Abstract
Plant-associated bacteria are of great interest because of their potential use in phytoremediation. However, their ability to survive and promote plant growth in metal-polluted soils remains unclear. In this study, a soilborne Cd-resistant bacterium was isolated and identified as Enterobacter sp. strain EG16. It tolerates high external Cd concentrations (Cd2+ MIC, >250 mg liter−1) and is able to produce siderophores and the plant hormone indole-3-acetic acid (IAA), both of which contribute to plant growth promotion. Surface biosorption in this strain accounted for 31% of the total Cd accumulated. The potential presence of cadmium sulfide, shown by energy-dispersive X-ray (EDX) analysis, suggested intracellular Cd binding as a Cd response mechanism of the isolate. Cd exposure resulted in global regulation at the transcriptomic level, with the bacterium switching to an energy-conserving mode by inhibiting energy-consuming processes while increasing the production of stress-related proteins. The stress response system included increased import of sulfur and iron, which become deficient under Cd stress, and the redirection of sulfur metabolism to the maintenance of intracellular glutathione levels in response to Cd toxicity. Increased production of siderophores, responding to Cd-induced Fe deficiency, not only is involved in the Cd stress response systems of EG16 but may also play an important role in promoting plant growth as well as alleviating the Cd-induced inhibition of IAA production. The newly isolated strain EG16 may be a suitable candidate for microbially assisted phytoremediation due to its high resistance to Cd and its Cd-induced siderophore production, which is likely to contribute to plant growth promotion.
INTRODUCTION
Heavy metal contamination has become one of the most serious environmental problems in recent years. Metal extraction activities are major sources for heavy metals in the environment (1). Cadmium is highly toxic to plants, animals, microorganisms, and humans even at quite low concentrations (2, 3). Although Cd is non-redox active, it can cause oxidative stress by generating reactive oxygen species (ROS) (4), which can lead to DNA damage (5), inhibit the DNA mismatch repair system (6), and disrupt the synthesis of nucleic acids and proteins (7).
Phytoremediation, a low-cost and eco-friendly technology for the decontamination of heavy-metal-polluted soils, uses plants to absorb, accumulate, and detoxify heavy metals in soil (3). However, the serious environmental stresses present in most cases of phytoremediation result in slow plant growth, low biomass production, and long time frames for remediation, limiting the usefulness of this technology (8). Beneficial plant-associated bacteria have been shown to protect plants from metal toxicity and promote plant growth. The plant growth-promoting (PGP) characteristics that most of these bacteria possess, including N2 fixation, siderophore production, the production of plant growth hormones, such as indole-3-acetic acid (IAA), and the reduction of ethylene synthesis by 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase, are likely to play key roles in enhancing plant growth in metal-polluted soils (9–14). The combination of plants and plant-associated bacteria has been considered an important component of phytoremediation technology (15–18). Notably, since PGP characteristics are part of the growth strategies of bacteria and/or rely on bacterial metabolism, they are likely to be affected under metal stress conditions (19–22), which would probably have an impact on phytoremediation.
Microbes have developed several metal resistance mechanisms in response to heavy metal stress, including intracellular and/or extracellular sequestration, efflux systems to export excess metal ions from bacterial cells, and the transformation of metals into a less toxic form by enzymatic detoxification (23–26). It has been widely accepted that in the model bacterium Cupriavidus metallidurans, these mechanisms appear to be cooperative, not metal specific, and are controlled by a complex regulatory network involving several clusters of genes and functions (1, 27). However, for other metal-resistant bacteria, especially some recently isolated species utilized in phytoremediation, investigations of such global regulatory metal response systems are still limited. Understanding of the survival and adaptive strategies of these plant-associated metal-resistant bacteria in response to heavy metals is important for better utilization of these bacteria in phytoremediation.
To address the research gaps mentioned above, we investigated the following questions relating to the physiological response of the metal-resistant bacterium Enterobacter sp. strain EG16 to Cd exposure. (i) What are the survival and adaptive strategies that EG16 uses in response to Cd toxicity? (ii) What PGP characteristics does EG16 have, and how are those characteristics affected by Cd exposure? (iii) Is EG16 suitable for microbially associated phytoremediation in metal-polluted soils?
MATERIALS AND METHODS
Isolation and identification of the bacterial strain.
The bacterial strain used in this study was isolated from the rhizosphere of Hibiscus cannabinus growing in multimetal-polluted tailings in Dabao Mountain, Guangdong, China. The soil quality parameters are shown in Table S1 in the supplemental material. The roots of three H. cannabinus plants were soaked in sterile tap water for 20 min and were washed several times to remove adherent soil. After incubation at 30°C for 30 min on a rotary shaker (250 rpm), serial dilutions of the soil suspension were prepared. To select for the dominant species with Cd resistance, 0.1 ml of the diluent was spread on the solid surface of nutrient broth (NB) medium (1% peptone, 0.5% beef extract, 0.5% NaCl, 0.2 mg liter−1 Fe) containing 20 mg liter−1 (179 μM) Cd2+, added as Cd(NO3)2, in petri dishes. The petri dishes were incubated for 3 days at 30°C. Visually distinct bacterial colonies were selected and were incubated twice in the same Cd-containing medium in order to select pure Cd-resistant bacterial strains. One of the bacterial strains was selected, and genotypic identification was carried out by amplification of partial nucleotide sequences of the 16S rRNA. PCR products were sequenced, identified by running BLASTn against NCBI's 16S rRNA sequence database, and rechecked with Ribosomal Database Project (RDP) tools at a 90% confidence threshold. The bacterial strain was identified as an Enterobacter sp. strain and was named EG16. This strain has been deposited in the Guangdong Culture Collection Centre of Microbiology as strain number GIMCC1.808.
Effect of Cd on bacterial growth.
To measure the effect of Cd on the growth of EG16, a freshly grown bacterial culture (1%) was incubated in NB medium either alone or supplemented with 5, 10, 50, 100, 150, 200, or 250 mg liter−1 Cd2+ added as Cd(NO3)2 (equivalent to 44.6, 89.3, 446, 893, 1,339, 1,786, or 2,232 μM Cd2+). The optical density at 600 nm (OD600) was recorded at intervals by use of a spectrometer (Shimadzu, Japan) until the culture reached the stationary phase (28 h). The half-maximal inhibitory concentration (IC50) and the MIC values were determined. To determine whether the Cd resistance of EG16 was inducible, EG16 was grown in the presence or absence of 100 mg liter−1 (893 μM) Cd2+ for 24 h, after which 1 ml of the bacterial culture from each treatment was separately incubated in 100 ml of fresh NB medium supplemented with 100 mg liter−1 Cd2+, and the OD600 was recorded every 4 h until the stationary phase was reached (28 h). Each experiment was conducted in triplicate.
Accumulation of Cd by EG16.
Heavy metal accumulation, including both biosorption and intracellular bioaccumulation, is often one of the heavy metal resistance mechanisms in microbes (28). Biosorption is a rapid process involving physical adsorption, ion exchange, and complexation at the cell surface, while bioaccumulation, which involves the transport of metals into bacterial cells by an active metabolism-dependent process, occurs more slowly (28, 29). To determine the accumulation mechanisms of EG16, a desorption experiment was performed (30). Bacterial cells grown in NB medium for 24 h were collected by centrifugation (8,000 rpm, 10 min, 4°C), resuspended (OD600, 1.0), added to fresh NB medium (1%) containing 100 mg liter−1 (893 μM) Cd2+, and incubated for 2 h. After centrifugation (12,000 rpm, 10 min, 4°C), the Cd concentration in the supernatant was determined and was used to calculate the total amount of Cd retained by the cells. Then the cells were harvested and were added to 20 ml of either sterile deionized water, 1.0 M NH4NO3, or 0.1 M EDTA. After 2 h of incubation (30°C, 180 rpm), supernatants were harvested by centrifugation (10,000 rpm, 10 min) and were analyzed for Cd using inductively coupled plasma optical emission spectrometry (ICP-OES) (Optima 5300 DV system; Perkin-Elmer Instruments, USA). Each experiment was conducted in triplicate.
TEM and EDX analysis.
For electron microscopy analysis, bacterial cells either were not pretreated with Cd or were pretreated as in the desorption experiment, with the Cd2+ concentration set at 100 or 200 mg liter−1 (equivalent to 893 or 1,786 μM Cd2+). The cells were then fixed in 4% glutaraldehyde, washed three times in phosphate buffer, fixed in 1% osmium tetroxide, washed again in phosphate buffer, dehydrated though a graded series of ethanol solutions (30%, 50%, 70%, 90%, and 100%), soaked in propylene oxide, and finally embedded in Epon 812 epoxy resin for ultrathin sections (Leica, Austria) and subjected to transmission electron microscopy (TEM) analysis (JEOL, Japan). Electron-dense granules found via TEM were further examined by energy-dispersive X-ray (EDX) analysis as performed by Holmes et al. (2). Each treatment was prepared with three replicates.
PGP characterization experiments.
Siderophores produced by EG16 were quantified by the method of Dimkpa et al. (31). Cells that had been cultured overnight were used to inoculate a siderophore-inducing medium (1%, vol/vol), as described previously by Alexander and Zuberer (32), with a range of metal addition treatments. Specifically, the medium either was not treated with Cd (−Cd) or was amended with 10, 100, or 1,000 μM Cd2+ (Cd10, Cd100, or Cd1000, respectively), added as CdCl2, in the presence (+) or absence (−) of 100 μM Fe3+, added as FeCl3. Thus, eight treatments were set up as follows: −Fe −Cd, −Fe +Cd10, −Fe +Cd100, −Fe +Cd1000, +Fe −Cd, +Fe +Cd10, +Fe +Cd100, and +Fe +Cd1000. After metal addition, cells were further cultured in the siderophore-inducing medium at 180 rpm for 48 h at 30°C, and cell viability was tested by plate counts. Then the cultures were centrifuged at 10,000 rpm for 10 min (4°C), and supernatants were collected by filtration using a 0.22-μm filter (MicroPES; Membrana, Germany). For the detection of siderophores, the filtered solution was mixed with a chrome azurol S (CAS) indicator solution (1:1, vol/vol), which was prepared by the method of Alexander and Zuberer (32). After a 60-min reaction period, absorbance was measured at 630 nm using a spectrometer (Shimadzu, Japan). The medium without bacterial inoculation was used as a control. Deferoxamine mesylate (DFOM; Sigma, USA) was used to prepare a standard curve (32). Each treatment was conducted in triplicate.
In order to estimate IAA production, EG16 was cultured in the eight metal-containing media described in the preceding section, without exogenous tryptophan supplementation. After centrifugation, 1 ml supernatant was mixed with 4 ml Salkowski's reagent (9) and was placed at room temperature (25°C) for 20 min before the absorbance at 535 nm was measured. The concentration of IAA in each culture medium was determined by comparison with a standard curve made using an IAA standard (Sigma, USA).
N2 fixation activity and ACC deaminase activity were determined by the methods of Belimov et al. (3) with some modifications. The minimal salts (SM) medium (containing, per liter, 1 g glucose, 1 g sucrose, 1 g Na acetate, 1 g Na citrate, 1 g malic acid, 1 g mannitol, 0.4 g KH2PO4, 2 g K2HPO4, 0.2 g MgSO4, 0.1 g CaCl2, 5 mg FeSO4, 2 mg H3BO3, 5 mg ZnSO4, 1 mg Na2MoO4, 3 mg MnSO4, 1 mg CoSO4, 1 mg CuSO4, and 1 mg NiSO4 [pH 6.4]) was supplemented with 0.5 g liter−1 ACC or 0.5 g liter−1 (NH4)2SO4 as a sole source of nitrogen to prepare SMC or SMN medium, respectively. Bacteria were incubated at 30°C for 5 days at 180 rpm in flasks containing 5 ml of a liquid SM, SMC, or SMN medium, and then absorbance was measured at 600 nm using a spectrometer (Shimadzu, Japan).
To determine the PGP effects of EG16 on H. cannabinus, an elongation assay was performed by the method of Belimov et al. (3) with modifications. For the uninduced and induced treatments, bacteria were first grown in NB medium in the absence or presence, respectively, of 100 mg liter−1 Cd2+ (equivalent to 0 or 893 μM Cd2+) for 24 h, then collected by centrifugation (6,000 rpm, 10 min, 4°C), and finally resuspended in sterile deionized water (OD600, 1.0 [equivalent to 108 CFU ml−1]). Six-milliliter quantities of the bacterial suspension or sterile deionized water (uninoculated control [CK]) was added to sterile glass petri dishes with filter paper. The bacterial suspensions and water were either left untreated or supplemented with 10 or 100 mg liter−1 Cd2+ added as Cd(NO3)2 (equivalent to 89.3 or 893 μM Cd2+). H. cannabinus seeds were surface sterilized using 10% H2O2 for 30 min, washed with sterile deionized water, and placed in the glass petri dishes mentioned above (so that the seeds were moistened but not submerged). Root and shoot lengths were measured after incubation for 7 days at 30°C in the dark. Three dishes (30 seeds per dish), as three replicates, were prepared for each Cd treatment.
Uptake of Fe and Cd by EG16 as affected by the interplay between Fe and Cd.
The amounts of Fe and Cd in bacterial cells harvested in siderophore quantification experiments were determined as follows: cells were washed twice in 0.1 M EDTA, dried, and weighed, and then heavy metals were extracted by nitric acid digestion, after which Fe and Cd concentrations were determined by ICP-OES (Optima 5300 DV system; Perkin-Elmer Instruments, USA).
Bacterial cultivation conditions for RNA-seq analyses.
For transcriptome sequencing (RNA-seq) analysis, bacterial cultures were pregrown in NB medium to mid-log phase (12 h) on a rotary shaker (180 rpm) at 30°C. One milliliter of each culture was then added to 100 ml of fresh NB medium containing either no added Cd (control treatment) or 100 mg liter−1 (893 μM) Cd2+, added as Cd(NO3)2, and the mixture was then incubated for another 24 h under the same conditions as before. Each treatment was carried out in five replicates. After the 24-h incubation period, bacterial cultures were divided into equal volumes to obtain samples to be used for RNA extraction and were pretreated by following the methods of Maynaud et al. (1). One-tenth volume of ice-cold stop buffer (5% phenol in ethanol) was added to each sample, and the mixture was centrifuged at 8,000 rpm and 4°C for 5 min. Supernatants were discarded, and the samples were recentrifuged for 1 min to remove all the liquid at the bottoms of the tubes. Cells were quickly frozen in liquid nitrogen and were stored at −80°C until RNA extraction.
RNA extraction, sequencing, and RNA-seq analyses.
Total RNA was extracted from bacterial pellets by the TRIzol method (Invitrogen, USA) and was purified using the RNeasy MinElute cleanup kit (Qiagen, Germany). To eliminate rRNA, the RiboMinus transcriptome isolation kit for RNA-seq (Invitrogen, USA) was used. Total-RNA and mRNA quantities and quality were assessed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) and an Agilent 2100 bioanalyzer with RNA Nano chips (Agilent Technologies, USA). The RNA integrity numbers (RIN) were 6.0, showing good RNA quality (see Fig. S1 in the supplemental material).
For RNA sequencing, a cDNA library was synthesized according to the manufacturer's instructions as follows: RNA fragments were copied into first-strand cDNA using SuperScript III first-strand synthesis SuperMix (Invitrogen, USA) with random hexamer primers, after which the second-strand cDNA was synthesized using a buffer, RNase H, deoxynucleoside triphosphates (dNTPs), and DNA polymerase I. For sequencing, 10 ng of cDNA was mixed with magnetic beads (Invitrogen, USA) and with the sequencing primers, followed by thorough mixing with 100 ml castor oil, before being loaded into the Ion OneTouch system (Invitrogen, USA). After 1.5 h of emulsion PCR, beads with PCR products were collected and were washed with 150 μl buffer. A 100-μl volume of the bead suspension was loaded onto the Ion 318 chip used for sequencing (Ion Torrent system; Thermo Fisher Scientific, USA).
Pretreatment of the raw sequencing data, removal of the adapter sequences, and quality value calculations were performed using Torrent Suite software, version 3.6 (Thermo Fisher Scientific, USA). The raw reads were subjected to a quality check using FastQC (version 3.4.1.1). Quality reads were obtained by trimming the raw reads at a minimum PHRED quality (Q) score of 20. The number of Q20 bases was 321.70 Mbp; the mean length of a sequence was 79 bp; and the longest read was 368 bp (see Table S2 in the supplemental material). Evaluating the quality of the loading density on the chip, we obtained a loading of 82.4%, with an enrichment of 100.0% (see Table S2). Reads that passed the quality filter were mapped on the reference genome of Enterobacter cloacae ATCC 13047 (BioCyc database [http://biocyc.org/]) using TopHat2. Data were normalized by calculating RPKM (reads per kilobase per million mapped reads) values for each gene so as to analyze the gene expression level. The differential expression between controls and Cd treatment was measured by the fold change (FC), calculated as (RPKM with Cd treatment)/(RPKM for the control).
Accession numbers.
The sequence of the partial 16S rRNA gene (1,377 bp) of Enterobacter sp. strain EG16 has been deposited in the GenBank database under accession number KP406619. The transcriptomic sequencing data have been deposited in the NCBI Sequence Read Archive (SRA) database under accession number SRP051960.
RESULTS
Effect of Cd on the growth of EG16.
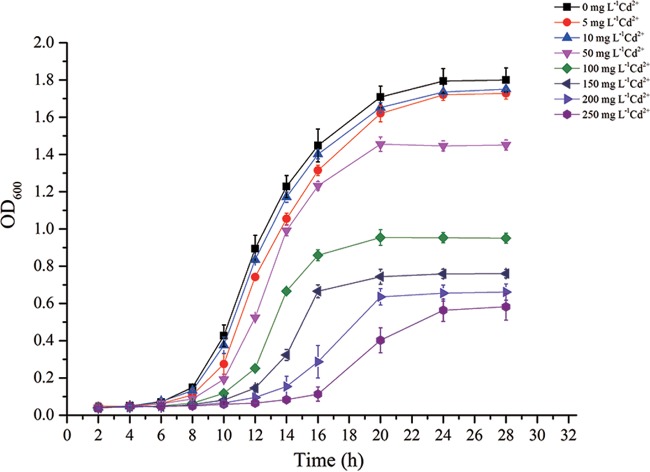
Strain EG16 was able to grow in NB medium containing a relatively high concentration of Cd (MIC, >250 mg liter−1 [2,232 μM] Cd2+) (Fig. 1). There was a threshold Cd2+ concentration of 100 mg liter−1 (893 μM Cd2+), below which the growth of EG16 was similar to that of the control with no added Cd and above which significant growth inhibition was observed. In addition, 100 mg liter−1 was near the IC50 of 118.7 mg liter−1 (1,060 μM Cd2+) calculated by the dose-response curve (Fig. 2). Accordingly, we used 100 mg liter−1 (893 μM) Cd2+ as the Cd concentration in subsequent experiments. Bacteria that had been preexposed to 100 mg liter−1 Cd2+ appeared to reach the exponential phase earlier, and had a higher growth rate, than uninduced bacteria (see Fig. S2 in the supplemental material), indicating that EG16 may have Cd resistance mechanisms that are induced under Cd stress.
FIG 1.
Growth pattern of EG16 exposed to a range of Cd concentrations (n = 3). The Fe content in the NB medium was 0.2 mg liter−1.
FIG 2.
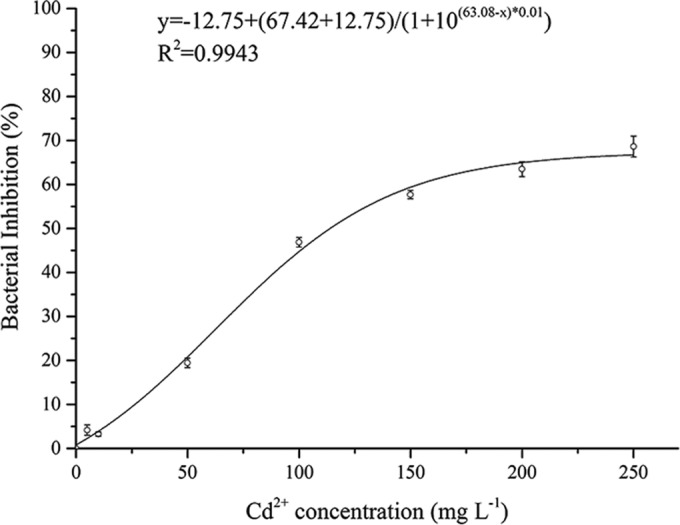
Dose-response curve of strain EG16 exposed to Cd (n = 3).
Mechanisms of Cd accumulation in EG16.
A desorption experiment was performed to determine the fractions of Cd adsorbed by different mechanisms. Metal ions released by sterile deionized water were regarded as the fraction adsorbed by physical adsorption, while those released by NH4NO3 contained both the fraction adsorbed by physical adsorption and that adsorbed by ion exchange. EDTA, however, releases all of the metal ions adsorbed by physical adsorption, ion exchange, and complexation (30, 33). In our study (see Fig. S3 in the supplemental material), 11% of Cd was released from EG16 cells by sterile deionized water, 22% by NH4NO3, and 31% by EDTA. Consequently, the fractions adsorbed by physical adsorption, ion exchange, and complexation were 11%, 11%, and 9%, respectively, and thus, these three mechanisms have similar importance in the biosorption of Cd by EG16. The fraction of total cell-accumulated Cd that was not desorbed by water, NH4NO3, or EDTA (about 70%) was regarded as the fraction transported into the cells, suggesting that intracellular accumulation is the dominant mechanism of Cd accumulation by EG16.
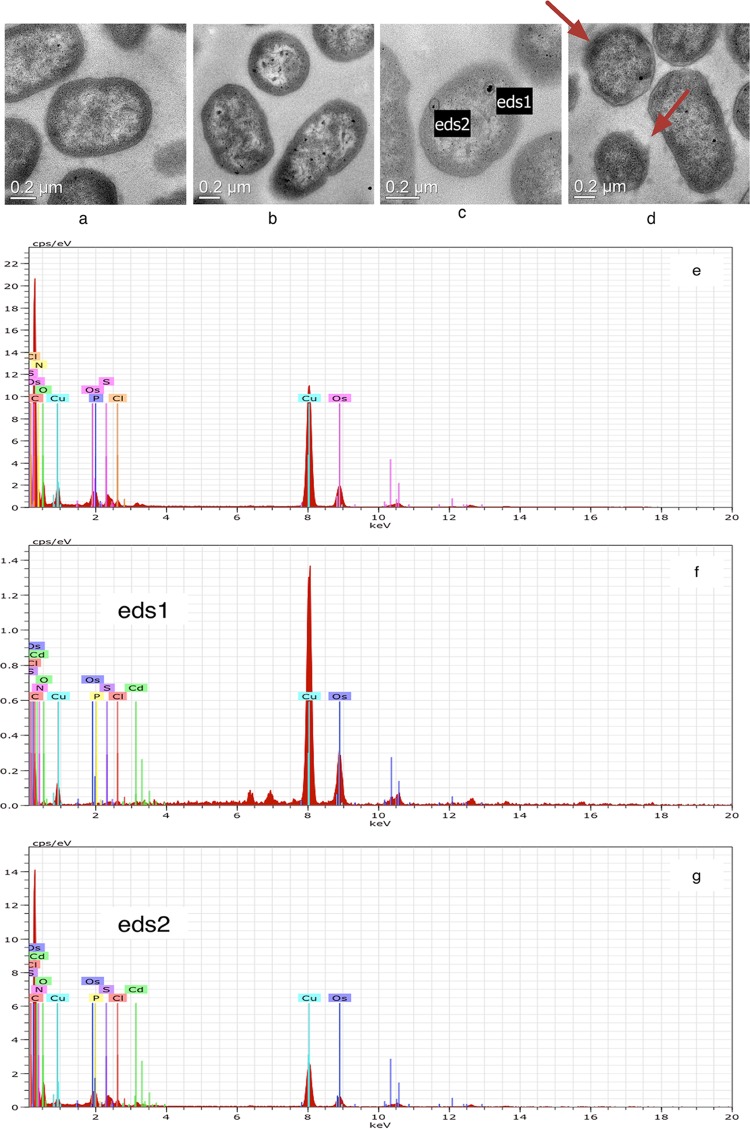
TEM was used to confirm the intracellular accumulation of Cd by EG16 cells. Ultrathin sections of cells grown without Cd exhibited normal morphology (Fig. 3a). Although detectable growth was also observed with the 200-mg liter−1 Cd2+ treatment, cells exhibited visually distorted morphologies with deformed cytoplasmic membranes (Fig. 3d, red arrows). Compared to the control, Cd-treated cells exhibited many dark electron-dense granules within the EG16 cells, suggesting Cd accumulation inside the cells (Fig. 3b, c, and d). Cells grown without Cd and two of the electron-dense granules found after the 100-mg liter−1 Cd2+ treatment (eds1 and eds2 [Fig. 3c]) were further tested by EDX analysis (Fig. 3e, f, and g, respectively). The results confirmed the presence of Cd in these electron-dense granules (0.35 atom% in eds1 [Fig. 3f] and 0.18 atom% in eds2 [Fig. 3g]), providing direct evidence for intracellular accumulation of Cd by EG16. Furthermore, the elemental analysis of eds1 and eds2 gave Cd/S ratios of ca. 1:1 and 1:3, respectively, suggesting the possibility of the presence of intracellular cadmium sulfide.
FIG 3.
TEM and EDX analyses of strain EG16 in the absence and presence of Cd. (a to d) TEM analyses. (a) Normal morphology of EG16 cells without accumulated Cd. (b and c) EG16 cells pretreated with 100 mg liter−1 Cd2+. eds1 and eds2 indicate electron-dense granules in an EG16 cell. (d) EG16 cells pretreated with 200 mg liter−1 Cd2+. Red arrows indicate cell regions with distorted morphologies and deformed cytoplasmic membranes. (e to g) EDX analyses of a cell without accumulated Cd (e), eds1 (f), and eds2 (g).
Effect of Cd on the PGP characteristics of EG16.
Our PGP characterization experiments showed that under Fe-deficient conditions, the presence of Cd significantly increased siderophore production in EG16 (P < 0.05), although no significant difference was observed between the three Cd treatments (Table 1). In Fe-containing media, no siderophore was detected, irrespective of the Cd concentration. However, IAA production decreased with increasing Cd exposure, and the inhibition of IAA synthesis by Cd was more severe in Fe-containing (siderophore-deficient) media than in media without Fe addition (Table 1). For detection of the N2 fixation and ACC deaminase activities of EG16, bacteria were incubated on SM medium (containing no nitrogen source) and SMC medium (containing ACC as the sole source of nitrogen). The absence of bacterial growth on these media indicated that EG16 has neither N2 fixation nor ACC deaminase activity (data not shown).
TABLE 1.
Siderophore and IAA production by EG16 grown in media containing various combinations of Cd and Fe concentrationsa
| Treatmentb | Production of: |
Bacterial growth (CFU ml−1 · 108) | |
|---|---|---|---|
| Siderophores (μM CFU−1 DFOM equivalent · 10−11) | IAA (μM CFU−1 · 10−12) | ||
| −Fe −Cd | 3.16 ± 0.32 B | 7.21 ± 0.10 A | 2.12 ± 0.13 B |
| −Fe +Cd10 | 7.08 ± 1.24 A | 7.74 ± 1.88 AB | 1.13 ± 0.14 D |
| −Fe +Cd100 | 7.68 ± 0.63 A | 2.32 ± 0.31 C | 0.95 ± 0.04 E |
| −Fe +Cd1000 | 7.74 ± 1.25 A | 1.95 ± 0.31 C | 0.75 ± 0.01 F |
| +Fe −Cd | ND | 1.34 ± 0.14 C | 2.38 ± 0.13 A |
| +Fe +Cd10 | ND | 6.07 ± 1.36 B | 1.48 ± 0.07 C |
| +Fe +Cd100 | ND | ND | 1.13 ± 0.10 D |
| +Fe +Cd1000 | ND | ND | 1.00 ± 0.01 DE |
Within columns, means not followed by the same letter are different at the 0.05 level by one-way analysis of variance with Duncan's correction (n = 3). ND, not detected.
−Fe, no Fe addition; −Cd, no Cd addition; +Fe, treatment with 100 μM Fe; +Cd10, +Cd100, or +Cd1000, treatment with 10, 100, or 1,000 μM Cd, respectively.
Interestingly, the PGP effects of EG16 on the growth of H. cannabinus were significant only in the medium to which a high concentration of Cd was added and which was inoculated with EG16 pretreated with 100 mg liter−1 Cd2+ (inducing treatment). When 100 mg liter−1 Cd2+ was added (see Fig. S4c in the supplemental material), the total root length and shoot length of the seedlings with the inducing treatment were 37.6% and 24.2% higher, respectively, than those for the uninoculated control (CK) (P < 0.01). However, PGP effects were slight or nonexistent (P > 0.05) when 10 mg liter−1 Cd2+ or no Cd was added (see Fig. S4a and b).
Uptake of Cd and Fe by EG16.
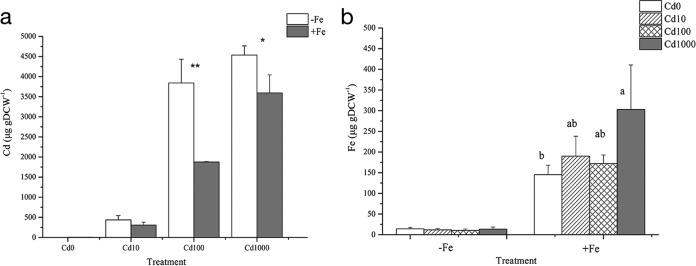
Bacterial cells were pretreated in media containing eight different combinations of Fe and Cd concentrations as described for the siderophore quantification experiment, and the contents of Fe and Cd in the cells were determined. For treatments with 100 μM or 1,000 μM Cd2+, the Cd content in EG16 was significantly higher when no exogenous Fe was added to the medium (−Fe) than when Fe was added (+Fe) (Fig. 4a). When Fe was added, EG16 cells exhibited a trend of increased contents of both Cd and Fe with increasing external concentrations of Cd (Fig. 4a and b). This result suggests that EG16 cells exposed to Cd have an increased requirement for Fe, which may help to counteract Cd toxicity (23).
FIG 4.
Effect of the interplay of Cd and Fe on their uptake by EG16. Shown are the Cd (a) and Fe (b) contents of EG16 cells grown in media with varying concentrations of Cd and Fe. (a) Asterisks indicate differences between the means compared, at the 0.01 (**) and 0.05 (*) levels, by an independent-sample t test (n = 3). (b) Different letters above bars indicate that the means differ at the 0.05 level by one-way analysis of variance with Duncan's correction (n = 3).
Identification of genes regulated by Cd in EG16.
In the RNA-seq analysis, 866 of 5,749 predicted EG16 genes (15%) were expressed significantly differentially (FC, ≥2 or ≤0.5) when exposed to Cd; 1.1% of genes were upregulated, and 13.9% were downregulated. In other words, the expression levels of most EG16 genes were unaffected when the strain was grown in a medium originally treated with 100 mg liter−1 (893 μM) Cd2+, showing that that level of Cd stress did not have a great impact at the level of global gene expression. Similar results were reported for two Mesorhizobium isolates, STM 2683T and STM 4661, exposed to Zn and Cd (1), for Saccharomyces cerevisiae exposed to several transition metals (34), and for Escherichia coli DH5α exposed to a mixture of heavy metals (35), where metals affected the expression levels of only a low proportion of genes. Such results are not unexpected in the case of metal-resistant strains, since they are able to maintain relatively normal growth while dealing with metal stress by regulating the portion of genes involved in metal response.
Highly responsive genes were divided into several categories based on the BioCyc database (http://biocyc.org/) (see Table S3 in the supplemental material). Specifically, 217 of 866 genes (25.1%) were involved in cell metabolic activities; 183 genes (21.1%) encoded proteins associated with cellular information transfer, including DNA-, RNA-, and protein-related processes; 47 genes (5.4%) encoded proteins involved in cell processes such as stress responses, cell division, and detoxification; 24 genes (2.8%) encoded transport-related proteins; 14 genes (1.6%) encoded regulators or enzymes involved in regulation; 1 gene (0.1%) encoded a protein related to cell structure; and the remainder of the genes encoded unclassified proteins (24.5%) or hypothetical proteins (19.4%) (see Table S3).
Of the 866 highly responsive genes, 40 that encoded proteins involved in energy metabolism were downregulated with Cd treatment, including 16 genes associated with aerobic or anaerobic respiration. Also significantly repressed were 35 genes related to amino acid biosynthesis, 30 genes involved in macromolecule biosynthesis or degradation, and 12 genes involved in cell division, all of which are processes that consume energy.
Five genes encoding stress-related proteins were induced in response to Cd (see Table S3 in the supplemental material). The ECL_03081 gene (ahpC), encoding alkyl hydroperoxide reductase subunit C, was upregulated 4.0-fold with Cd treatment. Alkyl hydroperoxide reductase participates in the control of endogenous peroxides and responses to elevated reactive oxygen species (ROS) levels, which can be enhanced by Cd stress (36). It has been reported that a defect in ahpC function leads to changes in the cell morphology, cell surface properties, biofilms, aggregation, and flocculation of the bacterium Azospirillum brasilense; all of these processes and characteristics are important for common adaptive responses to various stresses in bacteria (37), suggesting a critical role for ahpC in stress response. A zinc/cadmium/mercury/lead-transporting ATPase gene was 2.7-fold upregulated with Cd exposure. Metal-transporting ATPases are likely to be used by microorganisms to maintain nontoxic levels of metals in the cytoplasm (1). In addition, the ECL_01750, ECL_02612, and ECL_04763 genes, encoding proteins involved in responses to osmotic stress, starvation, and temperature extremes, were overexpressed with Cd treatment.
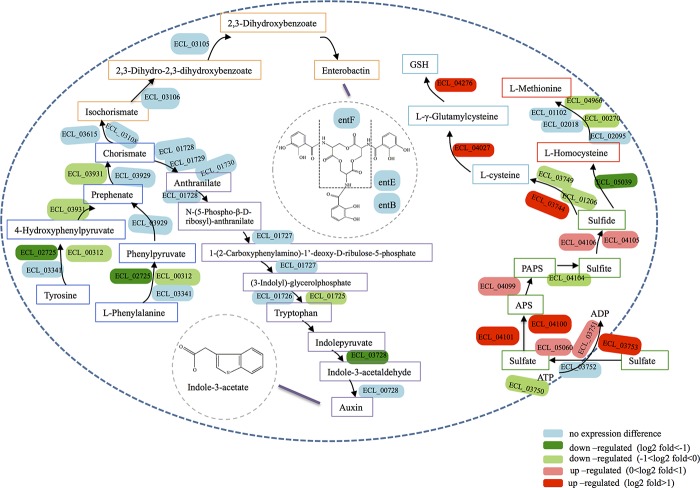
Several genes involved in intracellular sulfur metabolism were upregulated with Cd exposure, including genes encoding proteins participating in sulfate transport (cysP), sulfur assimilation (ECL_04101, ECL_04100, ECL_03744, and ECL_00157), and glutathione (GSH) biosynthesis (ECL_04276 and ECL_04027) (Fig. 5; see also Table S3 in the supplemental material). However, we detected significant downregulation of genes involved in the biosynthesis of Fe-S clusters (iscA and iscU) and of genes encoding proteins containing Fe-S clusters (such as ECL_05090, ECL_00918, ECL_04701, ECL_00555, and ECL_04602). On the other hand, ECL_01386 (encoding ferritin), ECL_04701 (encoding bacterioferritin-associated ferredoxin), and ECL_04773 (encoding a ferrous iron transport protein), all of which are involved in bacterial Fe acquisition and storage, were inhibited with Cd treatment (see Table S3). Interestingly, fur, which encodes the ferric uptake regulator, a transcriptional repressor that negatively regulates the uptake system for Fe (38), was downregulated, which acted to increase Fe uptake by EG16.
FIG 5.
Model of siderophore, IAA, sulfur, and GSH metabolic pathways in EG16 inferred from the genome of Enterobacter cloacae ATCC 13047. Colored text boxes represent the different metabolic pathways, with yellow for the siderophore biosynthesis pathway, purple for the IAA biosynthesis pathway, dark blue for the common part of the siderophore and IAA biosynthesis pathways, green for the sulfur metabolic pathway, light blue for the GSH biosynthesis pathway, and red for the l-methionine biosynthesis pathway.
DISCUSSION
Although EG16 has a relatively high Cd tolerance, our data showed widespread repression of the processes of both information transfer (transcription and translation) and metabolism with Cd exposure (see Table S3 in the supplemental material). Recent research on the transcriptional responses of two metal-tolerant Mesorhizobium isolates to Zn and Cd exposure showed repression of the translation machinery similar to what we observed (1). Another study found that E. coli shut down transcription and translation when exposed to Cd (39), suggesting that bacterial protein synthesis was inhibited by Cd exposure. The second largest functional group of genes that were significantly repressed consisted of those involved in metabolism, especially energy metabolism (40 of 217 genes), including a number of genes associated with aerobic and anaerobic respiration. In contrast to E. coli, which switches from aerobic to anaerobic respiration when exposed to Cd (39), strain EG16 appeared to promote energy conservation by lowering its overall respiration. A diminished need for respiration during growth arrest has been proposed as a microbial survival strategy (40). Other metabolic processes repressed were those that require high energy consumption, such as amino acid biosynthesis, macromolecule biosynthesis and degradation, and cell division, indicating that EG16 probably adopts an energy-conserving mode when exposed to Cd, a response similar to that of E. coli (39).
Energy conserved in the processes mentioned above was likely to be spent in producing stress-related proteins in response to Cd toxicity, as well as in importing sulfur and iron, which may become deficient upon Cd exposure. It has been reported that although some microbial genes are involved in common responses to all metals, genes required for specific metal tolerance fall into largely distinct clusters (41). In Saccharomyces cerevisiae, the functions of Cd-inducible genes are concentrated in the areas of chromatin modification, GSH biosynthesis, and responses to stress (34, 41). Induction of stress-related genes not only confirms the high Cd resistance of EG16 but also suggests a Cd response mechanism in this strain.
Sulfur is an essential element that plays several important roles in cells; it is incorporated into the amino acids cysteine and methionine, as well as into cellular cofactors, including glutathione, lipoic acid, and Fe-S clusters (42). Since Cd is one of the transition metals that have sulfur as their preferred ligand and show high reactivity with sulfhydryl groups (41), the binding of Cd to sulfur may lead to Cd toxicity but may also play an important role in metal detoxification by bacterial cells. In Enterobacter cloacae ATCC 13047, extracellular sulfate is taken up by sulfate transporters and is used in an assimilation pathway to produce sulfide (KEGG pathway database [http://www.kegg.jp/kegg/pathway.html]). Sulfide can then go into the cysteine/methionine/GSH biosynthesis pathway, as shown in Fig. 5. GSH is the main redox buffer of the cell and is necessary for resistance to oxidative stress and metal toxicity in microorganisms (43, 44). GSH contributes to metal detoxification either by binding metals using its sulfhydryl group or by protecting cells against metal-induced oxidative stress, or both (41, 43). Besides, sulfide can bind free Cd ions and might play a role in intracellular Cd binding. As shown in Fig. 5, we detected significant upregulation of genes involved in the GSH and sulfide biosynthesis pathways, while the l-methionine biosynthesis pathway was more or less suppressed. A number of genes encoding proteins containing Fe-S clusters were also inhibited with Cd treatment. It is likely that strain EG16 countered Cd toxicity by synthesizing more GSH and sulfide so as to bind and inactivate free Cd ions in cells. On the other hand, Cd binds to sulfur that is necessary for the synthesis of other sulfur-containing proteins (44), which might cause sulfur deficiency in cells. To defend against Cd toxicity, cells redirect sulfur metabolism by routing most of the assimilated sulfur into the synthesis of GSH and sulfide at the expense of other sulfur-containing proteins, as reported for Saccharomyces cerevisiae (41, 45, 46). Intracellular Cd binding by sulfur could be roughly proved in the Cd accumulation experiment and the TEM-EDX analysis, which showed the potential presence of cadmium sulfide.
Iron is the third most abundant of the metals found in enzymes, and about 5% of all E. coli proteins require Fe-S clusters (47, 48). The production of siderophores is one of the most common bacterial strategies for the acquisition of iron under Fe-limited conditions (11). Some metals, including Cd, have been found to compete with Fe for siderophore binding and to cause Fe deficiency in microbes, possibly associated with an inability to recycle siderophores complexed with other metals (23, 49–52). This phenomenon may be one of the mechanisms of metal toxicity in these organisms. In fact, genes encoding proteins involved in cellular Fe acquisition and Fe storage (ferritin, bacterioferritin-associated ferredoxin, and ferrous iron transport protein A) were found to be downregulated in our study, indicating that Cd exposure might have caused Fe deficiency in EG16 cells. According to Schalk et al. (21), under conditions of limited availability of an essential metal, various enzymes necessary for bacterial metabolism lose activity, compromising cell survival. Such a scenario may partially explain the widespread repression of metabolism observed in strain EG16. In addition, the expression of genes involved in cellular Fe uptake was altered in response to Cd-induced Fe deficiency. Downregulation of fur, which encodes a ferric uptake regulator protein, is likely to induce Fe uptake by regulating the synthesis of Fe metabolism-related proteins and complexes, such as siderophores (38). A recent study on E. coli described an additional role for ahpC as a regulator of iron metabolism, through its effect on the biosynthesis pathway of the siderophore enterobactin (53). It was found that deletion of ahpC caused decreased production of enterobactin. In our study, Cd exposure led to downregulation of fur and upregulation of ahpC, both of which changes contributed to increased production of siderophores. Meanwhile, we observed induced production of siderophores with Cd exposure (Table 1), as well as significantly higher Cd uptake in media to which no Fe was added (with siderophore production) than in those to which Fe was added (without siderophore production) (Fig. 4a). It was likely that the competition of Cd for siderophore binding caused Fe deficiency and increased Cd uptake in EG16 cells, resulting in increased bacterial siderophore production in response to this Cd-induced Fe deficiency. A similar stimulating effect of heavy metals on siderophore production in various metal-resistant bacteria has also been observed in several previous studies. For instance, the Pseudomonas aeruginosa strain KUCd1, which also showed high Cd resistance, was reported to increase siderophore production when exposed to Cd (20). Similarly, Braud et al. (54) found that the presence of Al, Cu, Ga, Mn, Ni, and Zn in the extracellular medium induced pyoverdine production in Pseudomonas aeruginosa PAO1, while Naik and Dubey (55) observed Pb-enhanced siderophore production when Pb-resistant Pseudomonas aeruginosa strain 4EA was cultured in a medium containing as much as 0.5 mM lead nitrate. In contrast, reduced siderophore production upon exposure to Cd (10 to 100 μM) was observed in Bacillus amyloliquefaciens NAR38.1 by Gaonkar and Bhosle (56), together with severe inhibition of bacterial growth. The authors suggested that the decrease in siderophore production was a result of the Cd sensitivity of the bacterium. Thus, induction of siderophore production may be a metal response mechanism in metal-resistant, but not metal-sensitive, bacteria for dealing with Fe deficiency caused by other toxic metals.
However, genes involved in the enterobactin biosynthesis pathway were not significantly affected (Fig. 5). For Enterobacter cloacae ATCC 13047, enterobactin can be synthesized from chorismate, which, in turn, is generated from tyrosine and phenylalanine (Fig. 5). Notably, although Cd toxicity caused strong repression of genes involved in amino acid biosynthesis, as mentioned above, the synthesis of tyrosine and phenylalanine was not significantly affected. Moreover, chorismate is also an upstream precursor of tryptophan, which is involved in the IAA biosynthesis pathway (Fig. 5). An interesting finding was that several genes involved in the chorismate and IAA biosynthesis pathways were repressed to various extents with Cd exposure (Fig. 5), and IAA production was also observed to decline significantly with increasing Cd exposure in the characterization experiments (Table 1). It seemed that EG16 preferentially distributed chorismate to the production of siderophores rather than to IAA when the synthesis of chorismate was impacted, thereby maintaining the enterobactin biosynthesis pathway under Cd exposure. Notably, the Salkowski colorimetric test that we used for IAA quantification is specific for IAA, indole-3-pyruvate (IPyA), and indole-3-acetamide (IAM) (57). Both IPyA and IAM are involved in the tryptophan-dependent IAA biosynthesis pathway; they are produced from tryptophan and are the precursors of IAA (9, 58). As shown in Fig. 5, genes involved in the IPyA pathway were identified in strain EG16. As a result, the IAA production we measured might include both IAA and its precursor IPyA, both of which are tryptophan dependent and showed inhibited synthesis. Reductions in the level of IAA production in the presence of metals have also been observed in several other studies (19, 31, 59) and were attributed either to a lower level of synthesis caused by metal stress or to degradation by IAA peroxidases, which can be induced by metal-catalyzed free radical formation (59, 60). The inhibition of IAA synthesis by Cd was more severe in Fe-containing (siderophore-deficient) media than in Fe-deficient media (Table 1). Similarly, Dimkpa et al. (59) reported an important role for siderophores in IAA production under conditions of metal stress. They surmised that the binding of metals by siderophores decreased free toxic metal concentrations, thereby alleviating the inhibitory effects of these metals on IAA synthesis.
When the bacteria were not suffering from Cd stress and showed normal growth, as shown in Fig. 1, there was no measurable PGP effect (see Fig. S4a and b in the supplemental material). However, in the medium containing a high Cd concentration (100 mg liter−1 Cd2+) that was inoculated with EG16 preexposed to Cd (see Fig. S4c, bars I), the stimulation of shoot and root elongation by EG16 was significant. It seemed that the PGP effect shown by the isolate under inducing conditions resulted from its Cd-induced response mechanisms (see Fig. S2 in the supplemental material) and contributed to retaining relatively normal growth and probably increasing siderophore production. Nevertheless, since the bacterial suspension had no nutrition, it might be difficult for the isolate to activate its Cd response mechanisms under uninduced conditions (UI), and thus, it showed no significant PGP effect under these conditions. As a result, the plants benefited from bacterial inoculation only in the case of Cd stress, just as Remans et al. (61) reported. In another study (3), inoculation of plant-associated bacteria in the absence of Cd also significantly increased the root lengths of seedlings, while such stimulation was more pronounced with Cd addition. Tripathi et al. (62) inoculated mung beans with the siderophore-producing Pb- and Cd-resistant Pseudomonas putida strain KNP9 and observed stronger growth-promoting effects in the presence of Pb and Cd than for the control without added metal. According to Remans et al. (61), plant-associated bacteria may possess characteristics that relieve stress and thus promote plant growth only under stress conditions. In the present study, induced production of siderophores under conditions of Cd exposure may be one such stress-relieving characteristic and seems to contribute to plant growth promotion.
In evaluating candidate plant-associated metal-resistant bacteria for use in phytoremediation, it is of great importance to understand their survival and adaptive strategies under conditions of metal stress. Bacteria belonging to the genus Enterobacter have been found to associate frequently with a large number of plant species, colonizing the rhizosphere and other plant parts (63–66). Moreover, several metal-resistant Enterobacter species, including some with PGP effects, have been isolated, and some of their characteristics in response to metals have been determined (67–72). However, investigations of their metal response systems at the global regulatory level are limited.
Here we characterize the transcriptomic response of an Enterobacter strain to metal stress. The newly isolated Cd-resistant Enterobacter sp. strain EG16 responded to high levels of Cd through regulation at the transcriptomic level. When exposed to Cd, the bacterium adopted an energy-conserving mode via regulation of genes involved in energy-consuming processes. This conserved energy was available to be spent on the production of stress-related proteins responding to Cd stress and on the import of sulfur and iron, both of which become deficient under conditions of Cd toxicity, indicating an alteration in priorities from growth to survival and adaptation to a new environment. Gene expression data also suggested that EG16 redirected sulfur metabolism to maintain its intracellular GSH level, which is important for microbial resistance to oxidative stress and metal toxicity. Increased siderophore production was one of the responses of strain EG16 to Cd stress as it coped with Cd-induced Fe deficiency in cells. This siderophore production may also help promote plant growth under the conditions of metal stress encountered in phytoremediation, possibly through the alleviation of Cd-induced inhibition of IAA production. Further research is needed to confirm the practical effect of strain EG16 on plant growth in Cd-polluted soils and to clarify the PGP characteristics of the strain during phytoremediation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Special Scientific Research Fund of the Environmental Public Welfare Profession of China (grant 201509037), the National Natural Science Foundation of China (grants 41225004 and 41403060), and the Jiangsu Provincial Natural Science Foundation (grant SBK201342355).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03689-15.
REFERENCES
- 1.Maynaud G, Brunel B, Mornico D, Durot M, Severac D, Dubois E, Navarro E, Cleyet-Marel J-C, Le Quéré A. 2013. Genome-wide transcriptional responses of two metal-tolerant symbiotic Mesorhizobium isolates to zinc and cadmium exposure. BMC Genomics 14:292. doi: 10.1186/1471-2164-14-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes JD, Smith PR, Evans-Gowing R, Richardson DJ, Russell DA, Sodeau JR. 1995. Energy-dispersive X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiella aerogenes. Arch Microbiol 163:143–147. doi: 10.1007/BF00381789. [DOI] [PubMed] [Google Scholar]
- 3.Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR. 2005. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem 37:241–250. doi: 10.1016/j.soilbio.2004.07.033. [DOI] [Google Scholar]
- 4.Stohs S, Bagchi D. 1995. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336. doi: 10.1016/0891-5849(94)00159-H. [DOI] [PubMed] [Google Scholar]
- 5.Mitra RS, Bernstein IA. 1978. Single-strand breakage in DNA of Escherichia coli exposed to Cd2+. J Bacteriol 133:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin YH, Clark AB, Slebos RJ, Al-Refai H, Taylor JA, Kunkel TA, Resnick MA, Gordenin DA. 2003. Cadmium is a mutagen that acts by inhibiting mismatch repair. Nat Genet 34:326–329. doi: 10.1038/ng1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitra RS. 1984. Protein synthesis in Escherichia coli during recovery from exposure to low levels of Cd2+. Appl Environ Microbiol 47:1012–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turgay O, Bilen S. 2012. The role of plant growth-promoting rhizosphere bacteria in toxic metal extraction by Brassica spp., p 213–237. In Anjum NA, Ahmad I, Pereira ME, Duarte AC, Umar S, Khan NA (ed), Environmental pollution, vol 21 The plant family Brassicaceae: contribution towards phytoremediation. Springer, Dordrecht, Netherlands. [Google Scholar]
- 9.Patten CL, Glick BR. 2002. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glick BR. 2005. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett 251:1–7. doi: 10.1016/j.femsle.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Rajkumar M, Ae N, Prasad MNV, Freitas H. 2010. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149. doi: 10.1016/j.tibtech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Dourado MN, Martins PF, Quecine MC, Piotto FA, Souza LA, Franco MR, Tezotto T, Azevedo RA. 2013. Burkholderia sp. SCMS54 reduces cadmium toxicity and promotes growth in tomato. Ann Appl Biol 163:494–507. doi: 10.1111/aab.12066. [DOI] [Google Scholar]
- 13.Habibi S, Djedidi S, Prongjunthuek K, Mortuza MF, Ohkama-Ohtsu N, Sekimoto H, Yokoyoma T. 2014. Physiological and genetic characterization of rice nitrogen fixer PGPR isolated from rhizosphere soils of different crops. Plant Soil 379:51–66. doi: 10.1007/s11104-014-2035-7. [DOI] [Google Scholar]
- 14.Prapagdee B, Chanprasert M, Mongkolsuk S. 2013. Bioaugmentation with cadmium-resistant plant growth-promoting rhizobacteria to assist cadmium phytoextraction by Helianthus annuus. Chemosphere 92:659–666. doi: 10.1016/j.chemosphere.2013.01.082. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Prasad MNV, Rajkumar M, Freitas H. 2011. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258. doi: 10.1016/j.biotechadv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Khonsue N, Kittisuwan K, Kumsopa A, Tawinteung N, Prapagdee B. 2013. Inoculation of soil with cadmium-resistant bacteria enhances cadmium phytoextraction by Vetiveria nemoralis and Ocimum gratissimum. Water Air Soil Pollut 224:1696. doi: 10.1007/s11270-013-1696-9. [DOI] [Google Scholar]
- 17.Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel WW, Fallmann K, Puschenreiter M. 2013. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194. doi: 10.1016/j.soilbio.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajkumar M, Sandhya S, Prasad MNV, Freitas H. 2012. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30:1562–1574. doi: 10.1016/j.biotechadv.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Kamnev AA, Tugarova AV, Antonyuk LP, Tarantilis PA, Polissiou MG, Gardiner PHE. 2005. Effects of heavy metals on plant-associated rhizobacteria: comparison of endophytic and non-endophytic strains of Azospirillum brasilense. J Trace Elem Med Biol 19:91–95. doi: 10.1016/j.jtemb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Sinha S, Mukherjee SK. 2008. Cadmium-induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol 56:55–60. doi: 10.1007/s00284-007-9038-z. [DOI] [PubMed] [Google Scholar]
- 21.Schalk IJ, Hannauer M, Braud A. 2011. New roles for bacterial siderophores in metal transport and tolerance. Environ Microbiol 13:2844–2854. doi: 10.1111/j.1462-2920.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 22.Dimkpa CO, Zeng J, McLean JE, Britt DW, Zhan J, Anderson AJ. 2012. Production of indole-3-acetic acid via the indole-3-acetamide pathway in the plant-beneficial bacterium Pseudomonas chlororaphis O6 is inhibited by ZnO nanoparticles but enhanced by CuO nanoparticles. Appl Environ Microbiol 78:1404–1410. doi: 10.1128/AEM.07424-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimkpa CO, Merten D, Svatoš A, Büchel G, Kothe E. 2009. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol 107:1687–1696. doi: 10.1111/j.1365-2672.2009.04355.x. [DOI] [PubMed] [Google Scholar]
- 24.Ma Z, Jacobsen FE, Giedroc DP. 2009. Coordination chemistry of bacterial metal transport and sensing. Chem Rev 109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nies DH. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 26.Nies DH. 1999. Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51:730–750. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- 27.Monsieurs P, Moors H, Van Houdt R, Janssen PJ, Janssen A, Coninx I, Mergeay M, Leys N. 2011. Heavy metal resistance in Cupriavidus metallidurans CH34 is governed by an intricate transcriptional network. Biometals 24:1133–1151. doi: 10.1007/s10534-011-9473-y. [DOI] [PubMed] [Google Scholar]
- 28.Srinath T, Verma T, Ramteke P, Garg S. 2002. Chromium(VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere 48:427–435. doi: 10.1016/S0045-6535(02)00089-9. [DOI] [PubMed] [Google Scholar]
- 29.Gadd GM. 1990. Heavy metal accumulation by bacteria and other microorganisms. Experientia 46:834–840. doi: 10.1007/BF01935534. [DOI] [Google Scholar]
- 30.Fang LC, Zhou C, Cai P, Chen WL, Rong XM, Dai K, Liang W, Gu JD, Huang QY. 2011. Binding characteristics of copper and cadmium by cyanobacterium Spirulina platensis. J Hazard Mater 190:810–815. doi: 10.1016/j.jhazmat.2011.03.122. [DOI] [PubMed] [Google Scholar]
- 31.Dimkpa C, Svatoš A, Merten D, Büchel G, Kothe E. 2008. Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can J Microbiol 54:163–172. doi: 10.1139/W07-130. [DOI] [PubMed] [Google Scholar]
- 32.Alexander D, Zuberer D. 1991. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45. doi: 10.1007/BF00369386. [DOI] [Google Scholar]
- 33.Li Y, Yue Q, Gao B. 2010. Adsorption kinetics and desorption of Cu(II) and Zn(II) from aqueous solution onto humic acid. J Hazard Mater 178:455–461. doi: 10.1016/j.jhazmat.2010.01.103. [DOI] [PubMed] [Google Scholar]
- 34.Jin YH, Dunlap PE, McBride SJ, Al-Refai H, Bushel PR, Freedman JH. 2008. Global transcriptome and deletome profiles of yeast exposed to transition metals. PLoS Genet 4:e1000053. doi: 10.1371/journal.pgen.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gómez-Sagasti MT, Becerril JM, Martin I, Epelde L, Garbisu C. 2014. cDNA microarray assessment of early gene expression profiles in Escherichia coli cells exposed to a mixture of heavy metals. Cell Biol Toxicol 30:207–232. doi: 10.1007/s10565-014-9281-6. [DOI] [PubMed] [Google Scholar]
- 36.Wang HW, Chung CH, Ma TY, Wong HC. 2013. Roles of alkyl hydroperoxide reductase subunit C (AhpC) in viable but nonculturable Vibrio parahaemolyticus. Appl Environ Microbiol 79:3734–3743. doi: 10.1128/AEM.00560-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wasim M, Bible AN, Xie Z, Alexandre G. 2009. Alkyl hydroperoxide reductase has a role in oxidative stress resistance and in modulating changes in cell-surface properties in Azospirillum brasilense Sp245. Microbiology 155:1192–1202. doi: 10.1099/mic.0.022541-0. [DOI] [PubMed] [Google Scholar]
- 38.Hantke K. 2001. Iron and metal regulation in bacteria. Curr Opin Microbiol 4:172–177. doi: 10.1016/S1369-5274(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 39.Wang A, Crowley DE. 2005. Global gene expression responses to cadmium toxicity in Escherichia coli. J Bacteriol 187:3259–3266. doi: 10.1128/JB.187.9.3259-3266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang DE, Smalley DJ, Conway T. 2002. Gene expression profiling of Escherichia coli growth transitions: an expanded stringent response model. Mol Microbiol 45:289–306. doi: 10.1046/j.1365-2958.2002.03001.x. [DOI] [PubMed] [Google Scholar]
- 41.Wysocki R, Tamás MJ. 2010. How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol Rev 34:925–951. doi: 10.1111/j.1574-6976.2010.00217.x. [DOI] [PubMed] [Google Scholar]
- 42.Scott C, Hilton ME, Coppin CW, Russell RJ, Oakeshott JG, Sutherland TD. 2007. A global response to sulfur starvation in Pseudomonas putida and its relationship to the expression of low-sulfur-content proteins. FEMS Microbiol Lett 267:184–193. doi: 10.1111/j.1574-6968.2006.00575.x. [DOI] [PubMed] [Google Scholar]
- 43.Grant CM. 2001. Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol Microbiol 39:533–541. doi: 10.1046/j.1365-2958.2001.02283.x. [DOI] [PubMed] [Google Scholar]
- 44.Helbig K, Grosse C, Nies DH. 2008. Cadmium toxicity in glutathione mutants of Escherichia coli. J Bacteriol 190:5439–5454. doi: 10.1128/JB.00272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fauchon M, Lagniel G, Aude JC, Lombardia L, Soularue P, Petat C, Marguerie G, Sentenac A, Werner M, Labarre J. 2002. Sulfur sparing in the yeast proteome in response to sulfur demand. Mol Cell 9:713–723. doi: 10.1016/S1097-2765(02)00500-2. [DOI] [PubMed] [Google Scholar]
- 46.Thorsen M, Lagniel G, Kristiansson E, Junot C, Nerman O, Labarre J, Tamas MJ. 2007. Quantitative transcriptome, proteome, and sulfur metabolite profiling of the Saccharomyces cerevisiae response to arsenite. Physiol Genomics 30:35–43. doi: 10.1152/physiolgenomics.00236.2006. [DOI] [PubMed] [Google Scholar]
- 47.Waldron KJ, Robinson NJ. 2009. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol 7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 48.Fontecave M. 2006. Iron-sulfur clusters: ever-expanding roles. Nat Chem Biol 2:171–174. doi: 10.1038/nchembio0406-171. [DOI] [PubMed] [Google Scholar]
- 49.Huyer M, Page WJ. 1988. Zn increases siderophore production in Azotobacter vinelandii. Appl Environ Microbiol 54:2625–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baysse C, De Vos D, Naudet Y, Vandermonde A, Ochsner U, Meyer J-M, Budzikiewicz H, Schäfer M, Fuchs R, Cornelis P. 2000. Vanadium interferes with siderophore-mediated iron uptake in Pseudomonas aeruginosa. Microbiology 146:2425–2434. doi: 10.1099/00221287-146-10-2425. [DOI] [PubMed] [Google Scholar]
- 51.Dimkpa CO. 2009. Microbial siderophores in rhizophere interactions in heavy metal containing environments. Ph.D. dissertation. University of Jena, Jena, Germany. [Google Scholar]
- 52.Dimkpa CO, Merten D, Svatoš A, Büchel G, Kothe E. 2009. Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol Biochem 41:154–162. doi: 10.1016/j.soilbio.2008.10.010. [DOI] [Google Scholar]
- 53.Ma L, Payne SM. 2012. AhpC is required for optimal production of enterobactin by Escherichia coli. J Bacteriol 194:6748–6757. doi: 10.1128/JB.01574-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braud A, Hoegy F, Jezequel K, Lebeau T, Schalk IJ. 2009. New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine-iron uptake pathway. Environ Microbiol 11:1079–1091. doi: 10.1111/j.1462-2920.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 55.Naik MM, Dubey SK. 2011. Lead-enhanced siderophore production and alteration in cell morphology in a Pb-resistant Pseudomonas aeruginosa strain 4EA. Curr Microbiol 62:409–414. doi: 10.1007/s00284-010-9722-2. [DOI] [PubMed] [Google Scholar]
- 56.Gaonkar T, Bhosle S. 2013. Effect of metals on a siderophore producing bacterial isolate and its implications on microbial assisted bioremediation of metal contaminated soils. Chemosphere 93:1835–1843. doi: 10.1016/j.chemosphere.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 57.Glickmann E, Dessaux Y. 1995. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patten CL, Blakney AJC, Coulson TJD. 2013. Activity, distribution and function of indole-3-acetic acid biosynthetic pathways in bacteria. Crit Rev Microbiol 39:395–415. doi: 10.3109/1040841X.2012.716819. [DOI] [PubMed] [Google Scholar]
- 59.Dimkpa CO, Svatos A, Dabrowska P, Schmidt A, Boland W, Kothe E. 2008. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 74:19–25. doi: 10.1016/j.chemosphere.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 60.Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK. 2007. Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci 12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Remans T, Thijs S, Truyens S, Weyens N, Schellingen K, Keunen E, Gielen H, Cuypers A, Vangronsveld J. 2012. Understanding the development of roots exposed to contaminants and the potential of plant-associated bacteria for optimization of growth. Ann Bot 110:239–252. doi: 10.1093/aob/mcs105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tripathi M, Munot HP, Shouche Y, Meyer JM, Goel R. 2005. Isolation and functional characterization of siderophore-producing lead- and cadmium-resistant Pseudomonas putida KNP9. Curr Microbiol 50:233–237. doi: 10.1007/s00284-004-4459-4. [DOI] [PubMed] [Google Scholar]
- 63.Farina R, Beneduzi A, Ambrosini A, de Camposa SB, Lisboa BB, Wendisch V, Vargas LK, Passaglia LMP. 2012. Diversity of plant growth-promoting rhizobacteria communities associated with the stages of canola growth. Appl Soil Ecol 55:44–52. doi: 10.1016/j.apsoil.2011.12.011. [DOI] [Google Scholar]
- 64.Kuklinsky-Sobral J, Araujo WL, Mendes R, Geraldi IO, Pizzirani-Kleiner AA, Azevedo JL. 2004. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ Microbiol 6:1244–1251. doi: 10.1111/j.1462-2920.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 65.Qiu Z, Tan H, Zhou S, Cao L. 2014. Enhanced phytoremediation of toxic metals by inoculating endophytic Enterobacter sp. CBSB1 expressing bifunctional glutathione synthase. J Hazard Mater 267:17–20. doi: 10.1016/j.jhazmat.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 66.Zakria M, Ohsako A, Saeki Y, Yamamoto A, Akao S. 2008. Colonization and growth promotion characteristics of Enterobacter sp. and Herbaspirillum sp. on Brassica oleracea. Soil Sci Plant Nutr 54:507–516. doi: 10.1111/j.1747-0765.2008.00265.x. [DOI] [Google Scholar]
- 67.Abbas SZ, Rafatullah M, Ismail N, Lalung J. 2014. Isolation, identification, characterization, and evaluation of cadmium removal capacity of Enterobacter species. J Basic Microbiol 54:1279–1287. doi: 10.1002/jobm.201400157. [DOI] [PubMed] [Google Scholar]
- 68.Banerjee G, Pandey S, Ray AK, Kumar R. 2015. Bioremediation of heavy metals by a novel bacterial strain Enterobacter cloacae and its antioxidant enzyme activity, flocculant production, and protein expression in presence of lead, cadmium, and nickel. Water Air Soil Pollut 226:91. doi: 10.1007/s11270-015-2359-9. [DOI] [Google Scholar]
- 69.Chien C-C, Huang C-H, Lin Y-W. 2013. Characterization of a heavy metal translocating P-type ATPase gene from an environmental heavy metal resistance Enterobacter sp. isolate. Appl Biochem Biotechnol 169:1837–1846. doi: 10.1007/s12010-012-0047-4. [DOI] [PubMed] [Google Scholar]
- 70.El-Deeb B, Gherbawy Y, Hassan S. 2012. Molecular characterization of endophytic bacteria from metal hyperaccumulator aquatic plant (Eichhornia crassipes) and its role in heavy metal removal. Geomicrobiol J 29:906–915. doi: 10.1080/01490451.2011.635764. [DOI] [Google Scholar]
- 71.Sinha A, Kumar S, Khare SK. 2013. Biochemical basis of mercury remediation and bioaccumulation by Enterobacter sp. EMB21. Appl Biochem Biotechnol 169:256–267. doi: 10.1007/s12010-012-9970-7. [DOI] [PubMed] [Google Scholar]
- 72.Holmes A, Vinayak A, Benton C, Esbenshade A, Heinselman C, Frankland D, Kulkarni S, Kurtanich A, Caguiat J. 2009. Comparison of two multimetal resistant bacterial strains: Enterobacter sp. YSU and Stenotrophomonas maltophilia ORO2. Curr Microbiol 59:526–531. doi: 10.1007/s00284-009-9471-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.