Abstract
Real-time quantitative PCR (qPCR) protocols specific to the reductive dehalogenase (RDase) genes vcrA, bvcA, and tceA are commonly used to quantify Dehalococcoides spp. in groundwater from chlorinated solvent-contaminated sites. In this study, loop-mediated isothermal amplification (LAMP) was developed as an alternative approach for the quantification of these genes. LAMP does not require a real-time thermal cycler (i.e., amplification is isothermal), allowing the method to be performed using less-expensive and potentially field-deployable detection devices. Six LAMP primers were designed for each of three RDase genes (vcrA, bvcA, and tceA) using Primer Explorer V4. The LAMP assays were compared to conventional qPCR approaches using plasmid standards, two commercially available bioaugmentation cultures, KB-1 and SDC-9 (both contain Dehalococcoides species). DNA was extracted over a growth cycle from KB-1 and SDC-9 cultures amended with trichloroethene and vinyl chloride, respectively. All three genes were quantified for KB-1, whereas only vcrA was quantified for SDC-9. A comparison of LAMP and qPCR using standard plasmids indicated that quantification results were similar over a large range of gene concentrations. In addition, the quantitative increase in gene concentrations over one growth cycle of KB-1 and SDC-9 using LAMP was comparable to that of qPCR. The developed LAMP assays for vcrA and tceA genes were validated by comparing quantification on the Gene-Z handheld platform and a real-time thermal cycler using DNA isolated from eight groundwater samples obtained from an SDC-9-bioaugmented site (Tulsa, OK). These assays will be particularly useful at sites subject to bioaugmentation with these two commonly used Dehalococcoides species-containing cultures.
INTRODUCTION
Microbially mediated reductive dechlorination plays a vital role in the bioremediation of the chlorinated ethenes tetrachloroethene (PCE) and trichloroethene (TCE). Under the appropriate conditions, PCE and TCE undergo sequential reductive dechlorination via hydrogenolysis to cis-1,2-dichloroethene (cDCE) and vinyl chloride (VC), respectively, finally forming the nontoxic end product ethene (ETH) (1). When reductive dechlorination is linked to growth, it is called organohalide respiration, a metabolism commonly associated with microbial taxa, like Dehalococcoides and Dehalobacter (2–9). Commercially available reductive dechlorinating mixed cultures (e.g., KB-1 and SDC-9) containing such strains are frequently used for the bioaugmentation of contaminated groundwater aquifers (10–12). In fact, in 2009, it was estimated that bioaugmentation with Dehalococcoides spp. had been used at several hundred sites in the United States (13). The growth of these strains in the field and in the laboratory is commonly monitored using real-time quantitative PCR (qPCR) targeting the genes vcrA, bvcA, and tceA, which code for distinct reductive dehalogenases (RDases) implicated in organohalide respiration (14). To date, a number of qPCR protocols with DNA binding dyes or TaqMan probes to quantify vcrA, bvcA, and tceA genes have been developed (2, 15–17). Although qPCR has been successful for monitoring reductive dechlorination, alternative methods would be advantageous for laboratories or practitioners without access to a real-time thermal cycler. Also, any method that is more economical and faster than qPCR would be beneficial.
Loop-mediated isothermal amplification (LAMP) is a novel molecular method recently developed for the specific detection of nucleic acids (18). LAMP is a one-step amplification reaction that amplifies a target DNA sequence using four to six primers. The Bst large-fragment DNA polymerase has strand displacement activity and helicase-like activity, allowing it to unwind and amplify DNA strands in a temperature range of 60 to 65°C (18). Because LAMP is rapid, sensitive, and specific and occurs isothermally, it has emerged as an alternative to PCR-based methods in a wide variety of applications. For example, many LAMP assays have been developed for testing foodborne bacterial pathogens and fungal contaminants (for a review, see reference 19). Recently, LAMP primer sets have been developed and tested for the detection of plasmids pXO1 and pXO2, which impart infectious properties to several strains of Bacillus anthracis (20–22). LAMP can also be used to detect RNA viruses. A reverse transcription step is used to convert the RNA from viruses, such as HIV-1 or the Ebola virus, to DNA (23, 24).
In 2014, LAMP primer sets were developed for vcrA and the Dehalococcoides 16S rRNA gene (25). In that study, a field-deployable approach for harvesting biomass from samples of groundwater bioaugmented with SDC-9 was described. The direct amplification of templates with LAMP was performed using the handheld Gene-Z platform. Detection limits of <107 gene copies/liter were reported (this is the generally accepted threshold for acceptable in situ dechlorination). However, larger volumes of groundwater were required when Dehalococcoides species numbers were <104 gene copies/liter.
Here, the objective was to evaluate if LAMP can be used for the quantification of Dehalococcoides mccartyi RDase genes (vcrA, bvcA, and tceA) in two commonly used commercial bioaugmentation cultures, KB-1 (from SiREM) and SDC-9 (from CB&I). This study involved DNA templates rather than direct amplification of harvested biomass. Quantification results (i.e., gene copies per liter values) were compared between LAMP and qPCR during one growth cycle to evaluate the effectiveness of LAMP as a tool to monitor the growth of Dehalococcoides spp. in KB-1 and SDC-9. Further, we used DNA templates isolated from eight groundwater samples to validate the quantification of the developed assays with LAMP on the Gene-Z platform (25). The data generated from the groundwater samples were also compared to data obtained using a real-time thermal cycler.
MATERIALS AND METHODS
Cultures and growth conditions.
All experiments were carried out in triplicate serum bottles (160-ml nominal volume) containing 100 ml (final volume) of culture and sealed with gray butyl rubber septa. After the microcosms were transferred into an anaerobic chamber, the KB-1 or SDC-9 inoculum (10 ml) and sterile mineral medium (90 ml) were added to the serum bottles using aseptic techniques. The bottles were capped with gray butyl rubber septa, removed from the anaerobic chamber, and sparged with 30% CO2–70% N2 to adjust the pH. During the growth cycle, the pH of each bottle was measured and adjusted to neutral using 1.0 M NaOH, as needed. The bottles were incubated quiescently, shielded from light, and kept at room temperature (∼22 to 24°C), and the liquid was kept in contact with the septum to minimize the loss of volatile compounds. The concentration of chlorinated ethenes was monitored by gas chromatography with a flame ionization detector (GC-FID), as previously described (1). All KB-1 serum bottles were amended with 10 μl of feed solution (1:10 dilution of neat TCE in methanol) to yield final concentrations of ∼23 μmol TCE and ∼112 μmol methanol in each bottle. The bottles were also amended with ethanol (∼44.0 mg/liter) each week if residual cDCE and VC were observed. An aliquot (1.0 ml) of culture fluid was removed on days 0, 7, 14, 21, 28, 35, 38, 41, and 44 for DNA extraction. DNA was isolated from 100-μl aliquots using the Mo Bio DNA isolation kit, per the manufacturer's protocol (Mo Bio Laboratories, Inc., Carlsbad, CA). All SDC-9 bottles were amended with ∼20 μmol VC, along with a 0.1-ml spike of 100 mM sodium lactate. DNA was extracted from 30-μl aliquots of 3 ml of culture fluid on days 0, 6, 22, 27, 32, and 40.
Groundwater samples.
Groundwater samples were obtained from a site in Tulsa, OK, which had been recently bioaugmented with SDC-9. Eight amber-colored glass bottles containing an ∼1.0-liter groundwater sample representative of monitoring wells 1 to 4 (MW1 to MW4) and injection wells 1 to 4 (IW1 to IW4) were bubble-wrapped and shipped overnight in a cooler packed with ice packs. Upon receipt, the bottles were stored at 4.0°C in the absence of light for the duration of testing. Groundwater samples (100.0 ml each) were filtered through a 0.22-μm-pore-size filter (EMD Millipore Corp., Billerica, MA) using a vacuum pump. Membranes were cut into 0.5-mm strips inside a petri dish with a no. 15 blade using aseptic technique, and these were added to 15.0-ml bead tubes supplied with the Mo Bio UltraClean water kit. DNA (1.5 ml) was extracted from this solution, according to the manufacturer's protocol. The DNA was precipitated by adding 150.0 μl of 5 M NaCl and 3.0 ml of absolute ice-cold ethanol and incubating for 30 min at 4°C. Following centrifugation (14,000 × g for 20 min at room temperature), the DNA pellet was rinsed with 70% ethanol, air dried, and suspended in 100 μl of distilled water (dH2O). The extracted DNA was immediately used for amplification or stored at −20°C for future use.
Preparation of plasmid standards.
Genomic DNA was extracted from 5 ml of KB-1 (SiREM, Guelph, ON, Canada) using the Mo Bio DNA isolation kit, per the manufacturer's protocol. The vcrA and the bvcA genes were amplified using PCR with previously described primers (17). The amplified templates were cloned into Escherichia coli DH5α using the pCR2.1 TOPO TA cloning vector (Invitrogen) to generate plasmid inserts. E. coli cultures were grown overnight in LB medium amended with 50 mg/ml ampicillin and 7.0% glycerol at 37°C. Plasmid standards for tceA were provided by Frank Löffler (University of Tennessee, Knoxville, TN). Plasmid inserts were extracted using 5 ml of E. coli culture and the Qiagen plasmid extraction kit. Gene copies were calculated as previously described (17). Serial dilutions of plasmid inserts from 3.16 × 108 plasmids/μl to ∼316 plasmids/μl for vcrA, 2.65 × 109 plasmids/μl to ∼265 plasmids/μl for bvcA, and 1.41 × 1010 plasmids/μl to 141 plasmids/μl for tceA were used as standards for the amplifications. By plotting the log of the calculated copy number against the cycle threshold (for qPCR) or threshold time (for LAMP) at which fluorescence for that sample crosses the threshold value, standard curves were obtained.
Design of LAMP primers.
The LAMP primer sets used for this study are listed in Table 1. FASTA files for the functional RDase genes vcrA (GenBank accession no. NC_013552.1, region 1187298 to 1188857), bvcA (GenBank accession no. NC_009455.1, region 834959 to 836509), and tceA (GenBank accession no. AY165309.1) of Dehalococcoides spp. were downloaded and aligned with the relevant environmental sequences in the NCBI nucleotide database to identify the conserved regions. Next, LAMP primer sets were designed for those regions using Primer Explorer V4 (https://primerexplorer.jp/e/). For the vcrA gene, two new LAMP primer sets, vcrA set A and vcrA set C, targeting the bp 857-to-1072 region, were designed and used along with vcrA set B, which was designed and tested previously (25). One primer set, bvcA set A, targeting the bp 895-to-1139 region, was designed for the bvcA gene. Similarly, tceA set A was designed to target the bp 882-to-1156 region of the tceA gene. Finally, NCBI nucleotide BLAST was used to determine the fidelity of the primer sets to the target sequences in environmental submissions to the database by setting the default expect value as 1 × 10−5.
TABLE 1.
LAMP primer sets designed and used in this study
| Target gene | Primer set | Primer name | Sequence (5′→3′) | GenBank accession no., region (bp) |
|---|---|---|---|---|
| vcrA | vcrA set A | F3 | GTAAGTTTTACGCGAGATGG | NC_013552.1, 1187298–1188857 (857–1072) |
| B3 | GTCATCGGCTGAGCTTTC | |||
| FIP | ACCCTCCCATTTTGGTACGCTTGTATGGTCCGCCACAT | |||
| BIP | AAGACAATTTTCTAATGCTGAGGGCATTTGGGATCTGCCAGGT | |||
| LF | CATCAGGTGGCGCTGAATC | |||
| LB | AGCTGCAAAATATTTTGGTGCTGG | |||
| vcrA set B (25) | F3 | ACTAATATATAAGAAAGCTCAGCC | NC_013552.1, 1187298–1188857 (652–886) | |
| B3 | TCTTATTGAGTTCTTGTGGTTG | |||
| FIP | GGTCAGGAACCTTGGGATAAATTTTGATGACTCTAGGAAAAGGAACA | |||
| BIP | AACTTTAAGGAAGCGGATTATAGCTATGGATTCACACTTTGTTGG | |||
| LF | CCTGGTCCACCTAATTCACTGTA | |||
| LB | ACTACAATGATGCAGAGTGGGTTA | |||
| vcrA set C | F3 | GTAAGTTTTACGCGAGATGG | NC_013552.1, 1187298–1188857 (857–1072) | |
| B3 | GTCATCGGCTGAGCTTTC | |||
| FIP | ACCCTCCCATTTTGGTACGCTTGTATGGTCCGCCACAT | |||
| BIP | AAGACAATTTTCTAATGCTGAGGGCATTTGGGATCTGCCAGGT | |||
| LF | CCATCAGGTGGCGCTGAA | |||
| LB | TGGTGCTGGTGGCGTT | |||
| bvcA | bvcA set A | F3 | ACAATGCCTTTACCAGAAGA | NC_009455.1, 834959 to 836509 (895–1139) |
| B3 | ACCGTATTTGGGGCTGAT | |||
| FIP | TCGGCCTCCATTAAAAGCCATTCTCTAGGGTGGTCATGT | |||
| BIP | ATCAAGGACTTGGTGGCGACCTTGTTCGGAAAGACACTCA | |||
| LF | AGGCAATCATACTTGAAGCGTC | |||
| LB | TGTGGGGACCTGGTGGT | |||
| tceA | tceA set A | F3 | GCCGTTTATTCCATTCATGG | AY165309.1 (882–1156) |
| B3 | GCATAGACTGGATGAAGGAA | |||
| FIP | ACATAATTGCTGGGAGAACCCG-TCGCATAGAGAGATAAGGCC | |||
| BIP | GCCATTCGTGGCGGCATATAT-CAGATTATGACCCTGGTGAA | |||
| LF | CTTTATGGACGCTATGAAGGTTCTA | |||
| LB | TCTTCCCTGCGGTCGCCATA |
qPCR and LAMP amplification.
Each 20-μl LAMP reaction mixture contained 1× isothermal amplification buffer (catalog no. B0537S; NEB), 1.4 mM deoxynucleoside triphosphates (dNTPs), 0.8 mM betaine, 6.0 mM MgSO4, 1.6 U of Bst 2.0 WarmStart (NEB), 0.8 μl of Syto82 orange fluorescent dye (Life Technologies, Inc., Grand Island, NY), 0.8 μl of Pluronic (Life Technologies, Inc.), 0.8 μl of bovine serum albumin, a 0.25 μM concentration of 10× primer mix, and balance water to make up 18 μl. The reaction mixtures were incubated at 63°C for 60 min for amplification. All TaqMan assays were set up as 20-μl reaction mixtures. Each 20-μl reaction mixture contained 10 μl of iTaq Universal super mix supplied by Bio-Rad, 1.2 μl of TaqMan probe, described previously (15, 17, 26, 27), and balance water to make up 18 μl. PCR amplifications were performed using cycling conditions of 95°C for 15 s, 60°C for 1 min, a slow ramp of 1% to 95°C for 15 s, and 60°C for 15 s. DNA templates and plasmid standards were added to each LAMP and qPCR mixtures as 2-μl aliquots. All qPCR primers and probes used in this study are listed in Table 2. All qPCR experiments were performed in the commercially available Chromo 4 PCR real-time thermal cycler. For KB-1 and SDC-9 templates, amplification with LAMP was carried out in the real-time thermal cycler, while amplification with groundwater templates was performed in both the Gene-Z platform (described below) and the real-time thermal cycler. Triplicate reactions for each test and positive and no-template controls were used for all experiments.
TABLE 2.
qPCR primers used in this study
| Target gene | Primer | Sequencea | Reference |
|---|---|---|---|
| vcrA | vcrA1022F | CGGGCGGATGCACTATTTT | 17 |
| vcrA1093R | GAATAGTCCGTGCCCTTCCTC | 17 | |
| vcrA1042Probe | FAM-CGCAGTAACTCAACCATTTCCTGGTAGTGG-TAMRA | 17 | |
| bvcA | bvcA925F | AAAAGCACTTGGCTATCAAGGAC | 17 |
| bvcA1017R | CCAAAAGCACCACCAGGTC | 17 | |
| bvcA977Probe | FAM-TGGTGGCGACGTGGCTATGTGG-TAMRA | 17 | |
| tceA | tceA1270F | ATCCAGATTATGACCCTGGTGAA | 26, 27 |
| tceA1336R | GCGGCATATATTAGGGCATCTT | 26, 27 |
FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
Gene-Z analysis of groundwater samples.
Inside the Gene-Z device, an array of 64 light-emitting diodes (LEDs), a bundle of optical fibers, and a single photodiode were used to measure fluorescence in real time (28). An iPod Touch (gen 5) was used to control the reaction temperature and time, start the device, stream data via Bluetooth connectivity, and sort, plot, store, and transmit results. Disposable chips were made by etching channels and wells into black acrylic (1.58 mm thick) via a 40-W CO2 laser (Full Spectrum). The etched chips were cleaned and prepared, as previously described (29). Briefly, the chips were cleaned with distilled water, soaked in 70% ethanol for 10 min, and dried for 10 min at 70°C. Once dry, primers were dispensed and dried in wells at 70°C for 5 min. The wells were enclosed with optical adhesive film (MicroAmp; Applied Biosystems), and the chips were stored at −20°C until use. The chips were cut into 8 reaction wells per sample, with 4 samples per chip (i.e., 32 reaction wells per chip with 20-μl reaction volume). Six chips were used to test groundwater samples, and two additional chips were used to test plasmid dilution standards. vcrA and tceA primers were each dispensed into three separate reaction wells per sample lane.
RESULTS
Amplification with LAMP primers and their application.
As stated previously, vcrA set B was previously designed and tested using templates obtained from groundwater spiked with SDC-9 (25). In that study, larger volumes of groundwater samples (1 to 4 liters) were required when the vcrA gene copies were <104 gene copies/liter. In this study, we developed two new LAMP primer sets (vcrA set A and vcrA set C) that exhibited faster LAMP threshold times that than of vcrA set B. For example, with 103.5 gene copy templates, the threshold times were 23.9 ± 0.4 min for vcrA set A, 21.2 ± 0.2 min for vcrA set C, and 28.3 ± 0.3 min for vcrA set B. Moreover, the new primer sets had an equivalent or better detection limit than that of vcrA set B (103.5 gene copies/reaction for vcrA set A, 102.5 gene copies/reaction for vcrA set C, and 103.5 gene copies/reaction for vcrA set B).
Here, the first set of experiments targeted vcrA in SDC-9 using both vcrA set A and vcrA set B. The second set of experiments involved vcrA set C, the most refined LAMP primer set for vcrA, with KB-1 templates.
Additionally, new primer sets were developed for the bvcA and tceA genes, and these were tested with a KB-1 template. One aim was to evaluate if the new LAMP primer sets could be used to track the growth of Dehalococcoides spp. in actively dechlorinating KB-1 and SDC-9 cultures over one growth cycle. As SDC-9 does not contain bvcA, this assay was not tested with this culture. qPCR was used as a control assay for all experiments. While the specificities of the new primer sets were not evaluated experimentally, LAMP reactions require six different primers for amplification and are thus unlikely to produce false positives.
Monitoring Dehalococcoides species growth in KB-1 and SDC-9 cultures.
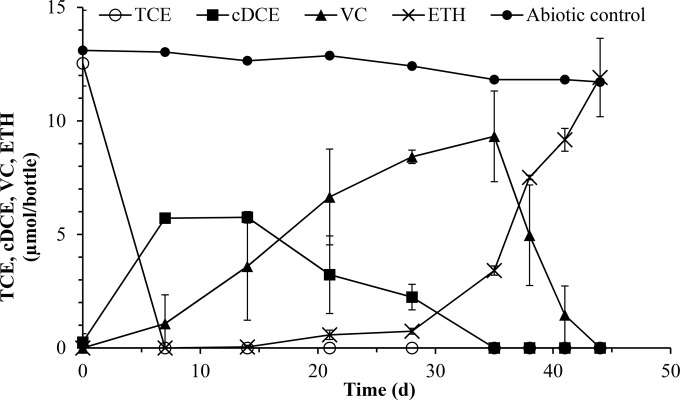
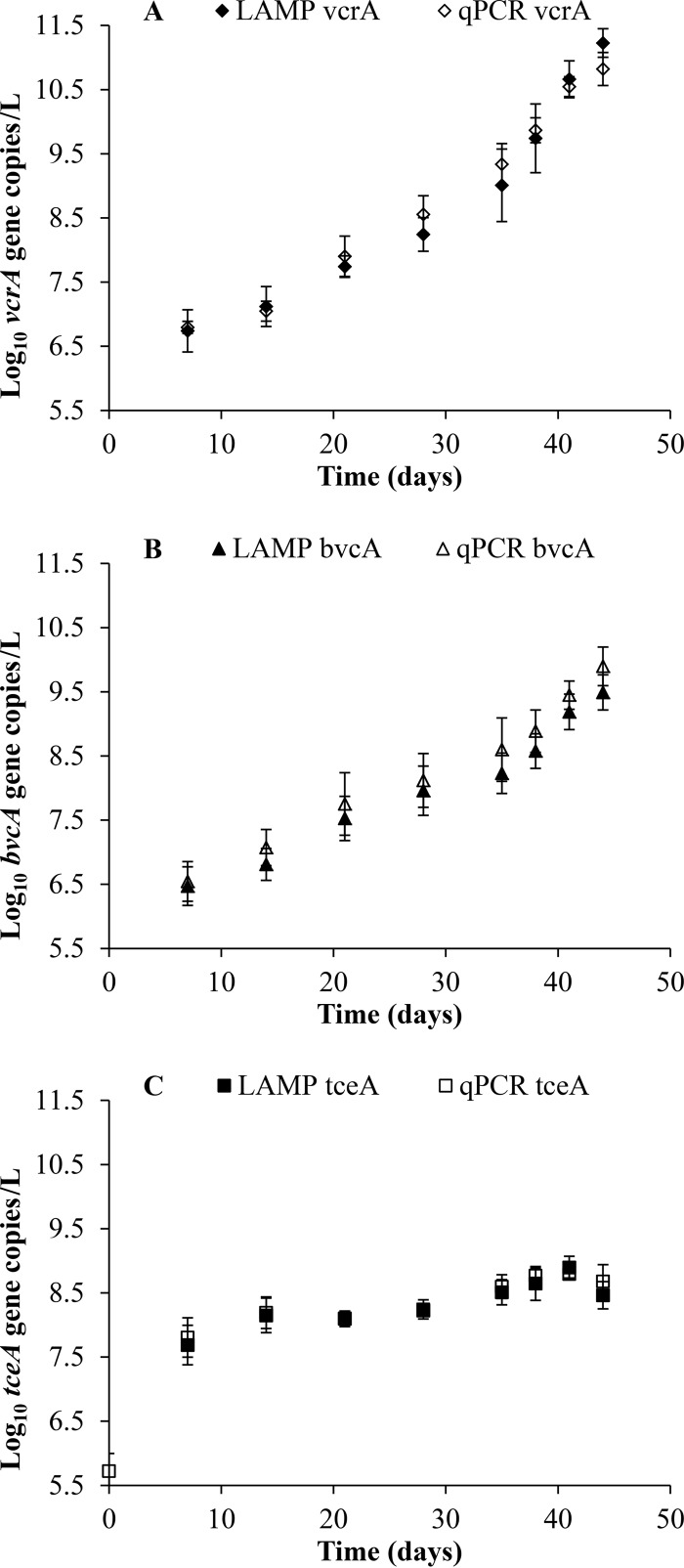
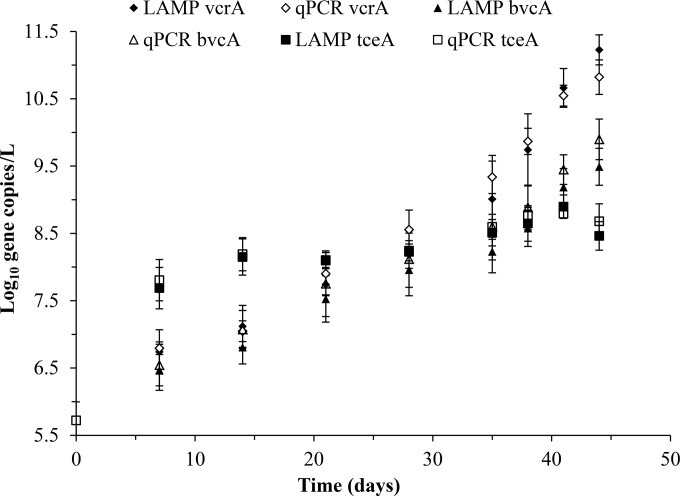
The mean masses of TCE, cDCE, VC, and ETH in triplicate KB-1 cultures and an abiotic control are shown in Fig. 1. As expected, TCE was reduced to cDCE and VC to ETH. cDCE accumulated and peaked at 7 days after inoculation, while VC peaked at ∼35 days before being rapidly degraded to ETH. Stoichiometric amounts of ETH accumulated at the end of the growth cycle. TCE, cDCE, and VC were not detected at the end of the 48-day incubation period. At each time point, the concentrations of two Dehalococcoides strains, VS and BAV1, were investigated using qPCR and LAMP targeting the vcrA and bvcA genes in DNA extracted from the KB-1 cultures. Figure 2 illustrates the gene copies of vcrA, bvcA, and tceA per liter in triplicate cultures of KB-1 while growing on TCE. We observed a comparable steady increase in the number of vcrA gene copies, from ∼5.8 × 106 gene copies/liter on day 7 to ∼6.4 × 109 gene copies/liter on day 38 using both LAMP and qPCR. This was followed by a more rapid increase between days 38 and 44, from ∼6.4 × 109 gene copies/liter to ∼1.1 × 1011 gene copies/liter, coupled with a significant reduction of VC to ETH. A similar trend was observed with the bvcA gene. Gene copy numbers steadily increased from 3.2 × 106 gene copies/liter at day 7 to 5.7 × 108 gene copies/liter at day 38, followed by a more rapid increase to 5.49 × 109 gene copies/liter at day 44. Gene copies of tceA increased to ∼1.4 × 108 gene copies/liter from day 0 to day 14, coupled to the reduction of TCE to cDCE and VC, which was then followed by a slight increase to ∼3.8 × 108 gene copies/liter on day 44.
FIG 1.
Mean masses of TCE, cDCE, VC, and ETH in triplicate KB-1 cultures and an abiotic control. The data are presented as the standard means and standard deviations.
FIG 2.
Gene copies of vcrA (A), bvcA (B), and tceA (C) per liter in triplicate cultures of KB-1 while growing on TCE. LAMP vcrA set C was used to target vcrA. The data are presented as the standard means and standard deviations.
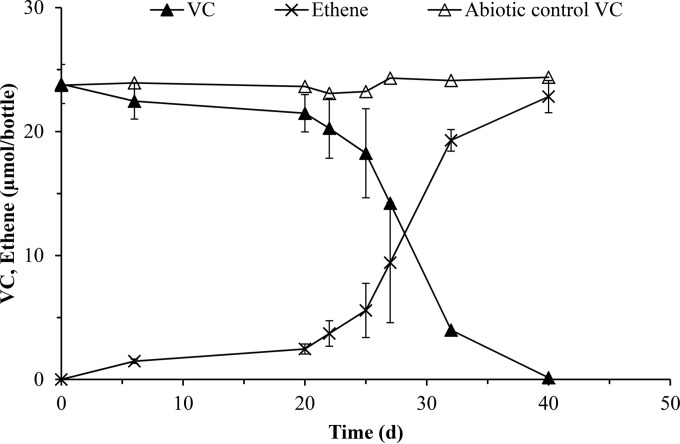
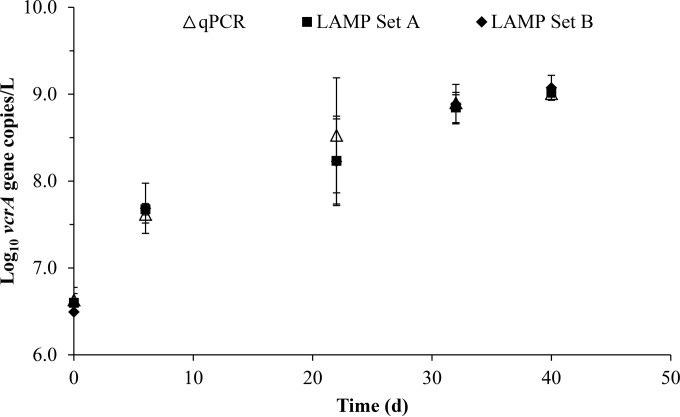
The growth of Dehalococcoides spp. in SDC-9 culture was investigated using the vcrA gene. The mean masses of VC and ETH in triplicate SDC-9 cultures and an abiotic control are shown in Fig. 3. Rapid reductive dechlorination of ∼24 μmol VC from days 20 to 40 was coupled to the stoichiometric accumulation of ETH. Figure 4 illustrates the vcrA gene copies per liter measured via two LAMP primer sets (vcrA set A and vcrA set B) and qPCR in the triplicate cultures. The vcrA gene copies steadily increased to ∼9.0 × 107 gene copies/liter. As VC rapidly dechlorinated to ETH, we observed that the vcrA gene copies increased from ∼9.0 × 107 gene copies/liter to ∼1.1 × 109 gene copies/liter.
FIG 3.
Mean masses of VC and ETH in triplicate SDC-9 cultures and an abiotic control. The data are presented as the standard means and standard deviations.
FIG 4.
vcrA gene copies per liter measured via qPCR and two LAMP assays (vcrA set A and vcrA set B) in triplicate cultures of SDC-9 during growth on VC. The data are presented as the standard means and standard deviations.
The mean gene copies of vcrA, bvcA, and tceA per liter in triplicate cultures of KB-1 while growing on TCE are shown in Fig. 5. Note that the y axis in Fig. 5 is a log scale, which does not start at zero, to illustrate the differences between vcrA, bvcA, and tceA concentrations. To elucidate the potential of LAMP as an alternate method to monitor Dehalococcoides spp. in commercial reductive dechlorinating cultures, the absolute quantification of each gene in a KB-1 template was determined using the two methods. Figure 6 shows a comparison of the mean gene copies (per liter) in vcrA, bvcA, and tceA in triplicate cultures of KB-1 while growing on TCE.
FIG 5.
Mean gene copies of vcrA, bvcA, and tceA per liter in triplicate cultures of KB-1 while growing on TCE. The data are presented as the standard means and standard deviations. Note that the y axis is a log scale and does not start at zero.
FIG 6.
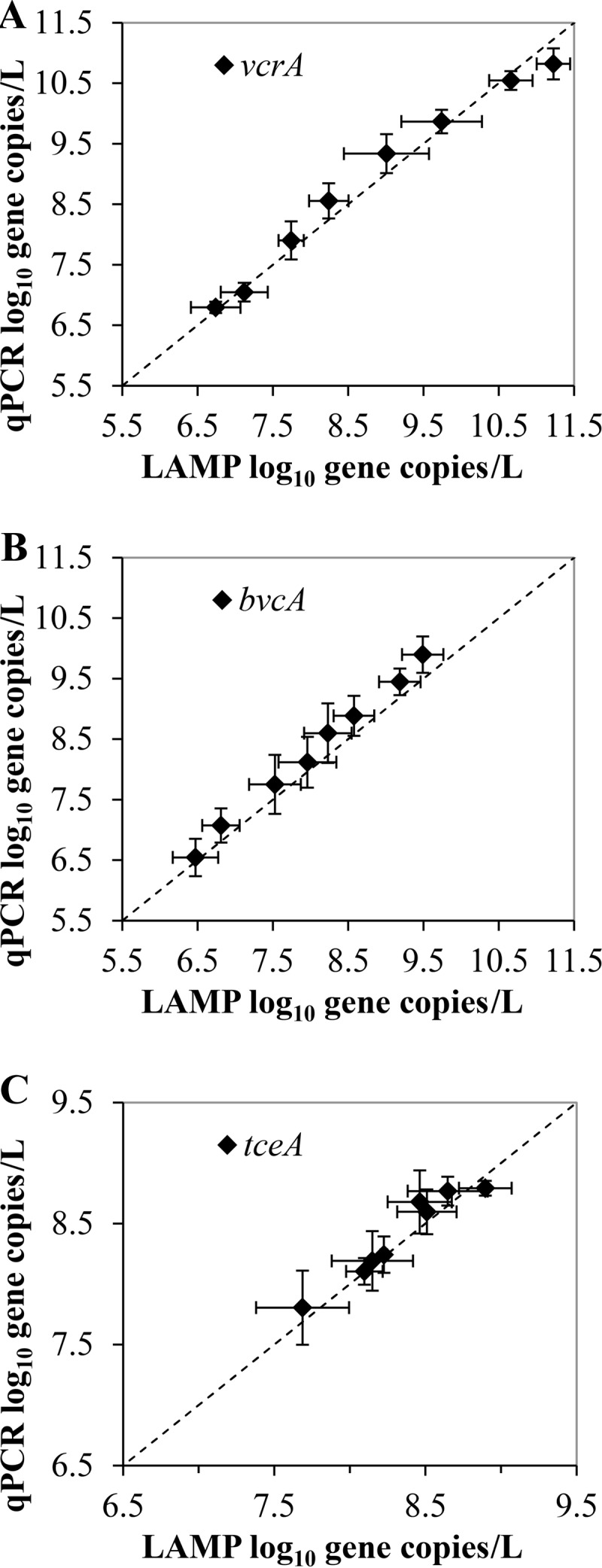
Comparison of vcrA (A), bvcA (B), and tceA (C) mean gene copies per liter in triplicate cultures of KB-1 while growing on TCE. The data are presented as the standard means and standard deviations (horizontal and vertical bars). The dashed line represents a 1:1 slope.
Validation of new LAMP assays with the Gene-Z platform using groundwater templates.
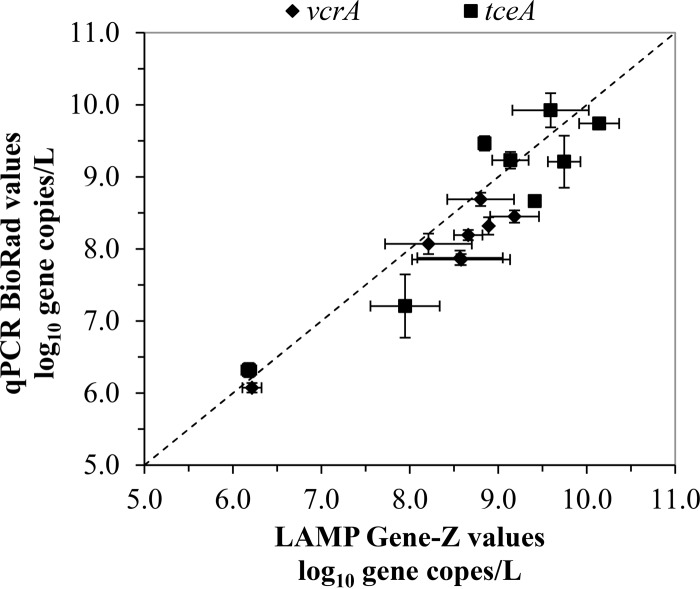
Nucleic acids extracted from groundwater from a previously bioaugmented and chlorinated solvent-contaminated site were used to validate the novel LAMP assays with the Gene-Z handheld device. The data obtained using the new LAMP assays on the Gene-Z device were compared to those obtained using qPCR on a real-time thermal cycler. Specifically, vcrA and tceA gene copies (per liter) from four monitoring wells and four injection wells (in triplicate) were compared using the new LAMP assays and qPCR (Fig. 7).
FIG 7.
Comparison of vcrA and tceA mean gene copies per liter in triplicate cultures or eight different groundwater DNA templates observed using qPCR on a real-time thermal cycler and the Gene-Z platform. The dashed line represents a 1:1 slope.
DISCUSSION
To date, LAMP primer sets have been developed for phylogenetic or functional genes of prokaryotes, eukaryotes, and viruses to detect target sequences in templates extracted from a variety of environmental matrices, such as air, water, soil, fecal matter, and blood. For example, LAMP can be used for detection of the invA gene in all known 89 strains of Salmonella, a foodborne bacterial pathogen causing salmonellosis (30, 31). LAMP was also applied to detect Cryptosporidium oocysts, which cause cryptosporidiosis, using the functional gene gp60 (32). Similarly, LAMP primer sets for the detection of viral pathogens, such as HIV-1 and Ebola virus, have also been described (23, 24). Another application of LAMP has been the identification of organisms in beef contaminated with ostrich meat. The LAMP assay successfully identified ostrich meat contamination of up to 0.01% using direct-cell amplification from swabs (33). These examples demonstrate the versatility of LAMP in terms of its application to human health, environmental, and food microbiology. In this study, LAMP was applied for the rapid quantification of the key biomarker RDase genes vcrA, bvcA, and tceA. These biomarker genes are important for monitoring the activity of Dehalococcoides spp. in groundwater during natural attenuation, biostimulation, and bioaugmentation at chlorinated solvent-contaminated sites.
The growth patterns observed in this study are characteristic of cultures, such as KB-1 and SDC-9, when amended with TCE and VC, respectively. KB-1 is highly enriched in a few unique D. mccartyi strains, which are capable of catabolic growth using cDCE and VC as electron acceptors for reductive dechlorination (34). Typically, the individual cells of such strains carry one copy of the vcrA or bvcA gene coding for the two distinct vinyl chloride reductases (35–37). However, neither vinyl chloride reductase is capable of growth-linked metabolic reduction of TCE to cDCE or VC. When amended with TCE, initial growth is cometabolic and often slower (4, 6, 9). As a result, the increase in observed vcrA and bvcA gene copies is faster and more discernible when cDCE and VC are being dechlorinated. In contrast, the tceA gene codes for trichloroethene reductive dehalogenase, which is responsible for the reductive dechlorination of TCE to cDCE and VC (38). An initial increase in the tceA gene copies coupled to rapid reduction of TCE to cDCE and VC was observed in all KB-1 microcosms, but as TCE was depleted, the increase in tceA gene copies was substantially less. This is indicative of growth of D. mccartyi strains with the tceA gene. However, the abundance of the tceA gene within the Dehalococcoides genus is more widespread than that of the vcrA and bvcA genes, and strains that carry the tceA gene may also carry the vcrA or bvcA gene (6, 8, 17). Additionally, TCE dechlorination may also be driven by Geobacter strains in KB-1, along with Dehalococcoides spp. (39), which may explain the less-discernible growth pattern.
In this research, the novel LAMP assays for vcrA and tceA were validated using DNA extracted from groundwater samples. We compared the quantification results, i.e., gene copies per liter values, for both genes obtained using qPCR on a real-time thermal cycler with LAMP on the Gene-Z platform.
Overall, similar values were obtained for each gene on the two platforms, indicating that quantification with LAMP on the Gene-Z platform is a viable alternative to qPCR. For some data points, the Gene-Z platform yielded slightly higher values than those of qPCR, which may be attributed to the difference in the fluorescence-sensing mechanisms of the two platforms. This issue will be examined in more detail in future studies. The new LAMP primer sets were able to detect quantities of <107 gene copies/liter, the accepted limit for natural attenuation.
LAMP offers two key advantages over qPCR. First, the LAMP primer sets described here may be used with a variety of several less-expensive platforms (with diverse types of detection mechanisms), which include real-time turbidimeters, microfluidic chips (e.g., Gene-Z), and electrochemical or ultrasonic sensors (25, 40–43). Besides being cheaper, these platforms are more-accessible alternatives to qPCR thermal cyclers. Second, these platforms use different reaction chemistries (e.g., they produce significant visible fluorescence or postreaction electrochemical changes) to detect amplified target sequences and thus can be more economical than qPCR. In time-limited studies, another potential advantage is that amplification during LAMP is faster than that with qPCR. With the primer sets and reaction chemistries described in this study, all LAMP reactions were complete in <1 h, which is significantly shorter than a typical qPCR run (>1.5 h). In summary, the development of LAMP assays for the detection of the RDase genes vcrA, bvcA, and tceA using alternative and potentially field-deployable platforms, such as the Gene-Z platform (25), will enable the rapid detection and quantification of Dehalococcoides gene biomarkers in groundwater from solvent-contaminated sites. Further, the development of LAMP assays specific to two commonly used commercially available cultures will facilitate the specific detection of these RDase genes at sites subject to bioaugmentation.
ACKNOWLEDGMENTS
We thank Yanlyang Pan (MSU) for helping with the GC-FID setup, Tiffany Stedtfeld (MSU) for her help with obtaining preliminary LAMP data, Simon Vainberg (CB&I) for supplying the SDC-9 culture, and Phil Dennis (SiREM) for supplying the KB-1 culture. We also thank Frank Löffler (UTK) for providing the tceA plasmid standard.
Funding Statement
Funding for this project was provided by the Strategic Environmental Research and Development Program (SERDP) under grant ER-2309.
REFERENCES
- 1.Freedman DL, Gossett JM. 1989. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol 55:2144–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cupples AM. 2008. Real-time PCR quantification of Dehalococcoides populations: methods and applications. J Microbiol Methods 72:1–11. doi: 10.1016/j.mimet.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Grostern A, Chan WWM, Edwards EA. 2009. 1,1,1-Trichloroethane and 1,1-dichloroethane reductive dechlorination kinetics and co-contaminant effects in a Dehalobacter-containing mixed culture. Environ Sci Technol 43:6799–6807. doi: 10.1021/es901038x. [DOI] [PubMed] [Google Scholar]
- 4.He J, Ritalahti KM, Yang K-L, Koeningsberg SS, Löffler FE. 2003. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62–65. doi: 10.1038/nature01717. [DOI] [PubMed] [Google Scholar]
- 5.Holliger C, Wohlfarth G, Diekert G. 1998. Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiol Rev 22:383–398. doi: 10.1111/j.1574-6976.1998.tb00377.x. [DOI] [Google Scholar]
- 6.Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, Müller JA, Fullerton H, Zinder SH, Spormann AM. 2013. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int J Syst Evol Microbiol 63:625–635. doi: 10.1099/ijs.0.034926-0. [DOI] [PubMed] [Google Scholar]
- 7.Maymó-Gatell X, Chien Y-T, Gossett JM, Zinder SH. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568–1571. doi: 10.1126/science.276.5318.1568. [DOI] [PubMed] [Google Scholar]
- 8.Sung Y, Ritalahti KM, Apkarian RP, Löffler FE. 2006. Quantitative PCR confirms purity of strain GT, a novel trichloroethene-to-ethene-respiring Dehalococcoides isolate. Appl Environ Microbiol 72:1980–1987. doi: 10.1128/AEM.72.3.1980-1987.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cupples AM, Spormann AM, McCarty PL. 2003. Growth of a Dehalococcoides-like microorganism on vinyl chloride and cis-dichloroethene as electron acceptors as determined by competitive PCR. Appl Environ Microbiol 69:953–959. doi: 10.1128/AEM.69.2.953-959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Major DW, McMaster ML, Cox EE, Edwards EA, Dworatzek SM, Hendrickson ER, Starr MG, Payne JA, Buonamici LW. 2002. Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environ Sci Technol 36:5106–5116. doi: 10.1021/es0255711. [DOI] [PubMed] [Google Scholar]
- 11.Vainberg S, Condee CW, Steffan RJ. 2009. Large-scale production of bacterial consortia for remediation of chlorinated solvent-contaminated groundwater. J Ind Microbiol Biotechnol 36:1189–1197. doi: 10.1007/s10295-009-0600-5. [DOI] [PubMed] [Google Scholar]
- 12.Steffan RJ, Vainberg S. 2013. Production and handling of Dehalococcoides bioaugmentation cultures, p 89–116. In Stroo HF, Leeson A, Ward CW (ed), Bioaugmentation for groundwater remediation. Springer, New York, NY. [Google Scholar]
- 13.Lyon DY, Vogel TM. 2013. Bioaugmentation for groundwater remediation: an overview, p 1–38. In Stroo HF, Leeson A, Ward CW (ed), Bioaugmentation for groundwater remediation. Springer, New York, NY. [Google Scholar]
- 14.Löffler FE, Ritalahti KM, Zinder SH. 2013. Dehalococcoides and reductive dechlorination of the chlorinated solvents, p 39–88. In Stroo HF, Leeson A, Ward CW (ed), Bioaugmentation for groundwater remediation. Springer, New York, NY. [Google Scholar]
- 15.Hatt JK, Löffler FE. 2012. Quantitative real-time PCR (qPCR) detection chemistries affect enumeration of the Dehalococcoides16S rRNA gene in groundwater. J Microbiol Methods 88:263–270. doi: 10.1016/j.mimet.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Lee PK, Macbeth TW, Sorenson KS Jr, Deeb RA, Alvarez-Cohen L. 2008. Quantifying genes and transcripts to assess the in situ physiology of “Dehalococcoides” spp. in a trichloroethene-contaminated groundwater site. Appl Environ Microbiol 74:2728–2739. doi: 10.1128/AEM.02199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritalahti KM, Amos BK, Sung Y, Wu Q, Koenigsberg SS, Löffler FE. 2006. Quantitative PCR targeting 16S rRNA and reductive dehalogenase genes simultaneously monitors multiple Dehalococcoides strains. Appl Environ Microbiol 72:2765–2774. doi: 10.1128/AEM.72.4.2765-2774.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. 2000. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niessen L, Luo J, Denschlag C, Vogel RF. 2013. The application of loop-mediated isothermal amplification (LAMP) in food testing for bacterial pathogens and fungal contaminants. Food Microbiol 36:191–206. doi: 10.1016/j.fm.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad F, Tourlousse DM, Stedtfeld RD, Seyrig G, Herzog AB, Bhaduri P, Hashsham SA. 2009. Detection and occurrence of indicator organisms and pathogens. Water Environ Res 81:959–980. doi: 10.2175/106143009X12445568399299. [DOI] [PubMed] [Google Scholar]
- 21.Herzog AB, Pandey AK, Reyes-Gastelum D, Gerba CP, Rose JB, Hashsham SA. 2012. Evaluation of sample recovery efficiency for bacteriophage P22 on fomites. Appl Environ Microbiol 78:7915–7922. doi: 10.1128/AEM.01370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao Y-M, Guo Y-C, Zhang X-E, Zhou Y-F, Zhang Z-P, Wei H-P, Yang R-F, Wang D-B. 2007. Loop-mediated isothermal amplification for rapid detection of Bacillus anthracis spores. Biotechnol Lett 29:1939–1946. doi: 10.1007/s10529-007-9472-9. [DOI] [PubMed] [Google Scholar]
- 23.Curtis KA, Rudolph DL, Owen SM. 2008. Rapid detection of HIV-1 by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP). J Virol Methods 151:264–270. doi: 10.1016/j.jviromet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Kurosaki Y, Takada A, Ebihara H, Grolla A, Kamo N, Feldmann H, Kawaoka Y, Yasuda J. 2007. Rapid and simple detection of Ebola virus by reverse transcription-loop-mediated isothermal amplification. J Virol Methods 141:78–83. doi: 10.1016/j.jviromet.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Stedtfeld RD, Stedtfeld TM, Kronlein M, Seyrig G, Steffan RJ, Cupples AM, Hashsham SA. 2014. DNA extraction-free quantification of Dehalococcoides spp. in groundwater using a handheld device. Environ Sci Technol 48:13855–13863. doi: 10.1021/es503472h. [DOI] [PubMed] [Google Scholar]
- 26.Aiello MR. 2003. Quantitative environmental monitoring of PCE dechlorinators in a contaminated aquifer and PCE-fed reactor. M.S. thesis Michigan State University, East Lansing, MI. [Google Scholar]
- 27.Johnson DR, Lee PK, Holmes VF, Alvarez-Cohen L. 2005. An internal reference technique for accurately quantifying specific mRNAs by real-time PCR with application to the tceA reductive dehalogenase gene. Appl Environ Microbiol 71:3866–3871. doi: 10.1128/AEM.71.7.3866-3871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stedtfeld RD, Tourlousse DM, Seyrig G, Stedtfeld TM, Kronlein M, Price S, Ahmad F, Gulari E, Tiedje JM, Hashsham SA. 2012. Gene-Z: a device for point of care genetic testing using a smartphone. Lab Chip 12:1454–1462. doi: 10.1039/c2lc21226a. [DOI] [PubMed] [Google Scholar]
- 29.Stedtfeld RD, Liu Y-C, Stedtfeld TM, Kostic T, Kronlein M, Srivannavit O, Khalife WT, Tiedje JM, Gulari E, Hughes M, Etchebarne B, Hashsham SA. 2015. Static self-directed sample dispensing into a series of reaction wells on a microfluidic card for parallel genetic detection of microbial pathogens. Biomedical Microdevices 17:1–12. doi: 10.1007/s10544-014-9904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohtsuka K, Yanagawa K, Takatori K, Hara-Kudo Y. 2005. Detection of Salmonella enterica in naturally contaminated liquid eggs by loop-mediated isothermal amplification, and characterization of Salmonella isolates. Appl Environ Microbiol 71:6730–6735. doi: 10.1128/AEM.71.11.6730-6735.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G, Brown EW, González-Escalona N. 2011. Comparison of real-time PCR, reverse transcriptase real-time PCR, loop-mediated isothermal amplification, and the FDA conventional microbiological method for the detection of Salmonella spp. in produce. Appl Environ Microbiol 77:6495–6501. doi: 10.1128/AEM.00520-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karanis P, Thekisoe O, Kiouptsi K, Ongerth J, Igarashi I, Inoue N. 2007. Development and preliminary evaluation of a loop-mediated isothermal amplification procedure for sensitive detection of Cryptosporidium oocysts in fecal and water samples. Appl Environ Microbiol 73:5660–5662. doi: 10.1128/AEM.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdulmawjood A, Grabowski N, Fohler S, Kittler S, Nagengast H, Klein G. 2014. Development of loop-mediated isothermal amplification (LAMP) assay for rapid and sensitive identification of ostrich meat. PLoS One 9:e100717. doi: 10.1371/journal.pone.0100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duhamel M, Mo K, Edwards EA. 2004. Characterization of a highly enriched Dehalococcoides-containing culture that grows on vinyl chloride and trichloroethene. Appl Environ Microbiol 70:5538–5545. doi: 10.1128/AEM.70.9.5538-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krajmalnik-Brown R, Hölscher T, Thomson IN, Saunders FM, Ritalahti KM, Löffler FE. 2004. Genetic identification of a putative vinyl chloride reductase in Dehalococcoides sp. strain BAV1. Appl Environ Microbiol 70:6347–6351. doi: 10.1128/AEM.70.10.6347-6351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMurdie PJ, Behrens SF, Müller JA, Göke J, Ritalahti KM, Wagner R, Goltsman E, Lapidus A, Holmes S, Löffler FE, Spormann AM. 2009. Localized plasticity in the streamlined genomes of vinyl chloride respiring Dehalococcoides. PLoS Genet 5:e1000714. doi: 10.1371/journal.pgen.1000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller JA, Rosner BM, Von Abendroth G, Meshulam-Simon G, McCarty PL, Spormann AM. 2004. Molecular identification of the catabolic vinyl chloride reductase from Dehalococcoides sp. strain VS and its environmental distribution. Appl Environ Microbiol 70:4880–4888. doi: 10.1128/AEM.70.8.4880-4888.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magnuson JK, Romine MF, Burris DR, Kingsley MT. 2000. Trichloroethene reductive dehalogenase from Dehalococcoides ethenogenes: sequence of tceA and substrate range characterization. Appl Environ Microbiol 66:5141–5147. doi: 10.1128/AEM.66.12.5141-5147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hug LA, Beiko RG, Rowe AR, Richardson RE, Edwards EA. 2012. Comparative metagenomics of three Dehalococcoides-containing enrichment cultures: the role of the non-dechlorinating community. BMC Genomics 13:327. doi: 10.1186/1471-2164-13-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh K, Ferguson BS, Eisenstein M, Plaxco KW, Soh HT. 2015. Integrated electrochemical microsystems for genetic detection of pathogens at the point of care. Acc Chem Res 48:911–920. doi: 10.1021/ar500456w. [DOI] [PubMed] [Google Scholar]
- 41.Mori Y, Kitao M, Tomita N, Notomi T. 2004. Real-time turbidimetry of LAMP reaction for quantifying template DNA. J Biochem Biophys Methods 59:145–157. doi: 10.1016/j.jbbm.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Rane TD, Chen L, Zec HC, Wang T-H. 2015. Microfluidic continuous flow digital loop-mediated isothermal amplification (LAMP). Lab Chip 15:776–782. doi: 10.1039/C4LC01158A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu G, Gunson RN, Cooper JM, Reboud J. 2015. Rapid ultrasonic isothermal amplification of DNA with multiplexed melting analysis–applications in the clinical diagnosis of sexually transmitted diseases. Chem Commun (Camb) 51:2589–2592. doi: 10.1039/C4CC08389J. [DOI] [PubMed] [Google Scholar]