vein-SMV margin plus SMA) margin. Studies were included in the present review provided that a comparative analysis of the postoperative and/or oncological outcomes was made between patients with versus without RHA who had PD. PD was mostly performed for malignant periampullary tumors; mainly pancreatic adenocarcinoma. Analysis for each article showed that the management of the RHA differed from center to another. The conservative approach was mostly used and the RHA was sacrificed in only 8% of patients. Preoperative embolization to increase liver blood flow through left hepatic artery has been described in six patients with tumor encasement of the RHA (18). This approach was safe and all patients could undergo PD after embolization. Other authors reported similarly hepatic artery embolization prior to PD, which they advocated as safe and effective (24-26). Regarding the postoperative outcome, the present study showed that postoperative mortality and overall morbidity were similar between patients with and without RHA. When focusing on pancreas-related morbidity, there was no significant difference in the incidences of POPF, DGE and postoperative hemorrhage between groups. Despite the preservation of the RHA in most cases, the rates of microscopic positive margin were also comparable between two groups with no impact of RHA on survival rates. In the context of pancreatic adenocarcinoma, Turrini et al. (14) similarly reported that PD in patients with RHA was safe, and overall survival did not differ from patients without RARHA. Nevertheless, two patients with encased RHA had poor survival and died 6 and 12 months after surgery. In Okada’s series (18), five of the eight cases (63%) who were positive for infiltration at the surgical margins, were in the RHA preservation group (vs. RHA resection); the authors concluded that it was technically and oncologically difficult to achieve sufficient surgical margins for pancreatic carcinoma in patients with RHA variation who undergoing PD, regardless of whether there is tumor abutment.
More importantly, anatomical courses of the RHA may have significant implications for the surgical procedure. In fact, Jah et al reported three different anatomical courses of the RHA (13). In the type 1, the RHA has a posterior route with respect of the head of the pancreas. In this situation, it is possible to preserve the RHA while performing a PD if the tumor is small with no involvement of the aberrant RHA. In other cases, the RHA may display an intraparenchymal course (type 2) or through the superior mesenteric vein (SMV) groove (type 3) requiring “en bloc resection” depending on the tumor site and the arterial involvement. The distance between the tumor and the root of the RHA could also interfere in surgical procedure and oncological outcome. Indeed, Okada et al. reported that the R1 resection rate was significantly higher for tumors located within 10 mm from the root of the RHA compared to tumors located 10 mm or more (78% vs. 6%, P=0.001) (18). The authors concluded that en bloc RHA resection should be performed in this situation to improve the rate of R0 resection. Thus, adequate multiphasic contrast-enhanced CT is fundamental to specify the relationship between the RHA and the head of the pancreas. In brief, the diagnosis of an arterial variation should be done preoperatively. The knowledge of an arterial variation prior to PD is mandatory to decrease the risk of vascular damage and to anticipate its intraoperative management. In a series of 78 patients, of whom 47 had a RHA, Turrini et al. (14) reported in 2010 that surgeons were more likely to identify RHA than radiologists on CT; nevertheless, almost nearly 50% of patients had RHA identified only during surgery.
Overall, the current literature review may have some limitations. All included studies were retrospective, although the risk of bias was limited by the comparative analysis between RHA and no RHA groups. The non-significance of the impact of RHA on postoperative and oncological outcomes may be related to the lack of power of each study. However, we found no statistical difference when globally comparing groups with vs. without RHA.
In particular, although the morbidity in the subgroup of patients in whom the RHAs was sacrificed, accidentally damaged or ligated was comparable with that in the no RHA group, the numbers in this subgroup were probably too small to detect possible differences in morbidity. The pre- or intra-operative management of the RHA was not specified in a non-negligible part of the patients; nevertheless, the RHA management was likely variable and obviously did not impact the postoperative and oncological outcomes. Few studies classified POPF, DGE and postoperative hemorrhage according to the international ISGPS criteria. Subsequently, the severity of postoperative complications could not be compared between groups. In addition, no study evaluated the morbidity according to the preservation or not of the RHA. Last and not least, there was no standardization of the histopathological analysis—consisting of multicolour inking by the surgeon of the three main resection margins: the “vascular” margin, i.e., the portal vein-SMV margin plus SMA, and the posterior margin—with the risk of underestimating the rates of R1 vascular margin. Indeed, recent studies on the standardization of histological examination showed that the vascular margin is more frequently invaded than previously thought, which worsens the prognosis (27). This may be especially true in the presence of a RHA that is usually travelling through the vascular margin. Nevertheless, in the studies selected for this review, the authors did not specify what margin was analysed and what minimal distance was selected to define R1 resection. As the rate of local recurrence after resection of pancreatic cancer based on autopsy findings was at odds with the R1 rate reported in series using a standardized pathological protocol for the examination of PD specimens, the impact of a RHA on resection margins and oncological outcome needs to be reappraised through standardized histological examination (28,29).
In conclusion, the RHA is an anatomical variation for which it is important to consider in pancreatic head surgery. Postoperative and oncological outcomes seemed unaffected by this variation provided that the RHA was identified and correctly managed intraoperatively. Nevertheless, the conclusions of these earlier studies should be reappraised through standardized histological examination, meaning that the impact of the RHA on postoperative and oncological outcome remained to be determined. Anyway, the few patients with clear RHA involvement seemed to have poor survival.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Perkins JD. Are we reporting the same thing? Liver Transpl 2007;13:465-6. [PubMed] [Google Scholar]
- 2.Ugurel MS, Battal B, Bozlar U, et al. Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Br J Radiol 2010;83:661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traverso LW, Freeny PC. Pancreaticoduodenectomy. The importance of preserving hepatic blood flow to prevent biliary fistula. Am Surg 1989;55:421-6. [PubMed] [Google Scholar]
- 4.Yamamoto S, Kubota K, Rokkaku K, et al. Disposal of replaced common hepatic artery coursing within the pancreas during pancreatoduodenectomy: report of a case. Surg Today 2005;35:984-7. [DOI] [PubMed] [Google Scholar]
- 5.Gaujoux S, Sauvanet A, Vullierme MP, et al. Ischemic complications after pancreaticoduodenectomy: incidence, prevention, and management. Ann Surg 2009;249:111-7. [DOI] [PubMed] [Google Scholar]
- 6.Biehl TR, Traverso LW, Hauptmann E, et al. Preoperative visceral angiography alters intraoperative strategy during the Whipple procedure. Am J Surg 1993;165:607-12. [DOI] [PubMed] [Google Scholar]
- 7.Michels NA. The hepatic, cystic and retroduodenal arteries and their relations to the biliary ducts with samples of the entire celiacal blood supply. Ann Surg 1951;133:503-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 1994;220:50-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stauffer JA, Bridges MD, Turan N, et al. Aberrant right hepatic arterial anatomy and pancreaticoduodenectomy: recognition, prevalence and management. HPB (Oxford) 2009;11:161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JM, Lee YJ, Kim CW, et al. Clinical implications of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy. World J Surg 2009;33:1727-32. [DOI] [PubMed] [Google Scholar]
- 11.Eshuis WJ, Olde Loohuis KM, Busch OR, et al. Influence of aberrant right hepatic artery on perioperative course and longterm survival after pancreatoduodenectomy. HPB (Oxford) 2011;13:161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perwaiz A, Singh A, Singh T, et al. Incidence and management of arterial anomalies in patients undergoing pancreaticoduodenectomy. JOP 2010;11:25-30. [PubMed] [Google Scholar]
- 13.Jah A, Jamieson N, Huguet E, et al. The implications of the presence of an aberrant right hepatic artery in patients undergoing a pancreaticoduodenectomy. Surg Today 2009;39:669-74. [DOI] [PubMed] [Google Scholar]
- 14.Turrini O, Wiebke EA, Delpero JR, et al. Preservation of replaced or accessory right hepatic artery during pancreaticoduodenectomy for adenocarcinoma: impact on margin status and survival. J Gastrointest Surg 2010;14:1813-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim PT, Temple S, Atenafu EG, et al. Aberrant right hepatic artery in pancreaticoduodenectomy for adenocarcinoma: impact on resectability and postoperative outcomes. HPB (Oxford) 2014;16:204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulpice L, Rayar M, Paquet C, et al. Does an aberrant right hepatic artery really influence the short- and long-term results of a pancreaticoduodenectomy for malignant disease? A matched case-controlled study. J Surg Res 2013;185:620-5. [DOI] [PubMed] [Google Scholar]
- 17.Rammohan A, Palaniappan R, Pitchaimuthu A, et al. Implications of the presence of an aberrant right hepatic artery in patients undergoing pancreaticoduodenectomy. World J Gastrointest Surg 2014;6:9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada K, Kawai M, Hirono S, et al. A replaced right hepatic artery adjacent to pancreatic carcinoma should be divided to obtain R0 resection in pancreaticoduodenectomy. Langenbecks Arch Surg 2015;400:57-65. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [DOI] [PubMed] [Google Scholar]
- 21.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761-8. [DOI] [PubMed] [Google Scholar]
- 22.Grützmann R, Rückert F, Hippe-Davies N, et al. Evaluation of the International Study Group of Pancreatic Surgery definition of post-pancreatectomy hemorrhage in a high-volume center. Surgery 2012;151:612-20. [DOI] [PubMed] [Google Scholar]
- 23.Amini N, Spolverato G, Kim Y, et al. Trends in Hospital Volume and Failure to Rescue for Pancreatic Surgery. J Gastrointest Surg 2015;19:1581-92. [DOI] [PubMed] [Google Scholar]
- 24.El Amrani M, Leteurtre E, Sergent G, et al. Pancreatic head carcinoma and right hepatic artery: embolization management-A case report. J Gastrointest Oncol 2014;5:E80-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cloyd JM, Chandra V, Louie JD, et al. Preoperative embolization of replaced right hepatic artery prior to pancreaticoduodenectomy. J Surg Oncol 2012;106:509-12. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto N, Kodama Y, Endo H, et al. Embolization of the replaced common hepatic artery before surgery for pancreatic head cancer: report of a case. Surg Today 2004;34:619-22. [DOI] [PubMed] [Google Scholar]
- 27.Verbeke CS, Leitch D, Menon KV, et al. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232-7. [DOI] [PubMed] [Google Scholar]
- 28.Verbeke CS. Resection margins and R1 rates in pancreatic cancer--are we there yet? Histopathology 2008;52:787-96. [DOI] [PubMed] [Google Scholar]
- 29.Hishinuma S, Ogata Y, Tomikawa M, et al. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg 2006;10:511-8. [DOI] [PubMed] [Google Scholar]