Abstract
Background
Targeting human epidermal growth factor receptor 2 (HER2) with trastuzumab in metastatic esophagogastric adenocarcinoma (EGA) improves survival. The impact of HER2 inhibition in combination with chemoradiotherapy (CRT) in early stage EGA is under investigation. This study analyzed the pattern of HER2 overexpression in matched-pair tumor samples of patients who underwent neoadjuvant CRT followed by surgery.
Methods
All patients with EGA who underwent standard neoadjuvant CRT followed by esophagectomy at the University of Florida were included. Demographics, risk factors, tumor features, and outcome data were analyzed. Descriptive statistics, Chi-square exact test, uni- and multivariate analyses, and Kaplan Meier method were used. HER2 expression determined by immunohistochemical (IHC) was scored as negative (0, 1+), indeterminate (2+) or positive (3+).
Results
Among 49 sequential patients (41 M/8 F) with matched-pair tumor samples, 9/49 patients (18%) had pathologic complete response (pCR), 10/49 had near pCR or not enough tumor (NET) to examine in the post- treatment samples. Patients with initial HER2 negativity demonstrated conversion to HER2 positivity after neoadjuvant CRT (7/30 cases; 23%). Baseline HER2 overexpression was more common in lower stage/node negative patients (67% in stages I, IIA vs. 33% in stages IIB, III) and did not correlate with treatment response or survival.
Conclusions
Although limited by a relatively small sample size, our study failed to demonstrate that baseline HER2 protein over-expression in EGA predicts response to standard CRT. However, our data suggested that HER2 was up regulated by CRT resulting in unreliable concordance between pre-treatment (pre-tx) and post-treatment (post-tx) samples. Pre-therapy HER2 expression may not reliably reflect the HER2 status of persistent or recurrent disease.
Keywords: Chemoradiation, human epidermal growth factor receptor 2 (HER2), esophagogastric adenocarcinoma (EGA)
Introduction
Human epidermal growth factor receptor 2 (HER2) is a member of the HER receptor family, which consists of four transmembrane glycoproteins (HER1-4). HER2 has no natural ligand and requires heterodimerization with other HER receptors to initiate the downstream signaling pathway. HER2 activation results in cell proliferation and survival via the RAS-MAPK pathway (1-3). HER2 is overexpressed in 10-44% of esophagogastric adenocarcinoma (EGA) (4-7). The prognostic significance of HER2 expression in EGA is unclear with conflicting results (8-11). Therapeutic inhibition of the HER2 receptor has improved outcomes when combined with chemotherapy in metastatic gastroesophageal junction (GEJ) and gastric adenocarcinoma overexpressing HER2 (12). Importantly, determination of HER2 overexpression for metastatic disease routinely utilizes molecular testing of either the initial endoscopic biopsy or the post-treatment (post-tx) resection specimen.
Treatment of localized EGA was historically surgery alone. Currently, the standard treatment for locoregional resectable esophageal and/or GEJ adenocarcinoma is trimodality therapy with neoadjuvant chemoradiotherapy (CRT) followed by surgery since combination therapy demonstrated improvement in survival compared to surgery alone (13,14). Inhibition of HER2 in localized EGA in the neoadjuvant setting is still under investigation. The Brown University Oncology Group conducted a pilot study—trastuzumab added to CRT in patients with HER2 overexpressed locally advanced adenocarcinoma of the esophagus. This study showed encouraging results with a 3-year survival of 47% (4). A phase III trial by RTOG evaluating the addition of trastuzumab to trimodality treatment of HER2 overexpressing esophageal adenocarcinoma is ongoing (Clinicaltrials.gov NCT01196390).
The objectives of our study were to determine the changes in HER2 expression pattern in matched-pair pre-treatment (pre-tx) and post-tx tissues, to determine the impact of CRT on HER2 expression and correlate pre-tx HER2 expression with response to CRT and survival. Additionally, we sought to provide a better understanding of HER2 expression in pre- and post-tx tissues and identify the most appropriate specimen (pre- vs. post-tx) for HER2 expression analysis in patients being considered for additional therapy when they develop recurrence.
Methods
We conducted a retrospective analysis of all patients who underwent neoadjuvant CRT followed by esophagectomy at the University of Florida from 2001 to 2011 in whom matched-pair tissue was available and intact. Forty-nine patients with distal esophageal or GEJ adenocarcinoma were identified through their enrollment in the UF GI Oncology Research Database and/or the presence of tumor samples in the UF Surgical Pathology database. The project was approved by the UF Health Institutional Review Board (IRB). Clinical and pathological data were obtained from the medical records.
HER2 immunohistochemical (IHC) staining
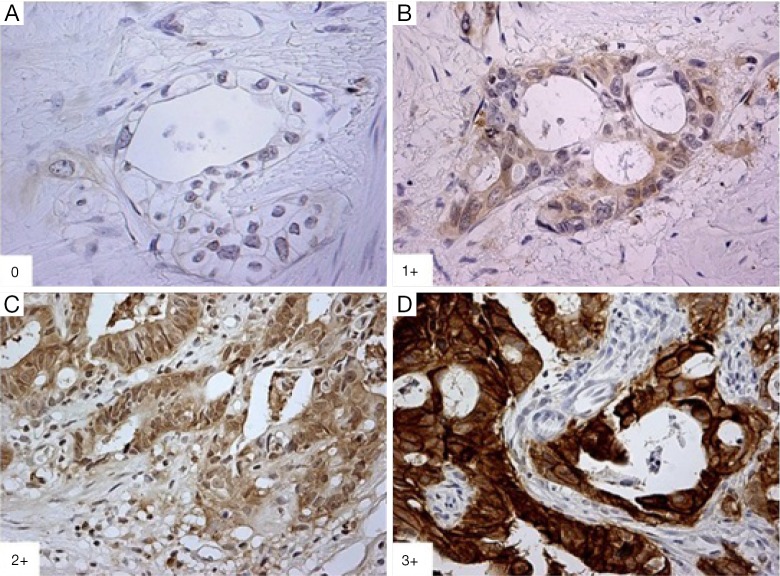
All available hematoxylin and eosin (H&E) stained slides of the pre-tx primary tumor biopsies and post-tx surgical specimens were reviewed with representative tumor blocks for each case selected. Formalin-fixed paraffin-embedded (FFPE) matched-pair tumor samples from the 49 patients were stained with HER2 monoclonal antibody (mouse monoclonal antibody; Invitrogen, Camarillo, CA, USA). The IHC results were interpreted using the standard validated scoring system for gastric cancer (Figure 1) consistent with previously published reports (12,15,16). IHC results were independently interpreted by three study pathologists (AA, LVD, and TZ), who were blinded to clinical data and pre-/post-tx tissue samples. In situ hybridization was not performed as part of this study.
Figure 1.
Immunohistochemistry of HER2 staining. (A) 0: negative (400×); (B) 1+: weak (400×); (C) 2+: moderate (100×); (D) 3+: strong positivity (100×). HER2, human epidermal growth factor receptor 2.
Statistical analysis
Descriptive statistics were used for summarizing demographic, clinical and pathological characteristics, as well as molecular expressions. Chi-square exact test was used to test the association of demographic and clinicopathological features, pre- and post-CRT HER2 expression with clinical characteristics. Kruskals’ Gamma coefficient was used to measure the association between pre- and post-tx HER2 expression. Odds ratio (OR) was used to measure the above association with recurrence and outcomes. Overall survival curves were compared by logrank test, and the corresponding cumulative survival rates were estimated using Kaplan-Meier method. All statistical analysis was performed using SAS 9.2 (SAS Institute Inc, NC, USA).
Results
A total of 49 patients were identified and included in this analysis. Mean age was 65.2 years (range, 39-87 years) and BMI was 29.4 (range, 16.9-52.0). The majority were men (84%), who were current or former smokers (80%), and had Barrett’s esophagus (78%). Tumor stage and other relevant demographics are summarized in Table 1. Treatment uniformly consisted of platinum-based chemotherapy (cisplatin and 5-fluorouracil) concurrent with standard doses, fractionation and volumes of external beam radiotherapy (approximately 50.4 Gy). Seventeen patients (35%) recurred with a median time to recurrence of 1.0 year and median survival time of 1.2 years (Table 1). Overall median survival for the entire cohort was 2.3 years with a median duration of follow up of 2.0 years.
Table 1. Summary of clinicopathological characteristics of patient with esophageal and GEJ adenocarcinoma who underwent neoadjuvant standard chemoradiation followed by esophagectomy.
| Characteristics | N=49 [%] |
|---|---|
| Mean ± SD | |
| Age | 65±10.9 |
| BMI | 29±6.5 |
| Gender | |
| Male | 41 [84] |
| Female | 8 [16] |
| cStage* | |
| I | 1 [2] |
| IIA | 19 [39] |
| IIB | 8 [16] |
| III | 21 [43] |
| Depth of invasion (cT) | |
| T1 | 3 [6] |
| T2 | 7 [14] |
| T3 | 33 [67] |
| T4 | 1 [2] |
| Tx | 5 [10] |
| Nodal involvement | |
| Positive | 29 [59] |
| Negative | 20 [41] |
| Tumor differentiation | |
| Well | 1 [2] |
| Moderate | 12 [28] |
| Poor | 30 [70] |
| Barrett’s esophagus | |
| Yes | 31[78] |
| No | 9 [22] |
| Smoking | |
| Current | 11 [23] |
| Former | 28 [57] |
| Never | 10 [20] |
| Recurrence | |
| Yes | 17 [35] |
| No | 26 [53] |
| Unknown | 6 [12] |
| Patient status | |
| Alive with no disease | 10 [20.4] |
| Alive disease unknown | 10 [20.4] |
| Died | 29 [59.2] |
*, TNM staging—per AJCC 6th Edition. GEJ, gastroesophageal junction.
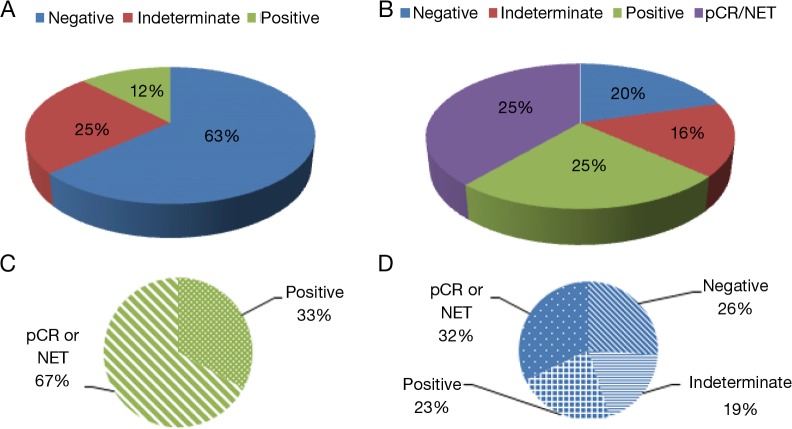
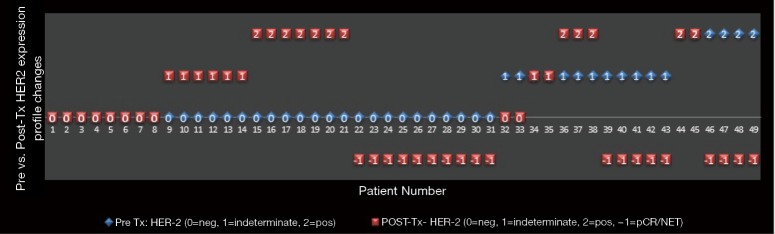
Patients with node negative disease had numerically more frequent baseline HER2 positivity compared to patients with node positive disease (20% vs. 6.9%; P=0.40) and were more likely to attain a pathologic complete response (pCR) after CRT (30% vs. 10.3%; P=0.15), although neither difference was statistically significant. Pre-tx HER2 expression was negative (0, 1+) in 63%, indeterminate (2+) in 25%, and positive (3+) in 12% of cases. Post-tx specimens revealed 18% (9/49) with pCR and another 20% (10/49) with a near pCR or not enough tumor (NET) tissue present to perform ancillary studies. Thirty out of 49 (61%) patients did not attain pCR or near pCR/NET. Of these patients, HER2 expression was found to be negative in 20% (10/49), indeterminate in 16% (8/49), and positive in 25% (12/49) of cases. The relationship of pre- and post-tx HER2 expression is shown in Table 2. After CRT, patient samples with initial pre-tx HER2 negativity maintained a negative expression in 26% (8/30), but became indeterminate in 20% (6/30) and demonstrated a positive expression in 23% (7/30) of patients. Patients with pre-tx HER2 positive expression attained pCR/NET in 67% (4/6) and maintained a positive expression profile in the remaining patients (2/6); none of them became negative or demonstrated indeterminate expression after CRT. Patients with indeterminate pre-tx HER2 expression demonstrated 41% pCR/NET, with cases of persistent disease demonstrating HER2 negative (17%), indeterminate (17%) or positive expression (25%) after CRT. There was only a small to moderate association between changes in pre- and post-tx HER2 expression (G=0.403; 95% CI, −0.147-0.953; P=0.185). HER2 expression patterns in matched pre- and post-tx samples are illustrated in Figures 2,3.
Table 2. Relationship between HER2 expressions in matched-pair samples.
| Pre CRT [n] | Post CRT [n] |
|||
|---|---|---|---|---|
| POS [12] | IND [8] | NEG [10] | pCR or NET [19] | |
| POS [6] | 2 | 0 | 0 | 4 |
| IND [12] | 3 | 2 | 2 | 5 |
| NEG [31] | 7 | 6 | 8 | 10 |
POS, positive, HER2 IHC 3+; IND, indeterminate, HER2 IHC 2+; NEG, negative, HER2 IHC 0, 1+ by IHC. HER2, human epidermal growth factor receptor 2; CRT, chemoradiotherapy; pCR, pathologic complete response; NET, not enough tumor to examine.
Figure 2.
HER2 expressions in pre- and post-treatment specimens. (A) HER2 expression in pre-tx specimens; (B) HER2 expression in post-tx specimens; (C) post-tx HER2 expression pattern in patients with pre-tx positive HER2 expression; (D) post-tx HER2 expression pattern in patients with pre-tx negative HER2 expression. Negative, HER2 expression 0 or 1+; indeterminate, HER 2 expression 2+; positive, HER2 expression 3+ by IHC. HER2, human epidermal growth factor receptor 2; pCR, pathologic complete response; NET, not enough tumor to examine; pre-tx, pre-treatment; post-tx, post treatment.
Figure 3.
Changes in HER2 expression pattern in matched pre and post CRT samples. Pre-tx (blue), initial diagnostic biopsy; post-tx (red), surgical resection specimen; Neg, HER2 expression 0 or 1+; Indeterminate, HER2 expression 2+; Positive, HER2 expression 3+ by IHC. HER2, human epidermal growth factor receptor 2; CRT, chemoradiotherapy; pCR, pathologic complete response; NET, not enough tumor to examine.
Neither pre- nor post-tx HER2 expression correlated significantly with treatment outcomes (Table 3). Univariate analysis did not show significant correlation of age, tumor stage and grade, smoking, presence of Barrett’s esophagus, or HER2 expression with survival. However, patients with node positive disease showed a trend toward worse survival (HR =2.03; 95% CI, 0.90-4.59; P=0.08).
Table 3. Analysis of pre- and post- treatment HER2 expression on overall survival.
| HER2 expression | HR | 95% CI for HR | P |
|---|---|---|---|
| Pre CRT | |||
| Negative | 1.000 | ||
| Intermediate | 0.584 | 0.234-1.460 | 0.2503 |
| Positive | 0.747 | 0.222-2.518 | 0.6385 |
| Post CRT | |||
| Negative | 1.000 | ||
| pCR/NET | 0.706 | 0.286-1.744 | 0.451 |
| Intermediate | 1.175 | 0.416-3.316 | 0.7607 |
| Positive | 0.367 | 0.112-1.203 | 0.0979 |
| Association between pre- and post-tx HER2 expression | G=0.403 | −0.147-0.953 | 0.1857 |
HER2, human epidermal growth factor receptor 2; CRT, chemoradiotherapy; pCR, pathologic complete response; NET, not enough tumor to examine.
Discussion
Our study demonstrated that HER2 expression patterns differ from baseline specimens in several post CRT samples. Importantly, 23% of pre-tx HER2 negative (IHC 0, 1+) expressing tumors were re-classified as HER2 positive (IHC3+) after CRT. None of the pre-tx HER2 positive samples (IHC3+) became negative after CRT and 67% of these patients attained pCR or not enough tumor to examine. Based on these findings, our study suggests upregulation of this receptor expression after concurrent CRT in EGA, which is not statistically significant likely due to the relatively small sample size. Discordance in HER2 expression has been noted in the literature. Pre-clinical and clinical studies have shown correlation of HER2 overexpression with chemoradioresistance in some solid tumors (17-19). In breast cancer, studies examining HER2 expression changes after neoadjuvant chemotherapy have demonstrated conflicting results, however, more commonly showed a gain of expression (20,21). A pre-clinical study reported by Cao et al. demonstrated HER2 upregulation in radioresistant breast cancer cell lines via NF-κB and inhibition of HER2 re-sensitizes the resistant cell lines to radiation (19).
In the MAGIC trial, Okines and colleagues investigated the effect of HER2 expression on prognosis and benefited from perioperative chemotherapy with epirubicin, cisplatin and fluorouracil (ECF) in patients with early EGA (22). HER2 positivity (IHC3+, or IHC2+/BDISH positive) was 10.9% among all patients. HER2 expression was evaluated by IHC and brightfield dual in situ hybridization (BDISH) in tissue microarrays. Paired samples were available for 179 of 503 patients (35.6%), and 87/179 received perioperative chemotherapy. Changes in HER2 status were noted in 11 cases among which up-regulation of HER2 expression (9 cases) was more common than loss of expression (2 cases). Three patients of the 11 discordant cases received perioperative chemotherapy. The authors interpreted these changes in HER2 status due to heterogeneous HER2 expression rather than a true change in the biology of the disease. HER2 status was not prognostic for survival nor predicted enhanced benefit from chemotherapy (22).
Our results did not show significant association of pre-tx HER2 expression with treatment response (i.e., pCR or near pCR) or post-tx HER2 expression. However, patients with pre-tx HER2 negativity were less likely to have a pCR or near pCR compared to those patients with initial HER2 overexpression (OR =0.24; 95% CI, 0.04-1.53; P=0.16). Although this difference is not statistically different, likely due to small sample size, the trend of patients with initial positive HER2 expression who attained pCR was clearly higher than those with initial negative HER2 expression (67% vs. 32%). Moreover, patients with clinically node negative disease were more likely to have HER2 overexpression and attain pCR compared to those with clinical node positive disease (30% vs. 10%).
Consistent with previous studies, our analysis showed a non-significant trend towards worse survival in node positive patients. In addition, no significant association was found between pre- or post-tx HER2 overexpression and survival. Although some studies suggest that HER2 overexpression in EGA is associated with poor prognosis, more contemporary studies have failed to show HER2 status as an independent prognostic factor (23-25). The conflicting results are possibly due to the difference in HER2 staining methodology and interpretation used prior to the validation of the HER2 IHC scoring system for gastric cancer described by Hoffman et al. in 2008 (15). Our results support the findings that HER2 overexpression in EGA is not independently associated with a poor prognosis.
In this study, HER2 overexpression was found in 12% of pre-tx samples, which is lower than that is described in the literature, however, FISH analysis was not performed on IHC2+ sample which may underestimate the determination of HER2 amplification by 10-15%. The relatively low rate of HER2 expression in pre-tx tissue from our current study is unlikely related to the smaller specimen size given the use of established criteria for scoring HER2 overexpression in biopsy samples, confirmation of adequate tumor tissue for analysis prior to IHC and consistent pathologist review of cases. Our previously reported analysis of patients with early stage EGA with Barrett’s esophagus taken directly to surgery without CRT demonstrated higher HER2 overexpression (38%) (11). In support of this, Yoon et al. reported that HER2 positivity was significantly associated with lower tumor grade and stage and the presence of adjacent Barrett’s esophagus (OR =1.8; 95% CI, 1.1-2.8; P=0.014) (26). The higher rate of HER2 overexpression in Barrett’s esophagus suggests that HER2 may play an important role in the progression of dysplasia to carcinoma sequence (27-30).
Limitations of our study include relatively small sample size, lack of ISH analysis on IHC2+ cases and a short follow up. The sample size contributed to non-statistically significant results although there were trends showing possible correlation between HER2 overexpression with node negative disease and pCR. A definition of positive HER2 expression in this study included only patients with HER3+ by IHC which could underestimate the clinical impact of HER2 overexpression on clinical outcomes (31-34), but is unlikely to alter the conclusions of this study. In addition, the analysis of post-tx HER2 expression was limited to patients who had residual disease after CRT and patients with not enough tissue after CRT (near pCR) could not be examined for HER2 expression.
To date, studies that examined the expression of HER2 in matched-paired tumor samples of an individual patient (initial diagnostic primary tumor biopsy specimens and surgical tumor resections after CRT) in EGA are limited. Our study demonstrated changes in HER2 expression pattern following CRT. This has clinical implications as therapeutic selection of anti-HER2 treatments is reserved for patients with demonstrated HER2 overexpression. Our study suggested 23% of HER2 negative patient demonstrated HER2 overexpression after CRT. Therefore, HER2 expression performed only on a pre-tx specimen could misidentify patients who became HER2 overexpressed after CRT when considering for systemic anti-HER2 therapies if they recur with metastatic disease. Therefore, HER2 testing on both pre- and post-tx specimens or re-biopsy of recurrence should be considered to avoid over or under treatment depending upon which tumor sample is chosen for molecular testing. Larger studies are needed to verify the changes in HER2 expression after neoadjuvant CRT in EGA, including the impact of whether radiotherapy or cytotoxic therapy or the combination, is accountable for the HER expression profile changes. If therapeutic HER2 inhibition during neoadjuvant therapy proves to be of clinical value, inhibition of this HER2 up-regulation may serve as an important therapeutic target for a subset of patients with HER2 negative initial assessment. Correlative analyses from the current ongoing prospective clinical trial testing the role of trastuzumab in the neoadjuvant CRT of EGA may offer further insights.
Conclusions
Changes in HER2 expressions in diagnostic biopsies compared to post-tx tumor resections are notable in this study and have the potential to impact therapeutic selection and clinical decision making in advanced disease patient management. Recurrent or residual tumor may require re-biopsy to confirm HER2 expression after neoadjuvant CRT. Consistent with the literature, our study demonstrated that baseline HER2 tumor expression does not have an impact on response to treatment nor is prognostic for survival; however, we acknowledge the limitations of a relatively small sample size. While ongoing studies are awaited to determine the potential therapeutic implications of anti-HER2 targeting in the neoadjuvant setting, the attention to the choice of sample selection for HER2 analysis for clinical decision making should be thoughtfully approached for patients who develop recurrence and are being considered for anti-HER2 therapy.
Acknowledgements
Funding: This study was supported by the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine and NIH (NCATS) CTSA grant UL1TR000064.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127-37. [DOI] [PubMed] [Google Scholar]
- 2.Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000;19:3159-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. Nat Rev Clin Oncol 2011;8:492-503. [DOI] [PubMed] [Google Scholar]
- 4.Safran H, Dipetrillo T, Akerman P, et al. Phase I/II study of trastuzumab, paclitaxel, cisplatin and radiation for locally advanced, HER2 overexpressing, esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys 2007;67:405-9. [DOI] [PubMed] [Google Scholar]
- 5.al-Kasspooles M, Moore JH, Orringer MB, et al. Amplification and over-expression of the EGFR and erbB-2 genes in human esophageal adenocarcinomas. Int J Cancer 1993;54:213-9. [DOI] [PubMed] [Google Scholar]
- 6.Fassan M, Ludwig K, Pizzi M, et al. Human epithelial growth factor receptor 2 (HER2) status in primary and metastatic esophagogastric junction adenocarcinomas. Hum Pathol 2012;43:1206-12. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Bandla S, Godfrey TE, et al. HER2 amplification, overexpression and score criteria in esophageal adenocarcinoma. Mod Pathol 2011;24:899-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begnami MD, Fukuda E, Fregnani JH, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol 2011;29:3030-6. [DOI] [PubMed] [Google Scholar]
- 9.Allgayer H, Babic R, Gruetzner KU, et al. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol 2000;18:2201-9. [DOI] [PubMed] [Google Scholar]
- 10.Grabsch H, Sivakumar S, Gray S, et al. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol 2010;32:57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan E, Alkhasawneh A, Duckworth LV, et al. EGFR family and c-Met expression profiles and prognostic significance in esophagogastric adenocarcinoma. J Clin Oncol 2013;31:abstr e15108. [DOI] [PMC free article] [PubMed]
- 12.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [DOI] [PubMed] [Google Scholar]
- 13.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797-805. [DOI] [PubMed] [Google Scholar]
- 16.Rüschoff J, Dietel M, Baretton G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch 2010;457:299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitzner M, Emons G, Kramer F, et al. A gene expression signature for chemoradiosensitivity of colorectal cancer cells. Int J Radiat Oncol Biol Phys 2010;78:1184-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akamatsu M, Matsumoto T, Oka K, et al. c-erbB-2 oncoprotein expression related to chemoradioresistance in esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2003;57:1323-7. [DOI] [PubMed] [Google Scholar]
- 19.Cao N, Li S, Wang Z, et al. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res 2009;171:9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Ven S, Smit VT, Dekker TJ, et al. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev 2011;37:422-30. [DOI] [PubMed] [Google Scholar]
- 21.Kinsella MD, Nassar A, Siddiqui MT, et al. Estrogen receptor (ER), progesterone receptor (PR), and HER2 expression pre- and post- neoadjuvant chemotherapy in primary breast carcinoma: a single institutional experience. Int J Clin Exp Pathol 2012;5:530-6. [PMC free article] [PubMed] [Google Scholar]
- 22.Okines AF, Thompson LC, Cunningham D, et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann Oncol 2013;24:1253-61. [DOI] [PubMed] [Google Scholar]
- 23.Janjigian YY, Werner D, Pauligk C, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol 2012;23:2656-62. [DOI] [PubMed] [Google Scholar]
- 24.Reichelt U, Duesedau P, Tsourlakis Mch, et al. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol 2007;20:120-9. [DOI] [PubMed] [Google Scholar]
- 25.Thompson SK, Sullivan TR, Davies R, et al. Her-2/neu gene amplification in esophageal adenocarcinoma and its influence on survival. Ann Surg Oncol 2011;18:2010-7. [DOI] [PubMed] [Google Scholar]
- 26.Yoon HH, Shi Q, Sukov WR, et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res 2012;18:546-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi E, Grisanti S, Villanacci V, et al. HER-2 overexpression/amplification in Barrett's oesophagus predicts early transition from dysplasia to adenocarcinoma: a clinico-pathologic study. J Cell Mol Med 2009;13:3826-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fassan M, Mastracci L, Grillo F, et al. Early HER2 dysregulation in gastric and oesophageal carcinogenesis. Histopathology 2012;61:769-76. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Nekarda H, Hoelscher AH, et al. Prognostic value of DNA ploidy and c-erbB-2 oncoprotein overexpression in adenocarcinoma of Barrett's esophagus. Cancer 1994;73:1785-94. [DOI] [PubMed] [Google Scholar]
- 30.Fléjou JF, Paraf F, Muzeau F, et al. Expression of c-erbB-2 oncogene product in Barrett's adenocarcinoma: pathological and prognostic correlations. J Clin Pathol 1994;47:23-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yano T, Doi T, Ohtsu A, et al. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep 2006;15:65-71. [PubMed] [Google Scholar]
- 32.Marx AH, Tharun L, Muth J, et al. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol 2009;40:769-77. [DOI] [PubMed] [Google Scholar]
- 33.Jeung J, Patel R, Vila L, et al. Quantitation of HER2/neu expression in primary gastroesophageal adenocarcinomas using conventional light microscopy and quantitative image analysis. Arch Pathol Lab Med 2012;136:610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bozzetti C, Negri FV, Lagrasta CA, et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer 2011;104:1372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]