Abstract
Dengue is an acute viral illness caused by RNA virus of the family Flaviviridae and spread by Aedes mosquitoes. Presenting features may range from asymptomatic fever to dreaded complications such as hemorrhagic fever and shock. A cute-onset high fever, muscle and joint pain, myalgia, cutaneous rash, hemorrhagic episodes, and circulatory shock are the commonly seen symptoms. Oral manifestations are rare in dengue infection; however, some cases may have oral features as the only presenting manifestation. Early and accurate diagnosis is critical to reduce mortality. Although dengue virus infections are usually self-limiting, dengue infection has come up as a public health challenge in the tropical and subtropical nations. This article provide a detailed overview on dengue virus infections, varied clinical manifestations, diagnosis, differential diagnosis, and prevention and treatment.
Keywords: Breakbone fever, cutaneous rash, dengue virus, dental and public health, hemorrhagic diathesis, oral manifestations
INTRODUCTION
The dengue virus, a member of the genus Flavivirus of the family Flaviviridae, is an arthropode-borne virus that includes four different serotypes (DEN-1, DEN-2, DEN-3, and DEN-4).[1,2] The World Health Organization (WHO) consider dengue as a major global public health challenge in the tropic and subtropic nations. Dengue has seen a 30-fold upsurge worldwide between 1960 and 2010, due to increased population growth rate, global warming, unplanned urbanization, inefficient mosquito control, frequent air travel, and lack of health care facilities.[3,4,5] Two and a half billion people reside in dengue-endemic regions[5] and roughly 400 million infections occuring per year, with a mortality rate surpassing 5–20% in some areas.[6] Dengue infection affects more than 100 countries, including Europe and the United States (USA).[7] The first reported case of dengue like illness in india was in Madras in 1780, the first virologically proved epidemic of DF in India occurred in Calcutta and Eastern Coast of India in 1963-1964.[8] Dengue virus infection presents with a diverse clinical picture that ranges from asymptomatic illness to DF to the severe illness of dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS).[4] Oral mucosal involvement is seen in approximately 30% of patients, although oral features are more frequently associated with DHF than with DF.[9] Dengue virus infection exhibit varied clinical presentation, hence, accurate diagnosis is difficult and relies on laboratory confirmation. The condition is usually self-limiting and antiviral therapy is not currently available. Supportive care with analgesics, hydration with fluid replacement, and sufficient bed rest forms the preferred management strategy.
ETIOPATHOGENESIS
DF is a severe flu-like infection that involves individuals of all age groups (infants, children, adolescents, and adults).[9] Transmission among human beings occurs by the mosquito Aedes aegypti and chiefly occurs during the rainy season.[10] The proposed etiologies for dengue virus infection are:
Viral replication, primarily in macrophages[11]
Direct skin infection by the virus[12]
Immunological and chemical-mediated mechanism induced by host–viral interaction.[12]
Dengue virus gains entry into the host organism through the skin following an infected mosquito bite. Humoral, cellular, and innate host immune responses are implicated in the progression of the illness and the more severe clinical signs occur following the rapid clearance of the virus from the host organism. Hence, the most severe clinical presentation during the infection course does not correlate with a high viral load.[13] Alterations in endothelial microvascular permeability and thromboregulatory mechanisms lead to an increased loss of protein and plasma. Proposed theories suggest that endothelial cell activation caused by monocytes, T-cells, the complement system, and various inflammatory molecules mediate plasma leakage. Thrombocytopenia may be related to alterations in megakaryocytopoiesis, manifested by infection of human hematopoietic cells and compromised progenitor cell growth. This may cause platelet dysfunction, damage, or depletion, leading to significant hemorrhages.[14,15]
Figure 1 depicts a diagramatic representation of the pathogenesis of dengue.
Figure 1.
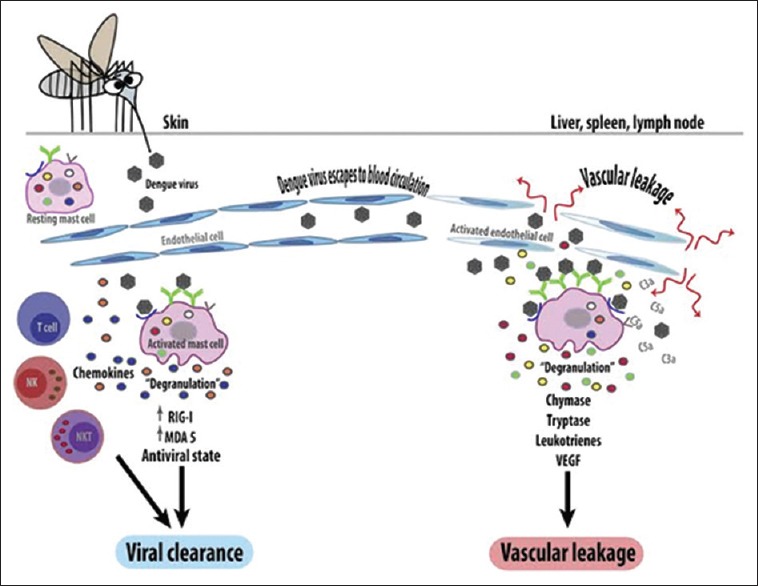
Pathogenesis of dengue virus infection
CLASSIFICATION
The WHO classifies DF into two groups: Uncomplicated and severe.[16,17] Severe cases are linked to excessive hemorrhage, organ impairement, or severe plasma escape, and the remaining cases are considered uncomplicated.[17]
According to the 1997 classification, dengue can be divided into undifferentiated fever, DF, and DHF.[18] DHF was further subdivided into grades I–IV.
Grade I: Only mild bruising or a positive tourniquet test
Grade II: Spontaneous bleeding into the skin and elsewhere
Grade III: Clinical sign of shock
Grade IV: Severe shock - feeble pulse, and blood pressure cannot be recorded.[19]
Here, grades III and IV comprise DSS.[17]
CLINICAL MANIFESTATIONS
Undifferentiated fever
This stage is seen mostly in the primary infection but may also occur following the initial secondary infection. Clinically, it is difficult to differentiate from numerous other viral diseases and often remains undiagnosed.
Dengue fever
DF follows both primary and secondary infections, and is most frequently encountered in adults and older children. Onset of symptoms is characterized by a biphasic, high-grade fever lasting for 3 days to 1 week.[20,21] Severe headache (mainly retrobulbar), lassitude, myalgia and painful joint, metallic taste, apetite loss, diarrhea, vomiting, and stomachache are the other reported manifestations. Dengue is also known as breakbone fever because of the associated myalgia and pain in joints.[16,22] Of patients with DF, 50–82% report with a peculiar cutaneous rash.[23,24] The initial rash is the result of capillary dilatation, and presents as a transient facial flushing erythema, typically occuring before or during the first 1–2 days of fever. The second rash is seen at 3 days to 1 week following the fever, and presents as a asymptomatic maculopapular or morbilliform eruption. Sometimes, individual lesions may merge and present as widespread confluent erythematous areas with pinpoint bleeding spots and rounded islands of sparing, giving a typical appearance of “white islands in a sea of red.”[23,25] The cutaneous rash is usually asymptomatic, and pruritis is reported only in 16-27% cases.[9,26] Bleeding episodes are infrequently seen in DF, although epistaxis and gingival bleeding, substantial menstruation, petechiae/purpura, and gastrointestinal tract (GIT) hemorrhage can occur.[20,27]
Dengue hemorrhagic fever
DHF is frequently seen during a secondary dengue infection. However, in infants it may also occur durring a primary infection due to maternally attained dengue antibodies.[28] The proposed diagnostic criteria for DHF includes:[29]
-
a.
Clinical parameters: Acute-onset febrile phase – high-grade fever lasting from 2 days to 1 week. Hemorrhagic episodes (at least one of the following forms): Petechiae, purpura, ecchymosis, epistaxis, gingival and mucosal bleeding, GIT or injection site, hematemesis and/or malena
Positive tourniquet and hepatomegaly.
-
b.
Laboratory parameters: Thrombocytopenia (platelet count <100,000/cu mm)
The hemorrhagic episodes in DHF are associated with multifactorial pathogenesis. Vasculopathy, deficiency and dysfunction of platelets and defects in the blood coagulation pathways are the attributed factors.[30] Decreased production of platelets[31,32] and increased destruction of platelets may result in thrombocytopenia in DHF.[33] The impaired platelet function causes the blood vessels to become fragile and this results in hemorrhage.[34]
The clinical course of DHF is characterized by three phases: Febrile, leakage, and convalescent phase. High-grade fever of acute onset along with constitutional signs and facial erythema characterizes the commencement of the febrile illness.[21] The initial febrile illness is marked by a morbilliform rash and hemorrhagic tendencies.[35] The fever persists for 2 days to 1 week and then drops to normal or subnormal levels when the patient either convalesces or advances to the plasma leakage phase.[36] High plasma escape cases are marked by frank shock with low pulse pressure, cyanosis, hepatomegaly, pleural and pericardial effusions, and ascites. Severe ecchymosis and gastrointestinal bleeding followed by epistaxis may also be noted in a few cases. Bradycardia, confluent petechial rashes, erythema, and pallor are seen during this phase.
Dengue shock syndrome
DSS is defined as DHF accompanied by a unstable pulse, narrow pulse pressure (<20 mmHg), restlessness, cold, clammy skin, and circumoral cyanosis. Progressively worsening shock, multiorgan damage, and disseminated intravascular coagulation account for a high mortality rate associated with DSS. The shock persists for a short span of time and the patient promptly recovers with supportive therapy.[37,38]
OROFACIAL FEATURES
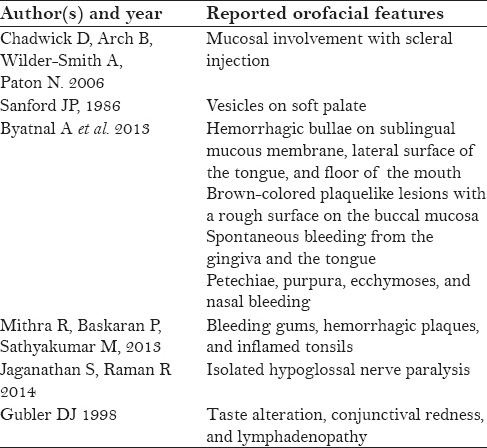
Oral features are infrequently seen in dengue virus infection and are more commonly associated with DHF. Erythema, crusting of lips, and ntongue and soft palatal vesicles constitute the prominent oral features in dengue virus infection. Chadwick et al.[26] reported higher cases involving the mucosa with scleral injection (90%), whereas Sanford noticed vesicular eruptions of the soft palate (>50%).[39] Byatnal et al., reported numerous hemorrhagic bullae on the sublingual mucous membrane, lateral surface of the tongue, and floor of the mouth. Brown-colored plaquelike lesions with a rough surface were seen on the buccal mucosa that showed bleeding on touch along with spontaneous bleeding from the gingiva and the tongue. Petechiae, purpura, ecchymoses, and nasal bleeding have also been reported.[40] Mitra et al. reported bleeding gums, hemorrhagic plaques, and inflamed tonsils in a dengue-infected patient.[41] Isolated hypoglossal nerve palsy following dengue infection is a rare occurence.[42] Taste alteration, conjunctival redness, and lymphadenopathy may also be reported in DF.[3] Table 1 depicts the reported orofacial features of dengue.
Table 1.
Summary of reported orofacial features in dengue[4]
DIAGNOSIS
Cautious attention should be directed at DF if a patient suffers from high fever within 2 weeks of being in the tropics or subtropics.[43] A decreased number of white blood cells (leukopenia), accompanied by a decreased number of platelet count (thrombocytopenia) and metabolic acidosis are the initial changes on laboratory examinations. Microbiological laboratory testing confirms the diagnosis of DF. Virus segregation in cell cultures, nucleic acid demonstration by polymerase chain reaction (PCR), and serological detection of viral antigens (such as NS1) or particular antibodies are the preferred microbiological assays.[5] Viral segregation and nucleic acid demonstration provide precise diagnosis, although the high cost limits the availability of these tests.
DIFFERENTIAL DIAGNOSIS
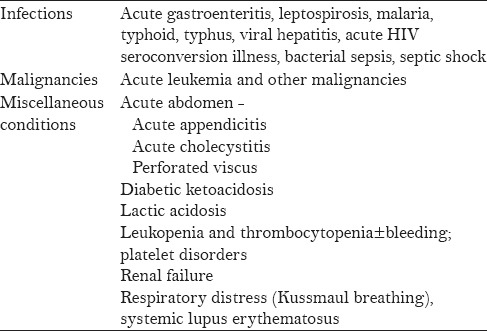
Broad differential diagnosis is considered in a patient presenting with fever and a rash similar to that seen in DF. Tables 2 and 3 present the varied clinical conditions that mimic the febrile and critical phase of dengue infection.
Table 2.
Conditions that mimic the febrile phase of dengue infection[4]

Table 3.
Conditions that mimic the critical phase of dengue infection[4]
MANAGEMENT OF DENGUE INFECTION
Fluid replacement and antipyretic therapy with paracetamol is the preferred therapy following the febrile phase. Care should be taken not to use other nonsteroidal antiinflammatory drugs.
Judicious fluid administration forms the mainstay of treatment during the critical phase of the infection. Normal saline, Ringer's Lactate, and 5% glucose diluted 1:2 or 1:1 in normal saline, plasma, plasma substitutes, or 5% albumin are the routinely administered fluids.
WHO guidelines summarize the following principles of fluid therapy:[44]
Oral fluid supplementation must be as plentiful as possible. However, intravenous fluid administration is mandatory in cases of shock, severe vomiting, and prostration (cases where the patient is unable to take fluids orally)
Crystalloids form the first-line choice of intravenous fluid (0.9% saline)
Hypotensive states that are unresponsive to boluses of intravenous crystalloids, colloids (e.g., dextran) form the second-line measures
If the patient remains in the critical phase with low platelet counts, there should be a serious concern for bleeding. Suspected cases of bleeding are best managed by transfusion of fresh whole blood.
DENTAL MANAGEMENT
Oral lesions are infrequently seen and are often misguided as platelet defects.[25] Significant hemorrhagic manifestations need platelet transfusions. In general, there is no need to give prophylactic platelets even at <20,000/cu mm. Prophylactic platelets may be given at a level of <10,000/cu mm in absence of bleeding manifestations. In case of systemic massive bleeding, platelet transfusion may be needed along with red cell transfusion. Liver functions should be monitored.
ADVANCED RESEARCHES
Control of mosquito (vector) transmission, development of dengue vaccine, and antiviral drugs constitute future directions with an aim to prevent and treat dengue infection.
Control of mosquito (vector) transmission can be done by keeping guppies (Poecilia reticulata) or copepods (doridicola agilis) in standing water, and infecting the mosquito population with bacteria of the Wolbachia genus.[43]
Due to the progressing transmission and enhancing severity of dengue infection, the necessity to develop a dengue vaccine has gained considerable importance. There is a worldwide public health need for a safe, effective, and economic tetravalent dengue vaccine. Complex pathology, the prerequisite to control four virus serotypes, and inadequate investment by vaccine designers have hindered vaccine advancement.[45]
Scrupulous attempts are aimed to develop antiviral drugs that can be used to manage DF and avoid the life-threatening episodes.[46,47]
CONCLUSION
Dengue has evolved as a global life-threatening public health concern, affecting around 2.5 billion individuals in more than 100 countries. The physician should be aware about the varied clinical manifestations of this condition and ensure an early and adequate treatment plan. Future directions to combat this dreadful disease aim at methods of mosquito control, development of vaccine, and antiviral drug regimen.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Halstead SB. Pathogenesis of dengue: Challenges to molecular biology. Science. 1988;239:476–81. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 2.Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis. 2007;30:329–40. doi: 10.1016/j.cimid.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Gubler DJ. Dengue and dengue Hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.New ed. Geneva, Switzerland: World Health Organization; 2009. World Health Organization (WHO). Dengue- Guidelines for Diagnosis, Treatment, Prevention and Control. [PubMed] [Google Scholar]
- 5.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: A continuing global threat. Nat Rev Microbiol. 2010;8(Suppl):S7–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linares EM, Pannuti CS, Kubota LT, Thalhammer S. Immunospot assay based on fluorescent nanoparticles for dengue fever detection. Biosens Bioelectron. 2013;41:180–5. doi: 10.1016/j.bios.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 7.San Martin JL, Brathwaite O, Zanbrano B, Solorzano JO, Bouckenooghe A, Dayan GH, et al. The epidemiology of dengue in the Americas over the last three decades: A worrisome reality. Am J Tropical Med Hyg. 2010;82:128–35. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta N, Srivastava S, Jain A, Chaturvedi UC. Dengue in India. Indian J Med Res. 2012;136:373–90. [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas EA, John M, Bhatia A. Muco-Cutaneous manifstations of dengue viral infection in Punjab. Int J Dermatol. 2007;46:715–19. doi: 10.1111/j.1365-4632.2007.03298.x. [DOI] [PubMed] [Google Scholar]
- 10.Arshad I, Malik FA, Hussain A, Shah SA. Dengue fever: Clinico-pathologic correlations and their association with poor outcome. Professional Med J. 2011;18:57–63. [Google Scholar]
- 11.Wu SJ, Grouard-Vigel G, Sun W, Mascola JR, Brachel E, Putvatana R, et al. Human skin langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–20. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- 12.Bhamarapravati N. Pathology and Pathogenesis of DHF. New Delhi: WHO Meeting; 1980. [Google Scholar]
- 13.Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine. 2011;29:7221–8. doi: 10.1016/j.vaccine.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Guzman MG, Kouri G. Dengue and dengue hemorrhagic fever in the Americas: Lessons and challenges. J Clin Virol. 2003;27:1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 15.Revised and Expanded ed. New Delhi: WHO; 2011. World Health Organization. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever. [Google Scholar]
- 16.Whitehorn J, Farrar J. Dengue. Br Med Bull. 2010;95:161–73. doi: 10.1093/bmb/ldq019. [DOI] [PubMed] [Google Scholar]
- 17.Geneva, Switzerland: World Health Organization; 2009. WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Part 1.1.6: Dengue case classification; pp. 10–2. [PubMed] [Google Scholar]
- 18.Ranjit S, Kissoon N. Dengue hemorrhagic fever and shock syndromes. Pediatr Crit Care Med. 2011;12:90–100. doi: 10.1097/PCC.0b013e3181e911a7. [DOI] [PubMed] [Google Scholar]
- 19.2nd ed. Geneva (Switzerland): World Health Organization; 1997. WHO. Clinical diagnosis. Chapter 2. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control; pp. 12–23. [Google Scholar]
- 20.Ahmed FU, Mahmood CB, Sharma JD, Hoque SM, Zaman R, Hasan MH. Dengue fever and dengue haemorrhagic fever in chidren the 2000 out break in Chittatong, Bangladesh. Dengue Bulletin. 2001;25:33–9. [Google Scholar]
- 21.Narayanan M, Aravind MA, Thilothammal N, Prema R, Sargunam CS, Ramamurty N. Dengue fever epidemic in Chennai-a study of clinical profile and outcome. Indian Pediatr. 2002;39:1027–33. [PubMed] [Google Scholar]
- 22.Chen LH, Wilson ME. Dengue and chikungunya infections in travelers. Curr Opin Infect Dis. 2010;23:438–44. doi: 10.1097/QCO.0b013e32833c1d16. [DOI] [PubMed] [Google Scholar]
- 23.Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117–22. doi: 10.1016/0738-081x(89)90034-5. [DOI] [PubMed] [Google Scholar]
- 24.Itoda I, Masuda G, Suganuma A, Imamura A, Ajisawa A, Yamada K, et al. Clinical features of 62 imported cases of dengue fever in Japan. Am J Trop Med Hyg. 2006;75:470–4. [PubMed] [Google Scholar]
- 25.Radakovic-Fijan S, Graninger W, Müller C, Hönigsmann H, Tanew A. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430–3. doi: 10.1067/mjd.2002.111904. [DOI] [PubMed] [Google Scholar]
- 26.Chadwick D, Arch B, Wilder-Smith A, Paton N. Distinguishing dengue fever from other infections on the basis of simple clinical and laboratory features: Application of logistic regression analysis. J Clin Virol. 2006;35:147–53. doi: 10.1016/j.jcv.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Kabra SK, Juneja R, Madhulika, Jain Y, Singhal T, Dar L, et al. Myocardial dysfunction in children with dengue haemorrhagic fever. Natl Med J India. 1998;11:59–61. [PubMed] [Google Scholar]
- 28.Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, et al. Dengue hemorrhagic fever in infants: Research opportunities ignored. Emerg Infect Dis. 2002;8:1474–9. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Institute of Communicable Diseases. Investigation and Control of Outbreaks: Dengue and Dengue Hemorrhagic Fever. 1997 [Google Scholar]
- 30.Chiu YC, Wu KL, Kuo CH, Hu TH, Chou YP, Chuah SK, et al. Endoscopic findings and management of dengue patients with upper gastrointestinal bleeding. Am J Trop Med Hyg. 2005;73:441–4. [PubMed] [Google Scholar]
- 31.La Russa VF, Innis BL. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin Haematol. 1995;8:249–70. doi: 10.1016/s0950-3536(05)80240-9. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld SJ, Young NS. Viruses and bone marrow failure. Blood Rev. 1991;5:71–7. doi: 10.1016/0268-960x(91)90037-d. [DOI] [PubMed] [Google Scholar]
- 33.Phanichyakarn P, Pongpanich B, Israngkura PB, Dhanamitta S, Valyasevi A. Studies on dengue hemorrhagic fever. III. Serum complement (C3) and platelet studies. J Med Assoc Thai. 1977;60:301–6. [PubMed] [Google Scholar]
- 34.Geneva: World Health Organization; 2001. World Health Organization. Dengue and dengue haemorrhagic fever. Chapter 6. WHO Report on Global Surveillance of Epidemic Prone Infectious Diseases. [Google Scholar]
- 35.Richards AL, Bagus R, Baso SM, Follows GA, Tan R, Graham RR, et al. The first reported outbreak of dengue hemorrhagic fever in Irian Jaya, Indonesia. Am J Trop Med Hyg. 1997;57:49–55. doi: 10.4269/ajtmh.1997.57.49. [DOI] [PubMed] [Google Scholar]
- 36.Srikiatkhachorn A, Krautrachue A, Ratanaprakarn W, Wongtapradit L, Nithipanya N, Kalayanarooj S, et al. Natural history of plasma leakage in dengue hemorrhagic fever: A serial ultrasonographic study. Pediatr Infect Dis J. 2007;26:283–90. doi: 10.1097/01.inf.0000258612.26743.10. [DOI] [PubMed] [Google Scholar]
- 37.Gurugama P, Garg P, Perera J, Wijewickrama A, Seneviratne SL. Dengue viral infections. Indian J Dermatol. 2010;55:68–78. doi: 10.4103/0019-5154.60357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shivpuri A, Shivpuri A. Dengue-An overview. Dent Med Probl. 2011;48:153–6. [Google Scholar]
- 39.Sanford JP. Harrison's Principles of Internal Medicine. 12th ed. Vol. 1. New York: McGraw-Hill; 1986. [Google Scholar]
- 40.Byatnal A, Mahajan N, Koppal S, Ravikiran A, Thriveni R, Parvathi Devi MK. Unusual yet isolated oral manifestations of persistent thrombocytopenia – A rare case report. Braz J Oral Sci. 2013;12:233–6. [Google Scholar]
- 41.Mithra R, Baskaran P, Sathyakumar M. Oral presentation in dengue hemorrhagic fever: A rare entity. J Nat Sci Biol Med. 2013;4:264–7. doi: 10.4103/0976-9668.107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaganathan S, Raman R. Hypoglossal nerve palsy: A rare consequence of dengue fever. Neurol India. 2014;62:567–8. doi: 10.4103/0028-3886.144501. [DOI] [PubMed] [Google Scholar]
- 43.Simmons CP, Farrar JJ, Nguyen V, Wills B. Dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. [Last accessed on 2012 Jun 13]. Available from: http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf . [PubMed]
- 45.Hombach J. Vaccines against dengue: A review of current candidate vaccines at advanced development stages. Rev Panam Salud Pública. 2007;21:254–60. doi: 10.1590/s1020-49892007000300011. [DOI] [PubMed] [Google Scholar]
- 46.Sampath A, Padmanabhan R. Molecular targets for flavivirus drug discovery. Antiviral Res. 2009;81:6–15. doi: 10.1016/j.antiviral.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noble CG, Chen YL, Dong H, Gu F, Lim SP, Schul W, et al. Strategies for development of dengue virus inhibitors. Antiviral Res. 2010;85:450–62. doi: 10.1016/j.antiviral.2009.12.011. [DOI] [PubMed] [Google Scholar]


