Abstract
Aim
The present study aims to estimate the relative importance of genetic and environmental factors for health-related quality of life (HRQL) measured by the 12-item Short-Form Health Survey (SF-12).
Methods
The study was based on two Danish twin cohorts (46,417 twin individuals) originating from the nationwide, population-based Danish Twin Registry. The twins were approached by a mailed-out questionnaire in 2002. The questionnaire included the SF-12, information on demographic factors, and questions on a variety of specific diseases. Heritability of the SF-12 includes the physical component summary (PCS) and the mental component summary (MCS); and etiologically important variance components were estimated using multivariate biometric models. The respondents were stratified into six groups, based on age and sex.
Results
A total of 33,794 (73%) individual twins responded to the survey. The SF-12 was completed by 29,619 individuals, which included 9,120 complete twin pairs. Overall, the best-fitting model explaining the variance of HRQL was the ACE model. The estimated heritability of the SF-12 was between 11% and 35%, whereas between 65% and 89% could be explained by unique environmental or stochastic factors in the different sex and age groups. The highest heritability was seen among older twins. In addition, the genetic correlation between MCS and PCS scores was low (0.07 and 0.23 for males and females, respectively) among younger and high (0.26 and 0.45 for males and females, respectively) in the oldest age group. Both the largest genetic influence on HRQL and the largest genetic overlap between the scores were seen in the oldest age group, which consisted of twins older than 55. The unique environmental correlation between MCS and PCS were generally negative.
Conclusion
The heritability of HRQL differs between different age groups. In general, most of the variance in the SF-12 summary components was determined by unique environmental factors.
Keywords: health-related quality of life, twin study, heritability, genetic models
The World Health Organization (WHO) defines quality of life (QL) as an ‘individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns’ (WHO, 1997, p. 1). Thus, QL is a broad-ranging concept that is affected by the individual’s physical health, psychological state, level of independence, social relationships, life fulfillment, and other factors. The focus of this article is the health-related quality of life (HRQL) that captures QL in the context of an individual’s mental and physical health.
HRQL can be assessed either descriptively or normatively by self-administered standardized psychometric tools. One of the most commonly used descriptive and generic measure for self-assessed HRQL is the 36-item short-form health survey (SF-36), which has eight dimensions, including physical functioning, physical role, bodily pain, general health, vitality, social functioning, emotional role, and mental health. A shorter version of the SF-36 is the 12-item Short-Form Health Survey (SF-12; Jenkinson & Layte, 1997; Jenkinson et al., 1997; Ware et al., 1996). The SF-12 was developed to provide a single-page HRQL that was suitable for population surveys. The SF-12 includes 12 items from the SF-36 that compose the physical component summary (PCS) and the mental component summary (MCS). The two dimensions, MCS and PCS, explain more than 80% of the variance in the original eight SF-36 scales (Bjorner et al., 2003;Ware et al., 1996). Both the SF-12 and the SF-36 are valid and reliable for determining general health outcomes in populations (Bjorner et al., 2003; Gandek et al., 1998; Jenkinson & Layte, 1997; Jenkinson et al., 1997, 2001; Ware et al., 1996). However, the different scales have also been used as screening tools for specific diseases. For instance, it has been shown that the MCS can be used for identifying people with anxiety and depression (Gill et al., 2007).
Previous studies on the heritability of QL and subjective well-being report heritability estimates of 36–50% (Bartels & Boomsma, 2009; Bartels et al., 2010). Although it is likely that some of the factors that determine general well-being also influence the self-rated health, QL and subjective well-being are much broader concepts that reflect a variety of other factors in addition to general health perception.
With regard to descriptive studies on self-rated health and HRQL, most studies use a simple item such as ‘What do you think about your current health status?’, ‘How do you consider your health in general?’, or ‘How, in general, is your health?’ This question is also included in the SF-12 as the first item (Table 1). In general, the genetic modeling of self-rated health based on this simple HRQL item showed that the best-fitting model was the ACE or the AE model, and the reported additive genetic effect explained between 8% and 63% of the variance (deMoor et al., 2007; Leinonen et al., 2005; Mosing et al., 2009, 2010; Romeis et al., 2000; Silventoinen et al., 2007; Svedberg et al., 2001, 2006). Interestingly, the Finnish and Swedish studies found that the variance of self-rated health was age dependent (Leinonen et al., 2005; Silventoinen et al., 2007; Svedberg et al., 2001). In addition, the reported HRQL shared genetic components with underlying diseases and level of function, thus supporting the hypothesis that the genetic impact on HRQL correlates with the genetics of the variety of underlying diseases and conditions that determine the subjective overall evaluation of people’s health (Leinonen et al., 2005). The heritability of HRQL has also previously been studied using the SF-36 on middle-aged male twins (Romeis et al., 2005). As with the other studies on HRQL, this study concluded that the best-fitting model was the AE model; however, it estimated that additive genetic factors explained only 17 to 33%of the variance of HRQL in that population.
TABLE 1.
Items and Respective Endorsements of the SF-12 Version 1
| Endorsements | ||||
|---|---|---|---|---|
| Items | Score | PCS | MCS | |
| 1 | In general would you say your health is: | Excellent (1) | 0 | 0 |
| Very good (2) | −1.31872 | −0.06064 | ||
| Good (3) | −3.02396 | 0.03482 | ||
| Fair (4) | −5.56461 | −0.16891 | ||
| Poor (5) | −8.37399 | −1.71175 | ||
| The following two questions are about activities you might do during a typical day. Does your health now limit you in these activities? If so, how much? | ||||
| 2 | Moderate activities, such as moving a table, pushing a vacuum cleaner, bowling, or playing golf: | Yes, limited a lot (1) | −7.23216 | 3.93115 |
| Yes, limited a little (2) | −3.4555 | 1.86840 | ||
| No, not limited at all (3) | 0 | 0 | ||
| 3 | Climbing several flights of stairs: | Yes, limited a lot (1) | −6.24397 | 2.68282 |
| Yes, limited a little (2) | −2.73557 | 1.42103 | ||
| No, not limited at all (3) | 0 | 0 | ||
| During the past 4 weeks, have you had any of the following problems with your work or other regular activities as a result of your physical health? | ||||
| 4 | Accomplished less than you would like: | Yes (1) | −4.61617 | 1.44060 |
| No (2) | 0 | 0 | ||
| 5 | Were limited in the KIND of work or other activities: | Yes (1) | −5.51747 | 1.66968 |
| No (2) | 0 | 0 | ||
| During the past 4 weeks, were you limited in the kind of work you do or other regular activities as a result of any emotional problems (such as feeling depressed or anxious)? | ||||
| 6 | Accomplished less than you would like: | Yes (1) | 3.04365 | −6.82672 |
| No (2) | 0 | 0 | ||
| 7 | Didn’t do work or other activities as carefully as usual: | Yes (1) | 2.32091 | −5.69921 |
| No (2) | 0 | 0 | ||
| 8 | During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)? |
Not at all (1) | 0 | 0 |
| A little bit (2) | −3.80130 | 0.90384 | ||
| Moderately (3) | −6.50522 | 1.49384 | ||
| Quite a bit (4) | −8.38063 | 1.76691 | ||
| Extremely (5) | −11.25544 | 1.48619 | ||
| The next three questions are about how you feel and how things have been during the past 4 weeks. For each question, please give the one answer that comes closest to the way you have been feeling. How much of the time during the past 4 weeks — | ||||
| 9 | Have you felt calm and peaceful? | All of the time (1) | 0 | 0 |
| Most of the time (2) | 0.66514 | −1.94949 | ||
| A good bit of the time (3) | 1.36689 | −4.09842 | ||
| Some of the time (4) | 2.37241 | −6.31121 | ||
| A little of the time (5) | 2.90426 | −7.92717 | ||
| None of the time (6) | 3.46638 | −10.19085 | ||
| 10 | Did you have a lot of energy? | All of the time (1) | 0 | 0 |
| Most of the time (2) | −0.42251 | −0.92057 | ||
| A good bit of the time (3) | −1.14387 | −1.65178 | ||
| Some of the time (4) | −1.61850 | −3.29805 | ||
| A little of the time (5) | −2.02168 | −4.88962 | ||
| None of the time (6) | −2.44706 | −6.02409 | ||
| 11 | Have you felt downhearted and blue? | All of the time (1) | 4.61446 | −16.15395 |
| Most of the time (2) | 3.41593 | −10.77911 | ||
| A good bit of the time (3) | 2.34247 | −8.09914 | ||
| Some of the time (4) | 1.28044 | −4.49055 | ||
| A little of the time (5) | 0.41188 | −1.95934 | ||
| None of the time (6) | 0 | 0 | ||
| 12 | During the past 4 weeks, how much of the time has your physical health or emotional problems interfered with your social activities (like visiting with friends, relatives, etc.)? |
All of the time (1) | −0.33682 | −6.29724 |
| Most of the time (2) | −0.94342 | −8.26066 | ||
| A good bit of the time (3) | −0.18043 | −5.63286 | ||
| A little of the time (4) | 0.11038 | −3.13896 | ||
| None of the time (5) | 0 | 0 | ||
The present study aims to determine the impact of genetic and environmental factors on the HRQL using the SF-12 in a nationwide, population-based cohort including twins of both sexes, aged from 20 to 71 years.
Methods
Twin Method
The present study was based on data from the Danish Twin Registry. In 2002, 46,417 twin individuals from the 1930–1982 twin cohorts were asked to participate in a nationwide twin survey (Skytthe et al., 2002). The survey included questions on HRQL (SF-12), demographic factors, and many different diseases. The accompanying letter explained that the aim of the study was about twins’ health in general. Zygosity assignment of same-sexed twins in the Danish Twin Registry was based on three or four questions concerning degree of similarity and mistaken identity (Christiansen et al., 2003; Kyvik et al., 1995). In case of disagreement between the twins or inconsistent answers, the zygosity was classified as unknown. If only one twin had answered, the zygosity diagnosis was based on that answer alone. The accuracy of the zygosity classification by the questionnaire method has been shown to be approximately 96% (Christiansen et al., 2003).
The SF-12
The SF-12 consists of 12 items that are scored in two dimensions: the PCS and MCS scores. The PCS score includes items on general health perception, physical functioning, reduction in physical activities as compared with the subjectively expected, and pain, whereas the MCS score includes questions on general health perception, mood, energy level, and reduction in social activities, work ability or general function explained by emotional factors. In order to estimate the SF-12 score, individual items are weighted with different endorsements and added to a constant (57.65693 and 60.58847, respectively) for PCS and MCS (Andrews, 2002; see Table 1). The median PCS and MCS have been shown to be 51 and 54, respectively (with standard deviation around 8) in a large Danish community sample (Bjorner et al., 2003).
Ethics
The questionnaire study was approved by the regional scientific ethical committees in Denmark and the Danish Data Protection Board.
Analysis
The SF-12 was scored using weighted endorsements (see Table 1), resulting in a PCS and a MCS score. Summary health statistic was reported as medians and 2.5 and 97.5 percentiles. Cronbach’s α was estimated in order to evaluate internal consistency of the SF-12.
Twin methodology makes the use of the fact that monozygotic (MZ) twins are genetically identical, whereas dizygotic (DZ) twins share 50% of their genes on average. In addition, it is assumed that twins are exposed to two different environmental factors, namely shared and unique, which is independent of zygosity. A higher correlation within MZ twin pairs as compared to DZ twin pairs reflects genetic influences.
Data on MZ pairs together with same-, and opposite-sexed DZ pairs were analyzed using bivariate Cholesky decomposition models. Because of the large dataset and previous findings, data were divided into three age groups (20–34, 35–54, and 55–71), treating additional effects of age and sex as covariates in a linear model. The total variances of disease liabilities to MCS and to PCS were partitioned into additive genetic effects (A), dominance genetic effects (D), common environmental effects (C), and random environmental effects (E) for each phenotype. MZ pairs share the same A and D genetic variances, whereas DZ pairs share one-half of the A variance and one-quarter of the D variance. The variance of C is the same for both MZ and DZ twin pairs. Because the effect of shared environment and genetic dominance is confounded and because the model only allows us to estimate three parameters, one has to fit data using either an ACE or ADE model. The choice between ACE and ADE models was done by comparing the interclass correlation coefficients. Reported statistics were the standardized parameter estimates from each group. The model allows the determination of the extent to which the covariance or phenotypic correlation (rp) can be explained by shared genetic and/or environmental factors. The genetic correlation (rg) indicates the amount of overlapping genes influencing the subscales, that is, genetic pleiotropy, and ranges from −1 to 1. An rg of 1 indicates that the same genes influence both subscales, whereas an rg of 0 indicates that there is no genetic overlap between them. Positive or negative genetic correlation reflects positive or negative associations between the subscales.
Descriptive statistics were analyzed in Stata. Variance component summary scores were assessed using Mx (Neale & Maes, 2004). Mx Scripts were extensions of scripts found in the GenomEUtwin Mx-script library (www.psy.vu.nl/mxbib).
Results
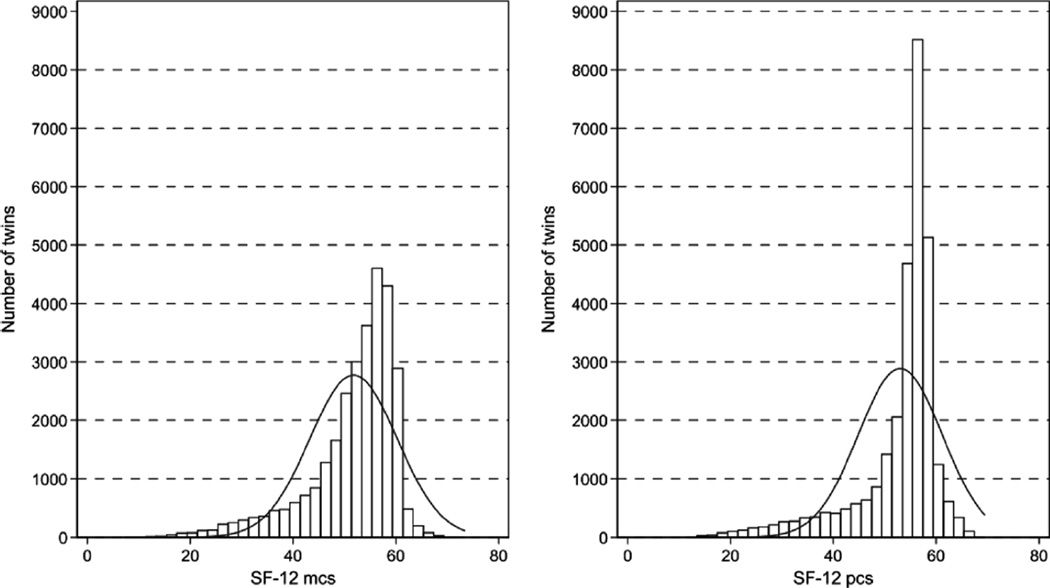
The overall response rate after one reminder was 73% in the 2002 Danish twin survey. The proportion of concordance for response among MZ pairs (60.5%) was higher than in DZ pairs (47.2%), a difference of 13.4% (95% CI: 11.9–14.8%, p < .0001). Same-sexed DZ twin pairs were more concordant for response than opposite-sexed DZ twin pairs, difference of 4.7% (95% CI: 3.2–6.2%, p < .0001). The concordance for response among females was higher than in males, difference of 13.9% (95% CI: 12.3–15.5%, p < .0001). The SF-12 was completed by 29,619 twin individuals with known twin status, and in 9,120 twin pairs both twins had responded to the SF-12. The characteristics of the twin cohort with regard to the SF-12 response are summarized in Table 2. Genetic modeling in Mx requires that data (after correcting for linear effects, i.e., age and sex) are normally distributed, and that the mean values and variance are similar across twins labeled as the ‘first twin’ and twins labeled as the ‘second twin’. The weighted endorsements in Table 1 have been constructed, so that the final SF-12 MCS and PCS scores should be approximately normally distributed (Bjorner et al., 2003). Figure 1 illustrates that this was not the case for our study, and correcting for linear effects did not improve this (data not shown). Transformation of data by a power of 4 or 5 could normalize MCS and PCS, respectively. We did, however, not transform the data because transformation might introduce bias on the correlation estimates. As shown in Table 2, the median SF-12 MCS and PCS scores differed slightly between complete and incomplete pairs. After correcting for age and gender, the residual variation was the same in complete and incomplete pairs (data not shown). Cronbach’s α was 0.78 and 0.83 for MCS and PCS, respectively, which is acceptable.
TABLE 2.
Characteristics of the Danish Twin Registry With Regard to the SF-12 Response
| Zygosity and sex | Number of twins |
Response to SF-12 (%) |
SF-12 MCS median (2.5–97.5th percentile) |
SF-12 PCS median (2.5–97.5th percentile) |
|---|---|---|---|---|
| All | 33,794 | 29,619 (88) | 54.4 (27.9–61.5) | 55.9 (27.9–62.4) |
| Females | 18,461 | 15,884 (86) | 53.5 (27.1–61.7) | 55.9 (26.7–62.7) |
| Males | 15,333 | 13,735 (90) | 55.0 (30.1–61.4) | 55.9 (30.3–62.1) |
| MZ | 9,000 | 8,076 (90) | 53.7 (27.1–61.3) | 56.3 (29.6–62.7) |
| MZ females | 5,024 | 4,471 (89) | 53.2 (25.6–61.3) | 56.3 (27.9–63.0) |
| MZ males | 3,976 | 3,605 (91) | 55.0 (29.3–61.2) | 56.1 (32.5–62.1) |
| ssDZ | 12,877 | 11,229 (87) | 54.7 (28.1–61.5) | 55.9 (27.5–62.3) |
| ssDZ females | 6,785 | 5,770 (85) | 53.5 (27.2–61.5) | 55.8 (26.1–62.6) |
| ssDZ males | 6,092 | 5,459 (90) | 55.1 (30.3–61.5) | 55.9 (29.8–62.0) |
| osDZ | 11,917 | 10,314 (87) | 54.6 (28.6–61.7) | 55.8 (27.1–62.3) |
| Twins from complete pairs | 22,744 | 20,197 (89) | 54.3 (28.4–62.5) | 55.9 (28.7–62.3) |
| Twins from incomplete pairs | 11,050 | 9,422 (85) | 54.6 (27.2–62.0) | 55.7 (26.4–62.5) |
Note: ssDZ = same-sexed dizygotic twin pairs; osDZ = opposite-sexed dizygotic twin pairs.
FIGURE 1.
Distribution of the SF-12 PCS and SF-12 MCS scores in the Danish Twin Cohort.
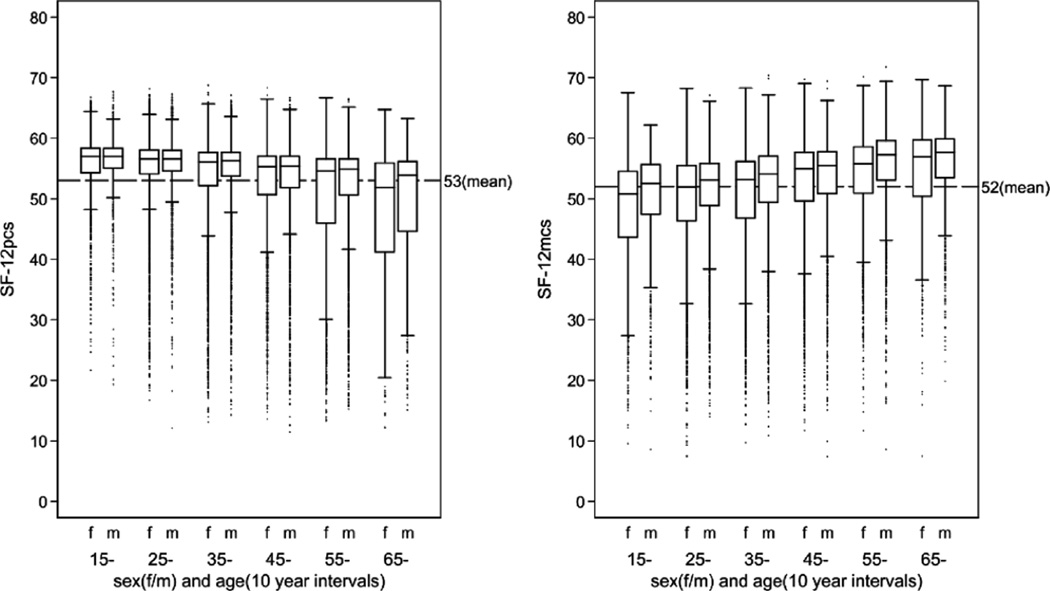
Based on the 29,619 responders who completed the SF-12 score, the median PCS score was 55.9, and the (2.5–97.5)th percentile range was 27.9–62.4. The median MCS score was 54.4, and the (2.5–97.5)th percentile range was 27.6–61.5. The SF-12 scores in males and females in 10-year-age groups are illustrated in Figure 2.
FIGURE 2.
SF-12 PCS and SF-12 MCS in the Danish Twin Cohort.
Overall, the interclass correlation coefficients were 0.297 (95% CI: 0.263–0.330) and 0.154 (95% CI: 0.129–0.178) for MCS in MZ and DZ twin pairs, and 0.246 (95% CI: 0.212–0.281) and 0.142 (95% CI: 0.118–0.167) for PCS in MZ and DZ twin pairs, respectively. Although the interclass correlation coefficients differed between gender and age groups, as presented in Tables 3 and 4, the general pattern was that MCS and PCS correlations in MZ twin pairs were between one and two times that of DZ twin pairs, indicating that the contribution of dominance genetic effects (D) would be minimal. Therefore, it was decided to use the ACE model.
TABLE 3.
Interclass Correlation Coefficients (95% CI) for SF-12 MCS
| Age groups | |||
|---|---|---|---|
| Zygosity and sex | 20–34 | 35–54 | 55–71 |
| MZ males | 0.276 (0.189–0.363) | 0.260 (0.175–0.345) | 0.389 (0.281–0.497) |
| MZ females | 0.380 (0.319–0.442) | 0.267 (0.195–0.338) | 0.396 (0.287–0.506) |
| ssDZ males | 0.185 (0.087–0.283) | 0.177 (0.098–0.256) | 0.119 (0.005–0.233) |
| ssDZ females | 0.097 (0.009–0.185) | 0.237 (0.173–0.300) | 0.188 (0.082–0.295) |
| osDZ | 0.099 (0.015–0.170) | 0.128 (0.071–0.186) | 0.193 (0.115–0.271) |
TABLE 4.
Interclass Correlation Coefficients (95% CI) for SF-12 PCS
| Age groups | |||
|---|---|---|---|
| Zygosity and sex | 20–34 | 35–54 | 55–71 |
| MZ males | 0.193 (0.100–0.285) | 0.266 (0.181–0.350) | 0.402 (0.296–0.508) |
| MZ females | 0.225 (0.155–0.296) | 0.307 (0.238–0.376) | 0.294 (0.171–0.417) |
| ssDZ males | 0.009 (0.000–0.119) | 0.180 (0.101–0.259) | 0.067 (0.000–0.186) |
| ssDZ females | 0.112 (0.025–0.199) | 0.150 (0.082–0.218) | 0.235 (0.133–0.337) |
| osDZ | 0.168 (0.094–0.241) | 0.197 (0.143–0.251) | 0.157 (0.077–0.238 |
For the bivariate Cholesky analysis, the 29,619 responding twins were divided into three age groups and adjusted for age and sex (Tables 5–7). In all age groups, the majority of the variance was explained by random environmental factors for both the SF-12 PCS and MCS scores. The common environmental effect was 0, the genetic effect explained from 11% to 35% of the variation in SF-12 MCS and PCS scores (Tables 5 and 6), and inter-scale correlations also varied in the different age and sex groups (Table 7). The pleiotropy was positive for the genetic effect; thus, the genes that influence the different subscales do so in the same direction, whereas the environmental correlations were mostly negative, indicating that the overlapping environmental factors work in different directions.
TABLE 5.
Estimates from the Bivariate ACE Model on SF-12 MCS in Different Age Groups
| Estimates in different age groups (95% CI) | ||||
|---|---|---|---|---|
| Factor | Sex | 20–34 | 35–54 | 55–71 |
| Genetic effect (h2) | Males | 0.23 (0.00 to 0.35) | 0.21 (0.21 to 0.34) | 0.35 (0.08 to 0.46) |
| Females | 0.30 (0.07 to 0.43) | 0.25 (0.00 to 0.33) | 0.30 (0.00 to 0.42) | |
| Common environmental effect (c2) | Males | 0.00 (0.00 to 0.30) | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.17) |
| Females | 0.00 (0.00 to 0.19) | 0.00 (0.00 to 0.00) | 0.00 (0.00 to 0.00) | |
| Unique environmental effect (e2) | Males | 0.77 (0.62 to 0.92) | 0.79 (0.72 to 0.86) | 0.65 (0.54 to 0.80) |
| Females | 0.70 (0.57 to 0.81) | 0.75 (0.69 to 0.86) | 0.70 (0.57 to 0.86) | |
| Effect of age (βage) | – | 0.57 (0.08 to 1.05) | 1.14 (0.68 to 1.59) | 1.85 (1.26 to 2.44) |
| Effect of sexa (βsex) | – | 2.00 (1.30 to 2.72) | 1.33 (0.85 to 1.81) | 1.43 (0.97 to 1.90) |
| Grand mean (μ) | – | 46.03 (43.62 to 48.37) | 44.65 (42.75 to 46.47) | 49.01 (45.58 to 51.80) |
Note:
Females are coded 0 and males are coded 1.
TABLE 7.
Correlations Between SF-12 MCS and PCS in Different Age Groupsa
| Correlations in different age groups (95% CI) | ||||
|---|---|---|---|---|
| 20–34 | 35–54 | 55–71 | ||
| Genetic correlation (rg) | Males | 0.23 (−0.14 to 0.79) | 0.06 (−0.16 to 0.32) | 0.45 (0.23 to 0.70) |
| Females | 0.07 (−0.13 to 0.33) | 0.25 (0.07 to 0.45) | 0.26 (−0.01 to 0.56) | |
| Unique environmental correlation (re) | Males | −0.29 (−0.35 to −0.22) | −0.12 (−0.18 to −0.06) | −0.08 (−0.25 to 0.05) |
| Females | −0.32 (−0.37 to −0.26) | −0.14 (−0.20 to −0.09) | 0.00 (−0.10 to 0.11) | |
Note:
The common environmental effect was fixed at 0 when estimating the correlations.
TABLE 6.
Estimates from the Bivariate ACE Model on SF-12 PCS in Different Age Groups
| Estimates in different age groups (95% CI) | ||||
|---|---|---|---|---|
| Factor | Sex | 20–34 | 35–54 | 55–71 |
| Genetic effect (h2) | Males | 0.11 (0.00 to 0.27) | 0.22 (0.00 to 0.35) | 0.32 (0.10 to 0.48) |
| Females | 0.18 (0.00 to 0.29) | 0.24 (0.01 to 0.35) | 0.26 (0.00 to 0.38) | |
| Common environmental effect (c2) | Males | 0.00 (0.00 to 0.18) | 0.00 (0.00 to 0.20) | 0.00 (0.00 to 0.13) |
| Females | 0.00 (0.00 to 0.23) | 0.00 (0.00 to 0.13) | 0.00 (0.00 to 0.29) | |
| Unique environmental effect (e2) | Males | 0.89 (0.72 to 1.00) | 0.77 (0.64 to 0.89) | 0.68 (0.52 to 0.83) |
| Females | 0.82 (0.70 to 0.95) | 0.76 (0.65 to 0.87) | 0.74 (0.60 to 0.88) | |
| Effect of age (βage) | – | −0.08 (−0.14 to −0.03) | −0.14 (−0.17 to −0.10) | −0.24 (−0.29 to −0.18) |
| Effect of sexa (βsex) | – | 0.10 (0.01 to 0.18) | 0.15 (0.12 to 0.19) | 0.08 (0.03 to 0.13) |
| Grand mean (μ) | – | 57.34 (55.74 to 58.96) | 58.51 (56.74 to 60.25) | 64.00 (60.33 to 68.08) |
Note:
Females are coded 0 and males are coded 1.
Discussion
This study is presently the largest unselected nationwide, population-based twin study on self-rated health. It lends further support to previous evidence of a moderate genetic contribution to HRQL. Overall, the impact of genetic factors ranged from 11% to 35% in different age and sex groups, and although there was a trend toward higher influence of genetic factors with increasing age, unique environmental factors explained most of the variance on self-assessed health and disease. Self-reported health has previously been shown to associate and correlate to underlying disease and function (Leinonen et al., 2005). Therefore, the finding of a low-to-moderate genetic impact on HRQL indicates that the dominating diseases and sources of discomfort on a population level are not heritable and therefore potentially avoidable.
Generally, self-rated health differs across age and sex groups. As shown in Figure 2, there was a reciprocal correlation between MCS and PCS with regard to the influences of age, so that physical well-being declines with increasing age, whereas mental well-being increases as the twins get older. The impact of genes and environment on self-rated health also differed across different age and sex groups (Tables 5 and 6), which is in agreement with previous twin studies on the genetics of HRQL (Leinonen et al., 2005; Silventoinen et al., 2007). Thus, we found that genes might be more important for mental and physical well-being in older age groups. However, there were wide confidence intervals on these estimates; and therefore, the finding is uncertain. There was only a small-to-moderate overlap of genetic and environmental factors between the SF-12 subscales (Table 7). The correlations were positive for the genetic effect and negative for the environmental effect. The negative environmental correlation was to be expected from the endorsements of the different SF-12 items (Table 1).
The SF-12 is a short version of the SF-36 and both have been shown to be valid, reliable, and general measures of HRQL (Bjorner et al., 2003). The estimated Cronbach’s α is acceptable. Therefore, it is not likely that the influence of random factors on the variance reflects random variation in filling in questionnaires.
Previous analysis of non-response in twins and singletons has shown that mainly socioeconomic variables and not health status differ between responders and nonresponders (Heath et al., 2001; Korkeila et al., 2001; Vink et al., 2004). In addition, previous studies on Danish twins indicate that self-reported health does not depend on adult social class and thus the estimated heritability was probably not biased by selection based on social class (Osler et al., 2007). Despite this, we cannot exclude that there could be bias due to non-response. However, the internal validity of the present findings was strengthened by the nationwide, population-based twin ascertainment procedure and by the large number of twins included. More than 73% of the twins answered the questionnaire, which is quite satisfactory (Skytthe et al., 2002). In addition, medians and variance for SF-12 estimates in twins correspond well to previous MCS and PCS estimates from the Danish population (Bjorner et al., 2003) indicating that the conclusions from the twin study also apply to singletons. The study was conducted among Scandinavians and cannot be uncritically generalized to other populations.
The biometric modeling used to estimate the genetic impact on HRQL does not identify gene–gene or gene–environment interaction. Thus, the results are only valid if it can be assumed that such interactions are not relevant for HRQL.
Most previous studies on HRQL in twins also found the ACE or the AE model to be the best-fitting model for explaining the impact of genes and environment. Also, most studies on twins aged from 20 to 71 years reported similar standardized parameters for genetic and environmental factors, as in the present study (Romeis et al., 2000, 2005; Silventoinen et al., 2007; Svedberg et al., 2001). As with the previous Finnish and Swedish twin studies, we found a trend toward higher genetic influence on HRQL in older age groups. Thus, the present study confirms the findings of previous studies in a larger population-based twin cohort. In conclusion, although there is a significant genetic influence, individual environmental factors explain most of the variation on self-assessed health and disease.
Acknowledgments
The program of twin research at the Danish Twin Registry has been supported through many years by funding from The University of Southern Denmark, The Danish Research Council, The Danish National Research Foundation, Helsefonden, The Danish Diabetic Association, The Danish Heart Foundation, NOVO Nordisk Foundation, the European Union Contract No. QLG2-CT-2002-01254, and the United States National Institute on Aging (P01-AG08761). The authors appreciate the voluntary participation by the twins.
References
- Andrews G. A brief integer scorer for the SF-12: Validity of the brief scorer in Australian community and clinic settings. Australian and New Zealand Journal of Public Health. 2002;26:508–510. doi: 10.1111/j.1467-842x.2002.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Bartels M, Boomsma DI. Born to be happy? The etiology of subjective well-being. Behavior Genetics. 2009;39:605–615. doi: 10.1007/s10519-009-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, Saviouk V, deMoor MH, Willemsen G, vanBeijsterveldt TC, Hottenga JJ, Boomsma DI. Heritability and genome-wide linkage scan of subjective happiness. Twin Research and Human Genetics. 2010;13:135–142. doi: 10.1375/twin.13.2.135. [DOI] [PubMed] [Google Scholar]
- Bjorner JB, Damsgaard MT, Watt T, Bech P, Rasmussen NK, Kristensen TS. Danish SF-36 manual —A health status questionnaire. Copenhagen: Lægemiddel industri foreningen; 2003. [Google Scholar]
- Christiansen L, Frederiksen H, Schousboe K, Skytthe A, Wurmb-Schwark N, Christensen K, Kyvik K. Age- and sex-differences in the validity of questionnaire based zygosity in twins. Twin Research. 2003;6:275–278. doi: 10.1375/136905203322296610. [DOI] [PubMed] [Google Scholar]
- deMoor MH, Stubbe JH, Boomsma DI, deGeus EJ. Exercise participation and self-rated health: Do common genes explain the association? European Journal of Epidemiology. 2007;22:27–32. doi: 10.1007/s10654-006-9088-8. [DOI] [PubMed] [Google Scholar]
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, Sullivan M. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. Journal of Clinical Epidemiology. 1998;51:1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- Gill SC, Butterworth P, Rodgers B, Mackinnon A. Validity of the mental health component scale of the 12-item Short-Form Health Survey (MCS-12) as measure of common mental disorders in the general population. Journal of Psychiatric Research. 2007;152:63–71. doi: 10.1016/j.psychres.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Heath AC, Howells W, Kirk KM, Madden PA, Bucholz KK, Nelson EC, Martin NG. Predictors of non-response to a questionnaire survey of a volunteer twin panel: Findings from the Australian 1989 twin cohort. Twin Research. 2001;4:73–80. doi: 10.1375/1369052012182. [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Chandola T, Coulter A, Bruster S. An assessment of the construct validity of the SF-12 summary scores across ethnic groups. Journal of Public Health Medicine. 2001;23:187–194. doi: 10.1093/pubmed/23.3.187. [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Layte R. Development and testing of the UK SF-12 (Short Form Health Survey) Journal of Health Services Research & Policy. 1997;2:14–18. doi: 10.1177/135581969700200105. [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, Stradling J. A shorter form health survey: Can the SF-12 replicate results from the SF-36 in longitudinal studies? Journal of Public Health Medicine. 1997;19:179–186. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- Korkeila K, Suominen S, Ahvenainen J, Ojanlatva A, Rautava P, Helenius H, Koskenvuo M. Nonresponse and related factors in a nation-wide health survey. European Journal of Epidemiology. 2001;17:991–999. doi: 10.1023/a:1020016922473. [DOI] [PubMed] [Google Scholar]
- Kyvik KO, Green A, Beck-Nielsen H. The new Danish Twin Register: Establishment and analysis of twinning rates. International Journal of Epidemiology. 1995;24:589–596. doi: 10.1093/ije/24.3.589. [DOI] [PubMed] [Google Scholar]
- Leinonen R, Kaprio J, Jylha M, Tolvanen A, Koskenvuo M, Heikkinen E, Rantanen T. Genetic influences underlying self-rated health in older female twins. Journal of the American Geriatrics Society. 2005;53:1002–1007. doi: 10.1111/j.1532-5415.2005.53319.x. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Pedersen NL, Martin NG, Wright MJ. Sex differences in the genetic architecture of optimism and health and their interrelation: A study of Australian and Swedish twins. Twin Research and Human Genetics. 2010;13:322–329. doi: 10.1375/twin.13.4.322. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Zietsch BP, Shekar SN, Wright MJ, Martin NG. Genetic and environmental influences on optimism and its relationship to mental and self-rated health: A study of aging twins. Behavior Genetics. 2009;39:597–604. doi: 10.1007/s10519-009-9287-7. [DOI] [PubMed] [Google Scholar]
- Neale MC, Maes HHM. Methodology for genetic studies of twins and families. London: Kluwer Academic; 2004. [Google Scholar]
- Osler M, McGue M, Christensen K. Socioeconomic position and twins’ health: A life-course analysis of 1266 pairs of middle-aged Danish twins. International Journal of Epidemiology. 2007;36:77–83. doi: 10.1093/ije/dyl266. [DOI] [PubMed] [Google Scholar]
- Romeis JC, Heath AC, Xian H, Eisen SA, Scherrer JF, Pedersen NL, True WR. Heritability of SF-36 among middle-age, middle-class, male-male twins. Medical Care. 2005;43:1147–1154. doi: 10.1097/01.mlr.0000183217.11811.bd. [DOI] [PubMed] [Google Scholar]
- Romeis JC, Scherrer JF, Xian H, Eisen SA, Bucholz K, Heath AC, True WR. Heritability of self-reported health. Health Services Research. 2000;35:995–1010. [PMC free article] [PubMed] [Google Scholar]
- Silventoinen K, Posthuma D, Lahelma E, Rose RJ, Kaprio J. Genetic and environmental factors affecting self-rated health from age 16–25: A longitudinal study of Finnish twins. Behavior Genetics. 2007;37:326–333. doi: 10.1007/s10519-006-9096-1. [DOI] [PubMed] [Google Scholar]
- Skytthe A, Kyvik K, Holm NV, Vaupel JW, Christensen K. The Danish Twin Registry: 127 birth cohorts of twins. Twin Research. 2002;5:352–357. doi: 10.1375/136905202320906084. [DOI] [PubMed] [Google Scholar]
- Svedberg P, Bardage C, Sandin S, Pedersen NL. A prospective study of health, life-style and psychosocial predictors of self-rated health. European Journal of Epidemiology. 2006;21:767–776. doi: 10.1007/s10654-006-9064-3. [DOI] [PubMed] [Google Scholar]
- Svedberg P, Lichtenstein P, Pedersen NL. Age and sex differences in genetic and environmental factors for self-rated health: A twin study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2001;56:S171–S178. doi: 10.1093/geronb/56.3.s171. [DOI] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Stubbe JH, Middeldorp CM, Ligthart RS, Baas KD, Boomsma DI. Estimating non-response bias in family studies: Application to mental health and lifestyle. European Journal of Epidemiology. 2004;19:623–630. doi: 10.1023/b:ejep.0000036814.56108.66. [DOI] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva: Author; 1997. WHOQOL: Measuring quality of life. [Google Scholar]