Abstract
Daptomycin is a potent cyclic lipopeptide antibiotic. It is widely used against various Gram-positive bacterial pathogens. Historically, a poor understanding of the transcriptional regulation of daptomycin biosynthesis has limited the options for targeted genetic engineering toward titer improvement. Here, we isolated a TetR family transcriptional regulator, DepR1, from the industrial producer Streptomyces roseosporus SW0702 using a biotinylated dptE promoter (dptEp) as a probe. The direct interaction between DepR1 and dptEp then was confirmed by electrophoretic mobility shift assays and DNase I footprinting assays. The deletion of depR1 led to a reduction in dptEp activity and the cessation of daptomycin production. The ΔdepR1 mutant produced less red pigment and failed to sporulate on R5 medium. This suggests that DepR1 plays a positive role in the control of morphological differentiation. Moreover, DepR1 was positively autoregulated by directly binding to its own promoter. This might account for the positive feedback regulation of daptomycin production. Based on these positive effects, genetic engineering by overexpression of depR1 raised daptomycin production and shortened the fermentation period both in flask and in fermentor.
INTRODUCTION
Daptomycin is a cyclic lipopeptide antibiotic produced by Streptomyces roseosporus via nonribosomal peptide synthases (1). It contains 13 amino acids and a straight-chain decanoic acid attached to the terminal amino group of Trp (2). In the presence of Ca2+, the lipid tail can insert into the bilayer membrane of pathogens. This results in potassium efflux, membrane depolarization, and eventual cell death (3). Daptomycin has been approved for the clinical treatment of diseases caused by drug-resistant Gram-positive bacterial pathogens. Such pathogens include vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, penicillin-resistant Streptococcus pneumoniae, and others (4–7).
Due to its importance in clinical medicine, several approaches have been employed for the production of daptomycin derivatives. These include semisynthetic modification (8, 9), chemoenzymatic synthesis (10, 11), and combinatorial biosynthesis (12–14), with the anticipation of altered pharmaceutical spectra and/or increased antimicrobial activities. In addition, many efforts have been made to increase daptomycin production. These have included random mutagenesis (15), in silico-based metabolic engineering (16, 17), and attempts to increase precursor supply (18). However, up to the present, the poor understanding of the transcriptional regulation of daptomycin biosynthesis has limited options for targeted genetic engineering toward titer improvement.
The gene cluster for daptomycin biosynthesis originally was identified in Streptomyces roseosporus NRRL 11379. Three putative cluster-situated transcriptional regulators, DptR1, DptR2, and DptR3 (1), also have been identified. We previously reported that the industrial strain S. roseosporus SW0702 also contained a similar daptomycin gene cluster with high identity to that in S. roseosporus NRRL 11379. In this industrial producer, we found that DptR1 was not involved in the regulation of daptomycin production (data not shown), and DptR2 was seen to be required for daptomycin production but did not seem to significantly affect the gene expression of the cluster (19). Similarly, DptR3 was shown to positively influence daptomycin production and dpt gene expression but also was reported to repress the expression of the adjacent gene, orf16, which encodes a putative ABC transporter ATP-binding protein (20).
It has been proposed that the whole daptomycin gene cluster is transcribed via a giant RNA molecule that is driven by the dptE promoter (dptEp) (12). We have previously used biotinylated dptEp as a probe to isolate transcriptional regulators that can interact with dptEp; thus, they may regulate daptomycin production. Using this strategy, we identified AtrA, a TetR family regulator, which acts as one of the regulators of a conserved A-factor-mediated transcriptional cascade. This pathway positively regulates daptomycin gene cluster expression, and a more than 2-fold increase of daptomycin production was achieved by targeted genetic engineering to delete arpA, encoding the conserved A-factor receptor (21).
Here, we show that DepR1, another TetR family regulator identified by dptEp affinity purification, binds directly to dptEp and positively regulates gene cluster expression and daptomycin production. We also find that DepR1 is positively autoregulated and involved in the morphological differentiation of S. roseosporus SW0702. Moreover, genetic engineering by the overexpression of depR1 shortened the fermentation period and raised daptomycin production in the fermentor.
MATERIALS AND METHODS
Bacterial strains and media.
Streptomyces roseosporus SW0702 was the industrial strain used for daptomycin production (19, 22) (China Center for Type Culture Collection [CCTCC] no. M2010136). Escherichia coli TG1 (Novagen) was used as a general cloning host. E. coli BL21(DE3) (Novagen) was used for protein expression. E. coli ET12567/pUZ8002 was used to introduce plasmids into Streptomyces (23).
The E. coli strains were cultured in LB medium. S. roseosporus strains were cultured on R5 medium (24) for morphological development and sporulation. MS medium (24) was used for conjugation. For shake-flask fermentation, 2% TSB (tryptic soybean broth) plus 5% polyethylene glycol 6000 (PEG6000) was used as a seed medium and YEME (24) was used as fermentation medium (19). For industrial fermentation, 3% TSB medium plus 3.5% potato starch was used as the primary seed medium. The secondary seed medium contained 6% potato starch, 2.9% soybean powder, 1.5% glucose, 0.08% (NH4)2Fe(SO4)2 · 6H2O, 0.72% molasses, and 0.1% defoamer GPE. The fermentation medium contained 7.2% potato starch, 1.2% yeast powder, 1.0% glucose, 0.08% (NH4)2Fe(SO4)2 · 6H2O, 0.72% molasses, and 0.1% defoamer GPE. Decanoic acid and methyl oleate (1:1, vol/vol) was used as the feeding medium (19).
Plasmid construction.
The plasmids and primers used in this work are listed in Table 1 and Table 2, respectively. The primer pair 1 and 2 was used to amplify depR1. The depR1 fragment was cloned into pET32a to get the expression plasmid pET32a-depR1. Primer pairs 3 and 4 as well as 5 and 6 were used to amplify the dptE and depR1 promoters, respectively. These then were cloned into pUC18 to get the plasmids pUC18-dptEp and pUC18-depR1p, respectively. The upstream and downstream regions of depR1 were amplified with primer pairs 7 and 8 as well as 9 and 10 and cloned into HindIII/XbaI and XbaI/EcoRI sites of pKC1139 (25), respectively, to get the plasmid pKC1139-depR1. The depR1 fragment, amplified with primers 11 and 12, was cloned into pIJ8661 (26) to get the overexpression plasmid pIJ8661-depR1. depR1 with the depR1 promoter fragment amplified using primers 13 and 14 was cloned into pSET152 (25) to get the complementation plasmid pSET152-depR1. dptEp and depR1p then were amplified with primer pairs 15 and 16 as well as 17 and 18 and cloned into pIJ8660 (27) to get plasmids pIJ8660-dptEp and pIJ8660-depR1p, respectively. All fragments were amplified with KOD plus-neo DNA polymerase (Toyobo) and then ligated into pTA2 (Toyobo) after dA (deoxyribosyladenine) addition with Taq polymerase (TaKaRa) for DNA sequencing. All fragments were ligated into vectors using T4 DNA ligase (Thermo Scientific). The plasmids pET32a, pUC18, and pTA2 and their derivatives were maintained with 100 μg/ml of ampicillin in E. coli cultures, and plasmids pKC1139, pSET152, and pIJ8660 and their derivatives were maintained with 50 μg/ml of apramycin in E. coli cultures. Moreover, 50 μg/ml of apramycin was used as the selection pressure for integration of plasmids pKC1139, pSET152, and pIJ8660 and their derivatives into the Streptomyces genome.
TABLE 1.
Plasmids used in this study
| Plasmid | Description | Reference or source |
|---|---|---|
| pTA2 | T vector | Toyobo |
| pUC18 | Cloning vector | Novagen |
| pUC18-dptEp | pUC18 containing dptE promoter | This study |
| pUC18-depR1p | pUC18 containing depR1 promoter | This study |
| pKC1139 | Temperature-sensitive shuttle vector for gene knockout in Streptomyces | 25 |
| pKC1139-depR1 | depR1 deletion plasmid | This study |
| pET-32a | E. coli protein expression vector | Novagen |
| pET-32a-depR1 | pET-32a containing depR1 open reading frame | This study |
| pSET152 | Integrated shuttle vector in Streptomyces | 25 |
| pSET152-depR1 | depR1 complementation plasmid | This study |
| pIJ8661 | Overexpression shuttle vector, integrative in Streptomyces | 26 |
| pIJ8661-depR1 | depR1 overexpression plasmid | This study |
| pIJ8660 | Promoter-probing plasmid containing egfp gene in Streptomyces | 27 |
| pIJ8660-dptEp | pIJ8660 containing dptE promoter | This study |
| pIJ8660-depR1p | pIJ8660 containing depR1 promoter | This study |
TABLE 2.
Primers used in this study

Construction of S. roseosporus strains.
The plasmid pKC1139-depR1 was introduced into S. roseosporus SW0702 by conjugation (24), as described previously. The ΔdepR1 mutant then was obtained using the in-frame deletion strategy (19). The plasmid pSET152-depR1, an integrative plasmid, then was introduced into the ΔdepR1 mutant to obtain the complementation strain. Plasmid pIJ8661-depR1, another integrative plasmid, was introduced into the wild type to obtain the overexpression strain. The integrative plasmids pIJ8660-dptEp and pIJ8660-depR1p were introduced into both the wild type and the ΔdepR1 mutant to investigate the activities of dptEp and depR1p, respectively.
Protein expression and purification from E. coli.
BL21(DE3), containing the expression plasmid pET32a-depR1, was grown at 37°C to an optical density at 600 nm (OD600) of 0.6 and then induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 16°C for 12 h. Cells were harvested and then disrupted by sonication. The His-tagged DepR1-TrxA fusion protein was purified on Ni2+-nitrilotriacetic acid (NTA) resin (Qiagen) as described by the manufacturer. The purified protein was confirmed by SDS-PAGE, and the concentration was determined using the Bradford method (28).
EMSA.
Electrophoretic mobility shift assays (EMSAs) were performed as described previously (29). The 5′-biotin-labeled probes were obtained by PCR from pUC18, pUC18-dptEp, and pUC18-depR1p using primers 19 and 20. Ten microliters of the binding reaction system contained 1 ng of biotin-labeled probes and the amounts of proteins indicated.
DNase I footprinting assays.
The DNase I footprinting assays were performed as previously described (29). Briefly, 5′-6-carboxyfluorescein (FAM)-labeled dptEp and depR1p were prepared by PCR from pUC18-dptEp and pUC18-depR1p with primers 20 and 21. 5′-FAM-labeled DNA probes were incubated with different quantities of DepR1 protein at 30°C for 30 min. A total of 0.01 U of DNase I was added for partial nuclease digestion.
GFP reporter assays.
Green fluorescent protein (GFP) reporter assays were carried out as previously described (30). Streptomyces strains were cultured in YEME medium (24). Mycelial cells were harvested every 24 h and resuspended in 1 ml of lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 5% glycerol, 1% Triton X-100). Cells then were disrupted by sonication, and the debris was removed by centrifugation at 4°C. Total protein concentration was determined by the Bradford method (28). Twenty micrograms of total protein was separated by SDS-PAGE for Western blotting (31) using a polyclonal anti-enhanced GFP (eGFP) antibody (Proteintech Group), while another 20 μg of protein was retained for Coomassie blue R250 staining as a control.
Scanning electron microscopy.
The morphology of S. roseosporus strains was observed by scanning electron microscopy (Hitachi S-3000N; Japan) as described previously (32). Briefly, equal numbers of mycelial cells were collected from TSB medium and spread on R5 medium. After 5 days, 1-cm2 agar blocks were glutaraldehyde fixed and sputter-coated with gold before examination.
Fermentation in fermentor.
Spores of S. roseosporus were prepared from the R5 medium. They then were cultured into 100 ml of the primary seed medium in flasks. The flasks were shaken on a rotary shaker at 250 rpm at 30°C for about 24 h. All 100 ml of the culture then was inoculated into 10 liters of the secondary seed medium in a 20-liter fermentor. This was cultured for a further 24 h with 300 rpm agitation and 10 liters/min air. After this, 1.2 liters of the secondary seed culture was inoculated into 30 liters of fermentation medium in a 50-liter fermentor. Cells were cultured for a further 7 to 8 days with 300 rpm agitation and 30 liters/min air. In this process, feeding medium was added at a rate of 100 ml/day when cell concentration reached 30%. All fermentors were manufactured by Zhenjiang Dongfang Biological Equipment Technologies Co., Ltd.
Daptomycin production and cell concentrations were measured every 24 h. The industrial fermentation medium included a number of insoluble components (yeast powder and potato starch). The cell concentration was measured as follows. One hundred milliliters of the fermentation medium was harvested at 24-h intervals and then was centrifuged at 4,000 rpm for 10 min. The volume of supernatant was measured with a graduated cylinder. The proportion of the deposit (containing the cell pellet and insoluble components) to the total volume was measured as the cell concentration.
HPLC analysis of daptomycin.
The shake-flask fermentation of S. roseosporus SW0702 and high-performance liquid chromatography (HPLC) analysis of daptomycin were performed as previously described (19). Daptomycin was analyzed by HPLC (1260 Infinity; Agilent Technologies) with a reverse-phase column (Zorbax 300SB-C18; 250 by 4.6 mm; Agilent Technologies) with solution A (0.05 M Na2HPO4, pH 3.15 ± 0.05) and solution B (100% acetonitrile) at a ratio of solution A to solution B of 67:33 with UV detection set at 214 nm. The flow rate was 1.0 ml/min. Forty microliters of cleared culture was injected directly for HPLC analysis.
Nucleotide sequence accession number.
Newly determined sequence data were deposited in GenBank under accession number KT626004.
RESULTS AND DISCUSSION
Direct and positive regulation of dptEp activities by DepR1.
To investigate the transcriptional regulatory mechanisms for daptomycin production, we isolated several transcriptional regulators, including AtrA, which interacted with dptEp (21). Among these proteins, another TetR family regulator, DepR1 (for dptEp-interactive regulator) (GenBank accession number KT626004), also was identified. TetR family regulators are composed of an N-terminal DNA binding domain and a C-terminal signal accepting domain. In Streptomyces, they represent the largest family involved in the regulation of responses to different environmental conditions and play key roles in antibiotic resistance. They also are involved in morphogenesis, biofilm formation, and nitrogen uptake (33, 34).
In the industrial daptomycin producer Streptomyces roseosporus SW0702, depR1 is colocalized with genes encoding an ABC transporter, an amidinotransferase, and an rRNA methyltransferase (Fig. 1A). Protein alignment shows that DepR1 is highly similar to a protein in S. griseus (WP_030854181.1) with a sequence identity of 86% (data not shown).
FIG 1.

DepR1 directly binds to the dptE promoter (dptEp). (A) Schematic diagram of depR1 and its neighboring genes in S. roseosporus. (B) depR1 was cloned into pET-32a. DepR1 was expressed and purified from E. coli BL21(DE3). (C) EMSA for DepR1 binding to dptEp. Biotin-labeled dptEp was used as a probe with a biotin-labeled vector as a negative control.
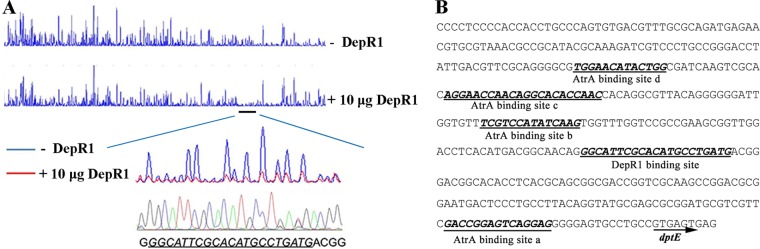
DepR1 was expressed and purified from E. coli BL21(DE3) (Fig. 1B). Electrophoretic mobility shift assays (EMSAs) showed that DepR1 could specifically bind to dptEp and that this shift became even more significant as the amount of DepR1 was increased (Fig. 1C), which also was seen in other studies of, e.g., AtrA-dptEp interaction (21). Moreover, one binding site of DepR1 on dptEp was identified by DNase I footprinting assays (Fig. 2A). After alignment with the DNA sequencing data, the binding sequence was determined. The binding site is 211 bp away from the start codon (GTG) of dptE (Fig. 2B). However, the binding sequence had no consensus with other TetR family regulators or apparent palindrome sequence, which often are recognized by TetR family regulators (35). However, this observation is not unique, since we had identified another TetR family regulator, AtrA, which binds to nonpalindrome sequence either on dptEp or its own promoter (21). These results confirmed DepR1 was a transcriptional regulator interacting directly with the promoter of the daptomycin gene cluster.
FIG 2.
Binding sequence of DepR1 on dptEp. (A) DNase I footprinting assays for determination of DepR1 binding site on dptEp. FAM-labeled dptEp was used as a probe. The protected region is underlined. (B) Binding sequence determination on dptEp. The binding sequences are shown with boldface, italics, and underlining. AtrA binding sites are also shown.
GFP reporter assays were used to detect the regulatory effects of DepR1 on dptEp. For this, depR1 was inactivated using an in-frame deletion strategy (Fig. 3). A reporter system was constructed by placing dptEp upstream of eGFP in a promoter-probing plasmid, pIJ8660, in Streptomyces (27) and introduced into both the wild type and the ΔdepR1 mutant. Western blot results showed that the eGFP expression levels had increased gradually in the wild type over the first 4 days during daptomycin production (Fig. 4 and 5B). The ΔdepR1 mutant showed reduction of the eGFP level for all days examined (Fig. 4). These results suggest that DepR1 positively regulates dptEp activity.
FIG 3.
Deletion of depR1. (A) pKC1139-depR1, containing the upstream and downstream regions of depR1, was used to delete depR1 using the in-frame deletion strategy. (B and C) PCR analysis of depR1 deletion. Primer 1 (p1), primer 2 (p2), primer 7 (p7), and primer 10 (p10) (as indicated) were used to verify depR1 deletion.
FIG 4.
GFP reporter assays for dptEp activities. Streptomyces mycelial cells were harvested every 24 h. Twenty micrograms of total protein was separated by SDS-PAGE for Western blotting and Coomassie blue R250 staining.
FIG 5.
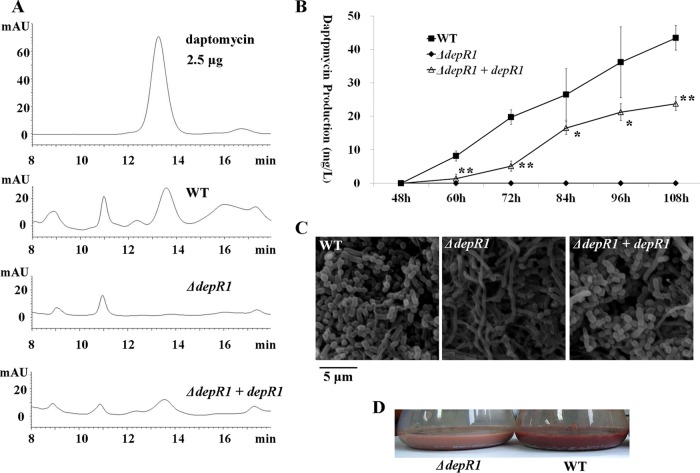
DepR1 is required for daptomycin production and morphological development. (A) HPLC analysis of daptomycin production. The wild type, the ΔdepR1 mutant, and the complementation strain all were cultured in YEME medium. Daptomycin production was measured on day 3. Purified daptomycin was used as a standard. (B) Daptomycin production of the ΔdepR1 mutant, complementation strain, and wild type. Streptomyces cells were cultured in YEME medium. Daptomycin production was measured every 12 h. The experiments were performed in triplicate. The standard deviations from three repeated experiments are shown. The t test results are shown as well: *, P < 0.05; **, P < 0.01. (C) Morphological development of the wild type, the ΔdepR1 mutant, and the complementation strain on R5 medium. Mycelial cells were collected from TSB medium and spread on R5 medium, and the spores of strains were observed using scanning electron microscopy after 5 days. (D) Fermentation of the ΔdepR1 mutant and wild type in YEME medium. Streptomyces cells were cultured in YEME medium for 4 days.
DepR1 is required for daptomycin production and morphological development.
Shake-flask fermentation was carried out to investigate the effects of DepR1 on daptomycin production. In the HPLC results, the wild-type strain produced 19.77 mg/liter of daptomycin on day 3, while no daptomycin was detected in the ΔdepR1 mutant. The complementation strain was able to restore daptomycin production, but not fully (Fig. 5A). Failure of full rescue of daptomycin production was seen in other studies (36, 37). It has been speculated to result from the genomic environments, since depR1 has been driven from depR1p at the pSET152 integrative site, the attB site, not its native locus. We also observed a gradual increase in daptomycin production in the wild type during the first 5 days or so, finally reaching 43.43 mg/liter. In contrast, no daptomycin production was observed in the ΔdepR1 mutant in any time period (Fig. 5B). Our results indicate that DepR1 is essential for daptomycin production, although dptEp still is functional in the absence of DepR1. Meanwhile, the S. roseosporus wild type could sporulate on R5 medium after 5 days. In contrast, the ΔdepR1 mutant showed deficiency in sporulation. This defect also could be rescued in the complementation strain (Fig. 5C). In addition, the ΔdepR1 mutant produced less pigment than did the wild type on YEME medium (Fig. 5D), suggesting DepR1 is required for both morphological differentiation and red pigment production.
Autoregulation of depR1 expression.
The TetR family transcriptional regulators often bind to their own promoters (35). Therefore, we checked whether DepR1 also regulated its own gene expression. EMSA results showed that DepR1 bound directly to its own promoter (depR1p) even at a low concentration (100 ng or 0.2 μM) (Fig. 6A). Furthermore, DNase I footprinting assays showed one protection site on depR1p by DepR1 (Fig. 6B). The binding sequence was determined after alignment with the DNA sequencing data and contained an imperfect palindrome sequence (Fig. 6C). We found that DepR1 binding motifs in depR1p and dptEp do not share common sequence motifs. The DepR1 binding site on depR1p is located in the intergenic region of depR1 and SRO2871 (encoding a putative NLP/P60-family protein) and is much closer to the start codon of depR1. This suggests that DepR1 regulates its own gene expression.
FIG 6.

DepR1 directly binds to the depR1 promoter (depR1p). (A) EMSA for DepR1 binding to depR1p. Biotin-labeled depR1p was used as a probe, and the negative control was a biotin-labeled vector. (B) DNase I footprinting assays for the determination of DepR1 binding site on depR1p. FAM-labeled depR1p was used as a probe. The protected region is underlined. (C) Binding sequence determination on depR1p. The binding sequence is shown in boldface and italics with underlining.
GFP reporter assays with a depR1p-egfp fusion showed that depR1p activities slowly increased during fermentation (Fig. 7). This was correlated with daptomycin production. Deletion of depR1 reduced the levels of eGFP expression driven from depR1p in all time frames examined (Fig. 7). This also suggests that DepR1 positively regulates its own gene expression.
FIG 7.

GFP reporter assays for depR1p activities. Streptomyces mycelial cells were harvested every 24 h. Twenty micrograms of total protein was separated by SDS-PAGE for Western blotting and Coomassie blue R250 staining.
Overexpressing depR1 improves daptomycin production.
Our results have demonstrated that DepR1 is a positive regulator for daptomycin production and morphological development. A depR1 overexpression strain then was constructed by introducing an extra copy of depR1 under the strong and constitutive promoter ermEp* in the wild type (26).
Shake-flask fermentations were carried out in three independent experiments. The HPLC results showed that the overexpression strain produced more daptomycin than the wild type across the time period from 60 h to 108 h. The final daptomycin production was 29% higher than that of the wild type by the late fermentation phase at 108 h (Fig. 8A). The overexpression strain and wild type then each were cultured in 50-liter fermentors. The cell concentration of the overexpression strain and the wild type was measured every 24 h during the fermentation process. Daptomycin production also was measured by HPLC over the same period. No apparent difference in cell concentrations was noted between the overexpression strain and the wild type (Fig. 8B). However, the daptomycin production of the depR1 overexpression strain gradually increased and reached its highest level (474 mg/liter) at 140 h, a level 41% higher than that of the wild type during the same time period (Fig. 8C). Moreover, the depR1 overexpression strain showed accelerated morphological differentiation and produced more red pigments on both R5 and YEME medium (data not shown).
FIG 8.

Overexpressing depR1 improves daptomycin production. (A) Daptomycin production profile in shake-flask fermentation. The overexpression strain and wild type were cultured in YEME medium. Daptomycin production was measured every 12 h. The experiments were performed in triplicate. The standard deviations from three repeated experiments are shown. t test results are shown: *, P < 0.05; **, P < 0.01. After the depR1 overexpression strain and wild type were cultured in the fermentor with the fermentation medium, cell concentration (B) and daptomycin production (C) were measured every 24 h.
Three putative transcriptional regulators, DptR1, DptR2, and DptR3, located within the daptomycin gene cluster, were identified previously. However, DptR1 is not involved in the regulation of daptomycin production (data not shown). Although both DptR2 and DptR3 are required for daptomycin production, they do not directly regulate the expression of the gene cluster (19, 20). This suggests that the daptomycin gene cluster does not contain the typical pathway-specific regulators within most other such clusters in secondary metabolites. To further understand the transcriptional regulatory mechanisms of daptomycin production, we used streptavidin-biotin interaction-based affinity purification to isolate the regulators binding to the promoter of the daptomycin gene cluster, dptEp (21). Using this approach, several DNA-binding proteins, including two TetR family transcriptional regulators, AtrA (21) and DepR1 (this study), were identified.
Here, we have demonstrated that DepR1 was bound to dptEp at a different site from that of AtrA (21) (Fig. 2B). This suggests that they mediate distinctive pathways in the regulation of daptomycin production. Although AtrA is a positive pleiotropic regulator for daptomycin and for morphological development, the overexpression of atrA alone has no effect on daptomycin production. The titer is improved only when the upstream A-factor receptor ArpA is removed (21). Here, DepR1 positively and directly regulates both dptEp activity and daptomycin production. This enables us to construct a strain with both enhanced daptomycin production and a shortened fermentation period by targeted genetic engineering via the overexpression of depR1. In addition, depR1 is positively autoregulated, as is also the case for atrA (21), and this might account for the persistent increase of depR1 expression during daptomycin production. This positive feedback regulation of depR1 expression also might be responsible, at least partially, for the sustainably increased daptomycin production.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (31370103, 81400511, and 31571284), the National Program on Key Basic Research Project (973 Program) (2012CB721005), and the National High Technology Research and Development Program of China (863 Program) (2012AA02A706).
We gratefully thank Chris Wood in the College of Life Sciences, Zhejiang University, for improving the manuscript.
REFERENCES
- 1.Miao V, Coeffet-Legal MF, Brian P, Brost R, Penn J, Whiting A, Martin S, Ford R, Parr I, Bouchard M, Silva CJ, Wrigley SK, Baltz RH. 2005. Daptomycin biosynthesis in Streptomyces roseosporus: cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology 151:1507–1523. doi: 10.1099/mic.0.27757-0. [DOI] [PubMed] [Google Scholar]
- 2.Debono M, Barnhart M, Carrell CB, Hoffmann JA, Occolowitz JL, Abbott BJ, Fukuda DS, Hamill RL, Biemann K, Herlihy WC. 1987. A21978C, a complex of new acidic peptide antibiotics: isolation, chemistry, and mass spectral structure elucidation. J Antibiot (Tokyo) 40:761–777. doi: 10.7164/antibiotics.40.761. [DOI] [PubMed] [Google Scholar]
- 3.Robbel L, Marahiel MA. 2010. Daptomycin, a bacterial lipopeptide synthesized by a nonribosomal machinery. J Biol Chem 285:27501–27508. doi: 10.1074/jbc.R110.128181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sader HS, Flamm RK, Farrell DJ, Jones RN. 2013. Daptomycin activity against uncommonly isolated streptococcal and other gram-positive species groups. Antimicrob Agents Chemother 57:6378–6380. doi: 10.1128/AAC.01906-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbeit RD, Maki D, Tally FP, Campanaro E, Eisenstein BI. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis 38:1673–1681. doi: 10.1086/420818. [DOI] [PubMed] [Google Scholar]
- 6.Tally FP, Zeckel M, Wasilewski MM, Carini C, Berman CL, Drusano GL, Oleson FB Jr. 1999. Daptomycin: a novel agent for Gram-positive infections. Expert Opin Investig Drugs 8:1223–1238. doi: 10.1517/13543784.8.8.1223. [DOI] [PubMed] [Google Scholar]
- 7.Tally FP, DeBruin MF. 2000. Development of daptomycin for gram-positive infections. J Antimicrob Chemother 46:523–526. doi: 10.1093/jac/46.4.523. [DOI] [PubMed] [Google Scholar]
- 8.Debono M, Abbott BJ, Molloy RM, Fukuda DS, Hunt AH, Daupert VM, Counter FT, Ott JL, Carrell CB, Howard LC, Boeck LD, Hamill RL. 1988. Enzymatic and chemical modifications of lipopeptide antibiotic A21978C: the synthesis and evaluation of daptomycin (LY146032). J Antibiot (Tokyo) 41:1093–1105. doi: 10.7164/antibiotics.41.1093. [DOI] [PubMed] [Google Scholar]
- 9.Hill J, Siedlecki J, Parr I, Morytko M, Yu X, Zhang Y, Silverman J, Controneo N, Laganas V, Li T, Lai JJ, Keith D, Shimer G, Finn J. 2003. Synthesis and biological activity of N-acylated ornithine analogues of daptomycin. Bioorg Med Chem Lett 13:4187–4191. doi: 10.1016/j.bmcl.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Grunewald J, Sieber SA, Mahlert C, Linne U, Marahiel MA. 2004. Synthesis and derivatization of daptomycin: a chemoenzymatic route to acidic lipopeptide antibiotics. J Am Chem Soc 126:17025–17031. doi: 10.1021/ja045455t. [DOI] [PubMed] [Google Scholar]
- 11.Kopp F, Grunewald J, Mahlert C, Marahiel MA. 2006. Chemoenzymatic design of acidic lipopeptide hybrids: new insights into the structure-activity relationship of daptomycin and A54145. Biochemistry 45:10474–10481. doi: 10.1021/bi0609422. [DOI] [PubMed] [Google Scholar]
- 12.Coeffet-Le Gal MF, Thurston L, Rich P, Miao V, Baltz RH. 2006. Complementation of daptomycin dptA and dptD deletion mutations in trans and production of hybrid lipopeptide antibiotics. Microbiology 152:2993–3001. doi: 10.1099/mic.0.29022-0. [DOI] [PubMed] [Google Scholar]
- 13.Mahlert C, Kopp F, Thirlway J, Micklefield J, Marahiel MA. 2007. Stereospecific enzymatic transformation of alpha-ketoglutarate to (2S,3R)-3-methyl glutamate during acidic lipopeptide biosynthesis. J Am Chem Soc 129:12011–12018. doi: 10.1021/ja074427i. [DOI] [PubMed] [Google Scholar]
- 14.Baltz RH. 2008. Biosynthesis and genetic engineering of lipopeptide antibiotics related to daptomycin. Curr Top Med Chem 8:618–638. doi: 10.2174/156802608784221497. [DOI] [PubMed] [Google Scholar]
- 15.Yu G, Jia X, Wen J, Lu W, Wang G, Caiyin Q, Chen Y. 2011. Strain improvement of Streptomyces roseosporus for daptomycin production by rational screening of He-Ne laser and NTG induced mutants and kinetic modeling. Appl Biochem Biotechnol 163:729–743. doi: 10.1007/s12010-010-9078-x. [DOI] [PubMed] [Google Scholar]
- 16.Huang D, Wen J, Wang G, Yu G, Jia X, Chen Y. 2012. In silico aided metabolic engineering of Streptomyces roseosporus for daptomycin yield improvement. Appl Microbiol Biotechnol 94:637–649. doi: 10.1007/s00253-011-3773-6. [DOI] [PubMed] [Google Scholar]
- 17.Huang D, Jia X, Wen J, Wang G, Yu G, Caiyin Q, Chen Y. 2011. Metabolic flux analysis and principal nodes identification for daptomycin production improvement by Streptomyces roseosporus. Appl Biochem Biotechnol 165:1725–1739. doi: 10.1007/s12010-011-9390-0. [DOI] [PubMed] [Google Scholar]
- 18.Liao G, Wang L, Liu Q, Guan F, Huang Y, Hu C. 2013. Manipulation of kynurenine pathway for enhanced daptomycin production in Streptomyces roseosporus. Biotechnol Prog 29:847–852. doi: 10.1002/btpr.1740. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Ren NN, Luo S, Chen XX, Mao XM, Li YQ. 2014. DptR2, a DeoR-type auto-regulator, is required for daptomycin production in Streptomyces roseosporus. Gene 544:208–215. doi: 10.1016/j.gene.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 20.Zhang QL, Chen Q, Zhuang S, Chen Z, Wen Y, Li JL. 2015. A MarR family transcriptional regulator, DptR3, activates daptomycin biosynthesis and morphological differentiation in Streptomyces roseosporus. Appl Environ Microbiol 81:3753–3765. doi: 10.1128/AEM.00057-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao XM, Luo S, Zhou RC, Wang F, Yu P, Sun N, Chen XX, Tang Y, Li YQ. 2015. Transcriptional regulation of the daptomycin gene cluster in Streptomyces roseosporus by an autoregulator, AtrA. J Biol Chem 290:7992–8001. doi: 10.1074/jbc.M114.608273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J, Chen XX, Xu YF, Wang B, Li Y, Gao XR. 2010. A streptomyces strain and its application in daptomycin production. China patent ZL 2010 1 0225300.2.
- 23.Paget MSB, Chamberlin L, Atrih A, Foster SJ, Buttner MJ. 1999. Evidence that the extracytoplasmic function sigma factor sigma is required for normal cell wall structure in Streptomyces coelicolor A3(2). J Bacteriol 181:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieser TBMJ, Butter MJ, Chater KF, Hopwood DA. 2000. Practical Streptomyces genetics. Int Microbiol 3:260–261. [Google Scholar]
- 25.Bierman M, Logan R, O'Brien K, Seno ET, Rao RN, Schoner BE. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 26.Liu SP, Yuan PH, Wang YY, Liu XF, Zhou ZX, Bu QT, Yu P, Jiang H, Li YQ. 2015. Generation of the natamycin analogs by gene engineering of natamycin biosynthetic genes in Streptomyces chattanoogensis L10. Microbiol Res 173:25–33. doi: 10.1016/j.micres.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Sun JH, Kelemen GH, Fernandez-Abalos JM, Bibb MJ. 1999. Green fluorescent protein as a reporter for spatial and temporal gene expression in Streptomyces coelicolor A3(2). Microbiology 145:2221–2227. doi: 10.1099/00221287-145-9-2221. [DOI] [PubMed] [Google Scholar]
- 28.Kruger NJ. 1994. The Bradford method for protein quantitation. Methods Mol Biol 32:9–15. [DOI] [PubMed] [Google Scholar]
- 29.Mao XM, Sun ZH, Liang BR, Wang ZB, Feng WH, Huang FL, Li YQ. 2013. Positive feedback regulation of stgR expression for secondary metabolism in Streptomyces coelicolor. J Bacteriol 195:2072–2078. doi: 10.1128/JB.00040-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao XM, Zhou Z, Hou XP, Guan WJ, Li YQ. 2009. Reciprocal regulation between SigK and differentiation programs in Streptomyces coelicolor. J Bacteriol 191:6473–6481. doi: 10.1128/JB.00875-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee EJ, Karoonuthaisiri N, Kim HS, Park JH, Cha CJ, Kao CM, Roe JH. 2005. A master regulator sigmaB governs osmotic and oxidative response as well as differentiation via a network of sigma factors in Streptomyces coelicolor. Mol Microbiol 57:1252–1264. doi: 10.1111/j.1365-2958.2005.04761.x. [DOI] [PubMed] [Google Scholar]
- 32.Du YL, Li SZ, Zhou Z, Chen SF, Fan WM, Li YQ. 2011. The pleitropic regulator AdpA(ch) is required for natamycin biosynthesis and morphological differentiation in Streptomyces chattanoogensis. Microbiology 157:1300–1311. doi: 10.1099/mic.0.046607-0. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Rodriguez A, Robledo-Casados I, Sanchez S. 2015. An overview on transcriptional regulators in Streptomyces. Biochim Biophys Acta 1849:1017–1039. doi: 10.1016/j.bbagrm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Cuthbertson L, Nodwell JR. 2013. The TetR family of regulators. Microbiol Mol Biol Rev 77:440–475. doi: 10.1128/MMBR.00018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos JL, Martinez-Bueno M, Molina-Henares AJ, Teran W, Watanabe K, Zhang X, Gallegos MT, Brennan R, Tobes R. 2005. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev 69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sprusansky O, Stirrett K, Skinner D, Denoya C, Westpheling J. 2005. The bkdR gene of Streptomyces coelicolor is required for morphogenesis and antibiotic production and encodes a transcriptional regulator of a branched-chain amino acid dehydrogenase complex. J Bacteriol 187:664–671. doi: 10.1128/JB.187.2.664-671.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu G, Tian Y, Yang H, Tan H. 2005. A pathway-specific transcriptional regulatory gene for nikkomycin biosynthesis in Streptomyces ansochromogenes that also influences colony development. Mol Microbiol 55:1855–1866. doi: 10.1111/j.1365-2958.2005.04512.x. [DOI] [PubMed] [Google Scholar]