Abstract
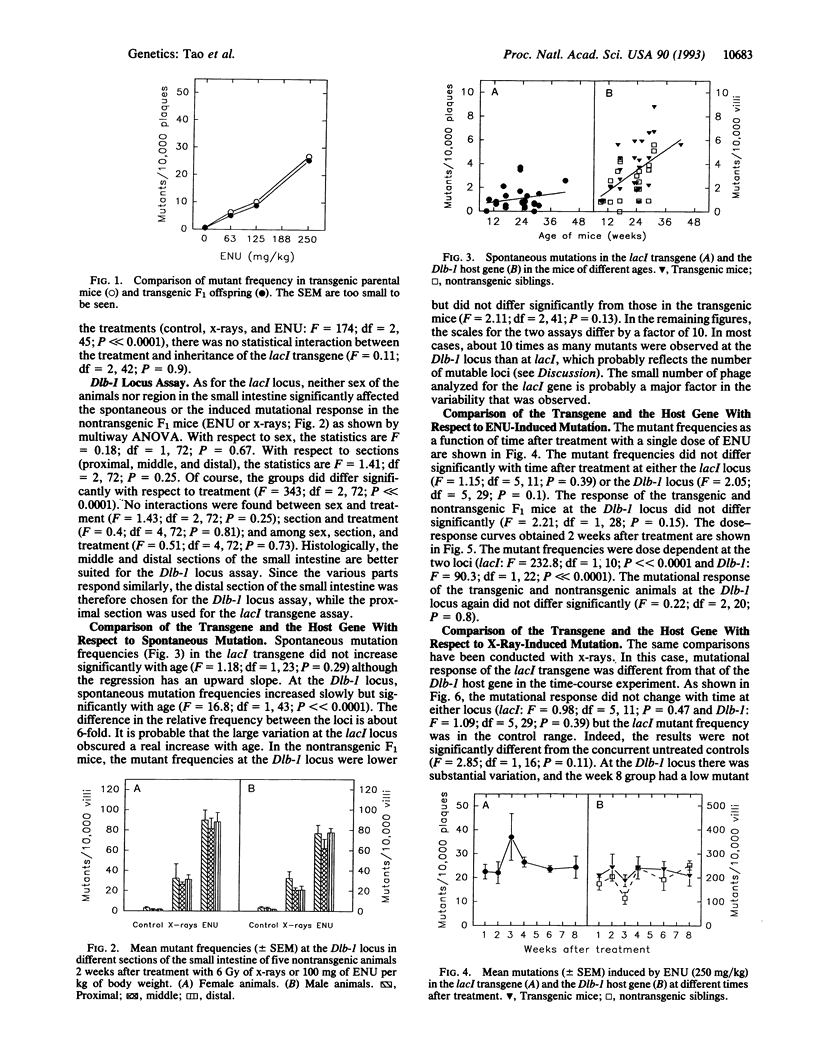
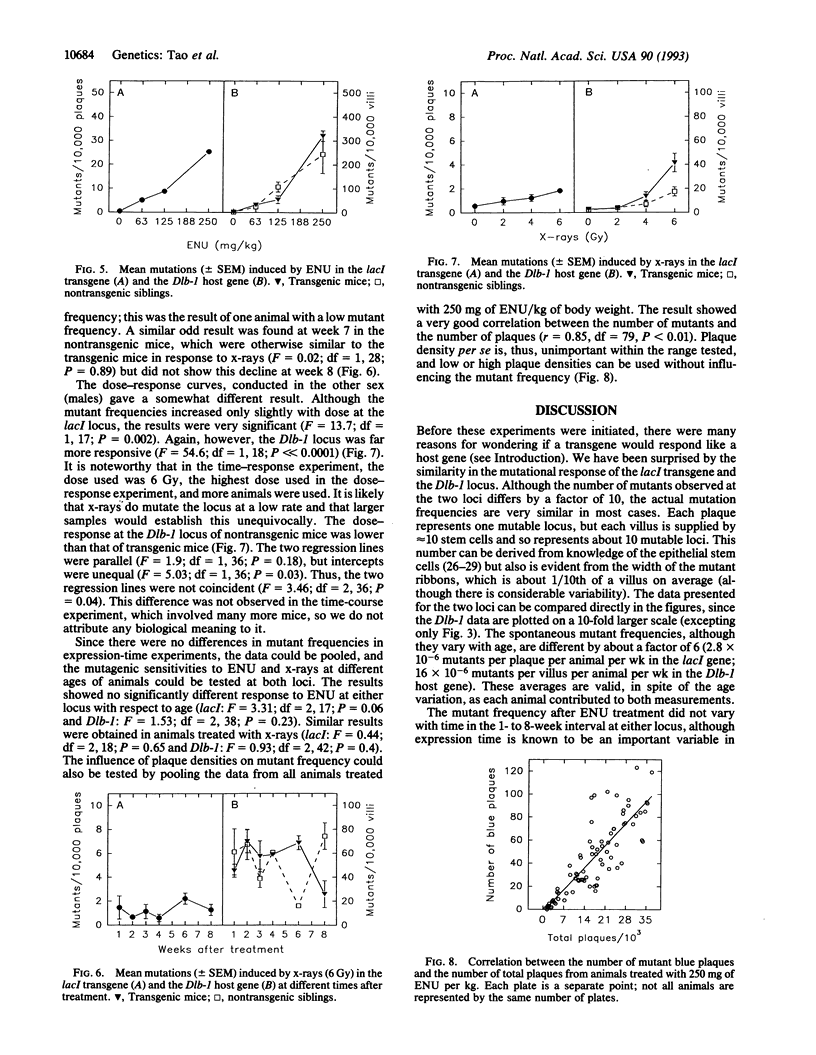
Somatic mutations can now be quantified in almost any cell type in mice carrying bacterial genes in a lambda phage shuttle vector. Mutations induced in vivo are detectable ex vivo, after packaging host-cell DNA into phage that are grown on suitable bacteria. However, the transgenic DNA differs from many host loci in several ways: it (i) is prokaryotic DNA, (ii) is present in multiple tandem copies, and (iii) is heavily methylated and probably not expressed. Thus, mutation of a transgene may not be a suitable model of the host loci, which are eukaryotic, unique, and expressed. To test the relevance of the transgene mutation model, the frequencies of the bacterial lacI+ to lacI- mutations induced in half of the small intestine were compared with the frequencies of the host Dlb-1b to Dlb-1a mutations induced in the other half. The loci responded similarly to ethyl nitrosourea (ENU) with respect to the animal's age and sex, sex of the parent transmitting the transgene, and expression time. ENU dose-response curves were similar. Furthermore, no difference was found at the Dlb-1 locus between transgenic and nontransgenic siblings. In contrast, x-rays induced few lacI mutations but many Dlb-1 mutations. Probably few large deletions are detectable at lacI, but many are detectable at Dlb-1. If so, an important class of mutation is not readily detected in these transgenic mice. With this exception, the transgene and host gene responded similarly in this somewhat limited trial, as is necessary if the transgenic mice are to be a useful model.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. D., Cran D. G., Barton S. C., Hettle S., Reik W., Surani M. A. Transgenes as probes for active chromosomal domains in mouse development. Nature. 1988 Jun 30;333(6176):852–855. doi: 10.1038/333852a0. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Wilson M. C. Delayed de novo methylation in teratocarcinoma suggests additional tissue-specific mechanisms for controlling gene expression. Nature. 1983 Jan 6;301(5895):32–37. doi: 10.1038/301032a0. [DOI] [PubMed] [Google Scholar]
- Gossen J. A., de Leeuw W. J., Tan C. H., Zwarthoff E. C., Berends F., Lohman P. H., Knook D. L., Vijg J. Efficient rescue of integrated shuttle vectors from transgenic mice: a model for studying mutations in vivo. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7971–7975. doi: 10.1073/pnas.86.20.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen J. A., de Leeuw W. J., Verwest A., Lohman P. H., Vijg J. High somatic mutation frequencies in a LacZ transgene integrated on the mouse X-chromosome. Mutat Res. 1991 Sep-Oct;250(1-2):423–429. doi: 10.1016/0027-5107(91)90198-w. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Harbers K., Schnieke A., Löhler J., Chumakov I., Jähner D., Grotkopp D., Hoffmann E. Germline integration of moloney murine leukemia virus at the Mov13 locus leads to recessive lethal mutation and early embryonic death. Cell. 1983 Jan;32(1):209–216. doi: 10.1016/0092-8674(83)90511-1. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Jones I. M., Burkhart-Schultz K., Strout C. L., Crippen T. L. Factors that affect the frequency of thioguanine-resistant lymphocytes in mice following exposure to ethylnitrosourea. Environ Mutagen. 1987;9(3):317–329. doi: 10.1002/em.2860090311. [DOI] [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Stewart C. L., Harbers K., Löhler J., Simon I., Jaenisch R. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982 Aug 12;298(5875):623–628. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- Kohler S. W., Provost G. S., Fieck A., Kretz P. L., Bullock W. O., Putman D. L., Sorge J. A., Short J. M. Analysis of spontaneous and induced mutations in transgenic mice using a lambda ZAP/lacI shuttle vector. Environ Mol Mutagen. 1991;18(4):316–321. doi: 10.1002/em.2850180421. [DOI] [PubMed] [Google Scholar]
- Kohler S. W., Provost G. S., Fieck A., Kretz P. L., Bullock W. O., Sorge J. A., Putman D. L., Short J. M. Spectra of spontaneous and mutagen-induced mutations in the lacI gene in transgenic mice. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7958–7962. doi: 10.1073/pnas.88.18.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S. W., Provost G. S., Kretz P. L., Dycaico M. J., Sorge J. A., Short J. M. Development of a short-term, in vivo mutagenesis assay: the effects of methylation on the recovery of a lambda phage shuttle vector from transgenic mice. Nucleic Acids Res. 1990 May 25;18(10):3007–3013. doi: 10.1093/nar/18.10.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Myhr B. C. Validation studies with Muta Mouse: a transgenic mouse model for detecting mutations in vivo. Environ Mol Mutagen. 1991;18(4):308–315. doi: 10.1002/em.2850180420. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J. F., Schmidt G. H., Paul D. Spontaneous mutation rate. Nature. 1991 Jul 18;352(6332):200–201. doi: 10.1038/352200a0. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Festing M. F., Wilkinson M. M. An allelic difference determines reciprocal patterns of expression of binding sites for Dolichos biflorus lectin in inbred strains of mice. J Embryol Exp Morphol. 1985 Jun;87:229–239. [PubMed] [Google Scholar]
- Ponder B. A., Schmidt G. H., Wilkinson M. M., Wood M. J., Monk M., Reid A. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985 Feb 21;313(6004):689–691. doi: 10.1038/313689a0. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. A comprehensive model of the crypts of the small intestine of the mouse provides insight into the mechanisms of cell migration and the proliferation hierarchy. J Theor Biol. 1987 Aug 21;127(4):381–391. doi: 10.1016/s0022-5193(87)80136-4. [DOI] [PubMed] [Google Scholar]
- Schmidt G. H., O'Sullivan J. F., Paul D. Ethylnitrosourea-induced mutations in vivo involving the Dolichos biflorus agglutinin receptor in mouse intestinal epithelium. Mutat Res. 1990 Feb;228(2):149–155. doi: 10.1016/0027-5107(90)90071-b. [DOI] [PubMed] [Google Scholar]
- Schnieke A., Harbers K., Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. 1983 Jul 28-Aug 3Nature. 304(5924):315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- Shawlot W., Siciliano M. J., Stallings R. L., Overbeek P. A. Insertional inactivation of the downless gene in a family of transgenic mice. Mol Biol Med. 1989 Aug;6(4):299–307. [PubMed] [Google Scholar]
- Uiterdijk H. G., Ponder B. A., Festing M. F., Hilgers J., Skow L., Van Nie R. The gene controlling the binding sites of Dolichos biflorus agglutinin, Dlb-1, is on chromosome 11 of the mouse. Genet Res. 1986 Apr;47(2):125–129. doi: 10.1017/s0016672300022953. [DOI] [PubMed] [Google Scholar]
- Winton D. J., Blount M. A., Ponder B. A. A clonal marker induced by mutation in mouse intestinal epithelium. Nature. 1988 Jun 2;333(6172):463–466. doi: 10.1038/333463a0. [DOI] [PubMed] [Google Scholar]
- Winton D. J., Gooderham N. J., Boobis A. R., Davies D. S., Ponder B. A. Mutagenesis of mouse intestine in vivo using the Dlb-1 specific locus test: studies with 1,2-dimethylhydrazine, dimethylnitrosamine, and the dietary mutagen 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline. Cancer Res. 1990 Dec 15;50(24):7992–7996. [PubMed] [Google Scholar]
- Winton D. J., Peacock J. H., Ponder B. A. Effect of gamma radiation at high- and low-dose rate on a novel in vivo mutation assay in mouse intestine. Mutagenesis. 1989 Sep;4(5):404–406. doi: 10.1093/mutage/4.5.404. [DOI] [PubMed] [Google Scholar]
- Winton D. J., Ponder B. A. Stem-cell organization in mouse small intestine. Proc Biol Sci. 1990 Jul 23;241(1300):13–18. doi: 10.1098/rspb.1990.0059. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Irwin M. The kinetics of villus cell populations in the mouse small intestine. I. Normal villi: the steady state requirement. Cell Tissue Kinet. 1982 Nov;15(6):595–609. doi: 10.1111/j.1365-2184.1982.tb01066.x. [DOI] [PubMed] [Google Scholar]



