Abstract
Among critically ill patients with lower respiratory tract (LRT)-confirmed influenza, we retrospectively observed worse 28-day clinical outcomes in upper respiratory tract (URT)-negative versus URT-positive subjects. This finding may reflect disease progression and highlights the need for influenza testing of both URT and LRT specimens to improve diagnostic yield and possibly inform prognosis.
Keywords: critically ill, influenza diagnosis, lower respiratory tract, upper respiratory tract
Influenza causes approximately 28 000 critical illness hospitalizations annually amongst US adults [1]. In 2009, the emergence of influenza A(H1N1)pdm09 virus raised awareness of the potential severity of influenza illness [2, 3].
In critically ill patients, influenza diagnostic yield is higher from lower respiratory tract (LRT) compared with upper respiratory tract (URT) specimens [4–6], although LRT testing is not performed routinely. Viral migration from the URT to LRT, reflecting disease progression [7], could cause falsely negative URT tests in patients with severe influenza disease. Indeed, influenza ribonucleic acid is present at higher levels and for longer periods in LRT compared with URT specimens [6, 8–11]. Thus, viral detection in the LRT, but not the URT, may indicate more prolonged or severe disease. It is unclear whether an association exists between URT influenza test result and clinical outcomes among patients with influenza confirmed in the LRT. We investigated whether intensive care unit (ICU) patients with LRT-confirmed influenza but negative URT influenza tests had different clinical outcomes compared with those with influenza confirmed in both the LRT and URT.
METHODS
We retrospectively identified subjects aged ≥18 years with diagnostic-confirmed influenza (at least 1 positive URT or LRT influenza test, by either direct immunofluorescence [DFA] or polymerase chain reaction [PCR]) admitted to an ICU at 2 hospitals between September 2009 and May 2014. We reviewed dates and results of influenza testing, types of specimens tested, and hospitalization course. Upper respiratory tract specimens were obtained by nasopharyngeal swab. Lower respiratory tract specimens were from bronchoalveolar lavage or washings, sputum induction, or endotracheal aspiration.
We considered a subject “URT-positive” or “LRT-positive” if either DFA or PCR of the site-appropriate specimen was positive. If a subject's only positive test was by LRT PCR, and URT testing was conducted by only DFA and not PCR, then the subject was excluded because there was not comparable testing of URT and LRT specimens (PCR is more sensitive than DFA [4]).
In primary analysis, we included only LRT-positive subjects who had a URT influenza test performed within 7 days prior, comparing URT-negative to URT-positive subjects. In sensitivity analysis, we compared URT-negative and URT-positive subjects among the following groups with confirmed influenza (including those who had no LRT test): (1) all subjects with a URT result; and (2) subjects with a URT result who required mechanical ventilation.
We compared URT-negative and URT-positive subjects, by Fisher's exact test, for the following: proportion breathing without ventilator assistance, proportion discharged alive from ICU, and proportion alive, all at 28 days after ICU admission. We generated Kaplan–Meier plots with censoring at 28 days for time to the equivalent outcome (breathing without ventilator assistance; discharge alive from ICU; death) and compared them using log–rank test. We analyzed data with SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
We identified 181 ICU subjects with confirmed influenza (Supplementary Figure 1). Influenza testing of the URT and LRT was performed in 170 (94%) and 64 (35%) subjects, respectively. Sixty-three subjects had a positive LRT influenza test; 52 of these had a concurrent URT test. Five subjects were excluded because their only positive test was LRT PCR and there was only URT DFA (no PCR) for comparison. We included the remaining 47 subjects in the primary analysis.
Demographic characteristics between the URT-negative and URT-positive groups were similar (Supplementary Table 1). All but 1 subject in each group required mechanical ventilation. Ninety-four percent of subjects received antiviral treatment. Of these, 1 URT-negative subject and 2 URT-positive subjects received antiviral treatment before URT sampling was completed, although the URT-negative subject had a negative URT DFA result before treatment initiation.
Lower respiratory tract testing was performed on the same day or shortly after URT testing. The median (interquartile range [IQR]) lapse in days between URT and LRT testing was 1 (IQR, 0–2). Lower respiratory tract testing (except for 1 subject) occurred after or on the same day as ICU admission.
Among the 47 subjects with a positive LRT influenza test and a comparable URT test, the latter was negative in 20 (43%) and positive in 27 (57%). By 28 days after ICU admission (Supplementary Table 2), URT-negative compared with URT-positive subjects were significantly less likely to breathe without ventilator assistance (40% vs 81%, P < .01) and significantly less likely to have been discharged alive from ICU (35% vs 70%, P = .02). The difference between the 2 groups in proportion alive at 28 days was not statistically significant (65% vs 81%, P = .31). Kaplan–Meier analyses (Figure 1) showed, in URT-negative compared with URT-positive subjects, significantly longer times to breathing without ventilator assistance (P < .01, log–rank) and discharge alive from ICU (P = .03, log–rank). There was a suggestion of shorter survival in the URT-negative group (P = .17, log–rank). Results of sensitivity analyses, which were similar to those of the primary analysis, are described in the Supplementary Material.
Figure 1.
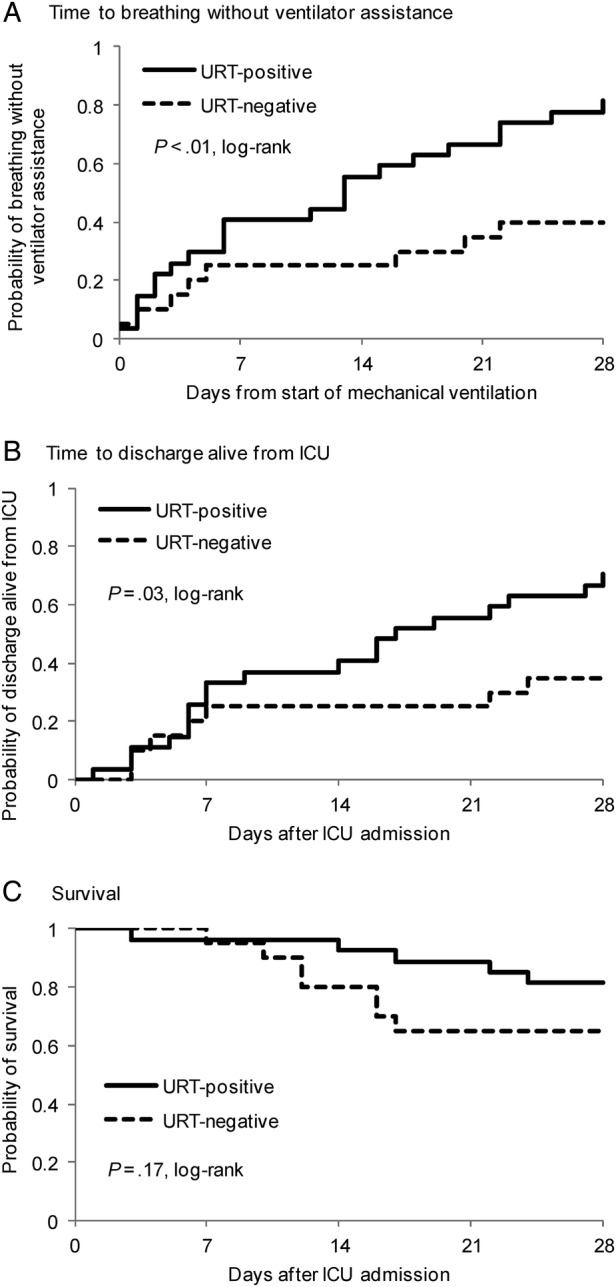
Kaplan–Meier plots of time to breathing without ventilator assistance, (A) time to discharge alive from intensive care unit (ICU), (B) and survival, (C) all to 28 days, according to upper respiratory tract (URT) influenza test result. Initial numbers at risk were 20 in the URT-negative group and 27 in the URT-positive group. P values are based on log–rank test.
DISCUSSION
To our knowledge, there are no reports comparing clinical outcomes of patients with influenza based on influenza test results. We hypothesized that patients with detectable virus in the LRT but not the URT have more severe disease than those with detectable virus in both the LRT and URT. We observed that amongst ICU subjects with LRT-confirmed influenza, URT-negative subjects had significantly worse 28-day clinical outcomes compared with URT-positive subjects.
In severely ill patients with suspected influenza, testing of URT specimens is frequently the first diagnostic maneuver. However, the diagnostic yield from URT specimen influenza tests is suboptimal; LRT testing is more sensitive [6]. This is supported by observations in our cohort: 20 subjects had negative URT and positive LRT results, whereas only 1 subject had positive URT and negative LRT results.
Critically ill influenza patients with negative URT tests may differ in important ways from those with a positive URT test. This could reflect the natural history of infection migrating from the URT to the LRT, causing more severe illness. The 2009 influenza A(H1N1)pdm09 strain appears to have a particular affinity for the LRT [12]. There may be different routes of transmission, wherein some are infected via direct inhalation of aerosols rather than via mucous membranes, thereby bypassing the URT. Alternatively, differences in outcomes might reflect delays in appropriate treatment after an initial URT test provides a negative result.
Expedited confirmation of influenza infection in ICU patients is important to direct management, avoid unnecessary tests and treatments while searching for an alternative diagnosis, and inform local infection control and global epidemiologic surveillance. Practices around diagnostic sampling and testing, including specimen collection site and assay type, have been variable. Lower respiratory tract specimen testing is especially prudent if the diagnosis has not yet been confirmed. Even when bronchoscopy cannot be performed, endotracheal aspiration may improve diagnostic yield over URT testing [13].
Technique and timing of specimen collection and delayed transport to the laboratory may influence influenza test results. Upper respiratory tract sampling by nasopharyngeal swab is subject to the operator's technique; LRT sampling depends on bronchoscopic technique or depth of endotracheal aspiration. In this study, URT and LRT specimens were not necessarily collected at the same time, but the lapse between specimen collections was generally short (median 1 day). Because almost all subjects who received antiviral treatment did so after URT testing was completed, antiviral treatment was unlikely to have influenced the results.
The results of this study should be interpreted in the context of its limitations. The small sample size limited our ability to detect significant differences in outcomes such as survival. Because not all ICU subjects with influenza underwent testing of both URT and LRT specimens, many subjects were excluded from the primary analysis. Lower respiratory tract testing was likely deferred for some in whom URT testing provided a positive result expeditiously. The 117 subjects with a positive URT result but no LRT result had similar outcomes (data not shown) to those with positive URT and LRT tests, so inclusion of these subjects in the primary analysis would have supported our findings. On the other hand, patients with a negative URT test and no LRT test did not have confirmed influenza and thus were excluded from the analysis; influenza may have been diagnosed in some if LRT sampling had been performed.
Although the retrospective nature of this study restricted our ability to compute, and adjust for, baseline illness severity scores, almost all subjects in our primary analytic cohort required mechanical ventilation, suggesting that the prevalence of respiratory failure was similar in the URT-negative and URT-positive groups. In a sensitivity analysis of subjects who required mechanical ventilation, the differences in clinical outcomes between URT-negative and URT-positive subjects were less striking but showed similar trends compared with the primary analysis.
Our results are not generalizable to patients with mild influenza disease. Although the interval between symptom onset and test date may impact diagnostic yield, we could not accurately determine symptom onset for all subjects.
CONCLUSIONS
In this study, URT testing failed to detect over 40% of cases of LRT-confirmed influenza, highlighting the importance of LRT testing for improving diagnostic yield and possibly informing prognosis. With the limitations of this retrospective study in mind, the results are worthy of further investigation; a prospective study to confirm the findings would entail simultaneous URT and LRT PCR testing of all persons in the ICU with suspected influenza.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Judith Tarselli and Dr. David Hooper for providing infection control records.
Disclaimer. The funding source had no role in the conduct of the research or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. The project described was funded by award number T32 AI007433 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Ortiz JR, Neuzil KM, Shay DK et al. . The burden of influenza-associated critical illness hospitalizations. Crit Care Med 2014; 42:2325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynfield R, Davey R, Dwyer DE et al. . Outcomes of influenza A(H1N1)pdm09 virus infection: results from two international cohort studies. PLoS One 2014; 9:e101785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nin N, Soto L, Hurtado J et al. . Clinical characteristics and outcomes of patients with 2009 influenza A(H1N1) virus infection with respiratory failure requiring mechanical ventilation. J Crit Care 2011; 26:186–92. [DOI] [PubMed] [Google Scholar]
- 4.Blyth CC, Iredell JR, Dwyer DE. Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 361:2493. [DOI] [PubMed] [Google Scholar]
- 5.Rice TW, Rubinson L, Uyeki TM et al. . Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med 2012; 40:1487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López Roa P, Rodríguez-Sánchez B, Catalán P et al. . Diagnosis of influenza in intensive care units: lower respiratory tract samples are better than nose-throat swabs. Am J Respir Crit Care Med 2012; 186:929–30. [DOI] [PubMed] [Google Scholar]
- 7.Kuiken T, Taubenberger JK. Pathology of human influenza revisited. Vaccine 2008; 26:D59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee N, Chan PK, Wong CK et al. . Viral clearance and inflammatory response patterns in adults hospitalized for pandemic 2009 influenza A(H1N1) virus pneumonia. Antivir Ther 2011; 16:237–47. [DOI] [PubMed] [Google Scholar]
- 9.Hui DS, Lee N, Chan PK. Clinical management of pandemic 2009 influenza A(H1N1) infection. Chest 2010; 137:916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh E, Luo RF, Dyner L et al. . Preferential lower respiratory tract infection in swine-origin 2009 A(H1N1) influenza. Clin Infect Dis 2010; 50:391–4. [DOI] [PubMed] [Google Scholar]
- 11.Piralla A, Pariani E, Rovida F et al. . Segregation of virulent influenza A(H1N1) variants in the lower respiratory tract of critically ill patients during the 2010–2011 seasonal epidemic. PLoS One 2011; 6:e28332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munster VJ, de Wit E, van den Brand JM et al. . Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science 2009; 325:481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Ayala E, Hill S et al. . Diagnosis of 2009 influenza A H1N1: diagnostic utility of blind endotracheal aspirate in intubated patients with false negative real-time reverse transcriptase polymerase chain reaction assays from nasopharyngeal samples. American Thoracic Society, 2010: A2623. Available at: http://dx.doi.org/10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A2623 Accessed 28 January 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


