Abstract
Microglia are the resident immune cells of the brain, which are important therapeutic targets for regulating the inflammatory responses particularly neurodegeneration in the aging human brain. The activation, chemotaxis and migration of microglia are regulated through G-protein coupled receptors by chemokines such as stromal cell-derived factor (SDF)-1α and bioactive lysophospholipids such as lysophosphatidic acid (LPA). Potassium channels play important roles in microglial function and cell fate decisions; however, the regulation of microglial potassium channels has not been fully elucidated. Here we show reciprocal action of SDF-1α and LPA, on potassium currents through Kir2.1 channels in primary murine microglia. The potassium channel modulation is mediated by the same small GTPases, Rac and Rho that regulate the actin cytoskeleton. SDF-1α rapidly increased the Kir2.1 current amplitude and cell spreading. These effects were mimicked by dialysing the cells with constitutively active Rac1 protein, and they were blocked by inhibiting the phosphatidylinositol 3-kinase (PI3K) with wortmannin. In contrast, LPA and constitutively active RhoA decreased the Kir2.1 currents and stimulated cell contraction. Thus, SDF-1α and LPA regulate both the actin cytoskeleton and the Kir2.1 potassium channels through the same Rho GTPase signaling pathways. The inhibition of Kir2.1 with chloroethylclonidine produced cell contraction independently of chemokine action. This suggests that potassium channels are essential for the morphological phenotype and functioning of microglia. In conclusion, the small GTPases, Rac and Rho, modulate Kir2.1 channels and block of Kir2.1 channels causes changes in microglia morphology.
Keywords: microglia, Rho GTPases, PI3-kinase, potassium channel
Introduction
Microglia are the immune cells of the central nervous system (CNS) and provide the brain with a robust innate immune response (Harry and Kraft, 2012). Microglia, therefore, have a key role in the inflammatory state of the CNS associated with injury and pathological conditions. Microglia migrate to sites of injury, release cytokines and phagocytose dead neurons which are all key aspects of the immune response (Graeber et al., 2011; Kettenmann et al., 2011). However, because microglia also release neurotoxins, reactive oxygen species, and proinflammatory mediators, which can have negative effects, microglia can also cause a significant amount of damage during inflammation (Graeber et al., 2011; Skaper et al., 2012). It would not be therapeutically useful to completely suppress microglia because some of the inflammatory responses are beneficial. Therefore it is important to elucidate mechanisms which regulate recruitment and activation of microglial cells which may be specifically targeted.
There are many signals that modulate microglial migration and activation including chemokines and phospholipids. Stromal cell-derived factor-1 (SDF-1) or CXCL12, is an α-chemokine that exerts effects on the actin cytoskeleton (Murata et al., 2012; Wu et al., 2012) and is a potent chemoattractant for microglia (Lazarini et al., 2003). CXCR4 is upregulated in a number of brain diseases, such as Parkinson’s and in hypoxia which drives increased microglia migration and cytokine secretion at sites of injury (Wang et al., 2008; Shimoji et al., 2009). The only known receptor for SDF-1-α, CXCR4, is a seven transmembrane G protein-coupled receptor but little is known about the signaling mechanisms downstream from CXCR4 (Patrussi and Baldari, 2008). In addition, LPA is a versatile phospholipid that signals through G12/13 and Rho GTPase to stimulate actin remodeling including stress fiber formation and motility (Pellegrin and Mellor, 2007; Bernhart et al., 2010; Xiang et al. 2013).
Dynamic rearrangement of the actin cytoskeleton is the first step in microglial functions of migration and phagocytosis. The Rho family small GTPases, Rac1 and RhoA, coordinate many diverse cellular processes however, some of their most profound effects involve rearrangement of the actin cytoskeleton in response to chemotactic receptors during migration (Kolsch et al., 2008; Cain and Ridley, 2009; Guilluy et al., 2011). The coordinated and reciprocal action of Rac and Rho is necessary for regulating the proper polarization of a migrating cell (Charest and Firtel, 2007; Berzat and Hall, 2010). Several studies demonstrate that the small GTPase, Rac, is a downstream effector for phosphatidylinositide 3-kinases (PI3-kinases) which affects the rearrangement of the actin cytoskeleton in response to chemoattractants (Welch et al., 2003; Amano et al., 2010).
The ion channels expressed on microglia have been the topic of much research as the functional role of these channels is still not fully understood (Kettenmann et al., 1993; Walz et al., 1993; Schilling and Eder, 2011; Black and Waxman, 2012; Shepherd et al., 2012). One of the predominant currents observed in the microglia/macrophage cell lineage is an inwardly rectifying potassium current with biophysical properties typical of Kir2.1 (Newell and Schlichter, 2005; Schilling and Eder, 2007).
Here, we have examined the reciprocal modulation of microglial potassium currents by the chemokine SDF-1α and LPA through the signaling pathways involving Rac and Rho. We have tested whether microglia express Kir2.1 channels and have investigated the role of the inwardly rectifying potassium currents in microglial morphology.
Materials and Methods
Reagents
Recombinant murine colony stimulating factor-1 (CSF), SDF-1α (R&D Systems, Minneapolis, MN, USA), anti-CXCR4 antibody (fusin) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), Alexafluor 594-conjugated phalloidin, Alexfluor 594 anti-rabbit secondary antibody, AlexaFluor 488 or AlexaFluor 568 goat anti-rabbit secondary antibody (Invitrogen-Molecular Probes, Carlsbad, CA), FITC-conjugated F4/80 antibody (Serotec, Raleigh, NC), anti-Kir2.1 antibody (Alomone Labs, Jerusalem, Israel), Rac and Rho recombinant proteins (Cytoskeleton, Denver, CO), barium chloride (BaCl2), CEC, LPA and goat serum (Sigma, St. Louis, MO, USA), wortmannin and gramicidin A 50 mg/mL (Calbiochem, La Jolla, CA).
Purified Microglial Cultures
Highly purified microglia were prepared from 10 neonatal CD-1 mouse pups (P2–4) (Charles River Laboratories, Raleigh, NC). Briefly, cortices were minced in ice cold 0.25% trypsin and 100 U/mL DNase I (Ambion, Austin, TX). Cells were then triturated gently, agitated for 20 min at 37°C, triturated again, and agitated for a further 20 min. After dissociation, the mixture was pelleted (10 min at 400g), resuspended in DMEM, passed through a cell strainer (40-μm diameter mesh), and seeded in 75 cm2 flasks in 20 mL of culture medium (DMEM, 5% FBS, 5% HS, 1% Pen/Strep). After 10–12 days in culture, flasks were shaken at 225 rpm for 5 h at room temperature and floating cells were harvested. These cells were plated on either glass coverslips (Deutsche spielglas, Carolina Biological, Burlington, NC) for electrophysiology or tissue culture dishes. After 20 min in culture, the flasks or coverslips were shaken and media replaced with DMEM with 5% FBS, 5% HS, 1% Pen/Strep, and 5 ng/mL CSF with the exception that 24 h prior to experiments CSF was withdrawn and 12–14 h prior to experiments serum was withdrawn. The adherent cells (98–100% microglia as determined by immunostaining with F4/80) were incubated at 37°C, 5% CO2 for 2 days, and used for experiments between 3 and 10 days in culture.
Transfections
Microglial cells were transfected with plasmids encoding recombinant GTPases 18 h prior to recording using LIPOFECTAMINE 2000 in Opti-MEM 1 medium (Life Technologies, Inc., Rockville, MY). Rac constructs were a kind gift from Channing Der at the University of North Carolina in Chapel Hill and the Rho construct was a kind gift from Ian Whitehead at the University of Medicine and Dentistry of New Jersey. The plasmid constructs were cotransfected at a ratio of 1:10 with a plasmid encoding GFP (Clontech Labs, Palo Alto, CA). Successfully transfected cells were detected with UV fluorescence. Control currents were measured from cells transfected with GFP alone.
Electrophysiology
All current recordings were made at room temperature (20–25°C) with an Axopatch 200 amplifier (Molecular Devices, Sunnyvale, CA) using either gramicidin-perforated patches or conventional whole-cell recordings for dialysis of small GTPases. Electrodes (2–5 MΩ) were pulled from borosilicate glass tubing, heat-polished and filled with the pipette solution (in mM) 84 K-gluconate, 40 KCl, 10 HEPES, 2 BAPTA, 5 glucose, 1 MgCl2, 2 MgATP, 0.1 Na GTP, pH 7.2). After filling the tip of the pipette by capillary action, the patch pipette was backfilled with the pipette solution to which 75 μg/mL gramicidin had been added. The bath solution contained (in mM) 134 NaCl, 5 KCl, 10 HEPES, 1 CaCl2, 1 MgCl2, 5 glucose, pH 7.4. The bath and pipette solutions have a junction potential of 11 mV. The currents were recorded using pClamp software (Molecular Devices, Sunnyvale, CA) and normalized to cell capacitance in order to correct for differences in cell size. The average capacitance recorded for microglia was 22.1 ± 0.7 pF, n = 44 microglia cells. The inwardly rectifying currents that we recorded with our voltage protocol were very similar in amplitude and kinetics regardless of the pipette configuration. Recordings were analyzed using Clampfit software (Molecular Devices, Sunnyvale, CA). Data are presented as mean +/− s.e.m. of n experiments. Statistics were evaluated using either t test or an unpaired Student’s t test. Statistical significance was considered P<0.05.
Confocal Microscopy
A laser scanning confocal microscope (LSM 510, Carl Zeiss, Inc., Thornwood, NY) was used to image microglia morphology, actin cytoskeleton and Kir2.1 channel with an oil-immersion Plan-Apo 100 objective (NA1,4). Primary murine microglial cells were fixed with 4% paraformaldehyde for 10 min, washed twice with phosphate buffered saline, and permeabilized with 0.1% Triton X-100. Nonspecific interactions were blocked with either Superblock (Pierce, Rockford, IL) or 10% normal goat serum for 45 min and then washed twice. Cells were incubated overnight with primary antibody: phalloidin (1:100), CXCR4 receptors (1:200), Kir2.1 (1:200), and F4/80 (1:100). Cells were then washed and stained with the appropriate secondary antibody. Cells were excited with a 543 nm line of the laser while collecting emission with a 560-nm long pass filter or excited at 488 nm while collecting emission at 529 nm with a bandpass of 500–550 nm filter. Figures show representative of the majority of cells per treatment.
Results
SDF-1α Induces Actin Dependent Cell Spreading Through CXCR4 in Primary Murine Microglia
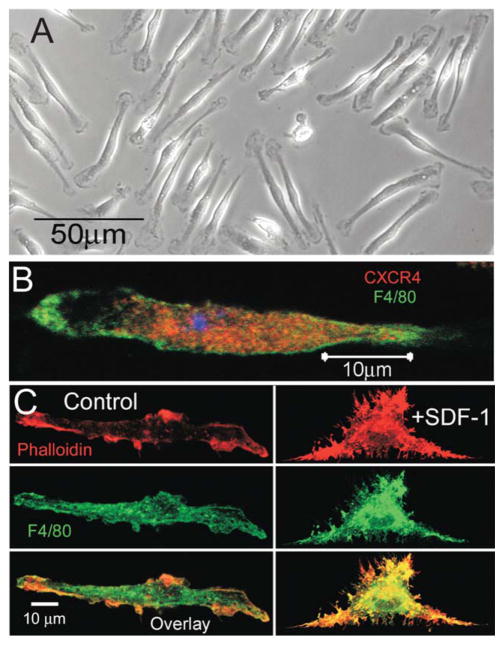
Freshly harvested cells plated with serum supplemented media and colony stimulating factor (CSF), a growth factor which stimulates microglial proliferation, exhibit a typical microglial bipolar phenotype shown in Fig. 1A. To further confirm that the primary cells plated were microglia, cells were stained with an antibody to a glycoprotein expressed on the surface membrane of cells of a macrophage lineage, F4/80 (green) (Fig. 1B). The presence of CXCR4, a G-protein coupled receptor and the only known receptor for SDF-1α, was detected by anti-CXCR4 antibody (red) staining on primary murine microglia as demonstrated in Fig. 1B. As SDF-1α activates microglia to produce the typical ramified phenotype, the effect of SDF-1α on microglial morphology was investigated by confocal microscopy of actin stained with Alexa-conjugated phalloidin (red) (Fig. 1C). Application of SDF-1α (100 ng/mL) to the cell media induced microglial spreading and production of numerous filapodia-like projections compared with untreated microglia. The bipolar shape of cells cultured in CSF, the concomitant staining with F4/80 (green) and CXCR4 and the morphological responses to SDF-1α all demonstrate microglial specificity of the culture Fig. 1C.
FIGURE 1.
SDF-1α induced cell spreading in microglia. A: Light microscope image showing the typical bipolar morphology of cultured primary murine microglia. B: Confocal image demonstrates the presence of CXCR4 receptors (red labeling, anti-CXCR4 antibody) on microglia (green labeling, FITC-conjugated F4/80). C: Confocal images showing that SDF-1α (100 ng/mL) induces cell spreading of microglia. Top panel shows actin filaments stained red with Alexafluor 594-conjugated phalloidin; middle panel shows green labeling of microglial marker FITC-conjugated F4/80 and the botton panel shows an overlay image of green labeling, FITC-conjugated F4/80 and red Alexafluor 594-conjugated phalloidin.
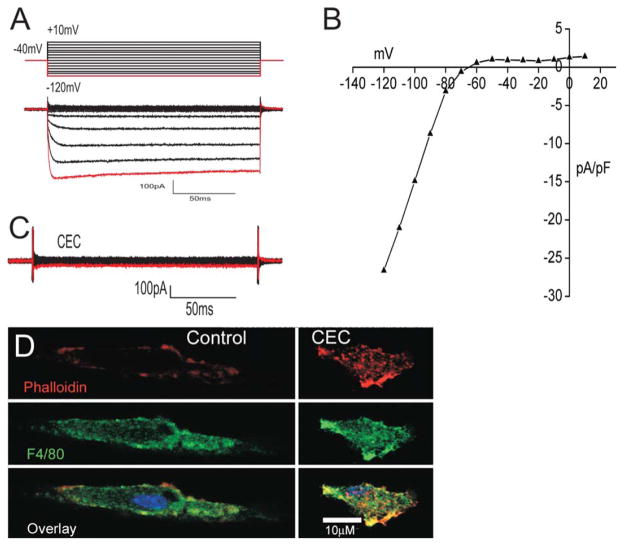
CEC Blocks a Kir2.1-Like Current and Induces Cell Contraction
The predominant current recorded by perforated patch-clamp from cultured primary murine microglia is an inwardly rectifying potassium current. A typical family of the currents evoked by a series of voltage steps is shown in Fig 2A. The red line in the voltage protocol corresponds to −120 mV which is also indicated by the red trace in the family of currents. The currents elicited by the protocol in Fig. 2 were typical of Kir2.1 channel currents with fast activation and deactivation kinetics. The current-voltage relationship is also typical of an inwardly rectifying potassium current like Kir2.1 (Fig. 2B). Application of BaCl2 (50 μM), which blocks Kir2.1 channels, reduced the current amplitude by an average of 86% (n = 5, data not shown). In addition, the channel was also completely inhibited by chloroethylclonidine (CEC; 100 μM), a direct blocker of Kir2.1 channels (Barrett-Jolley et al., 1999) (Fig. 2C, n = 5). The role of Kir2.1 channels in microglial morphology was tested using the Kir2.1 blocker CEC. Figure 2D demonstrates that CEC induced microglia to change from a bipolar morphology to a contracted phenotype lacking processes.
FIGURE 2.
The predominant current recorded in microglia is an inwardly rectifying potassium channel blocked by CEC. A: Protocol used and resultant family of currents elicited from microglia. The red trace corresponds to peak current at −120 mV. B: Current–voltage relationship shows a typical plot for an inwardly rectifying potassium channel current. C: Representative current traces after bath application of CEC (100 μM), (n = 5). D: Confocal images showing application of CEC (100 μM) induces contraction of microglia. Top panel shows actin stained red with Alexafluor 594-conjugated phalloidin; middle panel shows the microglia marker in green with FITC-conjugated F4/80 antibody and bottom panel shows an overlay image with yellow showing areas of colocalization.
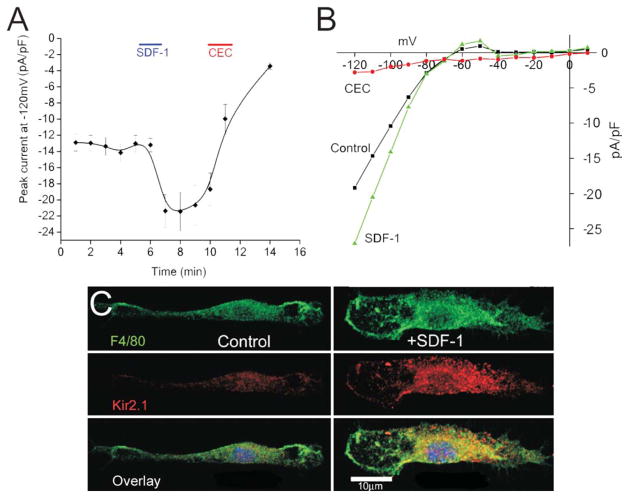
SDF-1 Induces an Instantaneous Increase in the Inward Rectifying Potassium Channel and Kir2.1 is Present on Primary Murine Microglia
Potassium currents of microglia cells measured by conventional whole-cell recording gradually decrease or rundown over time, probably due to dialysis of the pipette contents into the cell and washing away second messengers. To avoid rundown, the perforated-patch method was used to maintain the integrity of the intracellular milieu for the duration of the recordings. The effect of SDF-1α on the inwardly rectifying potassium current was tested. SDF-1α (100 ng/mL) increased the current density by ~40% (n = 7) within 2 min of bath application (Fig. 3A). Despite recording in the perforated-patch mode the current increase was transient with the increase in current amplitude returning to baseline level within minutes of recording. Application of the Kir2.1 channel blocker CEC (100 μM) completely blocked the current (Fig. 3A,B). A representative plot of the current–voltage relationship from a microglia cell is shown in Fig. 3B. As Kir2.1 channels have been proposed to be expressed on microglia and the biophysical and pharmacology is indicative of a Kir2.1 current, the presence of Kir2.1 channel proteins on microglia was tested using an immunofluorescent Kir2.1 monoclonal antibody. Confocal microscopy demonstrated the presence of Kir2.1 channel protein on primary murine microglia stimulated with SDF-1α (Fig. 3C). The lower panel shows the merged image of the macrophage cell lineage surface glycoprotein F4/80 antibody staining and immunofluorescent Kir2.1 antibody staining showing plasma membrane localization of Kir2.1. Although the immunohistochemistry shows the presence of the Kir2.1 channel protein, due to the lack of a more specific Kir2.1 channel blocker the currents have been referred to as the Kir2.1-like currents.
FIGURE 3.
SDF-1α induces a rapid, transient increase of Kir2.1-like current. A: Time course showing current (pA/pF) at −120 mV of microglia stimulated with 100 ng/mL SDF-1α (SDF-1 bar indicates time of bath application) and inhibition of the current by Kir2.1 inhibitor CEC (CEC bar indicates duration of bath application) using gramicidin-perforated patches (n = 7) (*P < 0.01). B: Current–voltage relationship from a typical microglia using the voltage protocol shown in figure 2 A (control, black; SDF-1α, green; CEC, red). C: Confocal images showing microglia stimulated with (right panels labeled SDF-1α) and without SDF-1α (left panels labeled control). Top panel shows the microglia marker FITC-conjugated F4/80 antibody in green; middle panel demonstrates the presence of Kir2.1 channels (red labeling) and bottom panel shows and overlay image of both membrane protein F4/80 (green) and Kir2.1 channel staining (red) with yellow showing areas of colocalization.
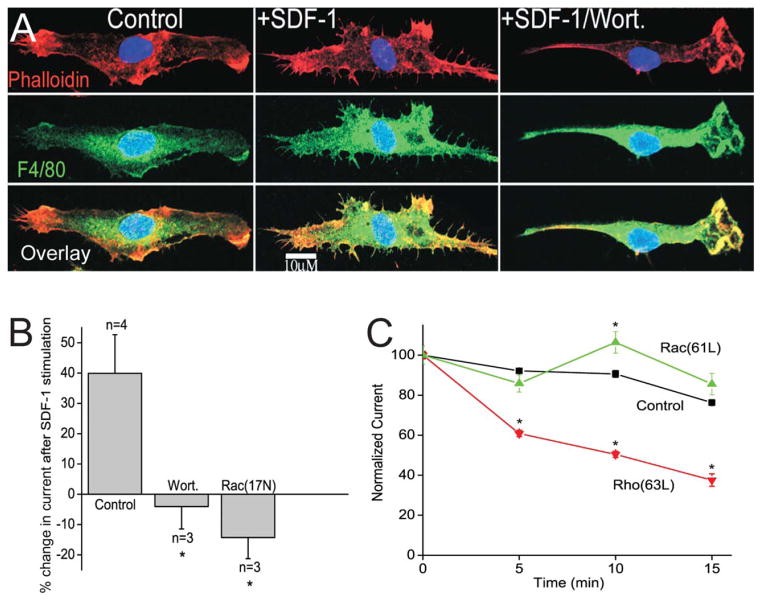
SDF-1α Signals Through PI3-kinase and Rac
PI3-kinase and Rac are effectors for some Gi coupled receptors and are known to modulate some potassium channels, however, little is known of their effect on Kir2.1. First the effect of a PI3-kinase inhibitor, wortmannin, was tested on the cytoskeletal changes and current increase induced by SDF-1α. The appearance of filopodia and cell spreading in response to SDF-1α was blocked in the presence of wortmannin (50 nM) (Fig. 4A). In addition, incubation of microglia in wortmannin (50 nM) completely blocked the increase in Kir2.1-like current by SDF-1α (Fig. 4B). As Rac can be activated by PI3-kinase, the effect of Rac on current density was tested by transiently transfecting DNA encoding dominant-negative Rac1 (17N) into primary microglia. Expression of Rac1 (17N) completely blocked SDF-1α stimulated increase of the inwardly rectifying current (Fig. 4B). For Rac (17N) transfected cells the current density was not significantly different from control cells; Rac (17N) transfected −18.8 ± 4 pA/pF, n = 3 compared with controls −22.2 ± 3 pA/pF, n = 3.
FIGURE 4.
SDF-1α signaling involves PI3-kinase and the small GTPase Rac. A: Left panels show a control untreated microglia, the middle panels shows SDF-1α (100 nM) treated microglia and the right panels show microglia treated with SDF-1α (100 nM) and wortmannin (50 nM). The top panels show actin stained red with Alexafluor 594-conjugated phalloidin; middle panel shows the microglia marker in green with FITC-conjugated F4/80 antibody and bottom panel shows an overlay image with yellow showing areas of colocalization. B: Percent change in Kir2.1-like current amplitude after exposure to SDF-1α (100 ng/mL). Bath application of wortmannin (50 nM) or expression of dominant-negative Rac (17N) prevented stimulation of the current by SDF-1α. C: Time course of change in normalized peak current in control cells (control, black, n = 6) and cells dialyzed with 6 μg/mL constitutively active Rac1 protein (61L, green, n = 6) or constitutively active RhoA protein (Rho 63L, red, n = 5) from the patch-pipette into the cell. The currents were normalized to the current amplitude at the start of the experiments to allow comparison between cells. *P < 0.05.
The involvement of Rac in SDF-1α signaling was further tested by dialysis of constitutively active proteins into the cell through the patch-pipette. Dialysis of constitutively active Rac1 protein (6 μg/mL) induced an acute, transient increase in current density compared with controls (Fig. 4C). Rundown of these currents is typical even when recording in the perforated-patch mode. After 10 min of recording the control current decreased by ~15%, however, dialysis of constitutively active Rac protein into the cell from the patch-pipette prevented rundown and resulted in a significant but transient increase in current density (n = 6) (Fig. 4C). The transitory nature of the current increase was also observed when microglia were stimulated with SDF-1α.
LPA Signals Through RhoA
The effect of Rac on ion channel modulation has been shown to be opposed by RhoA signaling in many cell types. The effect of RhoA on the microglia potassium currents was tested by the dialysis of constitutively active RhoA protein (6 μg/mL) into microglia through the patch-pipette. This resulted in a significant decrease in current amplitude (~65%, n = 5) compared with the normal rundown observed over a 15 min time period (~30%) (Fig. 4C). Consistent with this observation, transient transfection of DNA encoding for constitutively active RhoA protein into microglia resulted in a significant decrease current density, showing a decrease to −7 ± 3 pA/pF n = 3 compared with control cells 28 ± 6 pA/pF n = 3, P<0.05. Lysophosphatidic acid (LPA), is a bioactive lysophospholipid released from active platelets in response to brain injury. LPA stimulates G12/13 coupled receptors and activates the small GTPase Rho. Because Rac and Rho are known to have antagonistic effects on the actin cytoskeleton, we therefore postulated that LPA administration could modify microglial potassium currents and morphology in an opposite manner to SDF-1α. Figure 5A demonstrates that LPA (100 μM) induced microglia to change from a bipolar morphology to a contracted phenotype lacking processes. In addition, LPA application resulted in a significant decrease the current density by ~80% in microglia (n = 3). Figure 5B shows a typical family of current traces before (control) and after bath application of LPA (100 μM).
FIGURE 5.
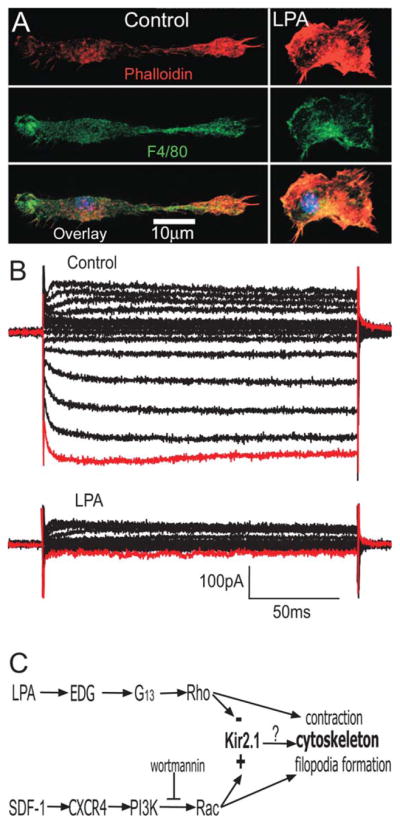
LPA induces contraction of the microglial cytoskeleton and decreases the current amplitude. A: Stimulation of microglia with LPA (100 μM) induces contraction of microglia. The top panels show actin stained red with Alexafluor 594-conjugated phalloidin; middle panel shows the microglia marker in green with FITC-conjugated F4/80 antibody and bottom panel shows an overlay image with yellow showing areas of colocalization. B: A family of potassium currents elicited from a microglia and the response to bath addition of LPA (100 μM). The red trace corresponds to peak current at −120 mV, n = 3, *P < 0.05. C: Diagrammatic summary of proposed signalling pathways regulating cytoskeleton and ion channel in microglia.
Discussion
SDF-1α Signals to Kir2.1-Like Channels and the Actin Cytoskeleton
Microglia, the immune effector cells of the CNS, migrate throughout the brain to sites of injury or disease to perform essential immune responses such as phagocytosis and the release of inflammatory mediators. Numerous studies of immune cells have demonstrated a link between potassium channel function and cell motility (Schwab et al., 2012). Indeed, inhibition of potassium channel, KCa3.1 has been shown to be sufficient to prevent migration in lung dendritic cells (Shao et al., 2011). However, the signaling link between chemotactic factors, potassium channel modulation and cytoskeletal rearrangement is unclear.
In this study, the signaling of two important agents that regulate the function of microglia have been investigated, the chemokine SDF-1α and the phospholipid lysophosphatidic acid (LPA). Primary murine microglia were used to show, for the first time, the regulation of Kir2.1-like potassium channels by SDF-1α signaling through PI3-kinase and the small GTPase Rac. Stimulation with SDF-1α also led to a dynamic change in the actin cytoskeleton to a ramified microglial phenotype. Conversely, the phospholipid LPA, which signals through G12/13 coupled EDG receptors remodels the actin cytoskeleton to form a compact, contracted phenotype through the small GTPase Rho and also rapidly inhibited the microglial potassium channels through a Rho-dependent process. Both the LPA and SDF-1α effects on Kir2.1 are more pronounced than the effects of dialysis of constitutively active Rac or Rho alone, this indicates that other factors may also play role in signaling the modulation of Kir2.1. Numerous studies demonstrate that the Rho family GTPases Rac1 and RhoA function in an antagonistic or reciprocal manner to modulate actin dynamics (Raftopoulou and Hall, 2004; Iden and Collard, 2008; Guilluy et al., 2011). Here, we have shown that they have a similarly reciprocal or opposing effect on microglial potassium channels. Consistent with this observation other ion channels have been shown to be modulated by small GTPases (Storey et al., 2002, 2006; Boyer et al., 2009).
In this study microglia transfected with dominant negative Rac showed no significant difference in current density. This is in contrast to Boyer et al. (2009) who described a ~200% increase in Kir2.1 currents of cardiac myocytes transfected with dominant negative Rac. These contrasting findings might reflect differential regulation of ion channels of microglia compared with cardiac ventricular myocytes. This study also shows that transfection of microglia with constitutively active Rho results in a decrease in current density. This is consistent with the findings of Jones (2003) who also described a decrease in current density of Kir2.1 in HEK293 cells transfected with constitutively active Rho.
Calcium-dependent potassium currents have been reported to increase in response to lysophospholipids in microglia (Schilling et al., 2002). In this study calcium dependent potassium currents were not observed perhaps because this is a different cell type and also as BAPTA was included in the patch pipette for the ruptured-patch whole-cell experiments. In this study, the inwardly rectifying potassium current was shown to have the same biophysical properties as Kir2.1 currents based on the electrophysiological characteristics and sensitivity to blockers such as BaCl2 and CEC, a direct blocker of Kir2.1 (Barrett-Jolley et al., 1999). Confocal microscopy confirmed that microglia express Kir2.1 channel protein by immunofluorescent staining with a specific monoclonal Kir2.1 antibody. This suggests that the current observed is likely to be predominantly a Kir2.1 channel current. Rho GTPases have been reported previously to rapidly regulate Kir2.1 channels (Jones, 2003) and the focus of other studies has been on changes in trafficking proteins to and from the surface membrane over a time course of hours (Boyer et al., 2009). Here we demonstrate rapid changes in Kir2.1 activity that is synchronous with changes in cell morphology.
There are several lines of evidence to suggest that Kir2.1 is important for cytoskeletal remodeling. Processes that depend on dynamic actin rearrangement are impaired when Kir2.1 is inhibited. For instance, inhibition of Kir2.1 by antisense RNA expression blocked myoblast fusion (Fischer-Lougheed et al., 2001). In addition, vascular smooth muscle cells negative for filamin-A, an actin-binding protein, showed decreased Kir2.1 current, decreased surface expression of Kir2.1 and more importantly, the myocytes remained rounded up and were unable to spread (Sampson et al., 2003). Actin-binding protein filamin-A binds both Kir2.1 and Rho family GTPases (Sampson et al., 2003; Ohta et al., 2006) therefore one possibility is that close proximity of Kir2.1 to Rho GTPases is important to organize cytoskeletal remodeling.
The data shown here demonstrates a transient response to SDF-1α, in this case transient Kir2.1 activation. Transient responses are commonly reported in studies on chemoattractant signaling, including actin polymerization, small GTPase activation and PIP3 accumulation (Ridley, 2011). PIP3 is produced by PI3-kinase and is metabolized by PTEN and by SHIP, and the activity of those enzymes can be regulated by many factors. During chemotaxis, transient activation of PI3-kinase, transient membrane localization of PIP3 and Rac to the leading edge has been observed (Cain and Ridley, 2009; Kuiper et al., 2011; Gerisch et al., 2012). The findings reported here suggest that at the leading edge CXCR4 receptors are activated by SDF-1α resulting in a transient accumulation of PIP3, Rac1 activation, and thus a transient increase in Kir2.1 current amplitude. At any given time a number of chemoattractive factors may influence a roving microglial cell and the transient nature of the response may provide a way of differentiating between spurious signals and a continuing chemoattractant signal.
The opposing modulation of Kir2.1 by SDF-1α and LPA and in microglia may reflect their different functions. SDF-1α is a chemoattractant which requires Rac dominated leading edge protrusion, whereas LPA enhances nondirectional chemokinetic movement which requires Rho activation and may play a role in retraction of the trailing end (Janetopoulos and Firtel, 2008; Schwab et al., 2012). The molecular effects downstream of Kir2.1 modulation on microglial function are still not fully known, although it may involve many factors that are dependent on changes in membrane potential. One example might be an increase in calcium influx through transient receptor potential channels (TRP) which are expressed on microglia and are controlled by membrane potential (Jiang et al., 2003; Konno et al., 2012). In addition, some signaling cascades are initiated by changes in membrane potential such as ERK activation in renal epithelial cells and activation of voltage sensitive phosphoinositde phosphatase in phagocytes (Iwasaki et al., 2008; Waheed et al., 2010).
In summary, this study shows that the chemoattractant factor SDF-1α and the phospholipid mediator LPA activate the small GTPases Rac and Rho respectively and signal to Kir2.1-like channels to regulate current density in primary microglia in vitro through the same Rho family GTPases that have been implicated by others in their regulation of cytoskeletal changes.
Acknowledgments
Grant sponsor: This work was supported by the NIH Intramural Research Program at the National Institute of Environmental Health Sciences, NIH, DHHS.
The authors are grateful to Channing Der and Ian Whitehead for providing Rho GTPase family constructs and to Susan Brunsson, University of North Carolina at Chapel Hill, and Chris McPherson, NIEHS, for technical assistance with the microglia preparation.
References
- Amano M, Nakayama M, Kaibuchi K. Rho-Kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Jolley R, Dart C, Standen NB. Direct block of native and cloned (Kir2.1) inward rectifier K+ channels by chloroethylclonidine. Br J Pharmacol. 1999;128:760–766. doi: 10.1038/sj.bjp.0702819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhart E, Kollroser M, Rechberger G, Reicher H, Heinemann A, Schratl P, Hallstrom S, Wintersperger A, Nusshold C, DeVaney T, Zorn-Pauly K, Malli R, Graier W, Malle E, Sattler W. Lysophosphatidic acid receptor activation affects the C13NJ microglia cell line proteome leading to alterations in glycolysis, motility, and cytoskeletal architecture. Proteomics. 2010;10:141–158. doi: 10.1002/pmic.200900195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzat A, Hall A. Cellular responses to extracellular guidance cues. EMBO J. 2010;29:2734–2745. doi: 10.1038/emboj.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Waxman SG. Sodium channels and microglial function. Exp Neurol. 2012;234:302–315. doi: 10.1016/j.expneurol.2011.09.030. [DOI] [PubMed] [Google Scholar]
- Boyer SB, Slesinger PA, Jones SVP. Regulation of Kir2.1 Channels by the Rho-GTPase, Rac1. J Cell Physiol. 2009;218:385–393. doi: 10.1002/jcp.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain RJ, Ridley AJ. Phosphoinositide 3-kinases in cell migration. Biol Cell. 2009;101:13–29. doi: 10.1042/BC20080079. [DOI] [PubMed] [Google Scholar]
- Charest PG, Firtel RA. Big roles for small GTPases in the control of directed cell movement. Biochem J. 2007;401:377–390. doi: 10.1042/BJ20061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Lougheed J, Liu J-H, Espinos E, Mordasini D, Bader CR, Belin D, Bernheim L. Human myoblast fusion requires expression of functional inward rectifier Kir2.1 channels. J Cell Biol. 2001;153:677–686. doi: 10.1083/jcb.153.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G, Schroth-Diez B, Muller-Taubenberger A, Ecke M. PIP3 Waves and PTEN dynamics in the emergence of cell polarity. Biophys J. 2012;103:1170–1178. doi: 10.1016/j.bpj.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. Febs Lett. 2011;585:3798–3805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: Another social network? Trends Cell Biol. 2011;21:718–726. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry GJ, Kraft AD. Microglia in the developing brain: A potential target with lifetime effects. Neurotoxicology. 2012;33:191–206. doi: 10.1016/j.neuro.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Murata Y, Kim YJ, Hossain MI, Worby CA, Dixon JE, McCormack T, Sasaki T, Okamura Y. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2008;105:7970–7975. doi: 10.1073/pnas.0803936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos C, Firtel RA. Directional sensing during chemotaxis. FEBS Lett. 2008;582:2075–2085. doi: 10.1016/j.febslet.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XP, Newell EW, Schlichter LC. Regulation of a TRPM7-like current in rat brain microglia. J Biol Chem. 2003;278:42867–42876. doi: 10.1074/jbc.M304487200. [DOI] [PubMed] [Google Scholar]
- Jones SVP. Role of the small GTPase rho in modulation of the inwardly rectifying potassium channel Kir2.1. Mol Pharmacol. 2003;64:987–993. doi: 10.1124/mol.64.4.987. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Banati R, Walz W. Electrophysiological behahior of microglia. Glia. 1993;7:93–101. doi: 10.1002/glia.440070115. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno M, Shirakawa H, Iida S, Sakimoto S, Matsutani I, Miyake T, Kageyama K, Nakagawa T, Shibasaki K, Kaneko S. Stimulation of transient receptor potential vanilloid 4 channel suppresses abnormal activation of microglia induced by lipopolysaccharide. Glia. 2012;60:761–770. doi: 10.1002/glia.22306. [DOI] [PubMed] [Google Scholar]
- Kuiper JWP, Sun CX, Magalhaes MAO, Glogauer M. Rac regulates PtdInsP(3) signaling and the chemotactic compass through a redox-mediated feedback loop. Blood. 2011;118:6164–6171. doi: 10.1182/blood-2010-09-310383. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- Murata K, Kitaori T, Oishi S, Watanabe N, Yoshitomi H, Tanida S, Ishikawa M, Kasahara T, Shibuya H, Fujii N, Nagasawa T, Nakamura T, Ito H. Stromal cell-derived factor 1 regulates the actin organization of chondrocytes and chondrocyte hypertrophy. Plos One. 2012;7:e37163. doi: 10.1371/journal.pone.0037163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Schlichter LC. Integration of K+ and Cl− currents regulate steady-state and dynamic membrane potentials in cultured rat microglia. J Physiol. 2005;567:869–890. doi: 10.1113/jphysiol.2005.092056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Hartwig JH, Stossel TP. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–U35. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- Patrussi L, Baldari CT. Intracellular mediators of CXCR4-dependent signaling in T cells. Immunol Lett. 2008;115:75–82. doi: 10.1016/j.imlet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Sampson LJ, Leyland ML, Dart C. Direct interaction between the actin-binding protein filamin-A and the inwardly rectifying potassium channel, Kir2.1. J Biol Chem. 2003;278:41988–41997. doi: 10.1074/jbc.M307479200. [DOI] [PubMed] [Google Scholar]
- Schilling T, Eder C. Ion channel expression in resting and activated microglia of hippocampal slices from juvenile mice. Brain Res. 2007;1186:21–28. doi: 10.1016/j.brainres.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Schilling T, Eder C. Amyloid-beta-induced reactive oxygen species production and priming are differentially regulated by ion channels in microglia. J Cell Physiol. 2011;226:3295–3302. doi: 10.1002/jcp.22675. [DOI] [PubMed] [Google Scholar]
- Schilling T, Repp H, Richter H, Koschinski A, Heinemann U, Dreyer F, Eder C. Lysophospholipids induce membrane hyperpolarization in microglia by activation of IKCa1Ca(2+)-dependent K+ channels. Neuroscience. 2002;109:827–835. doi: 10.1016/s0306-4522(01)00534-6. [DOI] [PubMed] [Google Scholar]
- Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- Shao ZF, Makinde TO, Agrawal DK. Calcium-activated potassium channel KCa3.1 in lung dendritic cell migration. Am J Respir Cell Mol Biol. 2011;45:962–968. doi: 10.1165/rcmb.2010-0514OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ, Loo L, Gupte RP, Mickle AD, Mohapatra DP. Distinct modifications in Kv2.1 channel via chemokine receptor CXCR4 regulate neuronal survival-death dynamics. J Neurosci. 2012;32:17725–17739. doi: 10.1523/JNEUROSCI.3029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji M, Pagan F, Healton EB, Mocchetti I. CXCR4 and CXCL12 expression is increased in the nigro-striatal system of Parkinson’s disease. NeurotoxRes. 2009;16:318–328. doi: 10.1007/s12640-009-9076-3. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Giusti P, Facci L. Microglia and mast cells: Two tracks on the road to neuroinflammation. FASEB J. 2012;26:3103–3117. doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- Storey NM, Gentile S, Ullah H, Russo A, Muessel M, Erxleben C, Armstrong DL. Rapid signaling at the plasma membrane by a nuclear receptor for thyroid hormone. Proc Natl Acad Sci USA. 2006;103:5197–5201. doi: 10.1073/pnas.0600089103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey NM, O’Bryan JP, Armstrong DL. Rac and Rho mediate opposing hormonal regulation of the ether-A-Go-Go-related potassium channel. Curr Biol. 2002;12:27–33. doi: 10.1016/s0960-9822(01)00625-x. [DOI] [PubMed] [Google Scholar]
- Waheed F, Speight P, Kawai G, Dan QH, Kapus A, Szaszi K. Extracellular signal-regulated kinase and GEF-H1 mediate depolarization-induced Rho activation and paracellular permeability increase. Am J Physiol Cell Physiol. 2010;298:C1376–C1387. doi: 10.1152/ajpcell.00408.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W, Ilschner S, Ohlemeyer C, Banati R, Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse-brain. J Neurosci. 1993;13:4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Li CX, Chen Y, Hao YT, Zhou W, Chen CH, Yu ZP. Hypoxia enhances CXCR4 expression favoring microglia migration via HRF-1 alpha activation. Biochem Biophys Res Commun. 2008;371:283–288. doi: 10.1016/j.bbrc.2008.04.055. [DOI] [PubMed] [Google Scholar]
- Welch HCE, Coadwell WJ, Stephens LR, Hawkins PT. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 2003;546:93–97. doi: 10.1016/s0014-5793(03)00454-x. [DOI] [PubMed] [Google Scholar]
- Wu X, Yu T, Bullard DC, Kucik DF. SDF-1 alpha (CXCL12) regulation of lateral mobility contributes to activation of LFA-1 adhesion. Am J Physiol Cell Physiol. 2012;303:C666–C672. doi: 10.1152/ajpcell.00190.2012. [DOI] [PubMed] [Google Scholar]
- Xiang SY, Dusaban SS, Brown JH. Lysophospholipid receptor activation of RhoA and lipid signaling pathways. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 2013;1831:213–222. doi: 10.1016/j.bbalip.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]