Abstract
Background
Elevated neutrophil-to-lymphocyte (NLR) and platelet-to-lymphocyte ratios (PLR) may represent markers of a suboptimal host immune response to cancer and have been shown to correlate with prognosis in multiple tumor types across different treatment modalities, including radiation therapy. Limited data suggest that NLR may predict for survival and disease control in patients receiving selective internal radiation therapy (SIRT). The correlation between clinical outcomes and change in NLR and PLR after SIRT has not been evaluated.
Methods
We retrospectively reviewed 339 consecutive patients with primary (n=37) or metastatic (n=79) liver cancer treated with SIRT from 2006 to 2014. Complete blood counts with differential were available for 116 patients both before and after (median, 29 and 20 days, respectively) SIRT. Survival and progression were calculated from date of initial SIRT. Patient and tumor characteristics evaluated for ability to predict overall survival (OS) and progression free survival (PFS) included pre- and post-treatment neutrophil, platelet, and lymphocyte counts (LCs), as well as NLR, PLR, and relative change in NLR and PLR. Cutoff values were determined for variables that were significant on multivariate analysis (MVA) for OS and/or PFS.
Results
Median follow-up of surviving patients was 12 months. Median OS was 8 months from SIRT and 20 months from date of liver metastasis diagnosis. Significant factors on univariate analysis (UVA) for both lower OS and PFS included higher post-treatment neutrophil count (NC), higher post-treatment NLR, higher liver tumor volume, higher percentage liver tumor burden, and worse Eastern Cooperative Oncology Group (ECOG) performance status. Significant factors on MVA for lower OS and PFS were ECOG performance status ≥2, higher liver tumor volume, higher pretreatment PLR, and increase in PLR after SIRT. Post-treatment increase in PLR >3-fold was the most predictive early marker for increased risk of death when compared with those whose PLR did not increase or increased <3-fold. Pretreatment PLR >78 was the most predictive serum marker associated with improved OS prior to therapy.
Conclusions
This is the largest study to evaluate the association between NLR and PLR with clinical outcomes in patients receiving SIRT, with results that confirm that pre- and/or post-treatment NLR and/or PLR are predictive of clinical outcomes. The largest increase in risk of death as well as local and extrahepatic disease progression was related to change in PLR, a datum not well reported in the literature. The impact of SIRT on blood count changes and the underlying implications of these ratios should be further characterized in a prospective study.
Keywords: Selective internal radiation therapy (SIRT), selective internal radiation-sphere (SIR-sphere), radioembolization, liver cancer
Introduction
Primary and secondary malignancies of the liver are estimated to affect nearly 700,000 individuals and result in up to 84,000 deaths in the US in 2015 (1,2). Selective internal radiation therapy (SIRT) has emerged as a standard therapy option for patients with unresectable primary liver cancer, including hepatocellular carcinoma (HCC) (3), as well as unresectable liver metastases, especially metastatic colorectal cancer (mCRC), and has been prospectively shown to significantly increase liver progression free survival (PFS) when SIRT is combined with mFOLFOX6 in the first-line setting (4). SIRT improves outcomes when combined with chemotherapy alone and has a favorable toxicity profile when compared with other liver-directed therapies (5,6).
Several factors have been found to be prognostic in patients undergoing SIRT, including performance status, presence of extrahepatic metastases, extent of liver involvement, liver function, and prior chemotherapy (7). Radiographic biomarkers have also been shown to have prognostic significance, including functional tumor volume, total lesion glycolysis, and 18F-FDG standardized uptake values on PET/CT (8,9). We report on a study designed to determine whether serum levels of differential blood counts and their ratios, as surrogates for host immune status, are prognostic in patients undergoing SIRT.
Since Rudolf Virchow postulated the relationship of the inflammatory response and the cancer life cycle, readily obtainable metrics of host antitumor immunity have been sought. The complete blood cell count (CBC) and the ratios of its components, along with other data gleaned from peripheral blood work, such as C-reactive protein and albumin, have been investigated for their utility as biomarkers in predicting outcomes in patients with a variety of malignant histologies. Several of these peripheral blood markers of systemic inflammation have been shown to correlate with tumor and nodal stage as well as number of metastases and, ultimately, to be prognostic for survival (10).
As generalized indicators of inflammation, these blood component ratios yield little insight into the specific mechanisms that connect the immune system to the outcomes with which these markers correlate. Several hypotheses have been put forward to explain the observations linking clinical outcome with these nonspecific metrics. It has been postulated that these values are biomarkers that represent absolute or relative lymphocytopenia, an ineffectual portfolio of overly broad inflammatory cytokines, or symptoms of nutritional, functional, and immunologic decline (11). Although the mechanisms that link immune function (and its second-hand indicators such as these lab values) with oncologic outcomes are the focus of intense ongoing research efforts, the clinical implications of several blood component ratios are becoming better appreciated.
Neutrophil-to-lymphocyte ratio (NLR) and/or changes in NLR have been shown to have independent prognostic value across histologies and in patients treated with multimodality therapy (11). Increasing platelet-to-lymphocyte ratio (PLR) has been found to be a significant negative predictor of survival in a meta-analysis of nearly 14,000 patients with various malignancies (12).
Researchers have reported a consistently strong association between NLR and PLR in patients with cancers of the liver who receive liver-directed therapies. Fan et al. (10) and Huang et al. (13) evaluated patients with HCC who were treated with transcatheter arterial chemoembolization and found that high pretreatment NLR and PLR were independent prognostic factors for overall survival (OS). High NLR and PLR indicated a worse prognosis along with vascular invasion, multiple tumors, and elevated α-fetoprotein levels in these patients. Among HCC patients who underwent curative resection, change in NLR (∆NLR) was an independent prognostic factor for OS and PFS (14).
For patients with mCRC treated either with systemic chemotherapy alone or with surgery and chemotherapy, pretreatment NLR >5 was the only independent predictor of worse survival (15). Patients who experienced a decline in NLR after therapy had better survival than those with persistently elevated NLR (15). Another study examining patients with mCRC who underwent hepatic resection found that elevated preoperative NLR was the sole significant predictor of recurrence on multivariable analysis (MVA); NLR >5 was associated with a greater than 2-fold increase in risk of death (16). Tohme (17) studied patients with unresectable mCRC treated with radioembolization. In this heavily pretreated population, pretreatment NLR >5 was associated with inferior survival following radioembolization when compared with pretreatment NLR ≤5 (5.6 and 10.6 months, respectively). The presence of extrahepatic disease, lung metastases, or high NLR was associated with worse survival on MVA (17). Our study is the first to examine the prognostic significance of ∆NLR and change in PLR (∆PLR) after SIRT in patients with unresectable primary or metastatic liver cancer.
Methods
Patients
This study included patients with unresectable primary or metastatic liver cancer treated with SIRT at the University of Maryland Medical Center (Baltimore, MD, USA) from 2006 to 2014. After obtaining Institutional Review Board approval, medical records from 339 patients who underwent SIRT were retrospectively reviewed. Inclusion criteria were: CBC and differential data available from both before and after SIRT and confirmation of diagnosis of primary or metastatic liver cancer by CT imaging, PET imaging, or pathology report, resulting in a study total of 116 patients.
We reviewed patient and treatment data including Eastern Cooperative Oncology Group (ECOG) performance status, Child-Pugh score, tumor histology, pretreatment tumor volumes, number of liver lesions, percent tumor burden, and CBC with differential data. Patients were followed until date of death or date of last follow-up, and tumor recurrence or progression was monitored.
Pre- and post-treatment NLR and PLR were calculated by dividing the absolute neutrophil count (NC) or the absolute platelet count (PC), respectively, by the absolute lymphocyte count (LC). ∆NLR and ∆PLR were calculated by subtracting the post-treatment NLR or PLR from the pretreatment NLR or PLR and dividing by the pretreatment NLR or PLR. The prognostic values of NLR and PLR were examined in correlation with OS and PFS, liver PFS, and extrahepatic PFS.
Patient characteristics are described in Table 1. The study included 52 (44.8%) men and 64 (55.2%) women. The majority of patients had metastatic liver tumors: 37 with HCC (31.9%), 30 with mCRC (25.8%), 18 with metastatic breast carcinoma (15.6%), 17 with pancreatic/bile duct carcinomas (14.7%), 8 with neuroendocrine carcinomas (6.8%), and 6 with other less common primary cancers, including sinonasal and laryngeal cancers (5.2%). The median age at diagnosis of liver primary or metastatic cancer was 60 years (range, 28-85 years).
Table 1. Patient demographics.
| Characteristic | n | Percent (%) |
|---|---|---|
| Sex | ||
| Male | 52 | 44.8 |
| Female | 64 | 55.2 |
| Primary tumor type | ||
| HCC | 37 | 31.9 |
| Colorectal | 30 | 25.8 |
| Breast | 18 | 15.6 |
| Pancreatic/bile duct | 17 | 14.7 |
| Neuroendocrine | 8 | 6.8 |
| Other | 6 | 5.2 |
| SIRT | ||
| Whole liver | 29 | 25 |
| Unilobar | 79 | 68 |
| Repeat same lobe | 8 | 7 |
| Lesion number | ||
| 1 | 16 | 13.8 |
| 2 | 10 | 8.6 |
| 3+ | 90 | 77.6 |
| Child-Pugh class | ||
| A | 93 | 80.2 |
| B | 23 | 19.8 |
| ECOG performance status | ||
| 0-1 | 108 | 93.1 |
| 2+ | 8 | 6.9 |
| Extrahepatic disease | ||
| Yes | 23 | 19.8 |
| No | 93 | 80.2 |
| Number of previous lines of chemotherapy | ||
| 0 | 73 | 62.9 |
| 1 | 17 | 14.7 |
| 2 | 11 | 9.5 |
| 3+ | 15 | 12.9 |
| % liver tumor burden | ||
| >25 (median) | 53 | 45.7 |
| ≤25 (median) | 63 | 54.3 |
HCC, hepatocellular carcinoma; SIRT, selective internal radiation therapy; ECOG, Eastern Cooperative Oncology Group.
The median intervals between the first blood draw and start of treatment and between the second blood draw and completion of treatment were 29 and 20 days, respectively. Median pretreatment NLR was 3.63, and median post-treatment NLR was 8.58. Median pretreatment PLR was 176.36, and median post-treatment PLR was 333.13.
Selective internal radiation therapy (SIRT)
All patients who received SIRT were screened and evaluated by a multidisciplinary tumor board. All SIRT patients had: (I) unresectable liver tumor; (II) limited or no extrahepatic metastasis; (III) ECOG performance status <3; and (IV) sufficient renal, hematologic, and hepatic function prior to undergoing SIRT. Before receiving SIRT, some patients were treated with chemotherapy (n=44) or attempted curative resection (n=23).
All patients received SIRT using yttrium-90 resin microspheres, with the prescribed activity calculated using the body surface area method and reduction in prescribed activity based on percent lung shunt as per standard protocol. SIRT was administered to the whole liver in a minority of patients, most of whom had mCRC liver metastases. Patients with bilobar disease, especially those with HCC, were most often treated in lobar fashion, with a minimum of 4 weeks between treatments to decrease post-treatment side effects. A few patients received repeat SIRT to the same lobe of the liver (n=8).
Treatment characteristics are described in Table 2. The majority of patients were treated in a sequential lobar fashion (n=64; 55.2%), and a minority received one unilobar SIRT treatment (n=28; 24.1%) or whole liver treatment (n=24; 20.7%). For patients receiving sequential lobar treatments, the average prescribed activities for first and second SIRT treatments were 33.9 and 20.7 mCi, respectively. Average prescribed and administered activities for SIRT treatments are included in Table 2. Activities prescribed or delivered for SIRT did not significantly correlate with OS or PFS.
Table 2. Treatment characteristics.
| Characteristic | Sequential | Unilobar | Whole liver | All | OS (P value) | Overall PFS | Intrahepatic PFS | Distant PFS |
|---|---|---|---|---|---|---|---|---|
| n | 64 | 28 | 24 | 116 | – | – | – | – |
| Percent (%) | 55.2 | 24.1 | 20.7 | 100 | – | – | – | – |
| Prescribed activity treatment 1 (mCi) | 33.9 | 35.5 | 46.6 | 36.90 | 0.615 | 0.916 | 0.946 | 0.850 |
| Delivered activity treatment 1 (mCi) | 34.2 | 36.4 | 47.5 | 37.46 | 0.597 | 0.795 | 0.918 | 0.863 |
| Dose delivered treatment 1 (Gy) | 55.6 | 54.5 | 53.7 | 54.97 | – | – | – | – |
| Prescribed activity treatment 2 (mCi) | 20.7 | – | – | – | 0.764 | 0.701 | 0.613 | 0.711 |
| Delivered activity treatment 2 (mCi) | 20.6 | – | – | – | 0.839 | 0.714 | 0.719 | 0.592 |
| Dose delivered treatment 2 (Gy) | 56.9 | – | – | – | – | – | – | – |
OS, overall survival; PFS, progression free survival.
Statistical analysis
Statistical analysis was performed using SAS software version 9.3 (SAS Institute Inc.; Cary, NC, USA). Cutoff values for NLR, PLR, ∆NLR, and ∆PLR were determined for variables that were significant on MVA for OS and/or PFS. We used the χ2 test to analyze categorical variables and the independent sample t-test to analyze continuous variables. The Kaplan-Meier method was also used to analyze OS and PFS inside and outside the liver. Variables significant in univariate analysis (UVA) were further analyzed in MVA to evaluate for prognostic significance using Cox proportional hazard regression.
Results
Overall survival (OS)
Median OS was 8 months (range, 1-81 months) from SIRT completion and 20 months (range, 4-184 months) from date of liver metastasis diagnosis. The median follow-up for surviving patients was 12 months, with significantly shorter median follow-up in patients with HCC (5.26 months) and significantly longer median follow-up in patients with neuroendocrine liver metastases (18.13 months).
Significant factors for OS on UVA and MVA are shown in Table 3. For UVA, factors associated with inferior OS included higher post-treatment NC, higher post-treatment NLR, higher liver tumor volume, higher percentage liver tumor burden, and higher ECOG performance status score. Significant factors on MVA for lower OS were ECOG performance status score ≥2, higher liver tumor volume, higher pretreatment PLR, and increase in PLR after SIRT. Any increase in PLR after treatment was associated with the highest increased risk of death [hazard ratio (HR): 1.285, CI, 1.037-1.592; P=0.022] on MVA.
Table 3. Univariate and multivariate analysis of factors associated with overall survival in patients receiving SIRT.
| Variable | Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| ECOG [0 & 1] | 0.022 | 0.003-0.185 | 0.0005 | 0.039 | 0.004-0.352 | 0.0039 | |
| ECOG [2] | 0.028 | 0.003-0.273 | 0.0021 | 0.064 | 0.0006-0.696 | 0.0239 | |
| Pre-treatment platelet | 0.999 | 0.996-1.001 | 0.309 | ||||
| Post-treatment platelet | 1 | 0.998-1.002 | 0.7323 | ||||
| Pre-neutrophil | 1.059 | 0.961-1.166 | 0.2499 | ||||
| Post-neutrophil | 1.151 | 1.071-1.238 | 0.0001 | ||||
| Pre-lymphocyte | 0.831 | 0.596-1.159 | 0.2753 | ||||
| Post-lymphocyte | 0.617 | 0.274-1.339 | 0.2437 | ||||
| Pre NLR | 1.049 | 0.974-1.13 | 0.2075 | ||||
| Post NLR | 1.014 | 1.005-1.024 | 0.0041 | 1.016 | 0.999-1.034 | 0.0662 | |
| ΔNLR | 0.997 | 0.983-1.012 | 0.6836 | ||||
| Pre PLR | 1.001 | 0.999-1.002 | 0.2624 | 1.003 | 1-1.005 | 0.0183 | |
| Post PLR | 1.001 | 1-1.001 | 0.0422 | ||||
| ΔPLR | 1.111 | 0.993-1.243 | 0.066 | 1.285 | 1.037-1.592 | 0.0219 | |
| Liver tumor volume | 1.01 | 1.001-1.002 | 0.0005 | 1.001 | 1-1.002 | 0.0086 | |
| Liver tumor burden % | 1.016 | 1.005-1.026 | 0.0042 | ||||
SIRT, selective internal radiation therapy; ECOG, Eastern Cooperative Oncology Group; NLR, neutrophil-to-lymphocyte; PLR, platelet-to-lymphocyte ratios.
Cutoff values for NLR, PLR, ΔNLR, and ΔPLR were established to determine thresholds most predictive for hazard of death or progression. Pretreatment PLR >78 was predictive for improved OS (HR: 0.36, CI, 0.19-0.7; P<0.001), whereas post-treatment PLR >290 was associated with inferior OS (HR: 3.45, CI, 1.17-10.17, P=0.017) Increase in PLR after treatment >3.67-fold was associated with an increased risk of death (HR: 2.26, CI, 1.2-4.28; P=0.01). The Kaplan-Meier curve for survival as a function of ΔPLR is shown in Figure 1.
Figure 1.
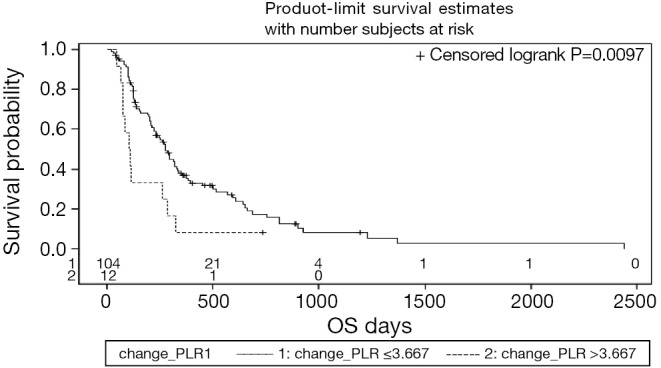
Kaplan-Meier overall survival curve of 116 patients after SIRT in correlation to ΔPLR. OS, overall survival; SIRT, selective internal radiation therapy; PLR, platelet-to-lymphocyte ratios.
Progression free survival (PFS)
Factors found to be significant for worse OS were also significant for worse PFS on UVA: higher post-treatment NC, higher post-treatment NLR, higher liver tumor volume, higher percentage liver tumor burden, and higher ECOG performance status. Higher post-treatment PLR was significant for worse PFS on UVA, whereas higher pretreatment PLR was significant on MVA for lower PFS. An increase in PLR after treatment was again associated with the highest increased risk of disease progression on MVA (Tables 4 and 5 show UVA and MVA for PFS, respectively.
Table 4. Univariate analysis of factors associated with progression free survival.
| Variable | Overall PFS HR | Overall PFS | Intrahepatic PFS | Distant PFS |
|---|---|---|---|---|
| ECOG 0,1 v ≥2 | 0.02 (0.01-0.19) | 0.001 | 0.005 | 0.001 |
| Pre-treatment platelet | 1 | 0.326 | 0.342 | 0.601 |
| Post-treatment platelet | 1 | 0.595 | 0.001 | 0.032 |
| Pre-neutrophil | 1 | 0.727 | 0.421 | 0.813 |
| Post-neutrophil | 1.12 (1.04-1.2) | 0.002 | <0.001 | 0.037 |
| Pre-lymphocyte | 0.81 | 0.190 | 0.139 | 0.047 |
| Post-lymphocyte | 0.51 | 0.087 | 0.841 | 0.930 |
| Pre NLR | 1.025 | 0.491 | 0.942 | 0.441 |
| Post NLR | 1.01 (1-1.02) | 0.008 | 0.214 | 0.246 |
| ΔNLR | 0.995 | 0.498 | 0.381 | 0.28 |
| Pre PLR | 1 | 0.222 | 0.473 | 0.936 |
| Post PLR | 1 (1-1.001) | 0.021 | 0.048 | 0.137 |
| ΔPLR | 1.1 | 0.060 | 0.071 | 0.013 |
| Liver tumor volume | 1 (1-1.002) | 0.001 | 0.033 | 0.055 |
| Liver tumor burden % | 1.01 (1-1.02) | 0.013 | 0.033 | 0.041 |
PFS, progression free survival; HR, hazard ratio; ECOG, Eastern Cooperative Oncology Group; NLR, neutrophil-to-lymphocyte; PLR, platelet-to-lymphocyte ratios.
Table 5. Multivariate analysis of factors associated with progression free survival in patients receiving SIRT .
| Variable | Overall PFS HR | 95% CI | Overall PFS | Intrahepatic PFS | Distant PFS |
|---|---|---|---|---|---|
| ECOG 0,1 v ≥2 | 0.310 | 0.003-0.276 | 0.0019 | 0.0012 | 0.0012 |
| Pre PLR | 1.002 | 1-1.003 | 0.0319 | – | – |
| ΔPLR | 1.178 | 1.054-1.318 | 0.0040 | 0.0271 | 0.0192 |
| Liver tumor volume | 1.001 | 1-1.002 | 0.0425 | 0.0425 | 0.0196 |
SIRT, selective internal radiation therapy; PFS, progression free survival; HR, hazard ratio; ECOG, Eastern Cooperative Oncology Group; NLR, neutrophil-to-lymphocyte; PLR, platelet-to-lymphocyte ratios.
Worse in-liver PFS was significantly related on MVA to ECOG performance status ≥2 (P=0.001), total tumor volume (P=0.043), and ΔPLR (P=0.027). Extrahepatic PFS was significantly related on MVA to ECOG performance status ≥2 (P=0.001), total tumor volume (P=0.02), and ΔPLR (P=0.019). The Kaplan-Meier curve for PFS as function of pre-treatment PLR is shown in Figure 2.
Figure 2.
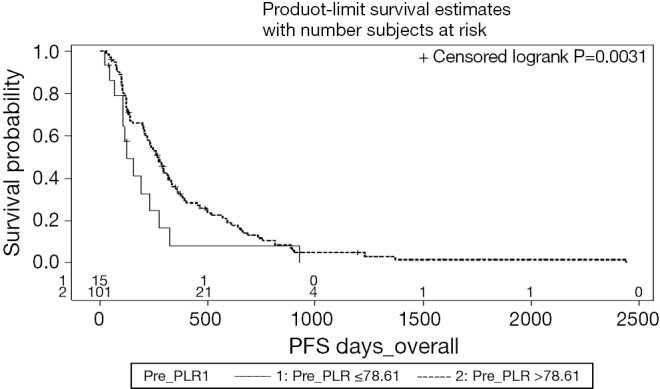
Kaplan-Meier progression free survival curve of 116 patients after SIRT in correlation with ΔPLR. SIRT, selective internal radiation therapy; PLR, platelet-to-lymphocyte ratios.
Cutoff values predictive of PFS were determined for ΔPLR and pretreatment PLR, given their significance on MVA. An increase in PLR >3.67 was associated with a hazard ratio of 2.21 (CI, 1.2-4.07; P=0.009) (Figure 3).
Figure 3.
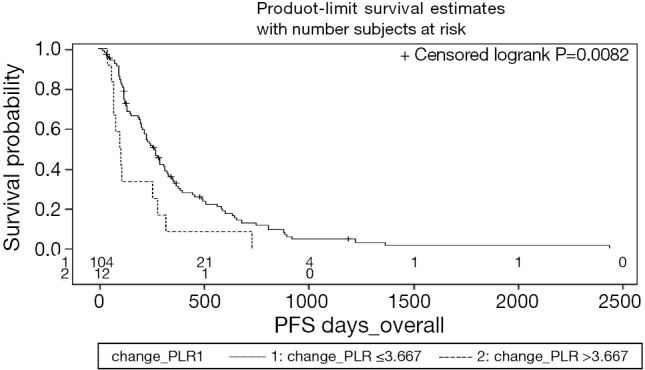
Kaplan-Meier progression free survival curve of 116 patients after SIRT in correlation with pre-treatment PLR. SIRT, selective internal radiation therapy; PLR, platelet-to-lymphocyte ratios.
Discussion
This study adds to the growing body of literature supporting NLR and PLR as predictors of clinical outcome and is the largest study to evaluate the correlation of NLR and PLR with survival and progression in patients receiving SIRT. In addition to commonly described SIRT patient and disease characteristics, such as performance status, percentage liver tumor burden, and absolute tumor volume, our results suggest that NLR, PLR, ∆NLR and ∆PLR may be strong predictive signals of outcome.
Increasing research focuses on awareness that the immune system plays a vital role in the efficacy of radiation therapy, including SIRT. SIRT has been shown to induce a serum proinflammatory cytokine response, including increased levels of IL-6 that can affect total leukocyte counts (18). The precise role of IL-6 in tumor biology and the clinical relevance of changes in its levels in cancer patients remain unknown; however, several studies suggest that serum IL-6 is a predictor of therapeutic effect induced by chemotherapy and radiotherapy (19). It is not surprising that we found, for example, that an increase in PLR early after treatment, which reflects a decrease in LC and/or increase in PC, was an independent predictor of worse survival. The potential for SIRT to stimulate an immune response and perhaps create a synergistic effect with novel immune modulators, such as immune checkpoint inhibitors, is the subject of ongoing investigation (20). Others have suggested that liver metastases in particular can have systemic immunosuppressive properties that limit the development of a robust antitumor response (21). Addressing this component of metastatic liver disease with SIRT may shift the balance between immune surveillance and tumor escape and thus create an opportunity to achieve better responses outside the liver.
If NLR or PLR reflects a snapshot of this immunologic balance, a shift in these ratios following treatment may be a sign that systemic-level tumor recognition and elimination is a possibility. The finding that ΔPLR is associated with extrahepatic PFS supports this hypothesis. However, although many patients experienced a decrease in NLR and/or PLR following SIRT, this did not correlate with clinical improvement in a statistically significant fashion. Outside of their association with clinical outcomes and prima facie meaning, the mechanistic importance of NLR and PLR is not well understood. It is theorized that neutrophilia and thrombocytosis are associated with increased vascular endothelial growth factor, matrix metalloproteinase, thrombospondin-1, and angiopoietin-1, among other agents that may enhance tumor survival, invasion, and metastasis (12,22). In addition, indistinct lymphocytopenia may reflect the explicit deficit of tumor antigen–specific effector lymphocytes. Alternatively, the relative proportion of blood component differentiation may reflect an altered and tumorigenic cytokine portfolio or simply the symptoms of nutritional, functional, and immunologic decline (10). Whatever the precise implications of an elevated NLR and/or PLR, their correlation with clinical outcome has been established in many studies and is further supported here.
Several limitations of our study include population heterogeneity (primary and metastatic tumors), potential selection bias, and lack of prospective data. However, the majority of these patients were enrolled on a prospective clinical trial, and their outcomes were followed prospectively. Another potential limitation of our study is that treatment was not uniform, because patients received whole liver, unilobar, or sequential lobar treatment and different prescribed radiation activities.
Others have described the independent prognostic significance of elevated NLR ≥5 in patients treated with radioembolization for liver cancer (23). This contrasts with our findings, in that elevated NLR did not emerge as an independent prognostic factor. This contrast may be indicative of the complexity and difference in immunologic response of liver-derived tumors between liver primary and metastatic tumors, because our patient cohort included both types of patients. Other studies support the prognostic value of NLR in patients with liver metastases (15); however, it could be that NLR is not as valuable in predicting survival in SIRT patients as in patients who received alternate liver cancer treatments.
Conclusions
This study has shown that any increase in PLR following SIRT is negatively prognostic of OS and disease response both inside and distant to the liver. In addition, our data suggest that an elevated pretreatment PLR can predict for disease outcomes before initiating SIRT. After ECOG performance status, increase in PLR was associated with the highest increased risk of death and overall disease progression. Pretreatment PLR may be useful in selecting patients for therapy, because it is also prognostic for progression and survival. Further research is needed to provide clarity in the mechanistic underpinnings of differences in the inflammatory response and resultant differences in NLR and PLR.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society, 2015:10-15. [Google Scholar]
- 2.Ananthakrishnan A, Gogineni V, Saeian K. Epidemiology of primary and secondary liver cancers. Semin Intervent Radiol 2006;23:47-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol 2014;20:4115-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbs P, Heinemann V, Sharma NK, et al. SIRFLOX: Randomized phase III trial comparing first-line mFOLFOX6 ± bevacizumab (bev) versus mFOLFOX6 + selective internal radiation therapy (SIRT) ± bev in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2015;33:abstr 3502. [DOI] [PubMed]
- 5.Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28:3687-94. [DOI] [PubMed] [Google Scholar]
- 6.Welsh JS, Kennedy AS, Thomadsen B. Selective internal radiation therapy (SIRT) for liver metastases secondary to colorectal adenocarcinoma. Int J Radiat Oncol Biol Phys 2006;66:S62-73. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastrointest Oncol 2015;6:134-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulec SA, Suthar RR, Barot TC, et al. The prognostic value of functional tumor volume and total lesion glycolysis in patients with colorectal cancer liver metastases undergoing 90Y selective internal radiation therapy plus chemotherapy. Eur J Nucl Med Mol Imaging 2011;38:1289-95. [DOI] [PubMed] [Google Scholar]
- 9.Haug AR, Tiega Donfack BP, Trumm C, et al. 18F-FDG PET/CT Predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med 2012;53:371-7. [DOI] [PubMed] [Google Scholar]
- 10.Fan W, Zhang Y, Wang Y, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PLoS One 2015:10:e0119312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil–lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One 2014;9:e101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang ZL, Luo J, Chen MS, et al. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. J Vasc Interv Radiol 2011;22:702-9. [DOI] [PubMed] [Google Scholar]
- 14.Peng W, Li C, Wen TF, et al. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. J Surg Res 2014;192:402-8. [DOI] [PubMed] [Google Scholar]
- 15.Kishi Y, Kopetz S, Chun YS, et al. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol 2009;16:614-22. [DOI] [PubMed] [Google Scholar]
- 16.Halazun KJ, Aldoori A, Malik HZ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol 2008;34:55-60. [DOI] [PubMed] [Google Scholar]
- 17.Tohme S, Sukato D, Chalhoub D, et al. Neutrophil-lymphocyte ratio is a simple and novel biomarker for prediction of survival after radioembolization for metastatic colorectal cancer. Ann Surg Oncol 2015;22:1701-7. [DOI] [PubMed] [Google Scholar]
- 18.Wickremesekera JK, Chen W, Cannan RJ, et al. Serum proinflammatory cytokine response in patients with advanced liver tumors following selective internal radiation therapy (SIRT) with (90)Yttrium microspheres. Int J Radiat Oncol Biol Phys 2001;49:1015-21. [DOI] [PubMed] [Google Scholar]
- 19.Petrini B, Andersson B, Strannegard O, et al. Monocyte release and plasma levels of interleukin-6 in patients irradiated for cancer. In Vivo 1992;6:531-4. [PubMed] [Google Scholar]
- 20.McNamara M. Radioembolization and ipilimumab in treating patients with uveal melanoma with liver metastases. ClinicalTrials.gov identifier NCT01730157. Available online: https://clinicaltrials.gov/ct2/show/NCT01730157. Accessed on July 19, 2015.
- 21.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 2013;24:1813-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soydal C, Keskin O, Kucuk ON, et al. Prognostic factors for prediction of survival of hepatocellular cancer patients after selective internal radiation therapy. Ann Nucl Med 2015;29:426-30. [DOI] [PubMed] [Google Scholar]
- 23.Sukato DC, Tohme S, Chalhoub D, et al. The prognostic role of neutrophil-to-lymphocyte ratio in patients with unresectable hepatocellular carcinoma treated with radioembolization. J Vasc Interv Radiol 2015;26:816-24.e1. [DOI] [PubMed]


