Abstract
Negative-sense RNA viruses, such as influenza, encode large, multidomain RNA-dependent RNA polymerases that can both transcribe and replicate the viral RNA genome1. In influenza virus, the polymerase (FluPol) is composed of three polypeptides: PB1, PB2 and PA/P3. PB1 houses the polymerase active site, whereas PB2 and PA/P3 contain, respectively, cap-binding and endonuclease domains required for transcription initiation by cap-snatching2. Replication occurs through de novo initiation and involves a complementary RNA intermediate. Currently available structures of the influenza A and B virus polymerases include promoter RNA (the 5′ and 3′ termini of viral genome segments), showing FluPol in transcription pre-initiation states3,4. Here we report the structure of apo-FluPol from an influenza C virus, solved by X-ray crystallography to 3.9 Å, revealing a new ‘closed’ conformation. The apo-FluPol forms a compact particle with PB1 at its centre, capped on one face by PB2 and clamped between the two globular domains of P3. Notably, this structure is radically different from those of promoter-bound FluPols3,4. The endonuclease domain of P3 and the domains within the carboxy-terminal two-thirds of PB2 are completely rearranged. The cap-binding site is occluded by PB2, resulting in a conformation that is incompatible with transcription initiation. Thus, our structure captures FluPol in a closed, transcription pre-activation state. This reveals the conformation of newly made apo-FluPol in an infected cell, but may also apply to FluPol in the context of a non-transcribing ribonucleoprotein complex. Comparison of the apo-FluPol structure with those of promoter-bound FluPols allows us to propose a mechanism for FluPol activation. Our study demonstrates the remarkable flexibility of influenza virus RNA polymerase, and aids our understanding of the mechanisms controlling transcription and genome replication.
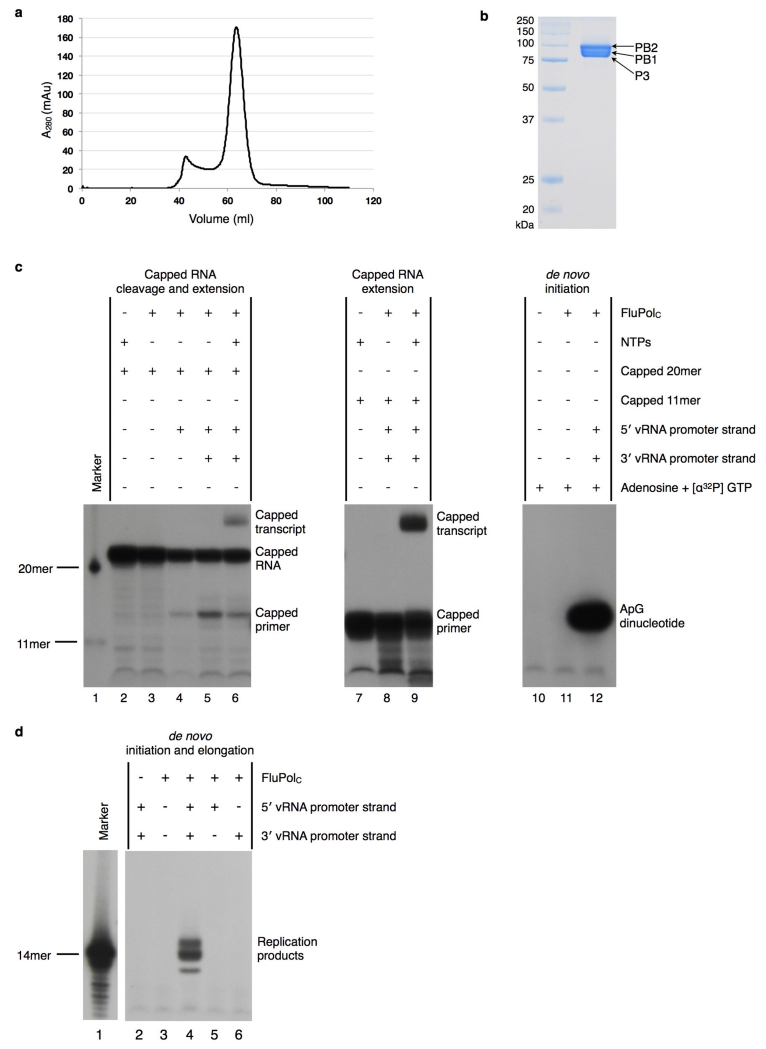
FluPol is a highly flexible protein complex; however, the conformational states it can adopt are uncharacterized. Understanding the nature of these conformational states is central to determining the regulatory mechanisms of this enzyme. To this end, we have determined the structure of FluPol from influenza C virus5 (FluPolC), in the absence of promoter RNA. We expressed all three individual subunits of FluPolC in insect cells by infection with a single baculovirus construct. FluPolC purified from this system was active in both replication and transcrip-tion initiation (Extended Data Fig. 1). We crystallized apo-FluPolC in two different crystal forms (Extended Data Table 1), and solved its structure at 3.9 Å (Extended Data Fig. 2) and 4.3 Å resolution.
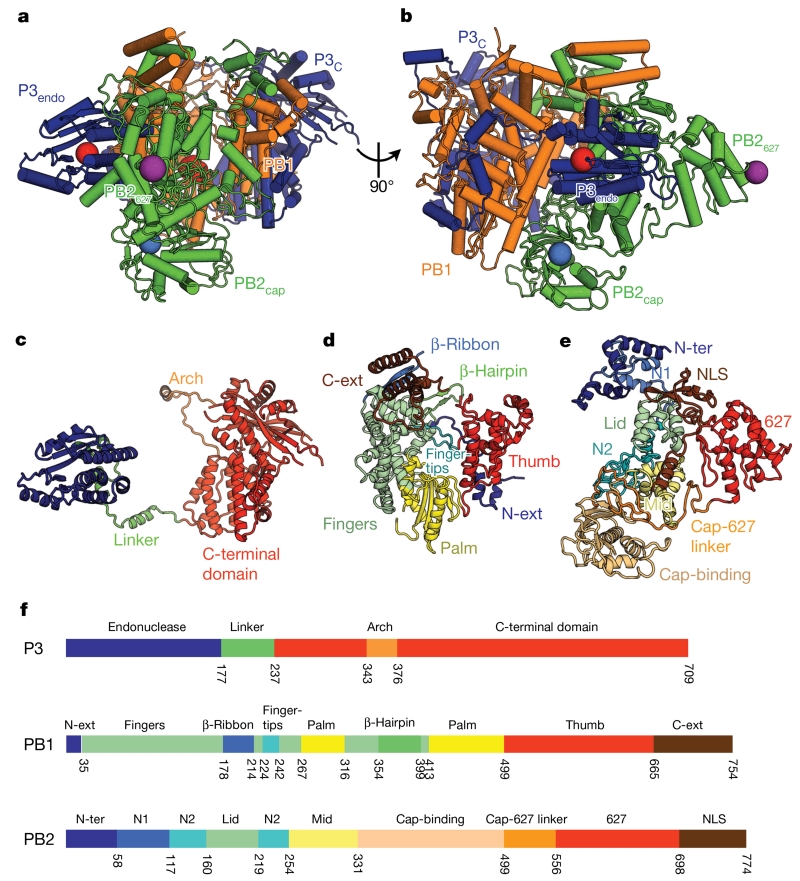
Our model of FluPolC (Fig. 1) comprises 711 of the 754 residues of PB1 (94.3%), 762 out of 774 for PB2 (98.4%) and 693 out of 709 for P3 (97.7%). FluPolC forms a relatively compact structure (Fig. 1a, b). P3 folds into two domains connected by a long linker (Fig. 1c): an amino-terminal endonuclease domain (P3endo) and a C-terminal domain (P3C), which sandwiches PB1 at the heart of the molecule. PB1 has the canonical right-hand-like polymerase fold, possessing palm, fingers and thumb subdomains with additional N- and C-terminal extensions (PB1N-ext and PB1C-ext) that facilitate interactions with the other subunits (Fig. 1d). The thumb of PB1 is reinforced by P3C. The priming loop of PB1, believed to facilitate de novo replication initiation4, is not visible in our structure and is probably disordered. PB2 stacks against one face of PB1, contacting both domains of P3. PB2 comprises 9 domains: the N-terminal PB1 interaction domain (PB2N-ter), PB2N1, PB2N2, PB2lid and PB2mid domains, a cap-binding domain (PB2cap), a linker domain (PB2cap-627 linker), the 627 domain (PB2627) and a C-terminal nuclear localization signal (NLS) (PB2NLS) domain (Fig. 1e).
Figure 1. Structure of FluPolC.
a, b, Two views of the structure of the FluPolC heterotrimer, coloured according to subunit (PB1, orange; PB2, green; P3, blue). The cap-binding pocket and endonuclease active site are shown as blue and red spheres, respectively. In a, the PB1 catalytic aspartates, residues 446 and 447, are also highlighted red. The position of PB2 residue 649 (equivalent to PB2 residue 627 in FluPolA) is marked by a purple sphere. c–e, Structures of FluPolC subunits P3 (c), PB1 (d) and PB2 (e), coloured and labelled by domain. f, Domain maps of each FluPolC subunit.
The fold of each FluPolC domain is very similar to its counterpart in FluPolA and FluPolB, even though the sequence identity between these polymerases is only ~30% (Extended Data Table 2). The average root mean squared deviation (r.m.s.d.) values of Cα atoms between equivalent superposed domains of FluPolC and FluPolA, or of FluPolC and FluPolB are 1.6 Å or 1.5 Å, respectively, demonstrating that the FluPol fold is conserved across influenza A, B and C viruses. All key active site residues within FluPolC are structur-ally conserved, and we confirmed, by mutation, that FluPolC shares common mechanisms with FluPolA (Extended Data Fig. 3a). The PB1 subunits of FluPol A, B and C belong to a structural grouping that most closely resembles the polymerases of Reoviridae and Cystoviridae/Flaviridae (Extended Data Fig. 3b).
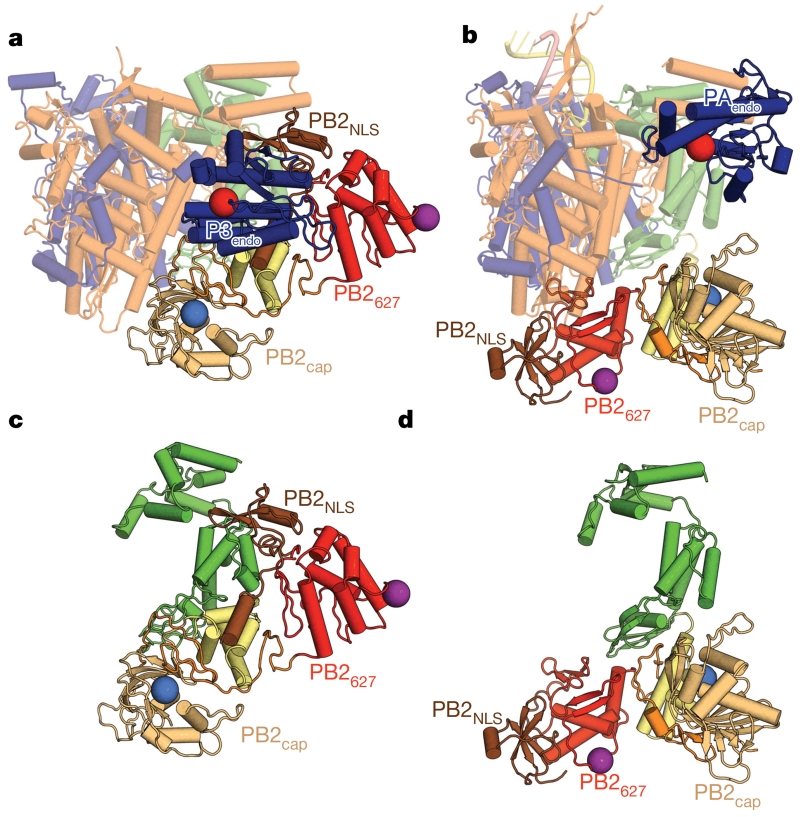
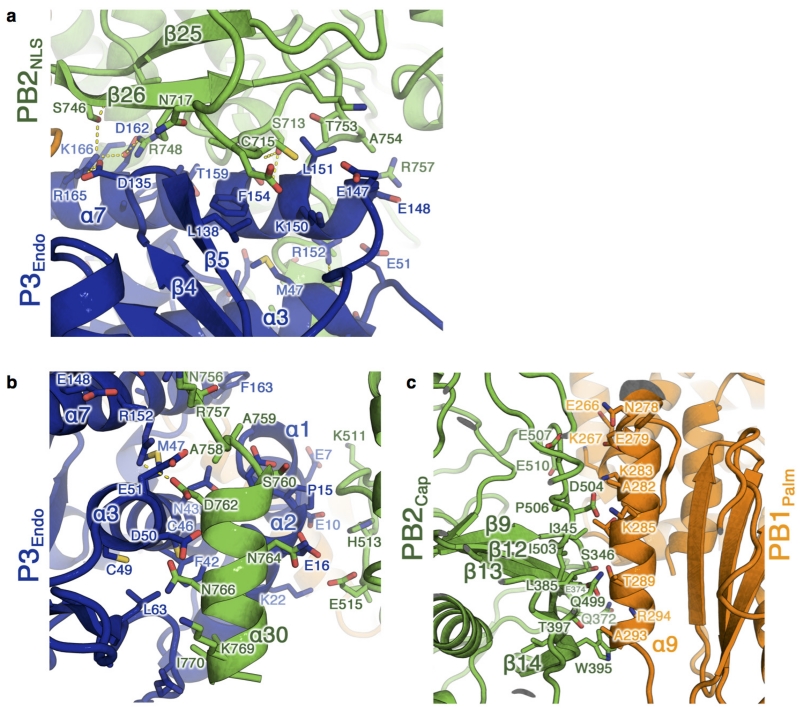
However, there are substantial differences between apo-FluPolC and the activated structures for promoter-bound FluPolA and FluPolB. Most striking are the position of P3endo and the arrangement of the C-terminal domains of PB2 (Fig. 2, Supplementary Video 1 and Extended Data Table 3). Thus, PB2627, which in FluPolA houses a crucial polymorphism (Glu627Lys) for the determination of viral host range and pathogenicity6, lies level with the endonuclease domain in the apo structure, (Fig. 2a), whereas in the activated structures it lies close to PB1palm (Fig. 2b). The PB2mid and PB2cap-627 linker domains are rearranged en bloc, by a rotation of 140° and a translation of 30 Å, between the apo and activated conformations. PB2cap also changes; in the apo structure, it is tucked in between the PB1palm and PB2cap-627 linker, while in the activated structures it does not extensively contact other domains. Finally, PB2NLS packs between PB1C-ter helix α23 and P3endo helix α7 in apo-FluPolC (Fig. 3a), but is near the base of the PB1palm in the activated structures (Fig. 2b). The movement of PB2NLS amounts to a rotation of 130° and a translation of 90 Å. The regions rearranged within PB2 match those that are disordered in the FluB2 structure4, lying immediately downstream of a conserved glycine (PB2 residue 255 in FluPolC).
Figure 2. Comparison of apo-FluPolC with promoter-bound FluPolA.
a, Apo-FluPolC, depicted in the same orientation and colouring as in Fig. 1b, but with the C-terminal domains of PB2 coloured as in Fig. 1e. Domains that do not change between apo and promoter-bound conformations are depicted as semi-transparent. b, Promoter-bound FluPolA, shown as in a. c, d, The PB2 subunits of apo-FluPolC (c) and promoter-bound FluPolA (d), depicted as in a and b.
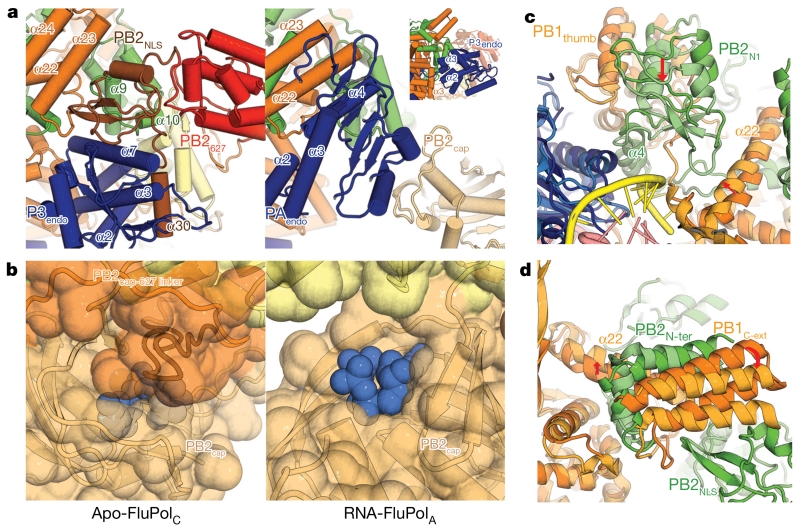
Figure 3. Critical changes between apo-FluPolC and promoter-bound FluPolA.
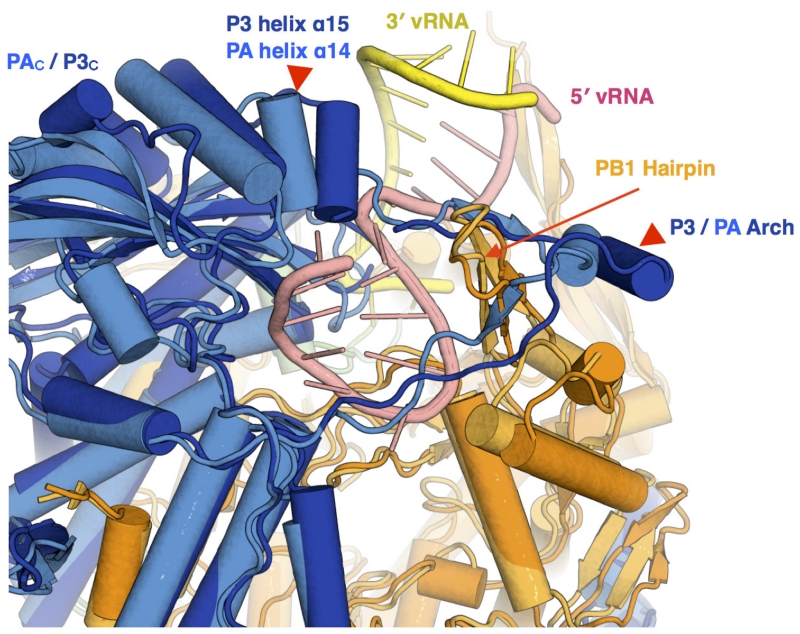
a, Equivalent views of FluPolC (left) and FluPolA (right), showing domain arrangement differences. The inset shows the arrangement of P3endo within the FluPolC structure. b, Close-up of the FluPolC (left) and FluPolA (right) cap-binding domains. PB2 residues 520–535 in FluPolC are coloured dark orange. The cap-binding pocket is shown with blue spheres. c, d, Two views of a superposition of apo-FluPolC and FluPolA, with FluPolC coloured as in Fig. 1 and FluPolA in lighter colours. 5′ and 3′ promoter RNAs in the FluPolA structure are coloured pink and yellow, respectively. Arrows highlight differences between the two conformations.
The buried area between PB2 and P3 (5,000 Å2) is more extensive in the apo than the activated, promoter-bound structures, reflecting new contacts between PB2 and P3endo (Fig. 3a and Extended Data Fig. 4a, b). Additionally, the extreme C terminus of PB2 is visible in our apo structure (Extended Data Fig. 4b), forming a helix (α30) that packs against the back face of P3endo (near P3 helices α2, α3 and α7). There are also many new contacts between PB2 and PB1 (Fig. 2 and Extended Data Fig. 4c).
One important consequence of the arrangement seen in apo-FluPolC is that the cap-binding pocket is occluded (Fig. 3b). PB2cap is folded in on the rest of the subunit, facing residues 520–535 from the PB2cap-627 linker domain. This is consistent with the observation that promoter RNA is required for FluPol cap-binding and endonuclease activity7,8. Thus, the structure we observe represents a closed, pre-activation state of FluPol and suggests that the viral RNA (vRNA) promoter causes the rearrangements necessary to form the activated structure.
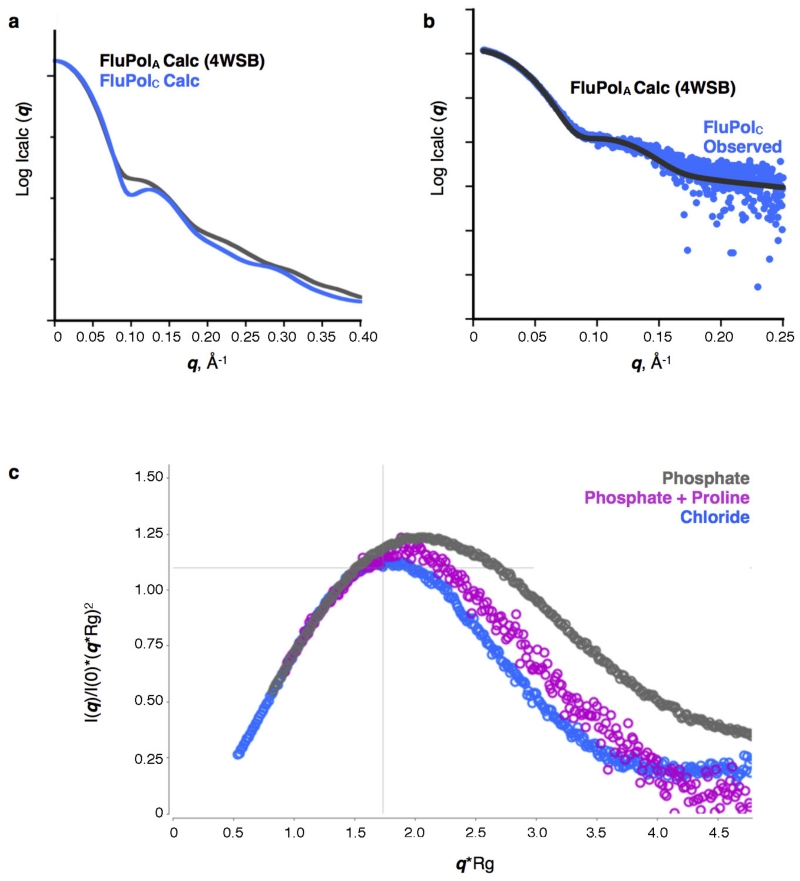
Alternatively, the structure of FluPolC could indicate a fundamental conformational difference between FluPols from different influenza viruses. To clarify this, we performed small angle X-ray scattering (SAXS) experiments with FluPolC, as these allowed us to distinguish between closed and activated FluPol conformations (Extended Data Fig. 5a). The observed scattering profiles from FluPolC were similar to a profile calculated from the FluPolA crystal structure, indicating that FluPolC can adopt the same activated conformation (Extended Data Fig. 5b). Thus, the change we see in the FluPolC crystal is not an influenza virus type difference. However, promoter RNA was not required for the activated conformation to be detected, indicating that changes between apo and promoter-bound structures need not exclusively be caused by RNA binding. This suggests that the energy barrier between different FluPol conformations is low. Indeed, when placed into a phosphate-based buffer, FluPolC adopted a currently uncharacterized conformation that was even more open than that of the promoter-bound structures (Extended Data Fig. 5c). These results suggest that FluPol may be poised between several different conformations, with only subtle environmental changes needed for a particular conformation to be favoured.
In line with this assessment, differences around the promoter-binding site between the apo-FluPolC and promoter-bound structures are small. Minor changes are evident around the pocket that binds the intra-base paired hook structure of the 5′ strand of the vRNA promoter (Extended Data Fig. 6); however, sequence alignments suggest that these differences are influenza-virus-type-specific. More interesting are the differences around the binding site for the 3′ strand of the vRNA promoter. In the apo structure, PB2 helix α4, PB2N1 and the associated region of PB1thumb lie ~5 Å further away from the polymerase core than in the promoter-bound structures (Fig. 3c). This change is transmitted to the neighbouring PB1C-ext–PB2N-ter interaction domain through PB1 helix α22, resulting in a 20° rotation of this domain between the apo and vRNA promoter-bound conformations (Fig. 3d). Since the PB1C-ext–PB2N-ter domain lies next to PB2NLS in apo-FluPolC (Fig. 3a), this rotation could trigger the movement of PB2NLS from its apo position, leading to the subsequent massive reorganization of FluPol after vRNA–promoter binding (Supplementary Video 2).
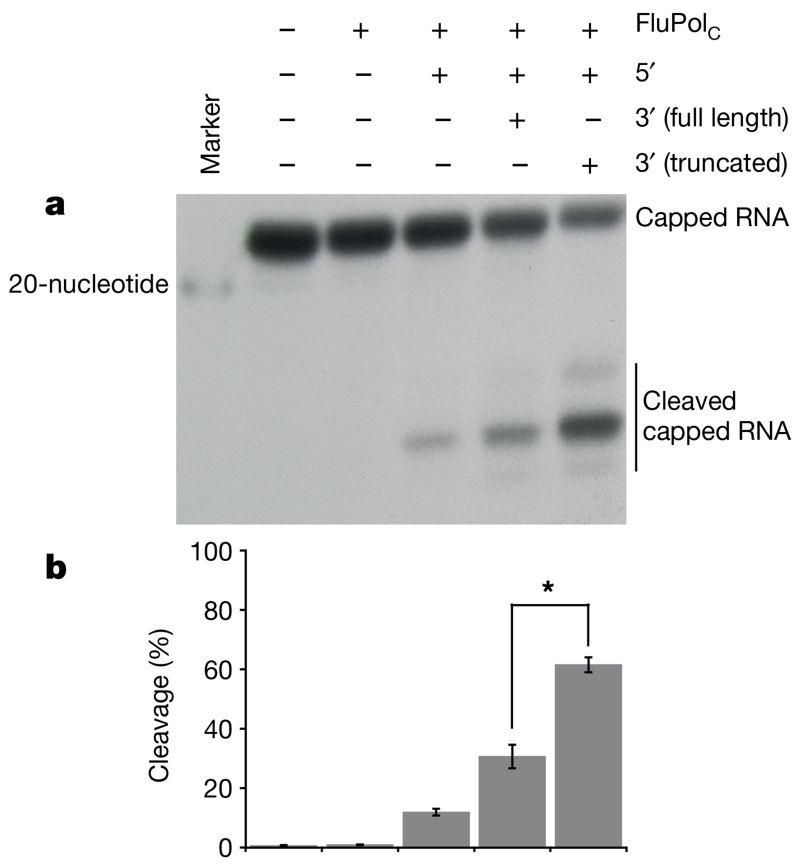
Notably, only one currently reported FluPol structure (FluB2) contains a fully ordered vRNA promoter (in the others, the 3′ vRNA strand is either truncated or partially disordered)4. However, this does not display a stable activated conformation, as the C-terminal two-thirds of PB2 are not resolved. We suggest that this is because initial binding of the vRNA promoter, into a resting position away from the active site, generates a dynamic equilibrium between closed and activated conformations. The activated structure is only seen when the 3′ end of the 3′ vRNA promoter strand is either not present or disordered3,4. Hence, the activated conformation might only be fully stabilized when this 3′ end is released from its resting position to enter the polymerase active site. To test this hypothesis, we compared the ability of a full-length or truncated (lacking four nucleotides at the 3′ end of the 3′ strand) vRNA promoter to stimulate FluPolC cap-dependent cleavage activity (Fig. 4). We reasoned that stabilization of an activated over a closed conformation would enhance capped-RNA cleavage, as the cap-binding pocket in PB2 becomes more accessible. In line with this, we observed a significant enhancement in capped RNA cleavage in the presence of the truncated promoter RNA (Fig. 4). The relative inefficiency of the full-length vRNA promoter to stimulate cleavage supports our assertion that initial promoter binding results in a closed/activated equilibrium. The mechanism behind this may involve the PB1 β-ribbon (177–212 in FluPolA)3, which is disordered in the apo-FluPolC structure, but adopts different conformations in the activated and FluB2 structures.
Figure 4. Capped-RNA cleavage assays with FluPolC.
a, Representative autoradiograph of a capped-RNA cleavage assay. In each reaction, radiolabelled capped RNA was incubated at 30 °C for 2 h with FluPolC and the indicated strands of the vRNA promoter. b, Quantification of cleavage, expressed as the percentage of cleaved to total RNA, from three replicates of this assay, performed with the same polymerase preparation. Mean cleavage percentage is plotted. Error bars show s.d. Asterisk indicates a significant difference between cleavage with full or truncated vRNA promoter (n = 3, P = 0.0003, two-tailed t-test).
In summary, we have solved the structure of the RNA polymerase from an influenza C virus in the absence of RNA, uncovering a closed conformation accessible to FluPol. Our structure explains the observation that FluPol in the absence of promoter RNA is unable to perform cap-snatching7,8, and we propose a mechanism for how vRNA promoter might bring about FluPol activation. However, the closed conformation captured here may have a wider functional relevance, because it could still be accessible to FluPol bound to a fully ordered vRNA promoter that does not enter the active site. Therefore, in the context of a non-transcribing viral ribonucleoprotein complex (RNP), containing FluPol, RNA and nucleoprotein, FluPol may well adopt this closed conformation. In addition, dependent on stabilization of the PB1 priming loop, the closed conformation that we observe might still allow de novo initiation, as this is not dependent on cap-snatching. Thus, the conformation that we observe, in addition to being a transcription pre-activation state, could be relevant during genome replication initiation. This would allow the activity of FluPol within an RNP to be regulated by other viral factors and host proteins9-12.
Our work underlines the tremendous flexibility of this protein complex. This flexibility offers an explanation for the differences between several low-resolution electron microscopy reconstructions of RNP-associated FluPol, as well as explaining why the promoter-bound structures do not fit well into these reconstructions13-16. Furthermore, since negative-sense RNA virus polymerases share a common organization, with a central polymerase core surrounded by various functional modular appendages1,17, the conformational flexibility revealed here might be a theme among all of these polymerases and not just particular to FluPol18.
METHODS
Protein expression and purification
The three subunits of the influenza C/Johannesburg/1/1966 virus polymerase (PB1: AAF89738, PB2: AAF89739, P3: AAF89737) were co-expressed in Sf9 cells from codon-optimized genes (GeneArt) cloned into a single baculovirus using the MultiBac system19. Expression and purification of FluPolC proceeded as previously described for FluPolA12, except that the gel filtration buffer used was 0.5 M NaCl, 25 mM HEPES-NaOH, pH 7.5 and 10% (v/v) glycerol. For crystallization and storage, protein purified in this buffer was supplemented with 0.5 mM TCEP, and 10 mM MgCl2 or 10 mM CaCl2.
Crystallization, data collection and structure determination
Crystals of FluPolC, belonging to two different space groups, grew from sitting-drop vapour-diffusion experiments at 20 °C20,21, set up using a protein:precipitant ratio of 2:1. In these experiments, 5 mg ml−1 protein was mixed with either 70% (v/v) Morpheus G2 (Molecular Dimensions), supplemented with 0%–1% 1 M NaOH, to generate P43212 crystals; or with crystal-seeds and 0.2 M NaCl, 0.1 M Na-HEPES, pH 7.5 and 25% (w/v) PEG 4000, for P212121 crystals. For heavy atom derivatization, P43212 crystals were soaked in a solution of gold(i) potassium cyanide dissolved in mother liquor, for 2–3 h at 20 °C. Crystals were cryo-protected using 25% (v/v) glycerol in crystallization buffer, before flash-cooling in liquid nitrogen, and data collection on beamlines I03 and I04 at the Diamond Light Source, Didcot, UK. The beam size was matched to the crystal size and data were collected on a Pilatus 6M detector at a wavelength of 0.9763 Å (tetragonal native), 1.0350 Å (tetragonal derivative) and 0.9795 Å (orthorhombic native). Data collection statistics are shown in Extended Data Table 1. Data were processed using Xia2 (ref. 22) and HKL2000 (ref. 23). Initial phases were obtained by single isomorphous replacement with anomalous scattering (SIRAS), using data from native P43212 and gold-derivatized crystals. The P43212 data used at this stage was collected earlier (at a wavelength of 0.8634 Å) than that subsequently used in refinement. Heavy atoms were located with SHELX24 and phases improved by two-fold non-crystallographic averaging (the crystallographic asymmetric unit contained two heterotrimers) and solvent flattening (solvent content 76%) using Phenix.autosol25. The tetragonal and the orthorhombic data were sharpened to 40 Å2 and 36 Å2, respectively. The P212121 crystals were solved by molecular replacement (program Phaser26), using the P43212 structure as the search model. As expected the orthorhombic crystals possessed four heterotrimers in the crystallographic asymmetric unit, allowing phase improvement using non-crystallographic symmetry (NCS) averaging and solvent flattening using general averaging program (GAP) (D.I.S. and J.M.G., unpublished observations). The published fragments of FluPolA (PDB accessions 4IUJ, 4AWH, 4CB4, 3A1G and 2VY7) were fitted by eye using Coot, which was used for all model building27. Comparison with the complete FluPolA and FluPolB structures (4WSB and 4WSA, respectively), aided by the anomalous scattering from the sulphur atoms as markers, allowed us to build and refine complete models for FluPolC. This provided a total of six independent views of the polymerase. Performing superpositions of these demonstrated that the molecule adopts a virtually identical conformation across all copies from both crystal forms (mean pairwise r.m.s.d. in Cα was 0.94 Å between all pairs of molecules across both space groups). Refinement (Extended Data Table 1) used BUSTER28 aided by NCS and initially phase restraints, and REFMAC29 with secondary structure restraints using PROSMART30.
SAXS experiments
SAXS measurements were performed on beamline B21 at Diamond Light Source, Didcot, UK. Samples were prepared onsite using a Shodex Kw-403 size exclusion column and Agilent HPLC. Approximately 40–60μl of sample were collected for SAXS at 20 °C using a sample to detector distance of 3.9 m and X-ray wavelength of 1 Å. Samples were exposed for 300 s in 10 s acquisition blocks. Images were corrected for variations in beam current, normalized for exposure time and processed into 1D scattering curves using GDA and DAWN. Buffer subtractions and all other subsequent analysis were performed with the program ScÅtter (http://www.bioisis.net/scatter). Samples were checked for radiation damage by visual inspection of the Guinier region as a function of exposure time.
Data analysis
Figures and videos were prepared using PyMOL (http://www.pymol.org) and Chimera31. Structural comparisons used SHP32.
Polymerase activity assays
For the cap-dependent cleavage and transcription assays, FluPolC (400 ng per reaction) was incubated for 2 h at 30 °C with or without (as indicated) NTPs (1 mM ATP, 0.5 mM each CTP/UTP/GTP), radiolabelled capped 20-nucleotide or 11-nucleotide RNAs, 0.6 μM each 5′ and 3′ vRNA promoter strands, in a reaction buffer containing 7.5 mM MgCl2, 1.0 mM TCEP, 2 U μl−1 RNasin (Promega), 20 mM HEPES-NaOH, pH 7.5, 100 mM NaCl and 5% (v/v) glycerol. For the de novo initiation and elongation assays, FluPolC (400 or 800 ng per reaction, as indicated) was incubated for 2–3 h at 30 °C with 2.5 mM adenosine and 0.075 μM [α-32P]GTP or 1 mM ATP, 0.5 mM each CTP/UTP, 0.1 mM GTP, 0.3 μM [α-32P]GTP and (as indicated) 0.6 μM each 5′ or 3′ vRNA promoter strands, in the same reaction buffer as above. The reaction volume was 4 μl for all reactions. Products were denatured by boiling (98 °C, 5 min) after the addition of formamide (4 μl) and separated on a denaturing 20% polyacrylamide gel, with the indicated size markers. Products were visualized by autoradiography. For all activity assays except the cleavage assays, the sequences of the promoter RNA oligonucleotides used were: 5′-AGCAGUAGCAAGGAG-3′ (5′ vRNA) and 5′-CUCCUGCUUCUGCU-3′ (3′ vRNA). The sequences of the RNAs used in the capped-RNA cleavage assays were 5′-AGCAGUAGCAAGGGG-3′ (5′), 5′-UAUACCCCUGCUUC-3′ (3′ truncated) or 5′-UAUACCCCUGCUUCUGCU-3′ (3′ full length).
Capped and radiolabelled RNA was produced by incubating 5′ diphosphate synthetic 20-nucleotide (5′-ppAAUCUAUAAUAGCAUUAUCC-3′)3,4 or 11-nucleotide (5′-ppGAAUACUCAAG-3′)33,34 RNA (Chemgenes), with [α-32P]GTP, vaccinia virus capping enzyme (NEB) and 2′-O-methyltransferase (NEB), following the manufacturer’s instructions. The resulting RNAs were gel purified before use in the above assays.
Extended Data
Extended Data Figure 1. Purification and characterization of FluPolC.
a, Elution profile of FluPolC, after affinity purification over IgG-sepharose, from a size-exclusion chromatography column. Eluted protein was detected by measuring the absorbance at 280 nm. b, Fractions corresponding to the major peak eluting from the size-exclusion chromatography column were mixed and analysed by SDS–PAGE on a 15% polyacrylamide gel, alongside the indicated molecular mass markers. Protein was visualized by Coomassie blue staining (PB1, 86.0 kDa; PB2, 87.2 kDa; P3, 81.9 kDa). c, Transcription and replication initiation assays. Lanes 2–6 test for transcription initiation. With the addition of vRNA promoter only (lanes 4 and 5), FluPolC can cleave a capped and radiolabelled 20-nucleotide RNA, demonstrating promoter-dependent endonuclease activity. Lane 6 shows that with the addition of NTPs, this capped primer can be extended to produce a capped transcript, thus demonstrating transcription initiation activity. This result is confirmed by lanes 7–9, which test for extension of a capped and radiolabelled 11-nucleotide RNA primer. Extension only takes place when the polymerase is supplied with NTPs and promoter RNA (lane 9). Lanes 10–12 assay for replication initiation. Lane 12 shows that FluPolC (400 ng per reaction) is able to synthesize ApG dinucleotide in a primer-independent manner. This demonstrates de novo replication initiation activity. Uncapped 20-nucleotide and 11-nucleotide primers are used as size markers in lane 1. The slow migration of the ApG dinucleotide compared to the markers is due to the lack of phosphate groups on the 5′ end of this product35. d, De novo initiation and elongation assay. FluPolC (800 ng) was incubated for 3 h with NTPs, [α-32P]GTP and 5′ or 3′ vRNA promoter strands, as indicated. In the presence of both promoter strands (lane 4), FluPolC is able to produce a full-length copy of the template (14 nucleotides, corresponding to the major band), demonstrating de novo replication initiation and elongation activity. The minor slower and faster bands may correspond to non-templated extension and premature termination products, respectively.
Extended Data Figure 2. Data and model quality.
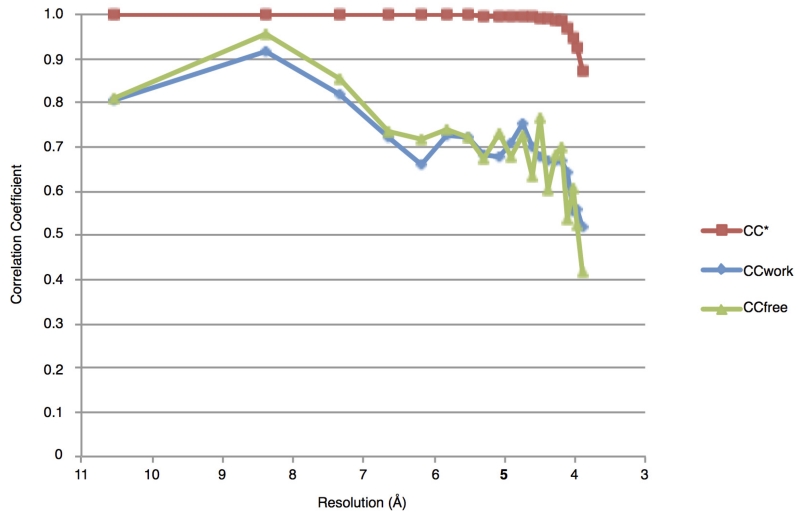
Plots of CC*, CCfree and CCwork against resolution, for the tetragonal crystal data set and model. The CC* statistic assesses the signal present in the data, while CCfree and CCwork provide an estimate of the agreement between data and model. A CC* value of 0.87 for the highest resolution shell indicates that these data contain useful information up to 3.9 Å. CCfree and CCwork are lower than CC*, showing that the model does not overfit the data.
Extended Data Figure 3. Functional and structural relationships of FluPolC.
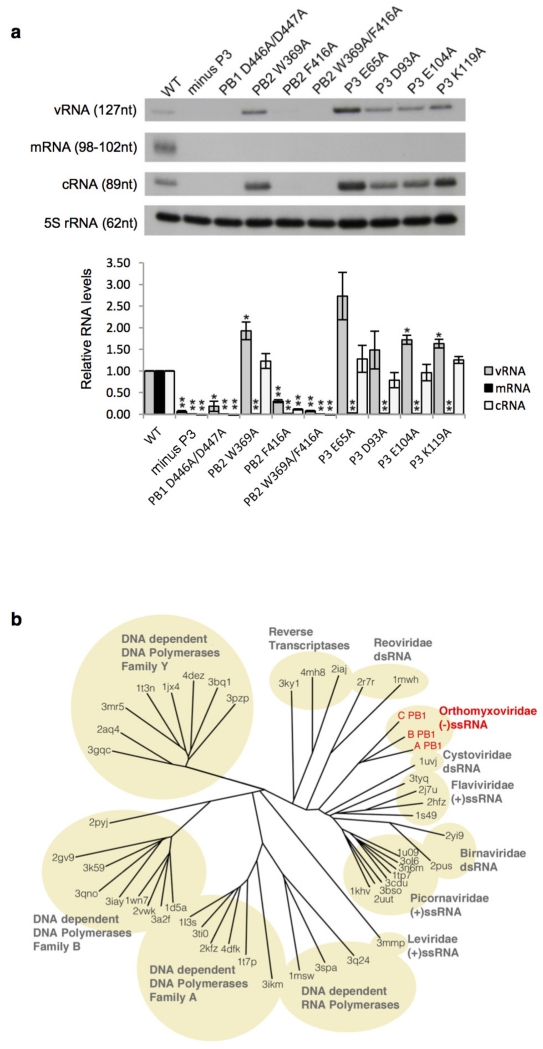
a, Effect of amino acid mutations in FluPolC on transcription and replication. Plasmids to express FluPolC subunits and NP along with a plasmid expressing a negative-sense CAT reporter gene flanked by the terminal non-coding sequences of the influenza C virus NS gene segment36 were transfected into 293T cells (ATCC). Total RNA was isolated using Trizol (Invitrogen) 30 h post transfection and viral RNAs were analysed by primer extension37 using the following primers: 5′-CGCAAGGCGACAAGGTGCTGA-3′ (for detection of vRNA, yielding a 127-nucleotide product) and 5′-ATGTTCTTTACGATGCGATTGGG-3′ (for detection of mRNA and complementary RNA (cRNA), yielding 98–102-nucleotide and 89-nucleotide products, respectively). Primer extension products were analysed by 6% PAGE. Quantification of primer extension analysis using phosphorimaging is shown below. The mean and s.d. of three experiments are shown. Asterisks indicate a significant difference from wild type (WT), which was set to 100% (* P < 0.05; ** P < 0.01, based on a two-sample t-test). A double mutation of two aspartic acids Asp446/Asp447, that align with Asp445/Asp446 of influenza A virus PB1 found to be critical for activity38, resulted in no detectable activity in the context of FluPolC. Mutation of amino acid residues in the PB2 cap-binding and P3 endonuclease domains that align with critical amino acid residues in FluPolA39-41 resulted in undetectable accumulation of viral mRNA although most of these mutants were still able to replicate. The exception was Phe416Ala in PB2 that inhibited both transcription and replication, suggesting that this mutation might not only affect cap-binding but overall PB2 folding, in agreement with previously observed inhibitory effects of mutations in the cap-binding domain on replication42. The requirement of these amino acid residues for mRNA synthesis is consistent with the hypothesis that FluPolC generates capped RNA primers for transcription initiation in a manner similar to that of FluPolA. b, Structure-based phylogenetic tree showing the relationship of PB1 from FluPolC (C PB1) to other right-handed polymerases. Pairwise comparisons were performed using SHP32 and a phylogenetic tree constructed using PHYLIP. The branches are identified by the PDB accession code of the polymerase.
Extended Data Figure 4. New subunit interfaces in apo-FluPolC.
a, b, Two views of the interaction interface between PB2NLS (green) and P3endo (blue). Predicted polar contacts between the subunits are shown as dotted yellow lines. c, The interface between PB2cap (green) and PB1palm (orange). In all panels, residues at the interface were calculated using the ‘Protein interfaces, surfaces and assemblies’ service PISA at the European Bioinformatics Institute (http://www.ebi.ac.uk/pdbe/prot_int/pistart.html)43.
Extended Data Figure 5. SAXS analysis of FluPolC.
a, Calculated solution-state SAXS profiles for the crystal structures of vRNA-FluPolA3 (activated conformation) and apo-FluPolC (closed conformation). A distinguishing difference between these profiles is the dip at q ~0.1 Å−1 in the FluPolC curve. b, Solution-state SAXS profile of FluPolC, without added promoter RNA, overlaid with the calculated curve for the vRNA-FluPolA structure3. The good match between these curves suggests that in this particular buffer (0.5 M NaCl, 25 mM HEPES-NaOH, pH 7.5, 5% (v/v) glycerol), FluPolC adopts the same globular conformation as the RNA bound state. c, Dimensionless Kratky plot of apo-FluPolC in the presence of 0.5 M NaCl, 25 mM HEPES-NaOH, pH 7.5 and 5% (v/v) glycerol (blue) or 100 mM KCl, 2% (w/v) sucrose and 100 mM sodium phosphate, pH 7.3, with (magenta) or without (grey) 200 mM proline. Cross-hairs denote the Guinier–Kratky point (1.732, 1.104), the peak position for an ideal, globular particle. As indicated by the upward shift of the peaks in the dimensionless Kratky plot, FluPolC is less globular in the presence of phosphate than it is in the 0.5 M NaCl buffer. This effect can be lessened if proline is also present, potentially owing to increased molecular crowding.
Extended Data Figure 6. Differences at the 5′ vRNA promoter binding site.
Superposition of apo-FluPolC (darker colours) and FluPolA (lighter colours) structures, with sites of interest labelled.
Extended Data Table 1. Data collection, phasing and refinement statistics.
| Tetragonal Native* | Tetragonal Derivative* | Orthorhombic Native* | |
|---|---|---|---|
| Data collection | |||
| Space group | P43212 | P43212 | P212121 |
| Cell dimensions | |||
| a, b, c (Å) | 185.66, 185.66, 598.22 | 184.22, 184.22, 598.75 | 107.28, 217.50, 597.75 |
| α, β, γ (°) | 90.00, 90.00, 90.00 | 90.00, 90.00, 90.00 | 90.00, 90.00, 90.00 |
| Resolution (Å) ** | 100.6 – 3.9 (4.0 – 3.9) | 127.3– 6.9 (7.1 – 6.9) | 80.9– 4.3 (4.4 – 4.3) |
| Rsym Or Rmerge | 0.196 (3.586) | 0.157 (0.665) | 0.204 (0.993) |
| I/σI | 13.2 (1.3) | 16.5 (1.8) | 5.5 (1.1) |
| Completeness (%) | 98.8 (96.9) | 99.1 (89.0) | 99.0 (93.1) |
| Redundancy | 21.2 (20.6) | 14.5 (3.7) | 3.3 (2.9) |
| CC1/2*** | (0.621) | (0.612) | (0.500) |
| Refinement | |||
| Resolution (Å) | 50.0 – 3.9 | 50.0 – 4.3 | |
| No. reflections | 90,335 | 90,390 | |
| Rwork/Rfree | 0.286/0.326 | 0.316/0.368 | |
| No. atoms | |||
| Protein | 34,720 | 69,371 | |
| Wilson B-factors (Å2) | |||
| Protein | 205 | 190 | |
| R.m.s deviations | |||
| Bond lengths (Å) | 0.008 | 0.009 | |
| Bond angles (°) | 1.12 | 1.24 |
The native data sets were each collected from a single crystal, whereas the derivative data set was produced by merging data from two crystals.
Highest resolution shell is shown in parentheses.
As described in ref. 44.
Extended Data Table 2. Sequence identities between subunits of FluPol from C/Johannesburg/1/1966 and those from A/Little yellow-shouldered bat/Guatemala/060/2010 or B/Memphis/13/2003, calculated using EMBOSS Stretcher45.
| FluPol | Sequence Identity with C (%) | |
|---|---|---|
| Subunit | A | B |
|
| ||
| PB1 | 38.4 | 40.8 |
| PB2 | 23.3 | 25.2 |
| P3 / PA | 25.4 | 25.6 |
Extended Data Table 3. Domain position differences between apo-FluPolC and promoter-bound FluPolA, calculated using SHP.
| Domain | Rotation (°) | Distance (Å) |
|---|---|---|
| PA Endo / P3Endo | 140 | 19 |
| PB2Mid and PB2cap-627 Linker | 141 | 29 |
| PB2Cap | 122 | 30 |
| PB2627 | 163 | 79 |
| PB2NLS | 134 | 93 |
Supplementary Material
Acknowledgements
We thank N. Naffakh for providing plasmids and I. Berger for providing us with the MultiBac system. The authors thank Diamond Light Source for beamtime (proposal mx8423), and the staff of the MX beamlines for assistance. K. Harlos also provided valuable technical assistance. We thank G. G. Brownlee for initiating this project and for his comments and continuous encouragement, and members of the Fodor, Grimes and Stuart laboratories, particularly A. te Velthuis, for discussions. This work was supported by Medical Research Council (MRC) grants MR/K000241/1 (to E.F.), G1100138 (to F.T.V.) and G1000099 (to D.I.S.), Wellcome Trust Studentship 092931/Z/10/Z (to N.H.), an MRC Studentship (to I.S.M.), and Wellcome Trust administrative support grant 075491/Z/04.
Footnotes
Online Content Methods, along with any additional Extended Data display items and Source Data, are available in the online version of the paper; references unique to these sections appear only in the online paper.
Supplementary Information is available in the online version of the paper.
Author Information Atomic coordinates and structure factors for the reported crystal structure have been deposited in the Protein Data Bank under accession numbers 5D98 and 5D9A.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of the paper.
References
- 1.Ortín J, Martin-Benito J. The RNA synthesis machinery of negative-stranded RNA viruses. Virology. 2015;479-480:532–544. doi: 10.1016/j.virol.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Fodor E. The RNA polymerase of influenza a virus: mechanisms of viral transcription and replication. Acta Virol. 2013;57:113–122. doi: 10.4149/av_2013_02_113. [DOI] [PubMed] [Google Scholar]
- 3.Pflug A, Guilligay D, Reich S, Cusack S. Structure of influenza A polymerase bound to the viral RNA promoter. Nature. 2014;516:355–360. doi: 10.1038/nature14008. [DOI] [PubMed] [Google Scholar]
- 4.Reich S, et al. Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature. 2014;516:361–366. doi: 10.1038/nature14009. [DOI] [PubMed] [Google Scholar]
- 5.Muraki Y, Hongo S. The molecular virology and reverse genetics of influenza C virus. Jpn. J. Infect. Dis. 2010;63:157–165. [PubMed] [Google Scholar]
- 6.Gabriel G, Fodor E. Molecular determinants of pathogenicity in the polymerase complex. Curr. Top. Microbiol. Immunol. 2014;385:35–60. doi: 10.1007/82_2014_386. [DOI] [PubMed] [Google Scholar]
- 7.Cianci C, Tiley L, Krystal M. Differential activation of the influenza virus polymerase via template RNA binding. J. Virol. 1995;69:3995–3999. doi: 10.1128/jvi.69.7.3995-3999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao P, Yuan W, Krug RM. Crucial role of CA cleavage sites in the cap-snatching mechanism for initiating viral mRNA synthesis. EMBO J. 2003;22:1188–1198. doi: 10.1093/emboj/cdg109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang S, et al. Cryo-EM structure of influenza virus RNA polymerase complex at 4.3 Å resolution. Mol. Cell. 2015;57:925–935. doi: 10.1016/j.molcel.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi A, Nagata K. De novo replication of the influenza virus RNA genome is regulated by DNA replicative helicase, MCM. EMBO J. 2007;26:4566–4575. doi: 10.1038/sj.emboj.7601881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterson D, Fodor E. Emerging roles for the influenza A virus nuclear export protein (NEP) PLoS Pathog. 2012;8:e1003019. doi: 10.1371/journal.ppat.1003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.York A, Hengrung N, Vreede FT, Huiskonen JT, Fodor E. Isolation and characterization of the positive-sense replicative intermediate of a negative-strand RNA virus. Proc. Natl Acad. Sci. USA. 2013;110:E4238–E4245. doi: 10.1073/pnas.1315068110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Area E, et al. 3D structure of the influenza virus polymerase complex: Localization of subunit domains. Proc. Natl Acad. Sci. USA. 2004;101:308–313. doi: 10.1073/pnas.0307127101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arranz R, et al. The structure of native influenza virion ribonucleoproteins. Science. 2012;338:1634–1637. doi: 10.1126/science.1228172. [DOI] [PubMed] [Google Scholar]
- 15.Coloma R, et al. The structure of a biologically active influenza virus ribonucleoprotein complex. PLoS Pathog. 2009;5:e1000491. doi: 10.1371/journal.ppat.1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA. Organization of the influenza virus replication machinery. Science. 2012;338:1631–1634. doi: 10.1126/science.1227270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin B, Kranzusch PJ, Rahmeh AA, Whelan SPJ. The polymerase of negative-stranded RNA viruses. Curr. Opin. Virol. 2013;3:103–110. doi: 10.1016/j.coviro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerlach P, Malet H, Cusack S, Reguera J. Structural insights into Bunyavirus replication and its regulation by the vRNA promoter. Cell. 2015;161:1267–1279. doi: 10.1016/j.cell.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bieniossek C, Imasaki T, Takagi Y, Berger I. MultiBac: expanding the research toolbox for multiprotein complexes. Trends Biochem. Sci. 2012;37:49–57. doi: 10.1016/j.tibs.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter TS, et al. A procedure for setting up high-throughput nanolitre crystallization experiments. I. Protocol design and validation. J. Appl. Crystallogr. 2003;36:308–314. [Google Scholar]
- 21.Walter TS, et al. A procedure for setting up high-throughput nanolitre crystallization experiments. Crystallization workflow for initial screening, automated storage, imaging and optimization. Acta Crystallogr. D. 2005;61:651–657. doi: 10.1107/S0907444905007808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winter G, Lobley CMC, Prince SM. Decision making in xia2. Acta Crystallogr. D. 2013;69:1260–1273. doi: 10.1107/S0907444913015308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 24.Sheldrick GM. Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr. D. 2010;66:479–485. doi: 10.1107/S0907444909038360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams PD, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mccoy AJ, et al. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 28.Bricogne G, et al. BUSTER version 2.11.5. Global Phasing Ltd.; 2011. [Google Scholar]
- 29.Murshudov GN, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholls RA, Long F, Murshudov GN. Low-resolution refinement tools in REFMAC5. Acta Crystallogr. D. 2012;68:404–417. doi: 10.1107/S090744491105606X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pettersen EF, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 32.Stuart DI, Levine M, Muirhead H, Stammers DK. Crystal-structure of cat muscle pyruvate-kinase at a resolution of 2.6 A. J. Mol. Biol. 1979;134:109–142. doi: 10.1016/0022-2836(79)90416-9. [DOI] [PubMed] [Google Scholar]
- 33.Brownlee GG, Fodor E, Pritlove DC, Gould KG, Dalluge JJ. Solid phase synthesis of 5′-diphosphorylated oligoribonucleotides and their conversion to capped m7Gppp-oligoribonucleotides for use as primers for influenza A virus RNA polymerase in vitro. Nucleic Acids Res. 1995;23:2641–2647. doi: 10.1093/nar/23.14.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung TD, et al. Biochemical studies on capped RNA primers identify a class of oligonucleotide inhibitors of the influenza virus RNA polymerase. Proc. Natl Acad. Sci. USA. 1994;91:2372–2376. doi: 10.1073/pnas.91.6.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng T, Sharps JL, Brownlee GG. Role of the influenza virus heterotrimeric RNA polymerase complex in the initiation of replication. J. Gen. Virol. 2006;87:3373–3377. doi: 10.1099/vir.0.82199-0. [DOI] [PubMed] [Google Scholar]
- 36.Crescenzo-Chaigne B, Naffakh N, van der Werf S. Comparative analysis of the ability of the polymerase complexes of influenza viruses type A, B and C to assemble into functional RNPs that allow expression and replication of heterotypic model RNA templates in vivo. Virology. 1999;265:342–353. doi: 10.1006/viro.1999.0059. [DOI] [PubMed] [Google Scholar]
- 37.Fodor E, et al. A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase inhibits endonucleolytic cleavage of capped RNAs. J. Virol. 2002;76:8989–9001. doi: 10.1128/JVI.76.18.8989-9001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas SK, Nayak DP. Mutational analysis of the conserved motifs of influenza A virus polymerase basic protein 1. J. Virol. 1994;68:1819–1826. doi: 10.1128/jvi.68.3.1819-1826.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dias A, et al. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature. 2009;458:914–918. doi: 10.1038/nature07745. [DOI] [PubMed] [Google Scholar]
- 40.Guilligay D, et al. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nature Struct. Mol. Biol. 2008;15:500–506. doi: 10.1038/nsmb.1421. [DOI] [PubMed] [Google Scholar]
- 41.Yuan P, et al. Crystal structure of an avian influenza polymerase PAN reveals an endonuclease active site. Nature. 2009;458:909–913. doi: 10.1038/nature07720. [DOI] [PubMed] [Google Scholar]
- 42.Fechter P, et al. Two aromatic residues in the PB2 subunit of influenza A RNA polymerase are crucial for cap binding. J. Biol. Chem. 2003;278:20381–20388. doi: 10.1074/jbc.M300130200. [DOI] [PubMed] [Google Scholar]
- 43.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Karplus PA, Diederichs K. Linking crystallographic model and data quality. Science. 2012;336:1030–1033. doi: 10.1126/science.1218231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rice P, Longden I, Bleasby A. EMBOSS: The European molecular biology open software suite. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.