Abstract
Longitudinal and transverse relaxations in the rotating frame, with characteristic time constants T1ρ and T2ρ, respectively, have potential to provide unique MRI contrast in vivo. On-resonance spin-lock T1ρ with different spin-lock field strengths and adiabatic T2ρ with different radiofrequency-modulation functions were measured in BT4C gliomas treated with Herpes Simplex Virus thymidine kinase (HVS-tk) gene therapy causing apoptotic cell death. These NMR tools were able to discriminate different treatment responses in tumor tissue from day 4 onwards. An equilibrium two-site exchange model was used to calculate intrinsic parameters describing changes in water dynamics. These results are consistent with destructive intracellular processes associated with cell death and the increase of extracellular space during the treatment. Furthermore association between longer exchange correlation time and decreased pH during apoptosis is discussed. In this study we demonstrated that T1ρ and T2ρ MR imaging are useful tools to quantify early changes in water dynamics reflecting treatment response during gene therapy.
Keywords: T1ρ, T2ρ, spin-lock, adiabatic, apoptosis, gene therapy, fast exchange
INTRODUCTION
Malignant gliomas are the most common primary tumors of the central nervous system. These tumors are often resistant to treatment and carry poor prognosis. Recent studies show that gene therapy may be an effective treatment (1–4). One of the most commonly tested approaches exploit the thymidine kinase enzyme (tk) from the Herpes Simplex Virus (HSV). This enzyme can metabolize intravenously administered ganciclovir (GCV) to produce a nucleotide analog that is selectively toxic for dividing cells (1, 3) which is very important in the brain with healthy neurons existing around the tumor (3, 5). It has been demonstrated that HSV-tk-ganciclovir has a therapeutic potential both in experimental and in clinical settings (2, 6, 7).
Magnetic resonance imaging (MRI) and spectroscopy (MRS) have been proven to be sensitive to cell death related processes in several different experimental disease models (8–14). MRI and MRS methods can detect treatment response in brain tumors at very early stages during cytotoxic treatment with anticancer drugs or irradiation (15), and provide information about tumor size, perfusion, molecular tumbling, chemical exchange, diffusion, and metabolite levels (16).
MRI permits proton relaxation studies which can be used to obtain information about water interactions and dynamics. The longitudinal relaxation time constant (T1) depends on the external magnetic field (B0), and it detects magnetic field fluctuations caused by molecular dynamics on the order of MHz, near of the Larmor precession frequency (ω0 = γB0 in which γ is the gyromagnetic ratio). The longitudinal relaxation time in the rotating frame (T1ρ) offers a valuable tool to study dipolar fluctuations in the tissue, arising from slow atomic motions in viscous liquids and proteins (17–20). The value of T1ρ is sensitive to molecular dynamics taking place on the order of kHz (ω1 = γB1, where B1 is the radiofrequency magnetic field), which is nearer to the frequencies at which much of the dipolar fluctuations occur in tissue (18, 21). It has been suggested that T1ρ predominantly reflects water-protein interactions in tissue and therefore, it can be used to generate a novel MRI contrast (8, 9). Furthermore, it has been shown that T1ρ is one of the earliest markers of the positive treatment response in gene therapy and chemotherapy (22–24), and it provides an indication of neural loss during neurodegenerative disorders (25).
Transverse relaxation in the rotating frame (T2ρ) can occur during a frequency-modulated pulse such as an adiabatic full-passage (AFP) pulse (26–28). The magnetization vector that initially lies in the x′y′-plane of the rotating frame will undergo rotation in the plane perpendicular to the sweeping effective magnetic field (Beff). The theoretical analysis of the T2ρ behavior shows that the range of spin dynamics to which T2ρ is sensitive extends to slow motion with correlation times (τc) up to ~ 10−4 s (29). Recently, this inherent property of the adiabatic Carr-Purcell (CP) pulse sequence was used to probe exchanging systems (27, 28) and was applied to the human brain to generate novel tissue contrast in MRI (25). Despite much progress in the understanding of T1ρ relaxation in NMR, theoretical descriptions and applications of transverse relaxation in the rotating frame T2ρ have been limited (29–32). It has been demonstrated that during AFP pulses, exchange-induced and dipolar relaxation contributions to T2ρ (T2ρ,ex and T2ρ,dd, respectively) depend on the choice of amplitude- and frequency-modulated functions used in the AFP pulses (26, 27, 33). Theory of the T2ρ,ex relaxation due to exchange between spins with a non-zero chemical-shift difference (δω ≠ 0, the anisochronous exchange) has been presented for the spin-lock (34) and time-dependent relaxation during adiabatic rotation (26, 28, 35). The dependence of the relaxation rate during AFP pulses on the modulation functions was recently used to generate tissue contrast in the human brain and to determine fundamental relaxation parameters in tissue (27).
The aim of this study was, for the first time, to investigate the sensitivity of adiabatic T2ρ measured with different pulse modulation functions to treatment response during glioma gene therapy. Furthermore, on-resonance spin-lock T1ρ with different spin-lock field strengths combined with T2ρ measurements were used to assess alterations in intrinsic relaxation parameters in tumor tissue during gene therapy. These alterations include: dipolar and exchange correlation times as well as relative populations of the exchanging sites, such as water associated with macromolecules and free water.
MATERIALS AND METHODS
Tumor model
BT4C gliomas were induced by implanting 104 HSV-tk positive cells into the corpus callosum of female BDIX rats (n = 14) as described previously (23, 24, 36). Rats in the treatment group (n = 9) were injected with Ganciclovir (GCV, 25 mg/kg, i.p., twice a day) for 8 days. Untreated tumor-bearing animals served as controls (n = 5) and they were injected with a saline solution. All animal experiments were approved by the ethical committee of the National Laboratory Animal Centre at the University of Kuopio.
Magnetic resonance imaging
MRI experiments were carried out in a 4.7 T horizontal magnet (Magnex Scientific Ltd., Yarnton, Oxfordshire, UK) equipped with actively shielded gradients (Magnex) interfaced to a UnityInova (Varian Inc, Palo Alto, CA, USA) console. A quadrature half-volume coil (Highfield Imaging, Minneapolis, MN, USA, loop diameter 28 mm) was used in transmit/receive mode. MR data were collected on alternate days. During MRI the animals were anesthetized with halothane (maintenance level of 1%) in N2O/O2 (75%:25%) and fixed into a custom-built head holder using teeth bar and ear pins.
To measure the tumor volume, T2-weighted spin echo multislice images were acquired with the following parameters: repetition time (TR) 2.5 s, echo time (TE) 65 ms, field of view 35 × 35 mm2, matrix size 128 × 256, slice thickness 1 mm.
T1ρ and T2ρ data were acquired from a single transversal slice positioned in the centre of the tumor based on volumetric T2-weighted images. On-resonance T1ρ was measured using a spin-lock pulse having variable length (SLT), following excitation by an adiabatic half passage (AHP) pulse having a constant length (Tp) equal to 4 ms (37). Four different spin-locking times (SLT = 12, 24, 48 and 96 ms) were used. Following the spin-lock pulse, a reverse AHP pulse (Tp = 4 ms) was used to return magnetization back to the +z′-axis. Then, after applying a crusher gradient, the prepared magnetization was readout using a fast spin echo imaging sequence (38). The acquisition parameters were: TR = 2.5 s, field-of-view = 25.6 × 25.6 mm2, matrix size = 64 × 128, slice thickness = 1.5 mm, 16 echoes with echo-spacing of 10 ms (38). In these experiments, the peak value of the B1(t) function (B1max) used in the AHP pulses was fixed at 0.4 G, while the amplitude of the spin-lock pulse was varied according to B1 = 0.02, 0.04, 0.06, 0.08, 0.1, 0.15, 0.2, 0.3, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4 G.
AFP pulses are especially well-suited for π rotations because they can provide broad bandwidth and accurate flip angles with high tolerance to spatial variations in radiofrequency (RF) field intensity. The present experiments were conducted with, adiabatic full-passage (AFP) pulses of the hyperbolic secant (HS) type which have modified amplitude and frequency modulation functions (HSn pulses) (39–41). HSn pulses are stretched versions of the original hyperbolic secant pulse (39), where n denotes the stretching factor (40, 41). As n becomes larger, the HSn pulse amplitude-modulation (AM) function becomes flatter, and progressively resembles that of the chirp pulse (e.g., the AM function flattens and the frequency sweep approaches linearity) (Figure 1). Thus, the time evolution of magnetization during a HSn pulse can change significantly with a change of n. For T2ρ experiments, a train of HSn pulses was placed after coherent excitation by an AHP pulse. To obtain data for estimating T2ρ values, the experiment was repeated with a variable number (m) of HSn pulses in the AFP pulse train (m = 4, 8, 16 and 32). Three separate T2ρ measurements were performed, each using HSn pulses with n = 1 (no stretching), 4, or 8. All HSn pulses were transmitted using B1max = 0.8 G and Tp = 3 ms. The MLEV-4 phase cycle was used (42). Because no time intervals occurred between the AFP pulses in the train, the signal decay was governed solely by T2ρ relaxation. The same fast spin echo imaging readout was used for the T2ρ and T1ρ measurements.
Fig. 1.
Amplitude (a) and frequency (b) modulation functions for three different AFP pulses, HS1, HS4 and HS8.
MR parameter maps were calculated using standard 2-parameter monoexponential relaxation formulae on a pixel-by-pixel basis. Regions of interests (ROI) covering the whole tumor were selected in control and in treated animals using T2-weighted images. ROIs from contralateral brain were selected to analyze the results of normal tissue.
Histology
After MRI scans, animals were sacrificed with CO2 and transcardially perfused with 0.9% NaCl for 10 min (30 ml/min), followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 10 min (30 ml/min). Fixed brains were removed from the skull, rinsed in phosphate-buffered saline, and cryoprotected (24 h in 20% glycerol in 0.02 M potassium phosphate-buffered saline) for cryosectioning. Histological sections (20 μm) were stained with the Nissl method, staining the ribonucleic acids within the cell body, rough endoplasmic reticulum and the nucleus, allowing for assessment of cytoarchitecture and cell counts. Cell counting was performed using Stereo Investigator software in a NeuroLucidia morphometry system (MicroBrightField, Colchester, VT). Only cells with intact, well-defined margins were included.
Theory
All MR signal intensities measured with the T1ρ and T2ρ techniques clearly exhibited monoexponential decay, within the spin-lock time range used in this study. Thus, for the interpretation of NMR data the dynamic processes were modeled with an equilibrium two-site exchange (2SX) approximation, comprising two water populations coupled by an equilibrium exchange: the water population associated with macromolecules (A), and the free water population (B).
The dipolar fluctuation contribution to the rotating frame longitudinal relaxation rate constant at one specific site (A or B) is given by (30, 33)
| [1] |
where
| [2] |
where r is the internuclear distance (1.58 Å), τc is the correlation time for populations A and B (τA and τB, respectively), ħ is Planck’s constant and I is the spin quantum number. In fitting, the free water correlation time τB was assumed to be 10−12 s.
For the case of fast chemical exchange (FXR) between identical spins having a chemical-shift difference δω, under continuous wave (cw) on-resonance spin-lock irradiation, the exchange rate constant R1ρ,ex is given by
| [3] |
Here, PA and PB are the fractional populations of the two sites A and B, which are related by PA + PB = 1, τex is the mean lifetime of the exchanging species at the two sites, and δω is the difference between chemical shifts of the two sites. To reduce the number of fitted parameters and keep fitting unambiguous, δω was assumed to be a constant value of 125 Hz. This was found to give the best fitting of the complete data set in initial test fittings with several different δω values between 0 and 2 kHz. The contributions of dipolar relaxation pathways and the anisochronous exchange to observed R1ρ (R1ρ,obs) are given by
| [4] |
The parameters, PA, τA, and τex, were estimated from the R1ρ,obs measurements using the Levenberg-Marquardt non-linear least-squares regression algorithm implemented in Matlab.
During the radiofrequency-modulated HSn pulses, the relaxation rate constant will be time dependent, i.e., R2ρ(t) (= 1/T2ρ(t)), due to the continuously varying magnitude and orientation of the effective field (Beff(t)) in the frequency-modulated rotating frame (28, 41). The time-dependent angle between the vector Beff and the +z′-axis is given by
| [5] |
where Δω(t) = (ω0 − ωRF(t)) and ωRF(t) is the time-dependent pulse frequency. The effective frequency ωeff(t) is
| [6] |
The contribution of dipolar interactions between identical spins to the rotating frame transverse relaxation rate constant is given by (27, 30, 33)
| [7] |
where kdd is given by Eq. [2].
Theory predicts R2ρ,ex to be dependent on the choice of amplitude- and frequency-modulation functions used in the AFP pulses via their α(t)- and ωeff(t)-dependencies for spin populations with different chemical shifts (δω ≠ 0, the anisochronous mechanism) (26, 28). Within the instantaneous approximation (34), the exchange-induced R2ρ relaxation in the FXR (τex−1 ≫ δω) is
| [8] |
and the observed R2ρ,obs is given by (43)
| [9] |
Statistical analysis
Results are shown as mean ± SEM. Statistically significant differences were estimated using Student’s t test. Results obtained using theoretical calculations are presented with 95% confidence intervals for the non-linear least-squares parameter estimates.
RESULTS
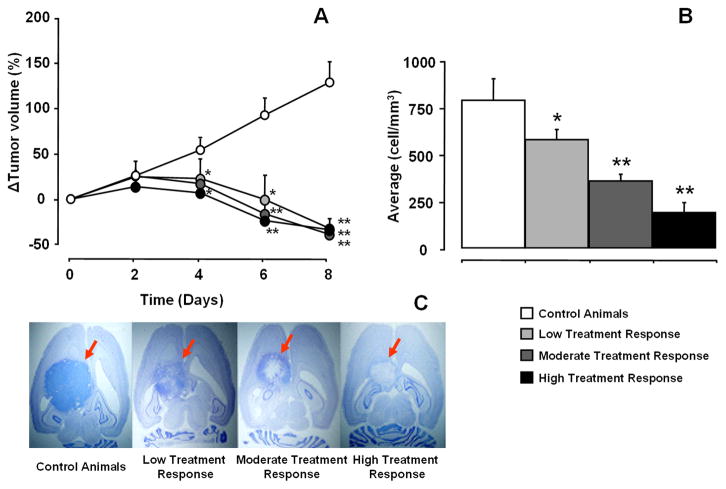
Animals showed variable response to treatment and were divided into three groups based on histology: low treatment response (< 30% of cell death, n = 2), moderate treatment response (cell death 30–75%, n = 5) and high treatment response group (> 75% of cell death, n = 2) (Fig. 2).
Fig. 2.
Relative tumor volumes (a) and average cell densities (cell/mm3) (b) counted from Nissl stained histological sections (c) on day 8. Tumors are indicated by red arrows. All values are given as mean ± SEM. Statistically significant difference for treated animals from the control animals were analyzed using Student’s paired t-test (* p < 0.05; ** p < 0.01).
Tumor volumes were measured from T2-weighted images on alternate days. Changes in tumor volume revealed a continuous growth in control group for 8 days (Fig. 2a). In treated animals, during first two days of the treatment, tumor volumes increased by 15–26% relative to their volumes on day 0. On the following days, tumor volumes decreased by 59–65%. From day 4, tumor volumes in all treatment groups were significantly different as compared to the control group. It should be noted that there was no statistically significant difference in reduction of tumor volume between low, moderate and high treatment response groups, in spite of clearly different cell densities assessed from histology (Fig. 2b and c). This result indicates that tumor volume alone was not a good marker of magnitude of the treatment response.
T1ρ and T2ρ maps from a high treatment response animal are shown in Figure 3. Relaxation times increased with increasing B1 and n in T1ρ and T2ρ measurements respectively, both in glioma and in normal brain tissue as also shown in previous studies (23, 27, 44). Before treatment, there was very little contrast between tumor and normal tissue in T1ρ and T2ρ maps, and also there was no difference between control group and treated groups. Treatment response was evident from day 4 onwards (Fig. 4). The initial increase of T1ρ on day 4 was practically independent of B1, but on treatment days 6 and 8 higher B1 values resulted in higher contrast. Similar behavior of sensitivity differences could be also seen in T2ρ maps with the increase of n of the HSn pulses. No changes in the relaxation time constants were detected in non-treated animals.
Fig. 3.
T1ρ maps (a) from a high treatment response animal with spin-lock field of 0.02, 0.8 and 1.4 G. T2ρ maps (b) from the same animal with HS1, HS4 and HS8 pulses on day 0 and day 8. Tumors are indicated by white arrows.
Fig. 4.
T1ρ values (ms) from treated and control animals with different B1 values (a: 0.02 G, b: 0.8 G and c: 1.4 G), and T2ρ values (ms) from treated and control animals calculated with different adiabatic full passage pulses during 8 days (d: HS1, e: HS4 and f: HS8) (● high,
 moderate and
moderate and
 low treatment response groups; ○ control animals). All values are given as mean ± SEM. Statistically significant differences for treated animals from the control animals were analyzed using Student’s paired t-test (* p < 0.05; ** p < 0.01).
low treatment response groups; ○ control animals). All values are given as mean ± SEM. Statistically significant differences for treated animals from the control animals were analyzed using Student’s paired t-test (* p < 0.05; ** p < 0.01).
Figure 5 shows measured R1ρ (= 1/T1ρ) values and the corresponding fitting obtained using the two-pool fast exchange model presented in Methods section (Eq. [4]). The model resulted in excellent fitting to our experimental data. On day 8 (Fig. 5b), R1ρ showed clearly different treatment response as compared to controls.
Fig. 5.

Calculated longitudinal relaxation rate constants R1ρ on day 0 (a) and day 8 (b) (● high,
 moderate and
moderate and
 low treatment response groups; ○ control animals; △ normal tissue). The points represent the experimental data and all values are given as mean ± SEM. The lines represent the fitting.
low treatment response groups; ○ control animals; △ normal tissue). The points represent the experimental data and all values are given as mean ± SEM. The lines represent the fitting.
Estimated parameters PA, τA, and τex, and the corresponding 95% confidence intervals are summarized in Fig. 6. There were no differences observed between groups in any of the fitting parameters before treatment or in the control group during the treatment. During the treatment with ganciclovir, the fractional population of the water pool associated with macromolecules (PA) decreased reflecting treatment response (Fig. 6b). Because PA + PB = 1, the fractional population associated with free water showed the opposite response, as free water increased during the treatment. On day 0, τA was ~ 4 × 10−9 s for control and treatment response groups, which is consistent with a previous study of human brain (33). For treated animals, τA increased to 1.0 × 10−8 ± 0.4 × 10−8 s in the high treatment response group (Fig. 6a). The exchange correlation time between pools A and B was ~ 8 × 10−5 s on day 0 and increased due to treatment effects (Fig. 6c).
Fig. 6.
Calculated parameters estimated from the R1ρ,obs measurements using the Levenberg-Marquardt non-linear least-squares regression algorithm (
 high,
high,
 moderate and
moderate and
 low treatment response groups;
low treatment response groups;
 control animals;
control animals;
 normal tissue). Results are presented with 95% confidence intervals.
normal tissue). Results are presented with 95% confidence intervals.
R2ρ values were calculated using parameters obtained from the R1ρ fitting (Fig. 7a–c). Calculated R2ρ values were slightly lower than experimental values and the difference increased with increasing n of the HSn pulses. To further investigate how treatment effects could be explained by our model we normalized R2ρ values by dividing them with pretreatment values. From Figure 7d–f it was evident that our modeling described well the changes caused by treatment despite a small discrepancy in absolute relaxation rate constants.
Fig. 7.
Calculated transverse relaxation rate constants R2ρ with HS1 (a), HS4 (b) and HS8 (c) AFP pulses and normalized R2ρ with HS1 (d), HS4 (e) and HS8 (f) AFP pulses (● high,
 moderate and
moderate and
 low treatment response groups; ○ control animals; △ normal tissue).
low treatment response groups; ○ control animals; △ normal tissue).
Discussion
Our T1ρ and T2ρ data showed excellent sensitivity to cell death in glioma during gene therapy. These NMR tools were able to detect a significant decrease of the tumor mass as observed from day 4 onwards and discriminate between different treatment responses as indicated by histological cell density calculations in tumor tissue. It should be noted that the measurements of tumor volumes were unable to differentiate between different treatment response groups on day 8.
In tumor tissue, both translational and rotational water mobility increases over time after treatment, and the magnitude of the change is related to the effectiveness of the therapy. This is caused by a subsequent reduction in cell density and an increase of the extracellular space. Changes in translational motion are most often probed non-invasively by water diffusion that is inversely correlated to the cell density (10, 45). Our results show that changes in rotational mobility and exchange can be measured by T1ρ and T2ρ from day 4 onwards with different B1 values and HSn pulses respectively, which is earlier than conventional T1 and T2 changes are detected in this model (24, 44). The sensitivity to the earliest apoptotic response to treatment was very similar for all HSn pulse shapes and over the B1 range used in this study. On the other hand, at days 6 and 8 when necrotic centres started to form in these tumors, higher B1 values and higher n of HSn pulses provided more contrast between normal and tumor tissue. These findings point to the different sensitivity of these techniques to various cellular and structural changes occurring during apoptotic and necrotic cell death.
The proposed simple two pool fast exchange model provided excellent fits for estimating R1ρ values, and thus, appears to reasonably describe alterations in water dynamics during gene therapy-induced treatment response. Of course any model which is used for such an analysis is a simplification of the complex interactions that water molecules experience in living tissue. The model parameters are therefore considered as apparent values that describe the weighted average of the multiple molecular processes. Nevertheless, some degree of clear correspondence with known features in responding tumors in this model can be found. An increase of free water and a decrease of water associated with macromolecule populations are consistent with shrinkage of the cells during apoptosis and cell death, both leading to increased extracellular volume (15, 46). Furthermore, the correlation time of water associated with macromolecules substantially increases, as intracellular space becomes more crowded. A part of the increase in exchange correlation time on day 8 could be associated with decreased pH known to take place during apoptosis (47). However, when considering the changes in exchange time, it should be noted that in our simplified model the exchange term includes all dynamic dephasing contributions, including not only chemical exchange but also diffusion in local field gradients. Furthermore, a fixed value for Δω was used in the fitting. It cannot be ruled out that possible change in Δω during disease progression could have contributed to the fitting results.
Unequivocal fitting of the 2SX model using the R2ρ data was not possible due to a limited amount of data points measured. For comparison, T2ρ values were simulated using parameters obtained from T1ρ fitting. The calculated values were slightly different from experimental R2ρ values and the difference slightly increased with the increase of n of the HSn pulses. Importantly, the normalized data showed that in spite of this discrepancy the model almost completely explained the changes due to treatment. Our data indicate that a treatment independent factor was not included in the present simple model. The most likely explanation for this discrepancy is the contribution of intermediate exchange, or possible magnetization transfer effects due to saturation of the macromolecular pool by the preparation pulses (48).
Relatively long preparation pulses or pulse trains with high B1 values have high specific absorption rate (SAR) of RF energy and could potentially lead to tissue heating. The SAR value was calculated using the conservative assumption that all RF energy was deposited into 10 g of tissue. The calculated SAR values for the longest spin-lock time of 96 ms were 3.0 W/kg and 34 W/kg for B1 of 0.4 G and 1.4 G, respectively, in cw-T1ρ measurements. In spite of high SAR values, similar and higher B1 fields in T1ρ measurements under identical conditions did not cause measurable heating effect in the brain tissue even when perfusion was reduced (49). In a previous study, the accuracy of measured T1ρ in detecting treatment response was high and comparable when using low and high B1 values at the same stages of treatment (44). This is consistent with the findings of this work, showing B1 independent T1ρ response in the earliest time points. T2ρ measurement with HSn pulses provides an alternative way of reducing delivery of RF energy to tissue, as the SAR of an HSn pulse train is always lower than that of a cw-type T1ρ experiment with equal maximum B1. An interesting option for future studies is the recently introduced adiabatic T1ρ measurement, which can also be performed with HSn pulse trains (33). It should be noted that both T1ρ and T2ρ MR imaging have already been applied to human studies (27, 33, 38). Therefore, our present findings support the idea that these novel MRI techniques may provide useful clinical MR imaging contrast for patients with glioma.
In summary, we were able to show that T1ρ and T2ρ can provide early evidence of glioma treatment efficacy in one of the most commonly used gene therapy models. Furthermore, intrinsic parameters describing changes in water dynamics were obtained from a simple 2SX model and were associated with known cellular and structural changes taking place during treatment. The use of T1ρ and T2ρ MR imaging has tremendous potential for monitoring early changes in tumors that undergo a treatment response.
Acknowledgments
This work was supported by Academy of Finland, Emil Aaltonen Foundation, Finnish Technology agency TEKES, and NIH grants P41 RR008079, R01 CA92004, and P30 NS057091. Maarit Pulkkinen’s technical expertise in animal handling and histology is greatly appreciated.
References
- 1.Ylä-Herttuala S. Gene therapy and brain tumours. In: Mikkelsen T, Bjerkvig R, Laerum OD, Rosenblum ML, editors. Brain Tumour Invasion: Biological, Clinical, and Therapeutic Considerations. Wiley–Liss, Inc; New York: 1998. pp. 435–445. [Google Scholar]
- 2.Ram Z, Culver KW, Oshiro EM, Viola JJ, DeVroom HL, Otto E, Long Z, Chiang Y, McGarrity GJ, Muul LM, Katz D, Blaese RM, Oldfield EH. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3(12):1354–61. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 3.Moolten FL. Drug sensitivity (“suicide”) genes for selective cancer chemotherapy. Cancer Gene Ther. 1994;1(4):279–87. [PubMed] [Google Scholar]
- 4.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256(5063):1550–2. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 5.Hakumäki JM, Poptani H, Sandmair AM, Ylä-Herttuala S, Kauppinen RA. 1H MRS detects polyunsaturated fatty acid accumulation during gene therapy of glioma: Implications for the in vivo detection of apoptosis. Nat Med. 1999;5(11):1323–7. doi: 10.1038/15279. [DOI] [PubMed] [Google Scholar]
- 6.Immonen A, Vapalahti M, Tyynelä K, Hurskainen H, Sandmair A, Vanninen R, Langford G, Murray N, Yla-Herttualä S. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: A randomised, controlled study. Mol Ther. 2004;10(5):967–72. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Poptani H, Puumalainen AM, Gröhn OH, Loimas S, Kainulainen R, Ylä-Herttuala S, Kauppinen RA. Monitoring thymidine kinase and ganciclovir-induced changes in rat malignant glioma in vivo by nuclear magnetic resonance imaging. Cancer Gene Ther. 1998;5(2):101–9. [PubMed] [Google Scholar]
- 8.Gröhn OH, Lukkarinen JA, Silvennoinen MJ, Pitkänen A, van Zijl PC, Kauppinen RA. Quantitative magnetic resonance imaging assessment of cerebral ischemia in rat using on-resonance T(1) in the rotating frame. Magn Reson Med. 1999;42(2):268–76. doi: 10.1002/(sici)1522-2594(199908)42:2<268::aid-mrm8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Sepponen RE, Pohjonen JA, Sipponen JT, Tanttu JI. A method for T1ρ imaging. J Comput Assist Tomogr. 1985;9(6):1007–11. doi: 10.1097/00004728-198511000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3(9):1457–66. [PubMed] [Google Scholar]
- 11.Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, Doran S. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360(9329):307–8. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- 12.Mardor Y, Roth Y, Lidar Z, Jonas T, Pfeffer R, Maier SE, Faibel M, Nass D, Hadani M, Orenstein A, Cohen JS, Ram Z. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res. 2001;61(13):4971–3. [PubMed] [Google Scholar]
- 13.Moffat BA, Chenevert TL, Meyer CR, McKeever PE, Hall DE, Hoff BA, Johnson TD, Rehemtulla A, Ross BD. The functional diffusion map: An imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia. 2006;8(4):259–67. doi: 10.1593/neo.05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakumäki JM, Poptani H, Puumalainen AM, Loimas S, Paljarvi LA, Ylä-Herttuala S, Kauppinen RA. Quantitative 1H nuclear magnetic resonance diffusion spectroscopy of BT4C rat glioma during thymidine kinase-mediated gene therapy in vivo: Identification of apoptotic response. Cancer Res. 1998;58(17):3791–9. [PubMed] [Google Scholar]
- 15.Kauppinen RA. Monitoring cytotoxic tumour treatment response by diffusion magnetic resonance imaging and proton spectroscopy. NMR Biomed. 2002;15(1):6–17. doi: 10.1002/nbm.742. [DOI] [PubMed] [Google Scholar]
- 16.Evelhoch JL, Gillies RJ, Karczmar GS, Koutcher JA, Maxwell RJ, Nalcioglu O, Raghunand N, Ronen SM, Ross BD, Swartz HM. Applications of magnetic resonance in model systems: Cancer therapeutics. Neoplasia. 2000;2(1–2):152–65. doi: 10.1038/sj.neo.7900078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ailion D, Slichter C. Observation of ultra-slow translational diffusion in metallic lithium by magnetic resonance. Phys Rev Lett. 1964;12:168–71. [Google Scholar]
- 18.Fischer MW, Zeng L, Majumdar A, Zuiderweg ER. Characterizing semilocal motions in proteins by NMR relaxation studies. Proc Natl Acad Sci U S A. 1998;95(14):8016–9. doi: 10.1073/pnas.95.14.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desvaux H, Berthault P. Study of dynamic processes in liquids using off-resonance RF irradiation. Progr NMR Spectrosc. 1999;35:295–340. [Google Scholar]
- 20.Korzhnev D, Billeter M, Arseniev A, Orekhov V. NMR studies of brownian tumbling and internal motions in proteins. Progr NMR Spectrosc. 2001;38:197–266. [Google Scholar]
- 21.Davis D, Perlman M, London R. Direct measurements of the dissociation-rate constant for inhibitor–enzyme complexes via the T1ρ and T2 (CPMG) methods. J Magn Reson B. 1994;104:266–75. doi: 10.1006/jmrb.1994.1084. [DOI] [PubMed] [Google Scholar]
- 22.Duvvuri U, Poptani H, Feldman M, Nadal-Desbarats L, Gee MS, Lee WM, Reddy R, Leigh JS, Glickson JD. Quantitative T1ρ magnetic resonance imaging of RIF-1 tumors in vivo: Detection of early response to cyclophosphamide therapy. Cancer Res. 2001;61(21):7747–53. [PubMed] [Google Scholar]
- 23.Gröhn OH, Valonen PK, Lehtimäki KK, Väisänen TH, Kettunen MI, Ylä-Herttuala S, Kauppinen RA, Garwood M. Novel magnetic resonance imaging contrasts for monitoring response to gene therapy in rat glioma. Cancer Res. 2003;63(22):7571–4. [PubMed] [Google Scholar]
- 24.Hakumäki JM, Gröhn OH, Tyynelä K, Valonen P, Ylä-Herttuala S, Kauppinen RA. Early gene therapy-induced apoptotic response in BT4C gliomas by magnetic resonance relaxation contrast T1 in the rotating frame. Cancer Gene Ther. 2002;9(4):338–45. doi: 10.1038/sj.cgt.7700450. [DOI] [PubMed] [Google Scholar]
- 25.Michaeli S, Öz G, Sorce DJ, Garwood M, Ugurbil K, Majestic S, Tuite P. Assessment of brain iron and neuronal integrity in patients with Parkinson’s disease using novel MRI contrasts. Mov Disord. 2007;22(3):334–40. doi: 10.1002/mds.21227. [DOI] [PubMed] [Google Scholar]
- 26.Sorce DJ, Michaeli S, Garwood M. The time-dependence of exchange-induced relaxation during modulated radio frequency pulses. J Magn Reson. 2006;179(1):136–9. doi: 10.1016/j.jmr.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Michaeli S, Gröhn H, Gröhn O, Sorce DJ, Kauppinen R, Springer JRCS, Ugurbil K, Garwood M. Exchange-influenced T2ρ contrast in human brain images measured with adiabatic radio frequency pulses. Magn Reson Med. 2005;53(4):823–9. doi: 10.1002/mrm.20428. [DOI] [PubMed] [Google Scholar]
- 28.Michaeli S, Sorce DJ, Idiyatullin D, Ugurbil K, Garwood M. Transverse relaxation in the rotating frame induced by chemical exchange. J Magn Reson. 2004;169(2):293–9. doi: 10.1016/j.jmr.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Atsarkin V, Khazanovich T. Effect of slow molecular motions on nuclear magnetic relaxation under “magic-angle conditions”. Sov Phys JETP. 1984;60:162–7. [Google Scholar]
- 30.Blicharski J. Nuclear magnetic relaxation in rotating frame. Acta Physica Polonica. 1972;A41(2):223–36. [Google Scholar]
- 31.Casieri C, De Luca F. Spin interaction filter in solid-state NMR imaging. J Magn Reson. 2002;159(1):62–7. doi: 10.1016/s1090-7807(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 32.De Luca F, De Vita E, Raza GH, Casieri C. The spin coherence relaxation in the rotating frame as a microscopy parameter for strongly coupled spin systems. J Magn Reson. 1999;139(1):126–31. doi: 10.1006/jmre.1999.1760. [DOI] [PubMed] [Google Scholar]
- 33.Michaeli S, Sorce DJ, Springer CS, Jr, Ugurbil K, Garwood M. T1ρ MRI contrast in the human brain: Modulation of the longitudinal rotating frame relaxation shutter-speed during an adiabatic RF pulse. J Magn Reson. 2006;181(1):135–47. doi: 10.1016/j.jmr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Abergel D, Palmer A., III On the use of the stochastic liouville equation in nuclear magnetic resonance: Application to R1ρ relaxation in the presence of exchange. Concepts Magn Reson A. 2003;19A(2):134–48. [Google Scholar]
- 35.Michaeli S, Sorce DJ, Garwood M. T1ρ and T2ρ adiabatic relaxations and contrasts. Current Analytical Chemistry 2007. 2008;4:8–25. [Google Scholar]
- 36.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. In vivo proton MR three-dimensional T1ρ mapping of human articular cartilage: Initial experience. Radiology. 2003;229(1):269–74. doi: 10.1148/radiol.2291021041. [DOI] [PubMed] [Google Scholar]
- 37.Gröhn OH, Mäkelä HI, Lukkarinen JA, DelaBarre L, Lin J, Garwood M, Kauppinen RA. On- and off-resonance T1ρ MRI in acute cerebral ischemia of the rat. Magn Reson Med. 2003;49(1):172–6. doi: 10.1002/mrm.10356. [DOI] [PubMed] [Google Scholar]
- 38.Gröhn HI, Michaeli S, Garwood M, Kauppinen RA, Gröhn OH. Quantitative T1ρ and adiabatic Carr-Purcell T2 magnetic resonance imaging of human occipital lobe at 4 T. Magn Reson Med. 2005;54(1):14–9. doi: 10.1002/mrm.20536. [DOI] [PubMed] [Google Scholar]
- 39.Silver M, Joseph R, Hoult D. Highly selective π/2 and π pulse generation. J Magn Reson. 1984;59:347–51. [Google Scholar]
- 40.Tannus A, Garwood M. Improved performance of frequency-swept pulses using offset-independent adiabaticity. J Magn Reson A. 1996;120:133–7. [Google Scholar]
- 41.Garwood M, DelaBarre L. The return of the frequency sweep: Designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153(2):155–77. doi: 10.1006/jmre.2001.2340. [DOI] [PubMed] [Google Scholar]
- 42.Levitt M, Freeman R, Frenkel T. Broadband heteronuclear decoupling. J Magn Reson. 1982;47:328–30. [Google Scholar]
- 43.Woessner DE. Nuclear transfer effects in nuclear magnetic resonance pulse experiments. J Chem Phys. 1961;35(1):41–8. [Google Scholar]
- 44.Kettunen MI, Sierra A, Närväinen MJ, Valonen PK, Ylä-Herttuala S, Kauppinen RA, Gröhn OH. Low spin-lock field T1 relaxation in the rotating frame as a sensitive MR imaging marker for gene therapy treatment response in rat glioma. Radiology. 2007;243(3):796–803. doi: 10.1148/radiol.2433052077. [DOI] [PubMed] [Google Scholar]
- 45.Valonen PK, Lehtimäki KK, Vaisanen TH, Kettunen MI, Gröhn OH, Ylä-Herttuala S, Kauppinen RA. Water diffusion in a rat glioma during ganciclovir-thymidine kinase gene therapy-induced programmed cell death in vivo: Correlation with cell density. J Magn Reson Imaging. 2004;19(4):389–96. doi: 10.1002/jmri.20026. [DOI] [PubMed] [Google Scholar]
- 46.Ross BD, Moffat BA, Lawrence TS, Mukherji SK, Gebarski SS, Quint DJ, Johnson TD, Junck L, Robertson PL, Muraszko KM, Dong Q, Meyer CR, Bland PH, McConville P, Geng H, Rehemtulla A, Chenevert TL. Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol Cancer Ther. 2003;2(6):581–7. [PubMed] [Google Scholar]
- 47.Wolf CM, Eastman A. Intracellular acidification during apoptosis can occur in the absence of a nucleus. Biochem Biophys Res Commun. 1999;254(3):821–7. doi: 10.1006/bbrc.1998.0132. [DOI] [PubMed] [Google Scholar]
- 48.Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10(1):135–44. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- 49.Grohn OHJ, Kettunen MI, Makela HI, Penttonen M, Pitkanen A, Lukkarinen JA, Kauppinen RA. Early detection of irreversible cerebral ischemia in the rat using dispersion of the magnetic resonance imaging relaxation time, T1rho. J Cereb Blood Flow Metab. 2000;20(10):1457–66. doi: 10.1097/00004647-200010000-00007. [DOI] [PubMed] [Google Scholar]