Abstract
Toxicity induced by radiation therapy is a curse for cancer patients undergoing treatment. It is imperative to understand and define an ideal condition where the positive effects notably outweigh the negative. We used a microarray meta-analysis approach to measure global gene-expression before and after radiation exposure. Bioinformatic tools were used for pathways, network, gene ontology and toxicity related studies. We found 429 differentially expressed genes at fold change >2 and p-value <0.05. The most significantly upregulated genes were synuclein alpha (SNCA), carbonic anhydrase I (CA1), X-linked Kx blood group (XK), glycophorin A and B (GYPA and GYPB), and hemogen (HEMGN), while downregulated ones were membrane-spanning 4-domains, subfamily A member 1 (MS4A1), immunoglobulin heavy constant mu (IGHM), chemokine (C-C motif) receptor 7 (CCR7), BTB and CNC homology 1 transcription factor 2 (BACH2), and B-cell CLL/lymphoma 11B (BCL11B). Pathway analysis revealed calcium-induced T lymphocyte apoptosis and the role of nuclear factor of activated T-cells (NFAT) in regulation of the immune response as the most inhibited pathways, while apoptosis signaling was significantly activated. Most of the normal biofunctions were significantly decreased while cell death and survival process were activated. Gene ontology enrichment analysis revealed the immune system process as the most overrepresented group under the biological process category. Toxicity function analysis identified liver, kidney and heart to be the most affected organs during and after radiation therapy. The identified biomarkers and alterations in molecular pathways induced by radiation therapy should be further investigated to reduce the cytotoxicity and development of fatigue.
Keywords: radiation therapy, toxicity, microarray, cancer, pathway analysis
1. Introduction
Regardless of its potential hazards, curative benefits of radiation have been reported in medicine, oncology in particular. It is routinely used in cancer treatment prior to surgery to help shrink the tumor, as a palliative therapy to relieve pain, pressure and other neurologic or obstructive symptoms, and post-surgery to kill any remaining cancer cells and to prevent tumor recurrence or in synergy with chemotherapy [1]. The three prime categories of radiation therapy (RT) based on the differences relating to the position of the radiation sources are external beam RT (teletherapy) where the radiation source is outside the body, sealed source RT (brachytherapy) employs sealed radioactive sources placed permanently or temporarily precisely in the area under treatment, and systemic unsealed source radioisotope therapy is given by oral ingestion of radioisotopes.
Localized RT, using ionizing radiation, is the most common therapeutic option recommended for the treatment of ~60% of non-metastatic cancer patients [2,3,4]. RT has led to high cure and improved survival rates; however, this is mitigated by the associated toxicities that decrease the quality of life for survivors as ~10% of patients suffer significant toxicity because of adverse side effects [5]. Additional risk factors include age, chemotherapy, anatomical variations and coexisting illnesses, for instance, diabetes and autoimmune diseases [6,7,8]. Extra caution and personalized or tailored palliative radiotherapy administration is essential for patients in the end-stage of life with terminal cancer [9].
Several ex vivo efforts to correlate radiation toxicity with cellular responses have been done. Studies have reported decreased survival of cultured skin fibroblasts [10], paradoxical decrease in radiation-induced apoptosis [11], and development of abnormal numbers of chromosome aberrations [12] in patients after radiation exposure. Still, the etiology of fatigue and severity of RT associated side effects during cancer treatment are not well understood. Some studies have correlated certain genes, pathways and molecular processes to fatigue [13,14]. It has been well demonstrated that irradiated dying cancer cells release tumor antigens. The extracellular antigens and dying tumor cells are engulfed by circulating bone marrow-derived antigen-presenting cells (APCs). Subsequent to antigen uptake, APCs move to lymph nodes, where they engage with helper T cells for post-stimulation and APC activation, and further stimulate the tumor specific cytotoxic T lymphocytes that could potentially clear tumor cells both at primary and metastatic sites [15]. Radiation-induced immune-modulation happens in two phases. First, radiation induces endogenous damage-associated molecular pattern (DAMP) molecules also known as alarmins and may include intracellular proteins, like heat-shock proteins, high-mobility group box 1 (HMGB1) and proinflammatory S100 proteins linked to inflammation and cancer [16] or non-protein molecules like ATP [17], uric acid [18], heparin sulfate and DNA [19]. The release of DAMP molecules are considered as “danger signals” and trigger stimulation and maturation of dendritic cells which can then present foreign antigens and cause stimulation of T lymphocytes. In this event, radiation normalizes tumor vasculature, increases tumor cells’ immune recognition and modulates the tumor cell phenotype. Radiation treatment can cause upregulation of chemokines and adhesion molecules, providing signals for T cells to be attracted to the tumor; and upregulation of Major Histocompatibility Complex molecules and tumor-associated antigens, making it easier for endogenous or immunotherapy-induced T cells to recognize and kill tumors via immunogenic modulation [20]. Second, amplification by abrogating immune checkpoint factors with simultaneous co-stimulation of effector factors can ultimately lead to the induction of multiple unique T-cell populations (antigen cascade) that can kill antigen disparate tumor cells at metastatic sites (systemic effect). It has been show that HMGB1, a nuclear non-histone chromatin-binding protein, is secreted at the late stages of cellular death and is also secreted by apoptotic tumor cells after chemotherapy or radiotherapy promoting antitumor responses [21,22].
We hypothesized that radiation induced toxicity in affected cells respond to DNA damage and have an abnormal transcriptional profile leading to alterations in key pathways. To comprehend the underlying mechanisms of radiation induced response, we adopted transcriptomics based molecular assessment of induced features during cancer treatment.
2. Results
The prime focus of this study was to determine the beneficial and deleterious effects of RT using transcriptional profiling and pathway prediction models.
2.1. Identification of Differentially Expressed Genes
We found 429 differentially expressed genes, 32 were up while the remaining 397 genes were downregulated, which were used to understand the molecular mechanism and processes involved in radiation therapy (Supplementary Table S1, Table 1, Figure 1). The most significantly upregulated genes were alpha synuclein (SNCA), carbonic anhydrase I (CA1), X-linked Kx blood group (XK), glycophorin A, and B (GYPA and GYPB), hemogen (HEMGN), 2,3-bisphosphoglycerate mutase (BPGM), ATP-binding cassette sub-family C member 13 (ABCC13), and ferrochelatase (FECH), while most downregulated genes were membrane-spanning 4-domains, subfamily A member 1 (MS4A1), immunoglobulin heavy constant mu (IGHM), chemokine (C-C motif) receptor 7 (CCR7), BTB and CNC homology 1 transcription factor 2 (BACH2), B-cell CLL/lymphoma 11B (BCL11B), paired box 5 (PAX5), lymphoid enhancer-binding factor 1 (LEF1), Fas apoptotic inhibitory molecule 3 (FAIM3), A2M antisense RNA 1 (A2M-AS1) and TNF receptor-associated factor 5 (TRAF5).
Table 1.
Top 20 upregulated and differentially expressed genes.
| S. No | Gene Symbol | Gene Title | Chromosome Location | Fold-Change | p-Value |
|---|---|---|---|---|---|
| 1 | SNCA | synuclein, alpha (non A4 component of amyloid precursor) | chr4q21 | 3.50 | 2.70 × 10−6 |
| 2 | CA1 | carbonic anhydrase I | chr8q21.2 | 3.30 | 9.12 × 10−7 |
| 3 | XK | X-linked Kx blood group | chrXp21.1 | 2.86 | 0.000510 |
| 4 | GYPB | glycophorin B (MNS blood group) | chr4q31.21 | 2.83 | 1.25 × 10−6 |
| 5 | HEMGN | hemogen | chr9q22.33 | 2.82 | 4.06 × 10−5 |
| 6 | GYPA | glycophorin A (MNS blood group) | chr4q31.21 | 2.67 | 1.13 × 10−5 |
| 7 | BPGM | 2,3-bisphosphoglycerate mutase | chr7q33 | 2.47 | 0.002613 |
| 8 | FAM46C | family with sequence similarity 46, member C | chr1p12 | 2.40 | 0.002343 |
| 9 | ABCC13 | ATP-binding cassette, sub-family C (CFTR/MRP), member 13, pseudogene | chr21q11.2 | 2.35 | 1.87 × 10−5 |
| 10 | FECH | ferrochelatase | 18q21.31 | 2.33 | 0.001334 |
| 11 | ISCA1 | iron-sulfur cluster assembly 1 | chr9q21.33 | 2.32 | 1.7 × 10−6 |
| 12 | CCDC176 | coiled-coil domain containing 176 | chr14q24.3 | 2.29 | 0.000566 |
| 13 | AHSP | alpha hemoglobin stabilizing protein | chr16p11.2 | 2.29 | 7.18 × 10−7 |
| 14 | YOD1 | YOD1 deubiquitinase | chr1q32.2 | 2.25 | 0.000749 |
| 15 | NUDT4//NUDT4P1 | nudix (nucleoside diphosphate linked moiety X)-type motif 4 | chr12q21//chr1p12-p13 | 2.19 | 6.08 × 10−5 |
| 16 | RHD | Rh blood group, D antigen | 1p36.11 | 2.18 | 1.02 × 10−7 |
| 17 | FGFR1OP2 | FGFR1 oncogene partner 2 | chr12p11.23 | 2.04 | 0.000354 |
| 18 | TSPO2 | translocator protein 2 | 6p21.1 | 2.03 | 6.71 × 10−13 |
| 19 | ITLN1 | intelectin 1 (galactofuranose binding) | 1q23.3 | 2.02 | 6.64 × 10−6 |
| 20 | KRT1 | keratin 1 | 12q13.13 | 2.01 | 0.002745 |
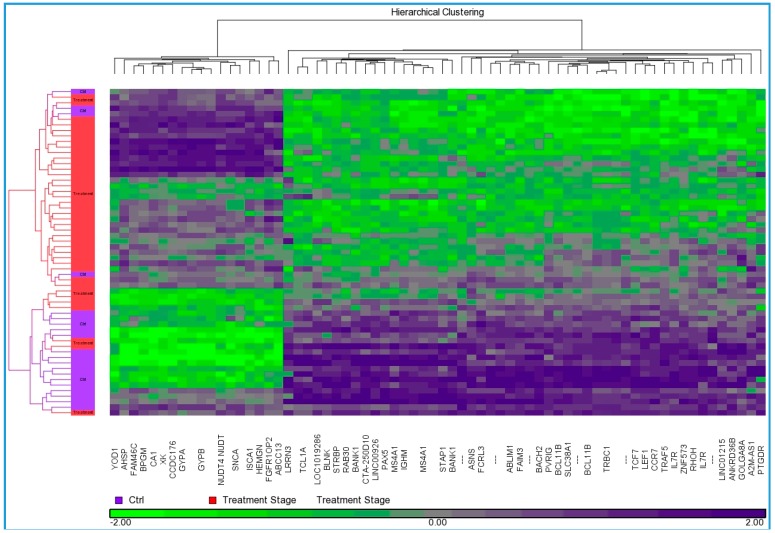
Figure 1.
Agglomerative average-linkage hierarchical clustering for differentially expressed genes between radiation treatment stage and controls. Dendrogram obtained using Partek GS 6.6 software shows the change in expression levels of genes (n = 429, 32-up and 397-downregulated) in RT treated cancer patients compared to untreated controls, Differentially Expressed Genes (DEGs) on X axis and treatment stage on Y axis. The cluster color represents the normalized expression level of a given gene in response to radiation treatment, Purple denotes upregulation and green denotes downregulation according the color scale.
2.2. Pathways and Networks Underlying Immune Dysfunction
Molecular pathway analysis of radiation treatment associated genes has predicted biofunctions and molecular networks which may be involved in the fatigue and cytotoxicity. Biofunctions including cell-mediated immune response, cell signaling, mineral metabolism, cellular function and maintenance were predicted to be significantly decreased while cell death and survival process were significantly activated (p-value = 1.76 × 10−7). Transcriptomic signatures displayed noteworthy disturbances in signaling pathways like calcium-induced T lymphocyte apoptosis (z-score = −3.31, Figure 2), and role of NFAT in regulation of the immune response (z-score = −3.77) were inhibited while apoptosis signaling (z-score = 2.23) and cytotoxic T lymphocyte-mediated apoptosis of target cells (z-score = 1.342) were predicted to be activated (Table 2). Cell death and survival process was predicted to be significantly increased/activated (cell death of immune cells, p-value = 1.76 × 10−7, z-score = 2.209; cell death of lymphocytes, p-value = 3.84 × 10−5, z-score = 2.017; apoptosis of leukocytes, p-value = 5.72 × 10−4, z-score = 2.424 and apoptosis of hematopoietic cell lines, p-value = 3.16 × 10−3, z-score = 2.489) (Figure 3). However, most of biofunctions were predicted to be decreased like cell-mediated immune response (T cell development, -homeostasis and -migration, p-value = 8.45 × 10−11, z-score = −2.867), cell signaling, and mineral metabolism (accumulation of Ca2+, mobilization of Ca2+, p-value = 5.72 × 10−4, z-score = −2.764), cellular function and maintenance (homeostasis of leukocytes, cellular homeostasis, p-value = 3.88 × 10−11, z-score = −3.122) (Supplementary Table S2).
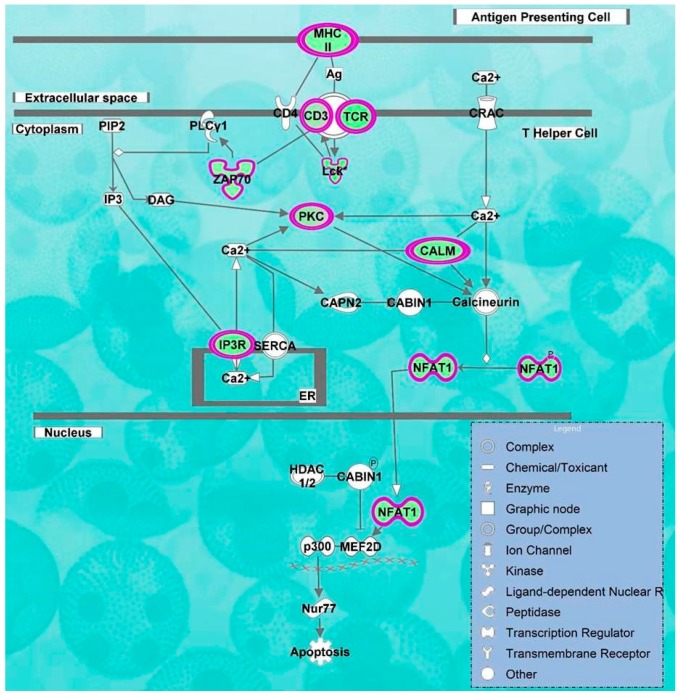
Figure 2.
Inhibition of Calcium-induced T Lymphocyte Apoptosis pathway. Based on overlap of identified DEGs to Calcium-induced T Lymphocyte Apoptosis pathways, IPA had predicted its inhibition. CD3, CAMK4, TRGV9, NFAT, ZAP70, LCK, PRKC, HLA-DOB, ITPR1 genes involved in this pathway were downregulated as shown by the purple circles. XXXX line indicate DNA strand. The white end arrow means “translocation” and the dark end arrow means “acts on”.
Table 2.
Top significant canonical pathways.
| Ingenuity Canonical Pathways | -log (p-Value) | z-Score | Molecules |
|---|---|---|---|
| Calcium-induced T Lymphocyte Apoptosis | 10.8 | −3.317 | CD247, CD3G, LCK, PRKCQ, CAMK4, TRGV9, ZAP70, NFATC2, HLA-DOB, PRKCH, ITPR1, CD3D, PRKCA |
| Role of NFAT in Regulation of the Immune Response | 10.5 | −3.771 | CD247, BLNK, FYN, CAMK4, PRKCQ, NFATC3,T RGV9, ITPR1, CD3D, CD3G, LCK, RRAS2, LAT, ZAP70, HLA-DOB, RCAN3, NFATC2, IKBKAP, ATM,ITK |
| iCOS-iCOSL Signaling in T Helper Cells | 10.3 | −3.000 | CD247, CAMK4, PRKCQ, NFATC3, TRGV9, ITPR1, CD3D, CD3G, LCK, ZAP70, LAT, NFATC2, HLA-DOB, PLEKHA1, ATM,ITK |
| CD28 Signaling in T Helper Cells | 9.61 | −3.317 | CD247, FYN, CAMK4, PRKCQ, NFATC3, TRGV9, ITPR1, CD3D, CD3G, LCK, ZAP70, LAT, NFATC2, HLA-DOB, ATM,ITK |
| PKCθ Signaling in T Lymphocytes | 8.64 | −2.324 | CD247,F YN, PRKCQ, NFATC3, TRGV9, MAP3K4, CD3D, CD3G, LCK,RRAS2, ZAP70, LAT, NFATC2, HLA-DOB, ATM |
| Phospholipase C Signaling | 8.01 | −3.606 | CD247,BLNK, PEBP1, FYN, CAMK4, PRKCQ, NFATC3, TRGV9, ITPR1, CD3D, RHOH, CD3G, LCK, RRAS2, LAT, ZAP70, NFATC2, PRKCH, PRKCA, ITK |
| Tec Kinase Signaling | 5.18 | −3.606 | FYN, PRKCQ, TRGV9, RHOH, STAT4, BLK, LCK, TXK, TNFRSF25, PRKCH, ITK, PRKCA, ATM |
| EIF2 Signaling | 4.62 | −2.828 | RPL22, RPS18, RPS4X, RPL10A, RPL14, RRAS2, RPS20, RPL5, RPL36, RPL18, EIF3L, RPS24, ATM |
| B Cell Receptor Signaling | 4.02 | −1.897 | BLNK, PAX5, ETS1, EBF1, CAMK4, PRKCQ, RRAS2, FOXO1, NFATC3, NFATC2, MAP3K4, ATM |
| PI3K Signaling in B Lymphocytes | 3.88 | −2.828 | CD81, BLNK, BLK, FYN, CAMK4, RRAS2, NFATC3, NFATC2, PLEKHA1, ITPR1 |
| fMLP Signaling in Neutrophils | 3.66 | −3.000 | CAMK4, PRKCQ, RRAS2, NFATC3, NFATC2, PRKCH, ITPR1, PRKCA, ATM |
| Apoptosis Signaling | 1.56 | 2.236 | PRKCQ, RRAS2, BIRC3, PRKCA, BCL2 |
| Cytotoxic T Lymphocyte-mediated Apoptosis of Target Cells | 3.84 | 1.342 | CD247, CD3G, TRGV9, CD3D, BCL2 |
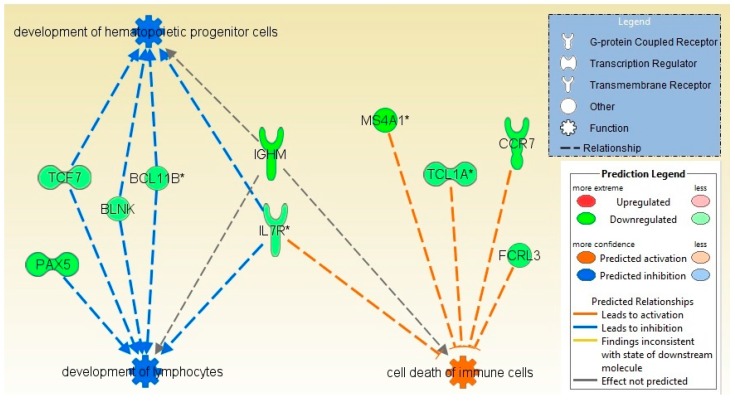
Figure 3.
Functional analysis and regulatory effect of differentially expressed genes. Using Ingenuity Pathways Analysis (IPA), Differentially Expressed Genes (DEGs) were overlaid on to the network to find a biological regulatory functional effect based on previously reported interactions in the literature. Function colored with blue denote inhibition and orange denotes activation; development of hematopoietic progenitor cells and development of lymphocytes were inhibited whereas cell death of immune cells as activated function.
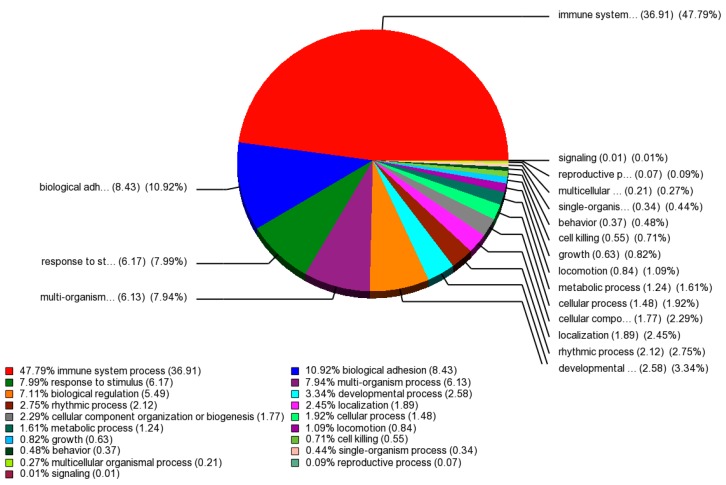
A Gene Ontology (GO) enrichment analysis was performed on those 429 differentially expressed probe sets that intersect on the basis of a p-value <0.05 and FC >2 between baseline and endpoint treatment groups. (Supplementary Table S3). The GO enrichment diagram illustrates functional groups that are significantly overrepresented in different categories (Figure 4). The most significantly overrepresented groups in the categories cellular component, molecular function, and biological process were “cell part”, “nucleic acid binding transcription factor activity”, and “immune system process”, respectively.
Figure 4.
Gene Ontology of differentially expressed genes. Pie Chart obtained using Partek GS 6.6 software shows the change in the biological process of the immune system, biological adhesion, response to stimulus, multi-organism process, biological regulation and developmental process as a significantly affected process.
2.3. Toxicity Function Analysis
IPA based toxicity function analysis predicted liver, kidney and heart to be most affected organs during and after radiation therapy (Table 3). Alteration in CCR7, IMPDH2, IL6ST, MYC, LPIN1, PDE7A and RORA was significantly associated with hepatotoxicity including liver damage, -hyperplasia, -inflammation, -steatosis, -fibrosis, -necrosis and -proliferation. Altered expression of AQP3, AAK1, BCL2, BIRC3, BNIP3, DDX17, FOXO1, ITPR1, MYC, PRKCA, SNCA, TNFRSF25, CCR7, MS4A1 and IMPDH2 was responsible for renal necrosis, nephrosis, nephritis, proliferation and kidney failure. Cardiotoxicity (cardiac proliferation, -arteriopathy, -necrosis, -infarction and heart failure) was found to be significantly associated with the following altered genes: CA1, FOXP1, NOG, ABCG1, CD47, DOCK9, MARCH6, PDE7A, PRKCH BNIP3 and MIAT during radiation therapy.
Table 3.
Functional annotations and molecules involved in toxicity (cardiotoxicity; hepatotoxicity; nephrotoxicity) resulting from radiation therapy.
| Functional Category | Function Annotations | p-Value | Molecules |
|---|---|---|---|
| Cardiotoxicity | |||
| Cardiac Proliferation | proliferation of cardiomyocytes | 1.07 × 10−1 | FOXP1, NOG |
| Cardiac Arteriopathy | coronary artery disease | 5.09 × 10−1 | ABCG1, CD47, DOCK9, MARCH6, PDE7A, PRKCH |
| Cardiac Necrosis/Cell Death | apoptosis of cardiomyocytes and ventricular myocytes | 5.36 × 10−1 | BNIP3, NOG |
| Heart Failure | chronic heart failure | 4.73 × 10−1 | CA1 |
| Cardiac Infarction | myocardial infarction | 1.00 × 10−1 | CD47, MIAT |
| Hepatotoxicity | |||
| Liver Damage | low and high grade chronic hepatitis C, chronic hepatitis C, hepatotoxicity | 1.92 × 10−2 | CCR7, IMPDH2, RASGRP1 |
| Liver Hyperplasia/Hyper-proliferation | inflammatory hepatocellular adenoma; hepatocellular carcinoma; growth of hepatocellular carcinoma; liver cancer | 7.47 × 10−2 | IL6ST, MYC, + 113 genes |
| Liver Inflammation/Hepatitis | inflammation of liver; steatohepatitis; chronic hepatitis C | 3.26 × 10−1 | CCR7, IMPDH2, LPIN1, PDE7A |
| Liver Steatosis | hepatic steatosis; steatohepatitis; nonalcoholic steatohepatitis | 1.85 × 10−1 | LPIN1, PDE7A, RORA |
| Liver Fibrosis | fibrosis of liver; activation, migration and proliferation of hepatic stellate cells | 1.39 × 10−1 | IL6ST, RORA, CCR7 |
| Liver Necrosis/Cell Death | cell death of liver cells; apoptosis of hepatocytes | 3.91 × 10−1 | BCL2, MYC |
| Liver Proliferation | proliferation of liver cells; proliferation of hepatocytes; proliferation of hepatic stellate cells | 2.24 × 10−1 | IL6ST, LY9, MYC |
| Nephrotoxicity | |||
| Renal Necrosis/Cell Death | apoptosis of kidney cell lines; apoptosis of podocytes; cell death of kidney cell lines; cell viability of kidney cell lines | 5.51 × 10−2 | AQP3,AAK1,BCL2, BIRC3, BNIP3, DDX17, FOXO1, ITPR1, MYC, PRKCA, SNCA, TNFRSF25 |
| Nephrosis | nephrosis; minimal change nephrotic syndrome; autosomal recessive steroid-resistant nephrotic syndrome; steroid dependent nephrotic syndrome | 2.43 × 10−1 | IMPDH2, MS4A1 |
| Renal Nephritis | IgA nephropathy; membranous glomerulonephritis; lupus nephritis | 1.13 × 10−1 | IMPDH2, MS4A1 |
| Renal Proliferation | proliferation of mesangial cells; proliferation of kidney cell lines | 3.96 × 10−1 | CCR7, HSP90AB1, KMT2A, SFPQ |
| Kidney Failure | end stage renal disease | 4.63 × 10−1 | IMPDH2, PDE7A |
3. Discussion
Up to 90% of cancer patients treated with radiation experience cumulative fatigue that is pervasive and affects quality of life [23]. Cancer treatment elicits inflammatory and stress response, mitochondrial function impairment, endocrine dysfunction and immune dysregulation. This induces a cascade of biological changes which get translated into cancer-related fatigue (CRF) manifested with alteration in skeletal muscle function contributing to physical disability along with cognitive and behavioral symptoms. The above-mentioned fatigue has a multifactorial etiology and is reported to be associated with neuroendocrine, metabolic, immune/inflammatory, and genetic biomarkers whose systemic identification will help to understand its etiology and develop better therapies to lessen CRF burden [24]. Though, fatigue felt by the patients might also be due to conditions like anaemia, thyroid dysfunction, pain, depression and sleep deprivation [25].
In our results, α-synuclein (SNCA) (p-value = 2.70 × 10−6 and fold change = 3.5) was one of the differentially expressed gene identified in the microarray meta-analysis, which was validated [5] and reported to be associated with neuroinflammation [26]. SNCA is primarily involved in diseases like Parkinson’s and hereditary amyloidosis. During localized radiation therapy, its upregulation activates inflammatory pathways and signals the associated cancer-related fatigue [5]. We also found upregulation of carbonic anhydrase I (CA1) in our results which is in accordance with the previously published reports [5]. CA1 is a cytosolic protein and belongs to the large family of zinc metalloenzymes. It is found at the highest level in erythrocytes. It functions by catalyzing the reversible hydration of carbon dioxide and participates in a variety of biological processes like respiration, maintenance of acid–base balance, bone resorption and calcification [27].
One of the most down regulated genes in our study was membrane-spanning 4-domains, subfamily A, member 1 (MS4A1). It encodes for CD20 protein, a B1 cell-surface antigen of human B lymphocytes and plays a role in hematopoietic cell activation [28]. Lower MS4A1 gene expression levels will possibly contribute to the lower CD20 protein expression [29]. Association of MS4A1 down regulation and fatigue intensification has been recently reported by Hsiao et al., suggesting that fatigue during RT may be related to impairment in B-cell immune response [30]. MS4A1 was included in molecular signatures that reflects a response to radiation in mice and humans [31]. Reportedly, MS4A1 has indirect interaction with CA1 and SNCA genes associated with cellular movement and immune cell trafficking [30]. Our gene ontology enrichment analysis revealed immune system process as the most significantly overrepresented group under biological process category that has been reported to be positively associated with CRF [32].
We found that apoptosis signaling and cytotoxic T lymphocyte-mediated apoptosis of target cells pathways were strongly associated with RT. Radiation induced stress activates p53-dependent apoptosis marked by increased formation of intracellular reactive oxygen species (ROS) and activation of stress responsive pathways like p38MAPK [33]. Radiation acts as an apoptotic stimuli and cause changes in the inner mitochondrial membrane permeability [34]. This leads to release of regulatory proteins such as cytochrome c which binds and activates Apaf-1, forming an “apoptosome” [35,36]. ATP activates apoptosome complex, which thereby activates procaspase-9. Smac/DIABLO and the serine protease, and HtrA2/Omi promote apoptosis by inhibiting IAP (inhibitors of apoptosis proteins) activity. During apoptosis, the mitochondria releases pro-apoptotic proteins like AIF (apoptosis-inducing factor), endonuclease G and CAD (caspase-activated DNase) [34].
Toxicity functional analysis revealed that radiation therapy does carry a huge price, i.e., at the cost of hepato- , cardio-, and nephrotoxicity. Cases of radiation-induced liver diseases are becoming frequent. Radioembolization may affect the normal liver parenchyma and produce pertinent toxic effects like cholecystitis, gastrointestinal ulceration, pneumonitis, and liver toxicity as a result of radiation of non-target organs [37]. Long-term radiation induced damage in different tissues possibly is a consequence of injury to microvascular endothelial cells causing their apoptosis. Irradiation causes oxidative damage to DNA (both mitochondrial and nuclear) and significantly depletes mitochondrial glutathione which further enhances in vitro as well as in vivo toxicity levels [38].
Accumulating evidences indicate that radiotherapy involving the heart can result in premature ischemic heart disease in cancer survivors especially in those who had concurrently received chemotherapy specifically with doxorubicin (adriamycin, a drug that can cause heart muscle damage). Cardiac complication and damage can manifest even years after high-dose radiation treatment [39]. Severe RT induced coronary artery disease complications include pericarditis, myocardial fibrosis (scarring), stenosis (narrowing), angina and extensive blockage, valvular injury and myocardial infarction [40,41]. The relative risk of death from a fatal myocardial infarction increased from 1.5 to 3.0 times in patients who have received mediastinal RT as compared to those who have not [42].
Radiation induced nephritis is a degenerative inflammatory disease affecting kidneys after exposure to radioactive substances or body irradiation. Administration of radiometal-labelled peptide conjugates or combined high dose chemotherapy and RT in stem cell transplantation reportedly increases nephrotoxicity [43]. Radiation exposure causes renal endothelial damage and other kidney diseases and so renal shielding during total body irradiation is perhaps protective [44].
There are some factors accounting for rare adverse radiation reactions. In few cases, radiation sensitivity can be credited to particular genetic mutations and includes autosomal recessive uncommon diseases like ataxia telangiectasia (AT) [45], AT-like disorder [46], Nijmegen breakage syndrome [47], and radiosensitivity with severe combined immunodeficiency [48]. Heterozygosity for mutations in ATM, the gene mutated in AT, may occur in 1% of individuals and has been reported to confer moderate sensitivity to radiation in tissue culture based studies [49]. In our differentially expressed gene list, we found ATM to be down-regulated (fold change = −2.06843, p-value = 1.73 × 10−7). A small number of adverse radiation reactions are linked with ATM mutations [50,51,52].
There are some scientific studies hinting towards safe use of alternative medicine like guarana, pineal hormone melatonin, curcumin, amifostine, etc. The extract of guarana, a highly caffeinated plant, was used to ease cancer related fatigue at a low cost [53,54]. Evidences had shown pineal hormone melatonin as radioprotective and can be used to reduce the oxidative injuries due to its free hydroxyl radical scavenging capacity [55,56,57]. Curcumin also has a radioprotection effect due to its capability to decrease oxidative stress and inflammatory responses, and also confers radiosensitization perhaps via the upregulation of genes responsible for apoptosis [58]. Multiple studies support the intrarectal application of amifostine (WR-2721/WR-1065), a phosphorothioate during external beam RT for prostate cancer for the prevention of radiation-induced rectal injury [59,60,61].
Radiations are an omnipresent stress to which all life forms are incessantly exposed in the environment. The more we turn towards nuclear power, the greater the concern for accidents, occupational risks or acts of radiological or nuclear terrorism [62]. Presently, the urban population’s exposure to radiation via medical diagnostics and other applications goes beyond their exposure to natural background radiation. Radiation is a double-edged sword: irradiation-induced DNA damage can halt cancer cell proliferation, but collateral radiation damage to adjacent tissues is always a concern. Despite its potential dangers (can even induce tumors and burn skin), the utility of localized radiation in medicine has fueled research and studies focusing on safety. There have been modern advancements in precision technology which lessens the radiation exposure to the healthy tissue, and, therefore, short radiation sessions with escalating doses are feasible for curative local radiation surgery especially in the case of oligometastases [63]. We need drugs (radioprotectors and radiosensitisers) that can protect normal cells while leaving malignant ones susceptible, and ideally, even sensitized to radiation therapy.
4. Materials and Methods
4.1. Patients and Samples
We retrieved global expression dataset for radiation toxicity studies from NCBI’s GEO database. Dataset were clearly divided into two groups: radiotherapy on cancer patients and radiation studies on healthy individuals or cell lines or mouse models. We focused on actual cancer cases over animal models or cell lines and provided sample information were used to classify case and control. To avoid variations owing to different tissue origins, we included single cancer type and focused on prostate cancer (GSE30174 [5]; GSE69961 [30]) having common platform (GPL570, HG-U133_Plus_2 array chip). To obtain a bigger cohort size for present meta-analysis study, we combined both the prostate cancer studies measuring the transcriptomics level of peripheral blood of patients receiving localized external beam radiation therapy at baseline, midpoints and endpoints.
4.2. Gene Expression Analysis
Transcription profiling of a total of 54,675 probe sets targeting ~47,000 gene transcripts in the human genome was done as described previously [64,65]. Partek Genomics Suite version 6.6 (Partek Inc., St. Louis, MO, USA) was used to import affymetrix .CEL files which were normalized using robust multiarray average (RMA) algorithm. Analysis of variance (ANOVA) was applied on the grouped data set to analyze mean expression level on a gene-by-gene basis and list of differentially expressed genes were generated using a Benjamini Hochberg’s false discovery rate (FDR), p-value <0.05 with fold change >2 as cut off. Disease and tissue type were two factors in ANOVA model and equal variance were assumed. Spearman’s correlation similarity matrix was used for 2-dimensional unsupervised average linkage hierarchical clustering and classification. Principal component analysis was used to assess overall variance in gene expression and represents cohesive tendency of samples with similar features. Samples clustered tightly together were analyzed and outliers were removed from study to reduce unwanted variance. Venn diagrams were generated to display genes that intersect or non-intersect between groups of differentially expressed genes.
4.3. Functional and Pathway Analysis
The principal microarray data analysis was done to detect biological pathways to demonstrate the utilities of more robust biomarker discovery methods for radiation cytotoxicity. Ingenuity Pathways Analysis tool (IPA, build version 338830M, Ingenuity Systems, Redwood City, CA, USA) was used to define biological networks, interaction and functional analysis among the differentially regulated genes in localized external beam radiation treated patients. IPA workflow comprised core and functional analysis and the Ingenuity Knowledge Base was used as a reference data set. Both direct and indirect molecular relationships were included in the analysis settings and significance of relationships was indicated by z-score and Fisher’s exact test p-values. Direction and ranking of pathways i.e., activated or inhibited, was determined by the number of uploaded molecules matching a canonical pathway. Network assembly to display significant biological functions was based on the interconnectivity of the uploaded molecules. We uploaded differentially expressed genes along with their p-values and fold changes, into the IPA tool for core analysis revealing associated genetic network, canonical pathway, and biofunctions.
4.4. Gene Set Enrichment Analysis
A broader understanding of global expression results is possible by grouping the genes of interest into biological processes, cellular component and molecular functions of the genes. Gene ontology (GO) enrichment study was done to functionally classify RT induced significant genes. The implication of this relationship amid transcriptomic data and canonical pathways were calculated by Fisher’s exact test and a cutoff enrichment score >3 (p-value < 0.05) was used to identify major overexpression of functional categories.
5. Conclusions
Holistic simultaneous measurement of global gene expression using microarray technology transformed the field of cancer biology, and we used it to determine the impact of radiation on cells. We found complex molecular mechanisms including cell cycle arrest, DNA repair and apoptosis involved in regulating the cellular responses to radiation. The most prominent symptom experienced during radiotherapy was fatigue, probably mediated by SNCA and CA1 overexpression, and which may serve as a useful biomarker to understand the mechanisms and pathways related to its development. Hence, an additional impetus for research is the need to develop structure based inhibitors that may have potential co-therapeutic relevance. Also, it is vital to comprehend the radiation dose and mechanisms of response to define an ideal condition where the positive benefits notably outweigh the harmful effects. The cancer patients undergoing RT must be aware of the possible delayed cardiotoxic, hepatotoxic and nephrotoxic effects so that they can adopt a healthy lifestyle and have long term follow up with their health providers. Radiation oncologists should operate on the principle that there is no totally safe radiation dose especially for the heart, and they should avoid direct cardiac radiation and keep the dose as low as possible. Clinicians should look into the deeper aspect of recognizing and advocating radiation-induced organ toxicities and other adverse effects in young adult cancer survivors.
Acknowledgments
This research was supported by Center of Excellence in Genomic Medicine Research and King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. We also acknowledge the National Plan for Science, Technology and Innovation (MAARIFAH)—King Abdulaziz City for Science and Technology—The Kingdom of Saudi Arabia—award number (10-BIO1073-03, 10-BIO1258-03 and 08-MED120-03) and Deanship of Scientific Research, King Abdulaziz University, Jeddah, Saudi Arabia—award number (HiCi-1434-117-2) for support.
Abbreviations
- GO
Gene ontology
- RT
Radiation therapy
- CA1
carbonic anhydrase I
- SNCA
a-synuclein
- APCs
antigen-presenting cells
- CRF
cancer related fatigue
- MS4A1
membrane-spanning 4-domains, subfamily A, member 1
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/2/250/s1.
Author Contributions
Study design (Sajjad Karim and Zeenat Mirza); Data retrieval and compilation (Sajjad Karim); Data analysis (Sajjad Karim, Zeenat Mirza and Mohammed H. Al-Qahtani); Manuscript writing (Sajjad Karim and Zeenat Mirza); Critical review of manuscript (Mamdooh Gari, Adeel G. Chaudhary, Adel M. Abuzenadah and Mohammed H. Al-Qahtani).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jones J.A., Lutz S.T., Chow E., Johnstone P.A. Palliative radiotherapy at the end of life: A critical review. CA Cancer J. Clin. 2014;64:296–310. doi: 10.3322/caac.21242. [DOI] [PubMed] [Google Scholar]
- 2.Jones C.U., Hunt D., McGowan D.G., Amin M.B., Chetner M.P., Bruner D.W., Leibenhaut M.H., Husain S.M., Rotman M., Souhami L., et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 3.Thompson I., Thrasher J.B., Aus G., Burnett A.L., Canby-Hagino E.D., Cookson M.S., D’Amico A.V., Dmochowski R.R., Eton D.T., Forman J.D., et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J. Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Rose J.N., Crook J.M. The role of radiation therapy in the treatment of metastatic castrate-resistant prostate cancer. Ther. Adv. Urol. 2015;7:135–145. doi: 10.1177/1756287215576647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saligan L.N., Hsiao C.P., Wang D., Wang X.M., St John L., Kaushal A., Citrin D., Barb J.J., Munson P.J., Dionne R.A. Upregulation of α-synuclein during localized radiation therapy signals the association of cancer-related fatigue with the activation of inflammatory and neuroprotective pathways. Brain Behav. Immun. 2013;27:63–70. doi: 10.1016/j.bbi.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chon B.H., Loeffler J.S. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136–143. doi: 10.1634/theoncologist.7-2-136. [DOI] [PubMed] [Google Scholar]
- 7.Robertson J.M., Clarke D.H., Pevzner M.M., Matter R.C. Breast conservation therapy. Severe breast fibrosis after radiation therapy in patients with collagen vascular disease. Cancer. 1991;68:502–508. doi: 10.1002/1097-0142(19910801)68:3<502::AID-CNCR2820680310>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Fleck R., McNeese M.D., Ellerbroek N.A., Hunter T.A., Holmes F.A. Consequences of breast irradiation in patients with pre-existing collagen vascular diseases. Int. J. Radiat. Oncol. Biol. Phys. 1989;17:829–833. doi: 10.1016/0360-3016(89)90074-6. [DOI] [PubMed] [Google Scholar]
- 9.Nieder C., Angelo K., Dalhaug A., Pawinski A., Haukland E., Norum J. Palliative radiotherapy during the last month of life: Predictability for referring physicians and radiation oncologists. Oncol. Lett. 2015;10:3043–3049. doi: 10.3892/ol.2015.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen J., Bentzen S.M., Overgaard J., Overgaard M. Relationship between the in vitro radiosensitivity of skin fibroblasts and the expression of subcutaneous fibrosis, telangiectasia, and skin erythema after radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1996;40:101–109. doi: 10.1016/0167-8140(96)01777-X. [DOI] [PubMed] [Google Scholar]
- 11.Crompton N.E., Miralbell R., Rutz H.P., Ersoy F., Sanal O., Wellmann D., Bieri S., Coucke P.A., Emery G.C., Shi Y.Q., et al. Altered apoptotic profiles in irradiated patients with increased toxicity. Int. J. Radiat. Oncol. Biol. Phys. 1999;45:707–714. doi: 10.1016/S0360-3016(99)00256-4. [DOI] [PubMed] [Google Scholar]
- 12.Barber J.B., Burrill W., Spreadborough A.R., Levine E., Warren C., Kiltie A.E., Roberts S.A., Scott D. Relationship between in vitro chromosomal radiosensitivity of peripheral blood lymphocytes and the expression of normal tissue damage following radiotherapy for breast cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2000;55:179–186. doi: 10.1016/S0167-8140(99)00158-9. [DOI] [PubMed] [Google Scholar]
- 13.Bokemeyer M., Ding X.Q., Goldbecker A., Raab P., Heeren M., Arvanitis D., Tillmann H.L., Lanfermann H., Weissenborn K. Evidence for neuroinflammation and neuroprotection in hcv infection-associated encephalopathy. Gut. 2011;60:370–377. doi: 10.1136/gut.2010.217976. [DOI] [PubMed] [Google Scholar]
- 14.Ronnback L., Hansson E. On the potential role of glutamate transport in mental fatigue. J. Neuroinflamm. 2004;1:22. doi: 10.1186/1742-2094-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed M.M., Guha C., Hodge J.W., Jaffee E. Introduction: Immunobiology of radiotherapy: New paradigms. Radiat. Res. 2014;182:123–125. doi: 10.1667/RR13849.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srikrishna G., Freeze H.H. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boeynaems J.M., Communi D. Modulation of inflammation by extracellular nucleotides. J. Investig. Dermatol. 2006;126:943–944. doi: 10.1038/sj.jid.5700233. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y., Evans J.E., Rock K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 19.Farkas A.M., Kilgore T.M., Lotze M.T. Detecting DNA: Getting and begetting cancer. Curr. Opin. Investig. Drugs. 2007;8:981–986. [PubMed] [Google Scholar]
- 20.Rotow J., Gameiro S.R., Madan R.A., Gulley J.L., Schlom J., Hodge J.W. Vaccines as monotherapy and in combination therapy for prostate cancer. Clin. Transl. Sci. 2010;3:116–122. doi: 10.1111/j.1752-8062.2010.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apetoh L., Ghiringhelli F., Tesniere A., Obeid M., Ortiz C., Criollo A., Mignot G., Maiuri M.C., Ullrich E., Saulnier P., et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki T., Hannani D., Poirier-Colame V., Ladoire S., Locher C., Sistigu A., Prada N., Adjemian S., Catani J.P., Freudenberg M., et al. Defective immunogenic cell death of hmgb1-deficient tumors: Compensatory therapy with tlr4 agonists. Cell Death Differ. 2014;21:69–78. doi: 10.1038/cdd.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofman M., Ryan J.L., Figueroa-Moseley C.D., Jean-Pierre P., Morrow G.R. Cancer-related fatigue: The scale of the problem. Oncologist. 2007;12:4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 24.Saligan L.N., Olson K., Filler K., Larkin D., Cramp F., Yennurajalingam S., Escalante C.P., del Giglio A., Kober K.M., Kamath J., et al. The biology of cancer-related fatigue: A review of the literature. Support. Care Cancer. 2015;23:2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X.S. Pathophysiology of cancer-related fatigue. Clin. J. Oncol. Nurs. 2008;12:11–20. doi: 10.1188/08.CJON.S2.11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao H.M., Zhang F., Zhou H., Kam W., Wilson B., Hong J.S. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of parkinson’s disease. Environ. Health Perspect. 2011;119:807–814. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sly W.S., Hu P.Y. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu. Rev. Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 28.Tedder T.F., Streuli M., Schlossman S.F., Saito H. Isolation and structure of a cDNA encoding the B1 (CD20) cell-surface antigen of human b lymphocytes. Proc. Natl. Acad. Sci. USA. 1988;85:208–212. doi: 10.1073/pnas.85.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokunaga T., Tomita A., Sugimoto K., Shimada K., Iriyama C., Hirose T., Shirahata-Adachi M., Suzuki Y., Mizuno H., Kiyoi H., et al. De novo diffuse large B-cell lymphoma with a CD20 immunohistochemistry-positive and flow cytometry-negative phenotype: Molecular mechanisms and correlation with rituximab sensitivity. Cancer Sci. 2014;105:35–43. doi: 10.1111/cas.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsiao C.-P., Reddy S.Y., Chen M.-K., Saligan L.N. Genomic profile of fatigued men receiving localized radiation therapy. Biol. Res. Nurs. 2015 doi: 10.1177/1099800415618786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dressman H.K., Muramoto G.G., Chao N.J., Meadows S., Marshall D., Ginsburg G.S., Nevins J.R., Chute J.P. Gene expression signatures that predict radiation exposure in mice and humans. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schubert C., Hong S., Natarajan L., Mills P.J., Dimsdale J.E. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav. Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Johnson T.M., Yu Z.X., Ferrans V.J., Lowenstein R.A., Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA. 1996;93:11848–11852. doi: 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalimuthu S., Se-Kwon K. Cell survival and apoptosis signaling as therapeutic target for cancer: Marine bioactive compounds. Int. J. Mol. Sci. 2013;14:2334–2354. doi: 10.3390/ijms14022334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chinnaiyan A.M. The apoptosome: Heart and soul of the cell death machine. Neoplasia. 1999;1:5–15. doi: 10.1038/sj.neo.7900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill M.M., Adrain C., Duriez P.J., Creagh E.M., Martin S.J. Analysis of the composition, assembly kinetics and activity of native apaf-1 apoptosomes. EMBO J. 2004;23:2134–2145. doi: 10.1038/sj.emboj.7600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maor Y., Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int. J. Hepatol. 2013;2013 doi: 10.1155/2013/815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kufe D.W., Pollock R.E., Weichselbaum R.R., Bast R.C., Jr., Gansler T.S., Holland J.F., Frei E. Holland-Frei Cancer Medicine. 6th ed. BC Decker; Hamilton, ON, USA: 2003. [Google Scholar]
- 39.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Bronnum D., Correa C., Cutter D., Gagliardi G., Gigante B., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 40.Pistevou-Gompaki K., Hatzitolios A., Eleftheriadis N., Boultoukas E., Ntaios G., Andronikidis I., Tzitzikas I. Evaluation of cardiotoxicity five years after 2D planned, non-simulated, radiation therapy for left breast cancer. Ther. Clin. Risk Manag. 2008;4:1359–1362. doi: 10.2147/tcrm.s2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeh E.T.H., Tong A.T., Lenihan D.J., Yusuf S.W., Swafford J., Champion C., Durand J.-B., Gibbs H., Zafarmand A.A., Ewer M.S. Cardiovascular complications of cancer therapy: Diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 42.Yusuf S.W., Sami S., Daher I.N. Radiation-induced heart disease: A clinical update. Cardiol. Res. Pract. 2011;2011 doi: 10.4061/2011/317659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoffel M.P., Pollok M., Fries J., Baldamus C.A. Radiation nephropathy after radiotherapy in metastatic medullary thyroid carcinoma. Nephrol. Dial. Transplant. 2001;16:1082–1083. doi: 10.1093/ndt/16.5.1082. [DOI] [PubMed] [Google Scholar]
- 44.Humphreys B.D., Soiffer R.J., Magee C.C. Renal failure associated with cancer and its treatment: An update. J. Am. Soc. Nephrol. 2005;16:151–161. doi: 10.1681/ASN.2004100843. [DOI] [PubMed] [Google Scholar]
- 45.Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D.A., Smith S., Uziel T., Sfez S., et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 46.Stewart G.S., Maser R.S., Stankovic T., Bressan D.A., Kaplan M.I., Jaspers N.G., Raams A., Byrd P.J., Petrini J.H., Taylor A.M. The DNA double-strand break repair gene hmre11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell. 1999;99:577–587. doi: 10.1016/S0092-8674(00)81547-0. [DOI] [PubMed] [Google Scholar]
- 47.Varon R., Vissinga C., Platzer M., Cerosaletti K.M., Chrzanowska K.H., Saar K., Beckmann G., Seemanova E., Cooper P.R., Nowak N.J., et al. Nibrin, a novel DNA double-strand break repair protein, is mutated in nijmegen breakage syndrome. Cell. 1998;93:467–476. doi: 10.1016/S0092-8674(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 48.Moshous D., Callebaut I., de Chasseval R., Corneo B., Cavazzana-Calvo M., Le Deist F., Tezcan I., Sanal O., Bertrand Y., Philippe N., et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell. 2001;105:177–186. doi: 10.1016/S0092-8674(01)00309-9. [DOI] [PubMed] [Google Scholar]
- 49.West C.M., Elyan S.A., Berry P., Cowan R., Scott D. A comparison of the radiosensitivity of lymphocytes from normal donors, cancer patients, individuals with ataxia-telangiectasia (A-T) and A-T heterozygotes. Int. J. Radiat. Biol. 1995;68:197–203. doi: 10.1080/09553009514551101. [DOI] [PubMed] [Google Scholar]
- 50.Hall E.J., Schiff P.B., Hanks G.E., Brenner D.J., Russo J., Chen J., Sawant S.G., Pandita T.K. A preliminary report: Frequency of A-T heterozygotes among prostate cancer patients with severe late responses to radiation therapy. Cancer J. Sci. Am. 1998;4:385–389. [PubMed] [Google Scholar]
- 51.Ramsay J., Birrell G., Lavin M. Testing for mutations of the ataxia telangiectasia gene in radiosensitive breast cancer patients. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1998;47:125–128. doi: 10.1016/S0167-8140(98)00014-0. [DOI] [PubMed] [Google Scholar]
- 52.Oppitz U., Bernthaler U., Schindler D., Sobeck A., Hoehn H., Platzer M., Rosenthal A., Flentje M. Sequence analysis of the atm gene in 20 patients with RTOG grade 3 or 4 acute and/or late tissue radiation side effects. Int. J. Radiat. Oncol. Biol. Phys. 1999;44:981–988. doi: 10.1016/S0360-3016(99)00108-X. [DOI] [PubMed] [Google Scholar]
- 53.Da Costa Miranda V., Trufelli D.C., Santos J., Campos M.P., Nobuo M., da Costa Miranda M., Schlinder F., Riechelmann R., del Giglio A. Effectiveness of guarana (paullinia cupana) for postradiation fatigue and depression: Results of a pilot double-blind randomized study. J. Altern. Complement. Med. 2009;15:431–433. doi: 10.1089/acm.2008.0324. [DOI] [PubMed] [Google Scholar]
- 54.Campos M.P., Hassan B.J., Riechelmann R., Del Giglio A. Cancer-related fatigue: A review. Rev. Assoc. Med. Bras. 2011;57:211–219. doi: 10.1590/S0104-42302011000200021. [DOI] [PubMed] [Google Scholar]
- 55.Vijayalaxmi, Reiter R.J., Tan D.X., Herman T.S., Thomas C.R., Jr. Melatonin as a radioprotective agent: A review. Int. J. Radiat. Oncol. Biol. Phys. 2004;59:639–653. doi: 10.1016/j.ijrobp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Shirazi A., Ghobadi G., Ghazi-Khansari M. A radiobiological review on melatonin: A novel radioprotector. J. Radiat. Res. 2007;48:263–272. doi: 10.1269/jrr.06070. [DOI] [PubMed] [Google Scholar]
- 57.Kucuktulu E. Protective effect of melatonin against radiation induced nephrotoxicity in rats. Asian Pac. J. Cancer Prev. 2012;13:4101–4105. doi: 10.7314/APJCP.2012.13.8.4101. [DOI] [PubMed] [Google Scholar]
- 58.Jagetia G.C. Radioprotection and radiosensitization by curcumin. Adv. Exp. Med. Biol. 2007;595:301–320. doi: 10.1007/978-0-387-46401-5_13. [DOI] [PubMed] [Google Scholar]
- 59.Ben-Josef E., Han S., Tobi M., Vargas B.J., Stamos B., Kelly L., Biggar S., Kaplan I. Intrarectal application of amifostine for the prevention of radiation-induced rectal injury. Semin. Radiat. Oncol. 2002;12:81–85. doi: 10.1053/srao.2002.31379. [DOI] [PubMed] [Google Scholar]
- 60.Zabbarova I., Kanai A. Targeted delivery of radioprotective agents to mitochondria. Mol. Interv. 2008;8:294–302. doi: 10.1124/mi.8.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simone N.L., Menard C., Soule B.P., Albert P.S., Guion P., Smith S., Godette D., Crouse N.S., Sciuto L.C., Cooley-Zgela T., et al. Intrarectal amifostine during external beam radiation therapy for prostate cancer produces significant improvements in quality of life measured by epic score. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:90–95. doi: 10.1016/j.ijrobp.2007.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amundson S.A. Functional genomics and a new era in radiation biology and oncology. BioScience. 2008;58:491–500. doi: 10.1641/B580606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dellas K. Does radiotherapy have curative potential in metastatic patients? The concept of local therapy in oligometastatic breast cancer. Breast Care. 2011;6:363–368. doi: 10.1159/000333115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mirza Z., Schulten H.J., Farsi H.M., Al-Maghrabi J.A., Gari M.A., Chaudhary A.G., Abuzenadah A.M., Al-Qahtani M.H., Karim S. Molecular interaction of a kinase inhibitor midostaurin with anticancer drug targets, S100A8 and EGFR: Transcriptional profiling and molecular docking study for kidney cancer therapeutics. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0119765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merdad A., Karim S., Schulten H.J., Jayapal M., Dallol A., Buhmeida A., Al-Thubaity F., Gari I.M., Chaudhary A.G., Abuzenadah A.M., et al. Transcriptomics profiling study of breast cancer from kingdom of Saudi Arabia revealed altered expression of adiponectin and fatty acid binding protein4: Is lipid metabolism associated with breast cancer? BMC Genom. 2015;16 doi: 10.1186/1471-2164-16-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.