Abstract
Background
Retinoic acid (RA), the main biologically active metabolite of vitamin A, is known to promote gut homing of lymphocytes, as well as various regulatory and effector immune responses. In contrast, the active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25D3), is predominantly immunosuppressive. Little is known about the effects of these vitamins on the recently identified innate lymphoid cells (ILCs).
Objective
We sought to characterize the effects of RA and 1,25D3 on human ILCs.
Methods
PBMCs were isolated from 27 non-selected blood donor buffy coats, and ILCs were sorted by FACS. ILC1, ILC2, and ILC3 cells were cultured for 5 days with RA, 1,25D3, and various cytokines known to activate ILCs (IL-2, IL-7, IL-12, thymic stromal lymphopoietin (TSLP), IL-25, and IL-33). Cytokines produced by ILCs were measured in culture supernatants, and surface receptor expression was analyzed by flow cytometry.
Results
RA acted synergistically with IL-2 and other activating cytokines to induce expression of the gut-homing integrin α4β7 in ILCs, as well as production of IL-5 and IL-13 in ILC2 cells, and IFN-γ in ILC1 and ILC3 cells. Expression of integrin α4β7 and cytokine production in ILCs stimulated with RA + IL-2 was increased at least 4-fold as compared to ILCs cultured with RA or IL-2 alone. In contrast, RA completely inhibited the IL-2-induced expression of cutaneous lymphocyte antigen (CLA) in ILCs. Moreover, addition of 1,25D3 to ILCs cultured with RA + IL-2 inhibited cytokine production and expression of integrin α4β7 by at least 30%.
Conclusions
RA and 1,25D3 have antagonistic effects on expression of effector cytokines and gut-homing integrin in human ILCs. The balance between these vitamins may be an important factor in the functioning of ILCs and the diseases in which ILCs are implicated, such as allergic inflammation.
Introduction
Innate lymphoid cells (ILCs) are emerging as important effectors of innate immunity, and are defined by three main features: their lymphoid morphology; the absence of recombination activating gene (RAG)-dependent antigen receptors; and a lack of myeloid cell and dendritic cell phenotypical markers [1]. The prototypical ILC populations are natural killer (NK) cells and lymphoid tissue-inducer (LTi) cells. Recently, several different ILC populations have been identified, which have distinct patterns of cytokine production that mirror the cytokine-secreting profiles of conventional T helper (Th) cell subsets [2]. For example, ILC1 cells produce IFN-γ in response to IL-12 combined with IL-2 or IL-18. These cells accumulate in inflamed intestine in individuals with Crohn’s disease [3]. In contrast, ILC2 cells produce predominantly IL-5 and IL-13, in response to IL-2 combined with IL-25, IL-33, or TSLP. These cells are enriched in the nasal polyps of patients with chronic rhinosinusitis, a condition frequently caused by allergies. Nasal polyp epithelial cells express TSLP, which enhances cytokine production in ILC2 cells [4, 5]. ILC2 cells are also present in healthy human skin, and are enriched in lesional skin from patients with atopic dermatitis (AD). Skin-resident ILC2 cells play a critical role in the development of AD in a murine model, and responses of these cells are dependent on TSLP [6]. Human ILC3 cells can be divided into NKp44+ and NKp44− subsets. In healthy human skin and blood, ILC3 cells are almost exclusively NKp44−, and appear to have a high degree of plasticity. Human NKp44− ILC3 cells can differentiate into NKp44+ ILC3 cells when stimulated with IL-23 and IL-1β, and under influence of IL-12 into ILC1 cells [3, 7]. The key functions of ILCs are thought to be preservation of epithelial integrity and tissue immunity throughout the body [8]. Vitamin A plays an important and pleiotropic role in immunity. It is exclusively provided through the diet, and vitamin A supplementation in deficient individuals improves the clinical outcome of multiple infectious diseases [9]. Retinoic acid (RA), the main biologically active metabolite of vitamin A, has been shown to modulate responses of various immune cells [10]. For example, RA enhances expression of the gut-homing integrin α4β7 in both murine and human B cells and CD4+ T cells [11–13]. Moreover, RA cooperates with TGF-β to promote the conversion of naive CD4+ T cells into Foxp3+ Treg cells in mice as well as humans [14, 15]. In contrast, RA has been shown to activate effector CD4+ T cells under proinflammatory conditions. The RA-retinoic acid receptor α (RARα) axis is essential for the production of the proinflammatory cytokines IFN-γ and IL-17A by Th1 and Th17 cells in response to infection [16]. Furthermore, RA enhances Th2 responses in human CD4+ T cells in vitro and in helminth-infected mice [12, 17–19]. A few recent studies have reported an effect of RA on murine ILCs. Mielke et al. showed that RA promotes production of IL-22 in ILC3 cells stimulated with IL-1β and IL-23 [20]. In addition, Van de Pavert et al. observed that fetal RA signaling controls the differentiation of LTi cells, which are a subset of ILC3 cells and crucial for the formation of secondary lymphoid organs. The authors established that maternal levels of dietary vitamin A control the size of secondary lymphoid organs and the efficiency of immune responses in the adult offspring [21].
Vitamin D is synthesized in the skin upon exposure to sunlight, and is provided through the diet. In its hormonally active form of 1,25-dihydroxyvitamin D3 (1,25D3), vitamin D is known to have a predominantly immunosuppressive effect [22]. Accordingly, low serum levels of vitamin D have been linked to a higher susceptibility to autoimmune and allergic diseases [23, 24]. 1,25D3 inhibits production of IFN-γ by human Th1 cells in vitro but has little direct effect on Th2 cells [25, 26]. However, 1,25D3 can inhibit allergen-specific Th2 cytokine responses indirectly by acting on dendritic cells (DCs) to reduce expression of the co-stimulatory molecule OX40 ligand (OX40L) and enhance production of TGF-β [27]. Furthermore, 1,25D3 promotes the differentiation of both IL-10+ and FoxP3+ Treg cells [28]. To our knowledge, the effect of 1,25D3 on ILCs has not yet been investigated.
In the present study, we hypothesized that RA and 1,25D3 influence human ILC responses. Because our primary interest is in allergy, we put emphasis on ILC2 cells. We observed that RA acts synergistically with IL-2 and other cytokines known to stimulate ILCs, to induce expression of the gut-homing integrin α4β7, as well as production of IL-5 and IL-13 in ILC2 cells, and IFN-γ in ILC1 and ILC3 cells. In contrast, 1,25D3 inhibited the effects of RA on cytokine production and expression of gut-homing integrin. Together, these data shed a light on the effect of two immunomodulatory vitamins on human ILC responses. The balance between these vitamins, which has shifted with industrialization, may be an important factor in the development of allergic inflammation and other chronic immune disorders in which ILCs play a role.
Materials and Methods
Blood donors and isolation of ILCs
Buffy coats were obtained from 27 non-selected, de-identified blood donors at the Massachusetts General Hospital Blood Donor Center (Boston, MA). The study was approved by the Institutional Review Board of Partners Healthcare (Boston, MA). Peripheral blood mononuclear cells (PBMCs) were isolated by means of density gradient centrifugation (Ficoll-Paque Plus; GE Healthcare). For depletion of T cells, monocytes, NK cells, B cells, and residual red blood cells, PBMCs were labeled with FITC-conjugated anti-CD3 (clone OKT3; BioLegend), anti-CD14 (M5E2), anti-CD16 (3G8), anti-CD19 (HIB19), and anti-CD235a (GA-R2) (all from BD Biosciences). Cells were washed and labeled with FITC selection cocktail (FITC Positive Selection kit), and magnetic D particles (Stemcell Technologies). After magnetic separation, the negative fraction was subsequently labeled with FITC-conjugated anti-CD3, anti-CD14, anti-CD16, and anti-CD19, as well as FITC-conjugated anti-CD1a (HI149), anti-CD11c (3.9), anti-CD94 (DX22), anti-CD123 (6H6), anti-FcεRIα (AER-37) (all from BioLegend), anti-CD4 (RPA-T4), anti-CD34 (581), anti-CD56 (NCAM16.2), anti-TCRαβ (T10B9.1A-31), anti-TCRγδ (B1) (all from BD Biosciences), and anti-BDCA2 (CD303; AC144; Miltenyi Biotec). Upon labeling with these lineage markers, cells were washed and labeled with V450-conjugated anti-CRTH2 (CD294; BM16; BD Biosciences), PE-conjugated anti-NKp44 (CD336; P44-8), APC-conjugated anti-c-Kit (CD117; 104D2) (both from BioLegend), and PE-Cy7-conjugated anti-CD127 (eBioRDR5; eBioscience). Cells were washed again and sorted with a FACSAria II instrument (BD Biosciences) (Supp. Fig. 1A). In a subset of experiments, anti-NKp44 was omitted from the labeling panel for sorting, and aliquots of sorted ILCs were labeled with anti-CRTH2, anti-c-Kit, anti-CD127, and PE-conjugated anti-ST2 (polyclonal; R&D Systems), before analysis by flow cytometry (Supp. Fig. 1B–C).
Culture of ILCs
Sorted ILC1, ILC2, and ILC3 cells were cultured in 96-well round-bottom plates for 5 days in StemSpan SFEM serum-free medium (Stemcell Technologies) with penicillin (100 U/ml) and streptomycin (100 μg/ml) (Life Technologies), but without additional supplements. ILCs were cultured with combinations of RA (all-trans retinoic acid; 10-fold dilutions from 0.5–500 nM, a concentration of 50 nM was used unless stated otherwise; Sigma), 1,25D3 (1α,25-Dihydroxyvitamin D3; 10-fold dilutions from 0.5–50 nM; Sigma), RAR antagonist (LE540; 1 μM; Wako Chemicals), RAR inverse agonist (BMS 493; 1 μM; Tocris Bioscience), IL-2 (10 U/ml), IL-7 (10 ng/ml), IL-12 (50 ng/ml), IL-25 (50 ng/ml), IL-33 (50 ng/ml), and TSLP (50 ng/ml) (all from R&D Systems). The number of plated cells per well ranged from 1,000–5,000 cells for ILC1 and ILC2 cells, and from 2,000–10,000 cells for ILC3 cells. PMA (10 ng/ml; Sigma) and calcium ionophore (250 ng/ml; Sigma) were added for the last 24 hours of culture [4].
Cytokine analysis and flow cytometry
After culture of ILCs, supernatants were harvested for cytokine analysis. IL-4, IL-5, IL-9, IL-13, IL-17A, and IFN-γ were measured with Cytometric Bead Array (CBA) Flex Sets (BD Biosciences). IL-22 was measured with Milliplex Immunology Multiplex Assay (EMD Millipore). The limits of detection were 1.4 pg/ml for IL-4, 1.1 pg/ml for IL-5, 3.1 pg/ml for IL-9, 0.6 pg/ml for IL-13, 0.3 pg/ml for IL-17A, 8 pg/ml for IL-22, and 1.8 pg/ml for IFN-γ.
Cytokine data shown in this paper were normalized to 2,000 ILCs per 200 μl culture volume. ILCs were labeled with FITC-conjugated anti-integrin β7 (FIB504), PE-conjugated anti-integrin α4 (CD49d; 9F10) (both from eBioscience), PE-Cy7-conjugated anti-integrin αE (CD103; Ber-ACT8; BioLegend), AF647-conjugated anti-CLA (HECA-452; BD Biosciences) or APC-conjugated anti-integrin β1 (CD29; MAR4; BD Biosciences), and violet cell viability dye (L34955; Life Technologies). In a subset of experiments, ILCs were labeled with cell viability dye, fixed and permeabilized (Fixation/Permeabilization solution kit; BD Biosciences), and labeled with PE-conjugated anti-Ki-67 (Ki-67; BioLegend). Flow cytometry was performed with an LSR II instrument (BD Biosciences). Data were analyzed using FlowJo software (TreeStar Inc.).
Data representation and statistical analysis
The boxplots shown in this paper represent first and third quartiles, minimum, maximum, and median values for each variable. The D’Agostino and Pearson omnibus normality test or Shapiro-Wilk normality test was used to assess whether data sets had a Gaussian distribution. Statistical significance was determined with the paired t-test if data were normally distributed, or with the Wilcoxon matched-pairs signed-rank test if data were not normally distributed. Prism 5 software (GraphPad) was used.
Results
RA acts synergistically with IL-2 and other cytokines to induce production of IL-5, IL-13, and IFN-γ in human ILCs
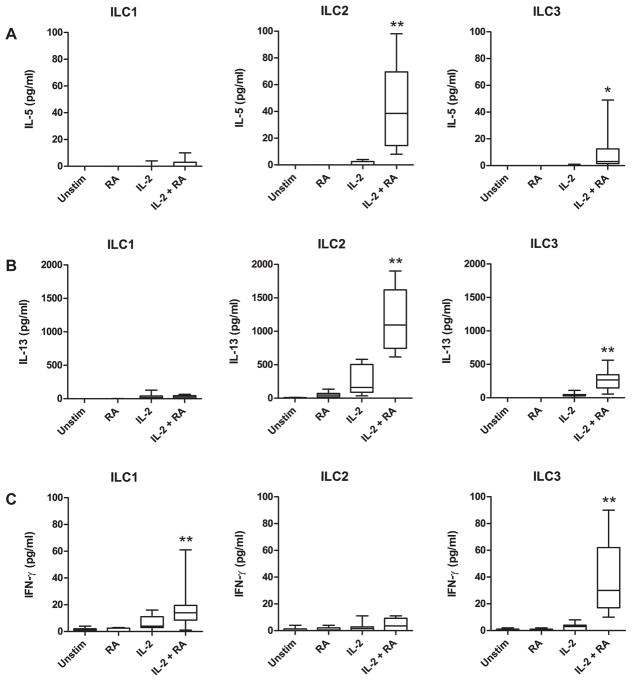
The gating strategy for the sorting of human ILCs from PBMCs was adapted from Bernink et al. [3] and is shown in Supp. Fig. 1A. ILC1 cells (Lin−CD127+CRTH2−c-Kit−NKp44−), ILC2 cells (Lin−CD127+CRTH2+), and NKp44− ILC3 cells (Lin−CD127+CRTH2−c-Kit+NKp44−), were readily detected. In agreement with a previous study, NKp44+ ILC3 cells (Lin−CD127+CRTH2−c-Kit+NKp44+) were very rare in healthy human peripheral blood [7]. Expression of the IL-33 receptor ST2 was observed on ILC2 cells, as well as on ILC3 cells (Supp. Fig. 1B–C). After sorting, ILC1, ILC2, and ILC3 cells were cultured with RA and various cytokines known to stimulate ILCs. We observed a synergistic effect of RA and IL-2 on the induction of IL-5, IL-13, and IFN-γ (Fig. 1A–C). IL-4, IL-9, IL-17A, and IL-22 were below detection limits (data not shown). Production of IL-5 in ILC2 cells, IL-13 in ILC2 and ILC3 cells, as well as IFN-γ in ILC1 and ILC3 cells, was 4–40 fold higher in cultures with RA + IL-2 than with RA or IL-2 alone (p<0.01). The effect of RA on cytokine production in ILCs was dose-dependent (Supp. Fig. 2).
Fig. 1.
RA acts synergistically with IL-2 to induce cytokine production in human ILCs. Concentrations of IL-5 (A), IL-13 (B), and IFN-γ (C) were measured in culture supernatants of ILC1, ILC2, and ILC3 cells incubated with the indicated stimuli. Cytokine data were normalized to 2,000 ILCs per 200 μl culture volume. Combined data of nine independent experiments and subjects are shown. * p<0.05, ** p<0.01 (cultures with IL-2 + RA vs. cultures with IL-2 or RA alone).
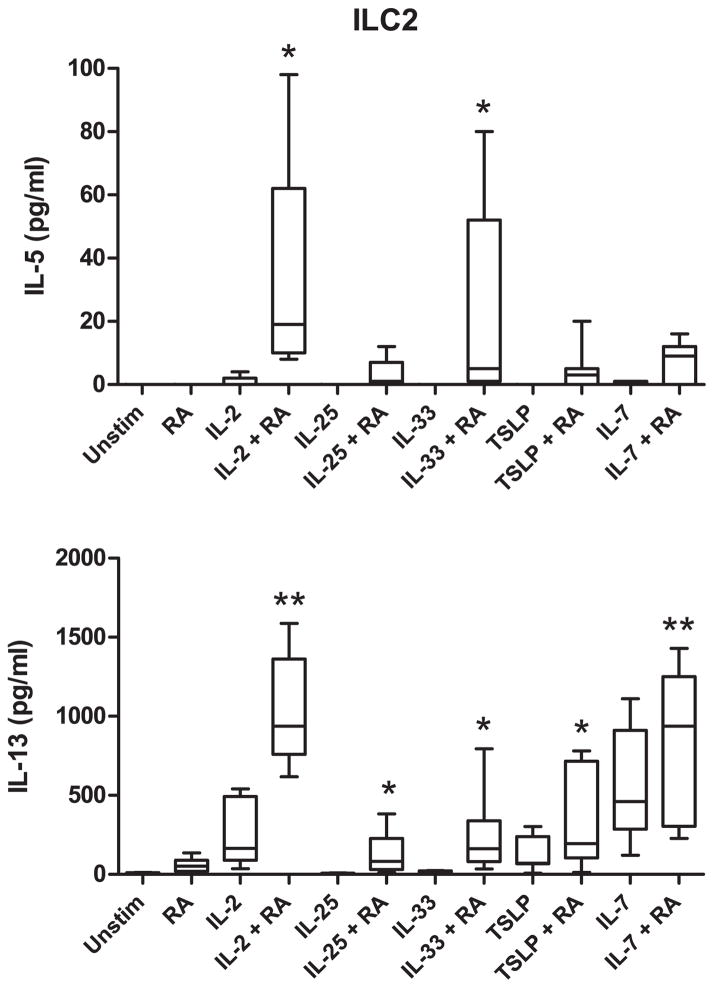
Because our primary interest was in the effect of RA on ILC2 cells and its potential role in allergic disease, we next tested combinations of RA with various cytokines known to activate this cell population. As shown in Fig. 2, RA significantly enhanced IL-5 production when combined with IL-2 or IL-33, whereas combinations of RA with IL-2, IL-25, IL-33, TSLP, or IL-7 led to an increase in production of IL-13. In contrast, RA enhanced the production of IFN-γ in ILC1 and ILC3 cells when combined with IL-2, but not with IL-12 or IL-7 (Supp. Fig. 3).
Fig. 2.
RA acts synergistically with activating cytokines to induce production of IL-5 and IL-13 in human ILC2 cells. Concentrations of IL-5 and IL-13 were measured in culture supernatants of ILC2 cells incubated with the indicated stimuli. Cytokine data were normalized to 2,000 ILCs per 200 μl culture volume. Combined data of seven independent experiments and subjects are shown. * p<0.05, ** p<0.01 (cultures with cytokine + RA vs. cultures with cytokine alone).
Lastly, we compared the magnitude of the synergistic effect of RA with that of conventional ILC1 and ILC2 agonists. Whereas IL-2 + RA induced a marked IFN-γ response in ILC1 cells, a combination of IL-2 + IL-12 was even more potent. In ILC2 cells, combinations of IL-2 + IL-25 and IL-2 + TSLP induced responses similar to IL-2 + RA, while IL-2 + IL-33 triggered higher production of IL-5 and IL-13 (Supp. Fig. 4).
RA acts synergistically with IL-2 and other cytokines to induce expression of the gut-homing integrin α4β7 in human ILCs
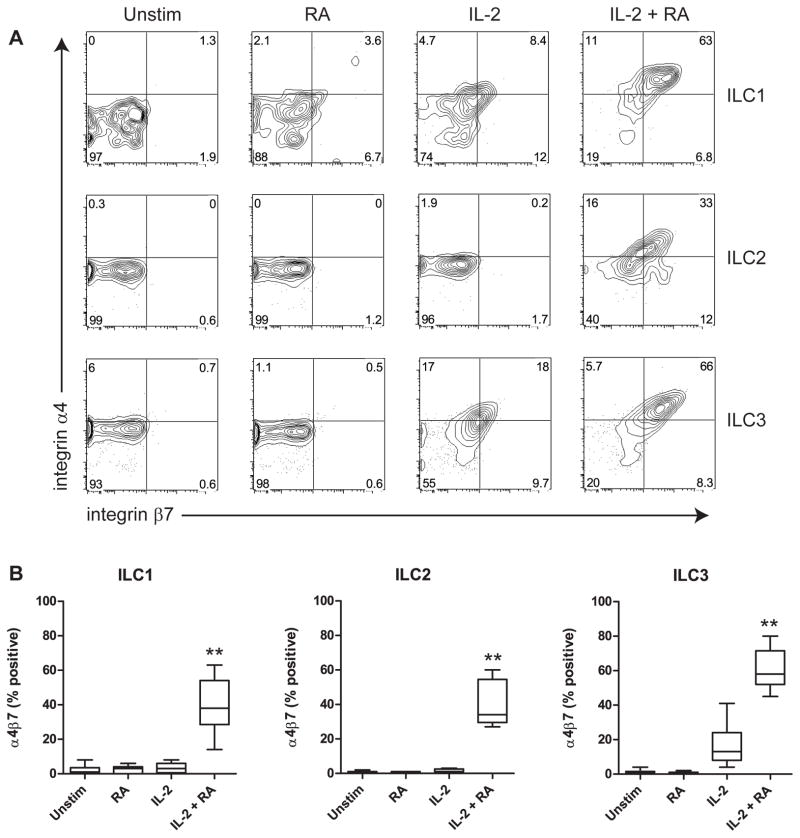
RA has previously been shown to induce expression of integrin α4β7 in human CD4+ T cells [12]. To investigate whether RA has a similar effect on human ILCs, we measured the expression of α4β7 by flow cytometry after ILC culture as described above. In all three ILC populations, we observed a very strong induction of α4β7 in cultures with RA + IL-2 (Fig. 3). In contrast, neither RA nor IL-2 alone had a significant effect, except in ILC3 cells, where IL-2 alone modestly induced α4β7. Similar to cytokine production, induction of α4β7 expression by RA was dose-dependent (Supp. Fig. 5). RA also synergized with IL-7 to enhance α4β7 expression in all three ILC populations, whereas RA induced α4β7 in ILC2 cells when combined with TSLP (Supp. Fig. 6A).
Fig. 3.
RA acts synergistically with IL-2 to induce expression of integrin α4β7 on human ILCs. A, Representative example of α4β7 expression on ILCs, gated on live cells. B, Expression of α4β7 was measured on ILCs incubated with the indicated stimuli. Combined data of nine independent experiments and subjects are shown. ** p<0.01 (cultures with IL-2 + RA vs. cultures with IL-2 or RA alone).
Of the individual integrin chains that constitute the α4β7 heterodimer, integrin α4 can also pair with integrin β1 to form α4β1, and integrin β7 can pair with integrin αE (CD103) to form αEβ7. We observed that in addition to integrins α4 and β7, also integrin β1 is induced in ILCs by RA in synergy with IL-2, as well as by IL-7 alone (Supp. Fig. 6B). In contrast, expression of integrin αE was not significantly induced by any of the used stimuli (data not shown). Whereas we were technically only able to assess expression of the individual integrin chains, it seems likely that expression of both α4β7 and α4β1 heterodimers is induced by RA + IL-2 in ILCs.
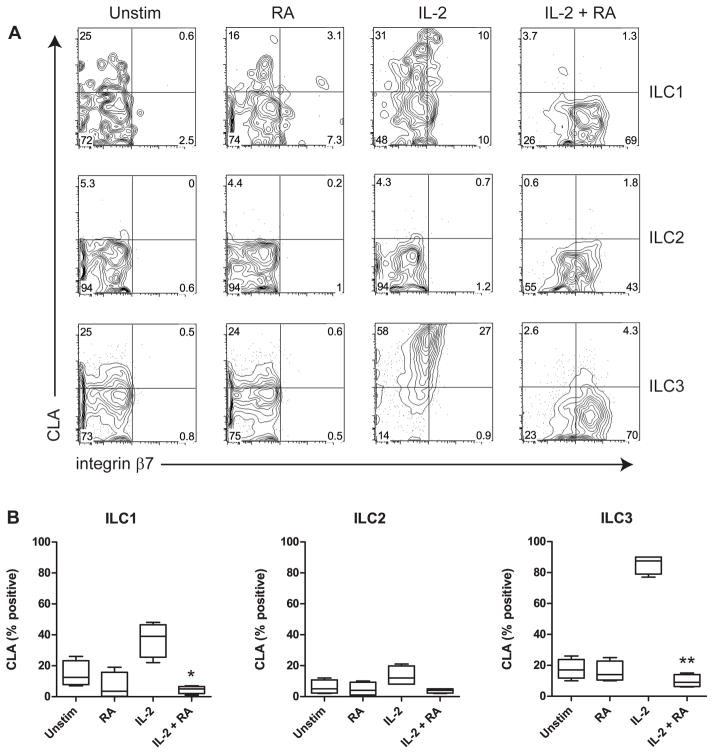
To further study the effect of RA on surface markers involved in homing to organs other than the gut, we also analyzed the expression of the skin-homing receptor cutaneous lymphocyte antigen (CLA) (Fig. 4). Interestingly, stimulation with IL-2 alone induced CLA expression in ILC1 cells and especially ILC3 cells, but addition of RA strongly decreased expression of CLA. Comparable results were found when IL-7 was used instead of IL-2 (Supp. Fig. 6C). Together, these data show that RA induces gut-homing integrin in human ILCs, whereas homing to the skin may be inhibited.
Fig. 4.
RA inhibits expression of CLA on human ILCs. A, Representative example of CLA and integrin β7 expression on ILCs, gated on live cells. B, Expression of CLA was measured on ILCs incubated with the indicated stimuli. Combined data of four independent experiments and subjects are shown. * p<0.05, ** p<0.01 (cultures with IL-2 + RA vs. cultures with IL-2 alone).
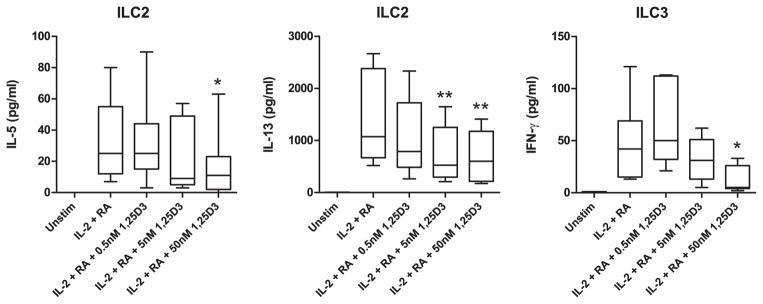
1,25D3 inhibits RA-induced cytokine production in human ILCs
1,25D3 has a predominantly immunosuppressive effect on human lymphocytes, and several groups have found an association between serum vitamin D levels and allergic disease [22, 24]. We investigated whether 1,25D3 could suppress RA-induced cytokine responses in human ILCs. Production of IL-5 and IL-13 in ILC2 cells stimulated with RA + IL-2, as well as production of IFN-γ in ILC3 cells, was inhibited by 1,25D3 in a dose-dependent manner (Fig. 5). The decrease in cytokine production did not appear to be due to non-specific effects of 1,25D3 on ILCs. Whereas IL-2 promoted ILC survival as well as proliferation, both RA and 1,25D3 did not significantly influence the proliferation or viability of ILCs (Supp. Fig. 7).
Fig. 5.
1,25D3 inhibits RA-induced cytokine production in human ILCs. Concentrations of IL-5, IL-13, and IFN-γ were measured in culture supernatants of ILC2 and ILC3 cells incubated with the indicated stimuli. Cytokine data were normalized to 2,000 ILCs per 200 μl culture volume. Combined data of seven independent experiments and subjects are shown. * p<0.05, ** p<0.01 (cultures with IL-2 + RA vs. cultures with IL-2 + RA + 1,25D3).
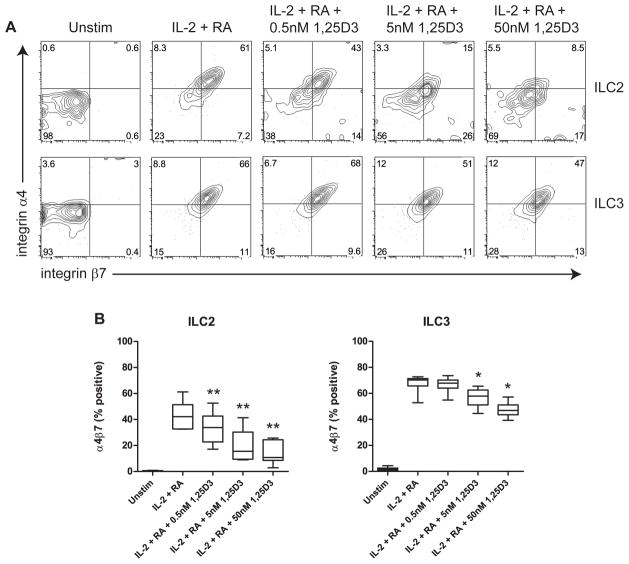
1,25D3 inhibits RA-induced expression of the gut-homing integrin α4β7 in human ILCs
In the same ILC cultures that were used for measurement of cytokine responses, we studied the effect of 1,25D3 on RA-induced expression of α4β7. In both ILC2 and ILC3 cells cultured with RA + IL-2, expression of α4β7 was significantly and dose-dependently inhibited by 1,25D3 (Fig. 6). In contrast, the inhibition of CLA expression by RA was not reversed by 1,25D3 (data not shown).
Fig. 6.
1,25D3 inhibits RA-induced expression of integrin α4β7 on human ILCs. A, Representative example of α4β7 expression on ILCs, gated on live cells. B, Expression of α4β7 was measured on ILC2 and ILC3 cells incubated with the indicated stimuli. Combined data of seven independent experiments and subjects are shown. * p<0.05, ** p<0.01 (cultures with IL-2 + RA vs. cultures with IL-2 + RA + 1,25D3).
Discussion
Allergic and other chronic immune-mediated diseases have become rapidly more prevalent in association with modernization and urbanization around the world [29–31]. There are myriad environmental changes suggested to partially explain this association. The ‘vitamin hypothesis’ posits that nutritional shifts accompanying these lifestyle changes, including increasing vitamin A sufficiency and vitamin D deficiency, may contribute to this trend by influencing conventional CD4+ T cell, B cell and basophil responses [22, 24, 32]. Our findings presented here suggest that ILCs may also be influenced in a pro-inflammatory manner by those changes.
The active metabolites of vitamins A and D have long been known to modulate responses of both innate and adaptive immune cells [22]. We observed that RA acts in synergy with IL-2 and other activating cytokines to induce production of effector cytokines in three recently identified types of human ILCs. In addition, RA combined with activating cytokines enhances the expression of the gut-homing integrin α4β7 in ILCs, whereas RA alone markedly inhibits the expression of the skin-homing receptor CLA. Our findings are in agreement with multiple papers reporting similar effects of RA on other lymphocytes, as well as DCs. For instance, RA induces production of Th1 and Th2 cytokines in CD4+ T cells [16–19]. Moreover, RA-induced expression of integrin α4β7 has been shown before in T cells and B cells [11–13]. Expression of CLA in human T cells has previously been reported to be downregulated by RA as well as 1,25D3 [33]. Whereas RA strongly decreased CLA expression in ILCs, we did not observe an inhibitory effect of 1,25D3 (data not shown).
A recent publication indicated that IL-13-producing ILC2 cells are enriched in the small intestinal lamina propria of vitamin A-deficient mice, whereas ILC3 cells are diminished [34]. Moreover, those authors observed that production of IL-13 in human Lin−CD127+c-Kit+ ILCs cultured with IL-7 and stem cell factor (SCF) was enhanced by a RAR inverse agonist (RAi). We tested the same RAi as well as a RAR antagonist in ILC2 cell cultures in combination with IL-2 or IL-7, but did not find any induction of IL-13 or IL-5 in these cells (Supp. Fig. 8). In contrast, stimulation with RA + IL-2 resulted in marked induction of IL-5 and IL-13 in ILC2 cells, and a combination of RA + IL-7 induced IL-13 production. An explanation for this discrepancy may be, that Spencer et al. used the c-Kit ligand SCF in their ILC cultures and therefore focused on the effect of RA on differentiating ILCs, whereas we studied fully differentiated, CRTH2+ ILC2 cells. It is possible that RA has profoundly different effects on ILC development and differentiation than on the effector response of mature ILCs. In addition, based on their gating strategy, ILC2 and ILC3 cells were co-cultured, leaving open the possibility of cell-cell interactions and effects of ILC3-secreted factors on ILC2 cells that we did not observe in our pure ILC2 cell cultures.
The ILC1 and ILC2 cells described in this paper exclusively produced type 1 (IFN-γ) and type 2 (IL-5, IL-13) cytokines, respectively, which suggests that these cells are fully committed. In contrast, human peripheral blood NKp44− ILC3 cells appear to be less differentiated. These cells possess characteristics of both ILC1 and ILC2 cells, as shown by their production of IFN-γ and IL-13, as well as expression of ST2. Moreover, production of the ILC3 cytokines IL-17A and IL-22 was below detection limits. It has been suggested previously that NKp44− ILC3 cells have a high degree of plasticity, which allows these cells to differentiate into NKp44+ ILC3 cells when stimulated with IL-23 and IL-1β, and into ILC1 cells when cultured with IL-12. Human NKp44+ ILC3 cells are mainly tissue-resident and express hallmark ILC3 genes [3, 7, 8]. Whereas the stimuli used in our study did not promote the expression of IL-17A and IL-22 in NKp44− ILC3 cells, the effects of RA and 1,25D3 on these cells were in line with those observed on ILC1 and ILC2 cells.
An important question is how activation of ILC2 cells by RA in synergy with IL-2 and other cytokines, likely to be present at sites of allergic inflammation, might take place in vivo, and what the consequences would be. Based on our sort data, the frequencies of ILC1, ILC2, and ILC3 cells in human PBMCs are approximately 0.01%, 0.015%, and 0.02%, respectively, which is consistent with published studies indicating that ILCs are mainly tissue-resident. ILC2 cells are abundantly present in human skin and lung, and constitute a minor ILC population in the gut, where ILC3 cells are the most prevalent [8]. Accumulation of ILC2 cells has been shown to occur in lesional skin of patients with AD [6, 35]. Moreover, ILC2 cells infiltrated the skin after intraepidermal allergen challenge in a house dust mite-allergic patient, where the cells produced IL-5 and IL-13 [35]. It appears that TSLP, IL-25 and IL-33 play important roles in the activation of ILC2 cells and their promotion of AD [6, 35]. In this context of chronic allergic inflammation, ILC2 cells are likely to be exposed to activating cytokines as well as RA, since it has been shown that skin-resident CD103− DCs constitutively produce RA [36].
AD is often the first manifestation of the ‘atopic march’, and approximately one-third of young children with moderate-to-severe AD develop food allergies, which are frequently participating triggers of the inflammation [37]. An important hallmark of AD is a defective epidermal barrier function, which allows the penetration of environmental triggers such as allergens, and their interaction with the immune system. In fact, the skin has been postulated to be an important route of sensitization to peanut allergen [38–40]. Recently, we observed that peanut protein induces expression of the enzyme retinaldehyde dehydrogenase 2 (RALDH2) and production of RA in human myeloid DC (unpublished data) [41]. A similar effect of peanut protein on skin-resident DC, in addition to their constitutive RA production, may contribute to an environment relatively rich in RA. In combination with activating cytokines, this could lead to production of IL-5 and IL-13 by skin-resident ILC2 cells, as well as homing of activated ILC2 cells to the gut. Here, ILC2 cells may cooperate with Th2 cells in orchestrating allergic inflammation after sensitization, as was recently shown in a murine model for peanut allergy [42].
The effects of 1,25D3 are generally immunosuppressive, and vitamin D deficiency has been linked to higher susceptibility for autoimmune and allergic diseases [22–24]. Recent studies have found that low serum vitamin D levels are correlated with both AD and food allergy [43, 44]. We observed that 1,25D3 inhibits RA-induced production of effector cytokines and expression of gut-homing integrin in human ILCs. ILC2 cells have been shown to enhance allergic inflammation, and two recent studies reported that ILC2 cells also facilitate sensitization to allergens [6, 35, 42, 45–47]. Inhibition of ILC2 cell activation and gut homing by 1,25D3 could therefore ameliorate allergic inflammation, particularly in the gastro-intestinal tract, and perhaps in this way play a role in prevention of allergic disease.
In summary, we have shown that the biologically active metabolites of vitamins A and D have profound effects on cytokine production and homing of human ILCs. Our data provide additional evidence for a mechanism by which alterations in nutrition and exposure to sunlight may influence immunity.
Supplementary Material
Acknowledgments
We would like to thank Ravi Mylvaganam and Christina Luo at the Massachusetts General Hospital flow cytometry core facility for their help with cell sorting and flow cytometry (shared instrumentation grant number 1S10RR023440-01A1). This study was supported by institutional funds.
Footnotes
Conflict of interest disclosure
The authors declare no conflict of interest.
References
- 1.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–75. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 2.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews Immunology. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 3.Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, Bemelman WA, Mjosberg JM, Spits H. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nature immunology. 2013;14:221–9. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 4.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature immunology. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 5.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teunissen MB, Munneke JM, Bernink JH, Spuls PI, Res PC, Te Velde A, Cheuk S, Brouwer MW, Menting SP, Eidsmo L, Spits H, Hazenberg MD, Mjosberg J. Composition of Innate Lymphoid Cell Subsets in the Human Skin: Enrichment of NCR ILC3 in Lesional Skin and Blood of Psoriasis Patients. The Journal of investigative dermatology. 2014 doi: 10.1038/jid.2014.146. [DOI] [PubMed] [Google Scholar]
- 8.Hazenberg MD, Spits H. Human innate lymphoid cells. Blood. 2014 doi: 10.1182/blood-2013-11-427781. [DOI] [PubMed] [Google Scholar]
- 9.Villamor E, Fawzi WW. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–64. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic Acid. J Immunol. 2014;192:2953–8. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 11.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Spiegl N, Didichenko S, McCaffery P, Langen H, Dahinden CA. Human basophils activated by mast cell-derived IL-3 express retinaldehyde dehydrogenase-II and produce the immunoregulatory mediator retinoic acid. Blood. 2008;112:3762–71. doi: 10.1182/blood-2008-01-135251. [DOI] [PubMed] [Google Scholar]
- 13.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 14.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–33. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 16.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–47. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wansley DL, Yin Y, Prussin C. The retinoic acid receptor-alpha modulators ATRA and Ro415253 reciprocally regulate human IL-5+ Th2 cell proliferation and cytokine expression. Clin Mol Allergy. 2013;11:4. doi: 10.1186/1476-7961-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson HD, Collins G, Pyle R, Key M, Weeraratna A, Deep-Dixit V, Nadal CN, Taub DD. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC immunology. 2006;7:27. doi: 10.1186/1471-2172-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broadhurst MJ, Leung JM, Lim KC, Girgis NM, Gundra UM, Fallon PG, Premenko-Lanier M, McKerrow JH, McCune JM, Loke P. Upregulation of retinal dehydrogenase 2 in alternatively activated macrophages during retinoid-dependent type-2 immunity to helminth infection in mice. PLoS Pathog. 2012;8:e1002883. doi: 10.1371/journal.ppat.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mielke LA, Jones SA, Raverdeau M, Higgs R, Stefanska A, Groom JR, Misiak A, Dungan LS, Sutton CE, Streubel G, Bracken AP, Mills KH. Retinoic acid expression associates with enhanced IL-22 production by gammadelta T cells and innate lymphoid cells and attenuation of intestinal inflammation. The Journal of experimental medicine. 2013;210:1117–24. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, Labao-Almeida C, Godinho-Silva C, Konijn T, Schooneman D, O’Toole T, Mizee MR, Habani Y, Haak E, Santori FR, Littman DR, Schulte-Merker S, Dzierzak E, Simas JP, Mebius RE, Veiga-Fernandes H. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–7. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature reviews Immunology. 2008;8:685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:217–26. doi: 10.1007/s12016-013-8361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muehleisen B, Gallo RL. Vitamin D in allergic disease: shedding light on a complex problem. The Journal of allergy and clinical immunology. 2013;131:324–9. doi: 10.1016/j.jaci.2012.12.1562. [DOI] [PubMed] [Google Scholar]
- 25.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987;84:3385–9. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. The Journal of nutrition. 1995;125:1704S–08S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 27.Kreindler JL, Steele C, Nguyen N, Chan YR, Pilewski JM, Alcorn JF, Vyas YM, Aujla SJ, Finelli P, Blanchard M, Zeigler SF, Logar A, Hartigan E, Kurs-Lasky M, Rockette H, Ray A, Kolls JK. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. The Journal of clinical investigation. 2010;120:3242–54. doi: 10.1172/JCI42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urry Z, Chambers ES, Xystrakis E, Dimeloe S, Richards DF, Gabrysova L, Christensen J, Gupta A, Saglani S, Bush A, O’Garra A, Brown Z, Hawrylowicz CM. The role of 1alpha, 25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur J Immunol. 2012;42:2697–708. doi: 10.1002/eji.201242370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eder W, Ege MJ, von Mutius E. The asthma epidemic. The New England journal of medicine. 2006;355:2226–35. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 30.Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. The Journal of allergy and clinical immunology. 2014;133:291–307. doi: 10.1016/j.jaci.2013.11.020. quiz 08. [DOI] [PubMed] [Google Scholar]
- 31.Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010;34:J168–77. doi: 10.1016/j.jaut.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff SC. “Vitamin hypothesis”: explanation for allergy increase? Blood. 2008;112:3535–6. doi: 10.1182/blood-2008-06-163089. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka K, Dimitroff CJ, Fuhlbrigge RC, Kakeda M, Kurokawa I, Mizutani H, Kupper TS. Vitamins A and D are potent inhibitors of cutaneous lymphocyte-associated antigen expression. The Journal of allergy and clinical immunology. 2008;121:148–57. e3. doi: 10.1016/j.jaci.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Jr, Wang J, Ramalingam TR, Bhandoola A, Wynn TA, Belkaid Y. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–7. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, Fallon PG, Ogg GS. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. The Journal of experimental medicine. 2013;210:2939–50. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–68. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 37.Bergmann MM, Caubet JC, Boguniewicz M, Eigenmann PA. Evaluation of food allergy in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2013;1:22–8. doi: 10.1016/j.jaip.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. The New England journal of medicine. 2003;348:977–85. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 39.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. The Journal of allergy and clinical immunology. 2009;123:417–23. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen KM, Maleki SJ, Sampson HA, Berin MC. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. The Journal of clinical investigation. 2014;124:4965–75. doi: 10.1172/JCI75660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiter B, Fleming E, Hurlburt BK, Maleki SJ, Shreffler WG. Peanut protein induces expression of RALDH2 in human dendritic cells in a TLR2-dependent manner. The Journal of allergy and clinical immunology. 2014;133:AB92. doi: 10.1016/j.jaci.2020.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu DK, Mohammed-Ali Z, Jimenez-Saiz R, Walker TD, Goncharova S, Llop-Guevara A, Kong J, Gordon ME, Barra NG, Gillgrass AE, Van Seggelen H, Khan WI, Ashkar AA, Bramson JL, Humbles AA, Kolbeck R, Waserman S, Jordana M. T helper cell IL-4 drives intestinal Th2 priming to oral peanut antigen, under the control of OX40L and independent of innate-like lymphocytes. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.29. [DOI] [PubMed] [Google Scholar]
- 43.Noh S, Park CO, Bae JM, Lee J, Shin JU, Hong CS, Lee KH. Lower vitamin D status is closely correlated with eczema of the head and neck. The journal of allergy and clinical immunology. 2014;133:1767–70. e6. doi: 10.1016/j.jaci.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 44.Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, Wake M, Vuillermin P, Martin P, Matheson M, Lowe A, Robinson M, Tey D, Osborne NJ, Dang T, Tina Tan HT, Thiele L, Anderson D, Czech H, Sanjeevan J, Zurzolo G, Dwyer T, Tang ML, Hill D, Dharmage SC. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. The journal of allergy and clinical immunology. 2013;131:1109–16. 16 e1–6. doi: 10.1016/j.jaci.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 45.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, Hendriks RW. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–16. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 46.Gold MJ, Antignano F, Halim TY, Hirota JA, Blanchet MR, Zaph C, Takei F, McNagny KM. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. The journal of allergy and clinical immunology. 2014;133:1142–8. doi: 10.1016/j.jaci.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 47.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–35. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.