Introduction
Allergic sensitization is a well-known risk factor for asthma development, morbidity and persistence. The patterns of allergens to which children become sensitized, i.e., the presence of sensitization to some but not other allergens, appear to be predictive of allergic disease development and prognosis beyond just the presence (vs. absence) of any allergic sensitization;1 however, the variety of allergens to which children can become sensitized is vast and dependent on climatic and domestic environments. Previous confines in study methodology for assessing sensitization to more than a handful of allergens (either because of difficulty of skin testing to many allergens or the amount of serum required for IgE testing) limited evaluation of sensitization profiles to common allergens. Also, it is becoming increasingly clear that assessing the specific allergens against which an individual develops IgE (i.e., component testing) can be more informative than assessing sensitization against crude allergen extracts for the purpose of primary care and specialist practice.2, 3 Recent advances in component testing using a microarray chip allow for the identification of IgE against 112 allergens with a relatively small amount of serum.4 The identification of sensitization patterns based on individual allergens and linking these to disease morbidity at an important age for asthma in young children could have important clinical implications.
In addition, discovering patterns of polysensitization can be challenging. Each patient is sensitized to very different allergens. If defining sensitization patterns using the individual observed IgE response to each allergen, the number of possible patterns can be large and increases at a geometric rate as the number of allergens increases. Alternatively, if defining sensitization patterns as presence or absence of sensitization to any allergen in a group, the variability in the sensitization to multiple allergens in the group is ignored. Latent class analysis (LCA) can overcome these challenges. LCA is a statistical method for identifying underlying subgroups within a population based on individuals’ responses to multiple observed variables.5 LCA assumes that the population consists of a finite number of groups and each group is characterized by a specific pattern of sensitization to multiple allergens. In each group, instead of classifying the sensitization to each allergen as presence or absence, the LCA estimates the probability of sensitization to each allergen for individuals belonging to that group. LCA has been widely used to identify phenotypes of asthma and asthma-like symptoms such as wheeze6, 7, 8, but it is less recognized for its application in identifying patterns of allergic sensitization from multiple individual allergens. The estimated patterns of allergic sensitization will allow modeling the effect of sensitization to a group of allergens on asthma, which is novel as the current research mostly focuses on the effect of sensitization to a single or small number of particular allergens or crude allergen extracts (e.g. Bla g 2 or German cockroach extract) on asthma.9
In New York City (NYC), asthma prevalence among children entering school varies by neighborhood from 3% to 19%.10 Through an employer-based, middle-income health insurance plan, we recruited 350 seven-eight year old children (57% asthma cases, 43% controls) into the NYC Neighborhood Asthma and Allergy Study (NAAS), representing in equal numbers high (HAPN) and low (LAPN) asthma prevalence neighborhoods for a case-control study. Of the 350 children, 246 have been followed prospectively to age 10–11. The analyses have suggested that cockroach and mouse allergens in dust were higher in HAPN than in LAPN homes; children exposed to cockroach allergens were more likely to be sensitized; and sensitization was associated with more frequent asthma symptoms.11, 12 In this paper, we used the data from the NAAS to identify patterns of allergic sensitization from the identification of IgE against 112 allergens. We hypothesized that distinct patterns of sensitization to allergens would be associated with asthma, allergic rhinitis and eczema morbidity and persistence among school age, preadolescent children in NYC.
Methods
Study cohort
The NYC NAAS is a case-control study of asthmatic children. Parents of 7 and 8-year old children were recruited through the Health Insurance Plan of New York, a provider used primarily by a middle-income population. Neighborhoods were selected based on zip code level asthma prevalence among 5-year old children as reported by the NYC Department of Health and Mental Hygiene (DOHMH).10 Asthma prevalence cut-points for LAPN (3–9%) and HAPN (11–19%) were selected to yield an approximately equal number of eligible families in each neighborhood category. All NYC neighborhoods in the Bronx, Brooklyn, Queens and Manhattan with asthma prevalence within these cut-points were selected for recruitment. Written consent was obtained. This study was approved by Columbia University’s Institutional Review Board.
Screening questionnaire and home visit
A brief telephone interview was administered to the parents during which the child’s eligibility was confirmed (age, insurance and residence). The screening questionnaire also included ascertainment of demographic information on the child and administration of the International Study of Asthma and Allergy in Childhood (ISAAC) questionnaire.13 Medication use for asthma was ascertained by the question, “Is your child currently taking any medication to treat or prevent wheezing, cough or other breathing problems, rhinitis or allergies”, followed by a question that queried on specific medications with a list of possibilities.14 Homes of willing families were visited during which a detailed questionnaire on the health history of the child, environmental exposures and socioeconomic and demographic information was administered. Dust samples were collected for allergen analyses and blood was collected for IgE analyses.
Asthma case definition
Children were classified as asthmatic if the parent reported at least one episode for at least one of the following for the child in the 12 months prior to administration of the questionnaire: 1) wheeze, 2) being woken at night by cough without having a cold, 3) wheeze with exercise or 4) report of medication use for asthma. Children who did not meet any of these criteria were classified as controls. For sensitivity analyses, controls were also compared to children with frequent symptoms of asthma, defined as in the past 12 months having any wheeze related symptom reported ≥ 4 times or sleep disturbed ≥ 1 time per week. Asthma cases with less frequent symptoms were excluded from the sensitivity analyses.
Parents of children were contacted annually by telephone, during which time a brief telephone survey was administered about the child’s health in the past year. Children were followed for up to three years after the baseline visit, with 246 followed for three years, 50 followed for two years, and 26 followed for one year. Children classified as asthmatic at baseline who also met those same criteria at the last follow-up (i.e., symptoms or medication use in the past year), were considered to have persistent asthma. Children classified as asthmatic at baseline that did not have asthma symptoms or medication use in the past year at the last follow-up were classified as not having persistent asthma.
Allergen measurements in the home
During the home visit a settled dust sample was collected from the child’s bed as described previously.11 Der f 1, Fel d 1, Can f 1, and Mus m 1 were measured by multiplex bead immunoassays.15 Bla g 2 was measured by ELISA (Indoor Biotechnologies, Charlottesville, VA).16 All results are based on the universal allergen standard curve.17 For results below the limit of detection (LOD), values of ½ LOD were used in analyses.
Serum IgE antibodies
IgE against a panel of 112 antigens was measured using the ISAAC multiplex panel array (Phadia/ThermoFisher, Uppsala, Sweden).4 Antigens with greater than 0.3 ISU-E (ImmunoCAP Specific IgE) units were considered positive. This cut-point has been demonstrated previously to be relatively concordant with the cut point of 0.35 IU/ml used with traditional ImmunoCap.18
Local neighborhood level variables
Children’s home addresses were geocoded and linked to a comprehensive geospatial demographic database described previously.19 The median household income in the surrounding 500M radius was determined for each home. Neighborhood asthma prevalence was based on previously described data from NYC DOHMH.10
Statistical analysis
Frequency and percentage of children with sensitization against each of the 112 allergens was calculated and compared between asthmatics and non-asthmatics. Latent class analysis (LCA) was used to identify patterns of allergic sensitization using the 26 most common allergens identified, against which at least 5% of the children in the study developed IgE. The number of sensitization patterns was determined by selecting the LCA model with a minimum value of Bayesian Information Criterion20, an information criterion that combines goodness of fit and parsimony of the model. The prevalence of sensitization patterns was estimated, as well as the probability of sensitization to each allergen in each pattern. The posterior probability of belonging to each of the sensitization patterns was calculated for each child. A categorical variable of sensitization pattern was generated assigning each child to the pattern with the highest posterior probability. The bivariable association was examined between this categorical variable of sensitization pattern and health history of the child, environmental exposures and socioeconomic and demographic information, allergen measure in the home, and local neighborhood level information, using Fisher’s exact test for categorical variables and using Kruskal-Wallis test for continuous variables. Variables that were significant in the bivariable analysis with a p-value less than or equal to 0.05 also were included in the multivariable analysis using a multinomial logistic regression model. Finally, the associations between the patterns of allergic sensitization and asthma, asthma-like symptoms, and other allergic diseases were examined using logistic regression adjusting for demographic and socioeconomic factors. The odds ratio (OR) and 95% confidence interval (CI) were reported. The statistical analysis was conducted using SAS 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
Descriptive statistics
Among the 350 children in the NAAS cohort, 332 had serum IgE measurements and were included in this study. Of the 332 children, 185 (56%) were males, 47 (14%) were white, 159 (48%) were black, 165 (50%) lived in HAPN, and 136 (41%) were non-asthmatic (Table 1).
Table 1.
Participant characteristics frequency (%) among the asthmatic and non-asthmatic children in the NYC Neighborhood Asthma and Allergy Study
| All n = 332 |
Asthmatic n = 196 |
Non-asthmatic n = 136 |
Chi-square test p-value |
|
|---|---|---|---|---|
| Race | ||||
| Black | 159 (48) | 98 (50) | 61 (45) | 0.38 |
| White | 47 (14) | 24 (12) | 23 (17) | |
| Others | 126 (38) | 74 (38) | 52 (38) | |
| Gender | ||||
| Males | 185 (56) | 111 (57) | 74 (54) | 0.69 |
| Females | 147 (44) | 85 (43) | 62 (46) | |
| Household income | ||||
| < 30,000 | 60 (18) | 39 (20) | 21 (15) | 0.24 |
| 30,000 – 60,000 | 118 (36) | 74 (38) | 44 (32) | |
| 60,000 – 90,000 | 83 (25) | 45 (23) | 38 (28) | |
| > 90,000 | 57 (17) | 28 (14) | 29 (21) | |
| Unknown | 14 (4) | 10 (5) | 4 (3) | |
| Birth order | ||||
| First | 93 (28) | 50 (26) | 43 (32) | 0.54 |
| Second | 115 (35) | 73 (37) | 42 (31) | |
| Third | 66 (20) | 39 (20) | 27 (20) | |
| Later than third one | 58 (17) | 34 (17) | 24 (17) | |
| Breastfed | ||||
| Yes | 229 (69) | 126 (64) | 103 (76) | 0.03 |
| No | 103 (31) | 70 (36) | 33 (24) | |
| Children given formula time | ||||
| Never | 102 (31) | 64 (33) | 38 (28) | 0.56 |
| < 1 month | 94 (28) | 55 (28) | 39 (29) | |
| 1 – 3 months | 67 (20) | 41 (21) | 26 (19) | |
| 3+ months | 69 (21) | 36 (18) | 33 (24) | |
| Daycare before age 2 | ||||
| Yes | 219 (66) | 59 (30) | 54 (40) | 0.07 |
| No | 113 (34) | 137 (70) | 82 (60) | |
| Neighborhood | ||||
| HAPN | 165 (50) | 99 (51) | 66 (49) | 0.91 |
| LAPN | 165 (50) | 96 (49) | 69 (51) | |
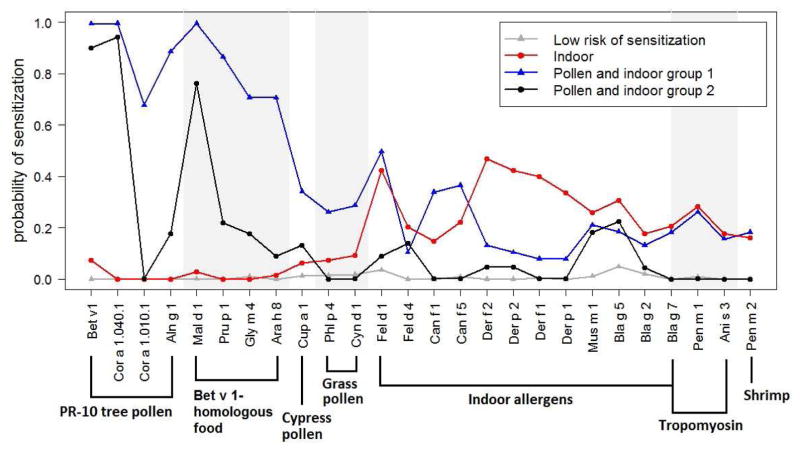
The frequencies of sensitization to the 26 most common allergens identified in this study together with their source and allergen family are shown in Table 2. The allergens were grouped based on allergological knowledge. Bet v 1 is the major allergen among PR-10 tree pollen, and was grouped together with Cor a 1.040.1, Cor a 1.010.1, and Aln g 1. Bet v 1-homologous food allergens, including Mal d 1, Pru p 1, Gly m 4, and Ara h 8, were grouped together. Cat (Fel d 1, Fel d 4), dog (Can f 1, Can f 5), dust mite (Der f/p 1, Der f/p 2), mouse (Mus m 1) and cockroach (Bla g 2, Bla g 5 and Bla g 7) define the indoor allergens. Bla g 7, Pen m 1 and Ani s 3 are allergens from the tropomyosin family. Cup a 1 and Phl p 4 are grass pollen. Among the 196 asthmatic children, 26% were sensitized to Bet v 1 (birch), 25% were sensitized to Cor a 1.040.1 (hazelnut), 24% were sensitized to Mal d 1 (apple), 19% were sensitized to Fel d 1 (cat), and 17% were sensitized to Pru p 1 (peach). Four of the above five most common allergens were from the PR-10 family. The proportion of sensitization to each of the 26 allergens was much lower in children without asthma - all lower than 10% except Fel d 1 (cat) with 14.7% of sensitization.
Table 2.
The frequency (%) of sensitization to the 26 most common allergens among the asthmatic and non-asthmatic children in the NYC Neighborhood Asthma and Allergy Study
| Category | Allergen | Source | Allergen family | All n = 332 |
Asthmatic n = 196 |
Non-asthmatic n = 136 |
|---|---|---|---|---|---|---|
| PR-10 tree pollen | Bet v 1 | Birch | PR-10 | 63 (19) | 50 (26) | 13 (10) |
| PR-10 tree pollen | Cor a 1.040.1 | Hazelnut | PR-10 | 59 (18) | 48 (25) | 11 (8) |
| PR-10 tree pollen | Cor a 1.010.1 | Hazelnut | PR-10 | 26 (8) | 22 (11) | 4 (3) |
| PR-10 tree pollen | Aln g 1 | Alder | PR-10 | 36 (11) | 28 (14) | 8 (6) |
| Bet v 1-homologous food | Mal d 1 | Apple | PR-10 | 57 (17.2) | 47 (24) | 10 (7) |
| Bet v 1-homologous food | Pru p 1 | Peach | PR-10 | 38 (12) | 33 (17) | 5 (4) |
| Bet v 1-homologous food | Gly m 4 | Soy bean | PR-10 | 33 (10) | 27 (14) | 6 (4) |
| Bet v 1-homologous food | Ara h 8 | Peanut | PR-10 | 30 (9) | 27 (14) | 3 (2) |
| Cypress pollen | Cup a 1 | Cypress | Pectate lyase | 23 (7) | 21 (11) | 2 (2) |
| Grass pollen | Phl p 4 | Timothy | Berberine bridge enzyme | 18 (5) | 14 (7) | 4 (3) |
| Grass pollen | Cyn d 1 | Bermuda grass | Grass group 1 | 21 (6) | 17 (9) | 4 (3) |
| Indoor allergens | Fel d 1 | Cat | Uteroglobin | 57 (17) | 37 (19) | 20 (15) |
| Indoor allergens | Fel d 4 | Cat | Lipocalin | 21 (6) | 16 (8) | 5 (4) |
| Indoor allergens | Can f 1 | Dog | Lipocalin | 23 (7) | 18 (9) | 5 (4) |
| Indoor allergens | Can f 5 | Dog | Arginine esterase/kallikrein | 31 (9) | 22 (11) | 9 (7) |
| Indoor allergens | Der f 2 | Dust mite | NPC2 family | 38 (12) | 27 (14) | 11 (8) |
| Indoor allergens | Der p 2 | Dust mite | NPC2 family | 34 (10) | 24 (12) | 10 (7) |
| Indoor allergens | Der f 1 | Dust mite | Cysteine protease | 31 (9) | 20 (10) | 11 (8) |
| Indoor allergens | Der p 1 | Dust mite | Cysteine protease | 26 (8) | 19 (10) | 7 (5) |
| Indoor allergens | Mus m 1 | Mouse | Lipocalin | 32 (10) | 24 (12) | 8 (6) |
| Indoor allergens | Bla g 5 | Cockroach | Glutathione S-transferase | 43 (13) | 30 (15) | 13 (10) |
| Indoor allergens | Bla g 2 | Cockroach | Aspartic protease | 22 (7) | 16 (8) | 6 (4) |
| Indoor/Tropomyosin | Bla g 7 | Cockroach | Tropomyosin | 21 (6) | 19 (10) | 2 (2) |
| Tropomyosin | Pen m 1 | Shrimp | Tropomyosin | 18 (5) | 18 (9) | 0 (0) |
| Tropomyosin | Ani s 3 | Anisakis | Tropomyosin | 18 (5) | 18 (9) | 0 (0) |
| Shrimp | Pen m 2 | Shrimp | Arginine kinase | 31 (9) | 20 (10) | 11 (8) |
Patterns of sensitization
The Bayesian Information Criterion selected a 4-class LCA model (Figure 1). In addition to the pattern with close to zero probability of sensitization to all 26 allergens (named “low risk of sensitization”), one pattern exhibited a high probability of sensitization to indoor allergens but a low probability of sensitization to pollen (named “indoor”), and two patterns exhibited sensitization to both pollen and indoor allergens (named “pollen and indoor group 1” and “pollen and indoor group 2”). The children in the “pollen and indoor group 1” had a high probability of sensitization to all allergens, especially the PR-10 tree pollen and plant food allergens that are cross-reactive to the birch tree pollen. In contrast, children in the “pollen and indoor group 2” were sensitized to fewer allergens with lower probability of sensitization and had a close to zero probability of sensitization to Cor a 1.010.1, the grass pollen (Phl p 4 and Cyn d 1), the dog allergens (Can f 1, Can f 5), the tropomyosin allergens (Bla g 7, Pen m 1 and Ani s 3) and shrimp (Pen m 2). By assigning children to the sensitization patterns with the highest posterior probabilities, the categorical variable of sensitization pattern had 206 children in the “low risk of sensitization” category, 66 in the “indoor” category, 38 in the “pollen and indoor group 1” category, and 22 in the “pollen and indoor group 2” category (Figure 1).
Figure 1.
Patterns of sensitization estimated using a 4-class Latent Class Analysis model with the data of the 26 most common allergenic proteins in the sample (n = 332).
Risk factors associated with patterns of sensitization
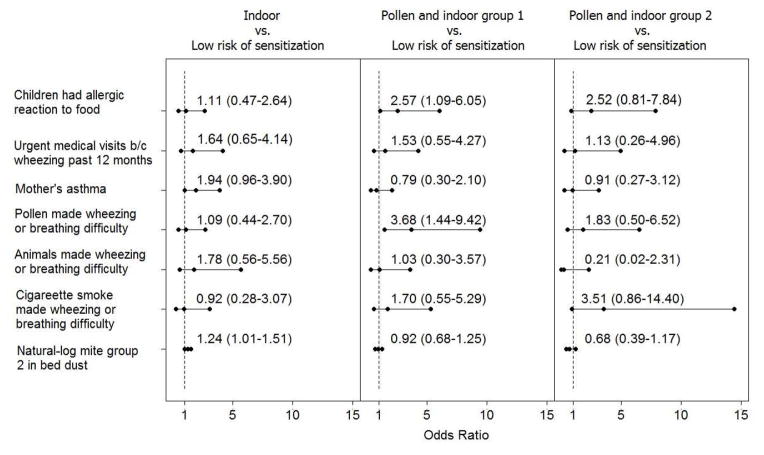
Bivariable analysis identified seven important risk factors that were associated with sensitization patterns. They were whether the parent reported that the child had an allergic reaction to foods (p < .001), whether child was taken to a doctor in a hurry because of wheezing in the past 12 months (p < .001), whether mother had asthma (p < .001), whether pollen (p < .001), animal (p < .001) or smoking (p < .001) made wheezing or breathing difficulty, and mite group 2 allergen measured in the bed dust (p = 0.031). The multivariable analysis using a multinomial logistic regression showed that, compared to children in the “low risk of sensitization” group, having reported allergy to food (OR=2.57 [95% CI: 1.09–6.05]) and having reported that pollen made the child wheeze or have difficulty breathing (OR=3.68 [95% CI: 1.44–9.42]) were associated with belonging to the “pollen and indoor group 1” sensitization pattern (Figure 2). Mite group 2 allergen in bed dust (OR=1.24 [95% CI: 1.01–1.51]) was a risk factor for belonging to the “indoor” sensitization pattern. The other covariates were not significantly associated with sensitization patterns in the multivariable analysis.
Figure 2.
Odds ratio estimates and 95% CIs examining the associations of health history, mother’s asthma, and the house dust mite allergen level with the patterns of sensitization.
Asthma and other allergic diseases by sensitization patterns
Table 3 shows the percentages of children belonging to each sensitization pattern stratified by the asthma (asthma, asthma with frequent symptoms, persistent asthma), asthma-like symptoms (being woken at night by cough without a cold, wheezing with exercise), and allergic diseases (ISAAC rhinitis and eczema). Children with asthma, asthma-like symptoms or other allergic diseases were more likely to belong to the patterns with sensitization to pollen or indoor allergens. Among the 136 non-asthmatic children, 15% were classified as “indoor”, 5% as “pollen and indoor group 1”, and 4% as “pollen and indoor group 2”; while among the 196 asthma children, 23% were classified as “indoor”, 16% as “pollen and indoor group 1”, and 9% as “pollen and indoor group 2”. The percentages of “indoor” and “pollen and indoor group 1” were even higher among the 123 children with persistent asthma, (24% and 20%, respectively) and among the 79 children with frequent symptoms of asthma (28% and 20%, respectively).
Table 3.
Frequency (%) of children belonging to each patterns of allergic sensitization among those with and without asthma, asthma-like symptoms and other allergic diseases
| n | Low risk of sensitization | Indoor | Pollen and indoor group 1 | Pollen and indoor group 2 | |
|---|---|---|---|---|---|
| Asthma | |||||
| No | 136 | 103 (76) | 21 (15) | 7 (5) | 5 (4) |
| Yes | 196 | 103 (53) | 45 (23) | 31 (16) | 17 (9) |
| Asthma with frequent symptomsa | |||||
| No | 136 | 103 (76) | 21 (15) | 7 (5) | 5 (4) |
| Yes | 79 | 35 (44) | 22 (28) | 16 (20) | 6 (8) |
| Persistent asthma | |||||
| No | 190 | 140 (74) | 29 (15) | 11 (6) | 10 (5) |
| Yes | 123 | 57 (46) | 30 (24) | 25 (20) | 11 (9) |
| Being woken at night | |||||
| No | 175 | 124 (71) | 30 (17) | 10 (6) | 11 (6) |
| Yes | 156 | 81 (52) | 36 (23) | 28 (18) | 11 (7) |
| Wheeze with exercise past 12 months | |||||
| No | 247 | 162 (66) | 47 (19) | 24 (10) | 14 (6) |
| Yes | 84 | 43 (51) | 19 (23) | 14 (17) | 8 (10) |
| Rhinitis last 12 monthsb | |||||
| No | 183 | 137 (75) | 27 (15) | 9 (5) | 10 (5) |
| Yes | 148 | 69 (47) | 39 (26) | 28 (19) | 12 (8) |
| Eczema last 12 monthsb | |||||
| No | 233 | 161 (69) | 42 (18) | 18 (8) | 12 (5) |
| Yes | 97 | 43 (44) | 24 (25) | 20 (21) | 10 (10) |
Frequent symptoms are defined as in the past 12 months having any wheeze related symptom reported ≥4 times or sleep disturbed ≥1 time per week. Individuals with less frequent symptoms of asthma were excluded.
determined by ISAAC
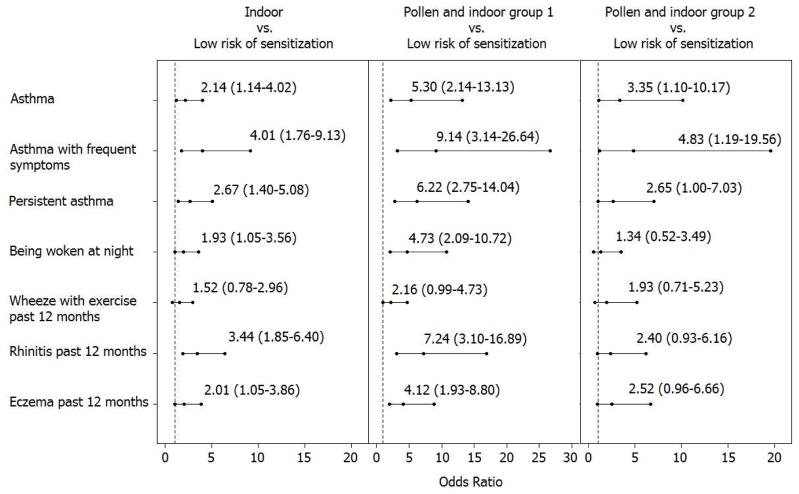
Adjusting for the demographic and socioeconomic factors in Table 1, compared to the “low risk of sensitization” category, children in the “pollen and indoor group 1” category had significantly higher odds of asthma (OR=5.30 [95% CI: 2.14–13.13]), asthma with frequent symptoms (OR=9.14 [95% CI: 3.14–26.64]), persistent asthma (OR=6.22 [95% CI: .75–14.04]), being woken at night by cough without a cold (OR=4.73 [95% CI: 2.09–10.72]), wheezing past 12 months (OR=2.16 [95% CI: 0.99–4.73]), ISAAC rhinitis past 12 months (OR=7.24 [95% CI: 3.10–16.89]), and ISAAC eczema past 12 months (OR=4.12 [95% CI: 1.93–8.80]) (Figure 3). Children with the “indoor” sensitization pattern also had significantly higher odds of asthma and other allergic diseases, except wheeze with exercise, but the effects were smaller compared to the “pollen and indoor group 1”. The odds ratios estimates for the “pollen and indoor group 2” were similar to those of the “indoor”, but the associations were only statistically significant for asthma which might be due to the small sample size in this group. Although the “pollen and indoor group 1” had the highest odds of asthma, asthma-like symptoms and other allergic diseases, their comparisons to the other two sensitization groups were not statistically significant, which again might be due to limited sample sizes in these sensitization groups.
Figure 3.
Odds ratio estimates and 95% CIs of asthma and other allergic diseases comparing children in each of the sensitization patterns to those who had low risk of sensitization to any of the 26 most common allergens, adjusting for demographic and socioeconomic factors.
Discussion
A few studies have characterized patterns of allergic sensitization on asthma diseases21, 22, 23, 23, 25, but many others have studied the asthma risk associated with the presence or absence of sensitization to particular allergens. In studying sensitization patterns, allergens are often grouped according to their origins and the known cross-reactivity. The sensitization to a group of allergens has been then summarized as presence or absence of sensitization to any allergen in the group or the total number of allergens sensitized to in the group, which ignores the variability in the sensitization to multiple allergens in the group.23 The application of LCA is novel, because it is a data driven method that identifies patterns of sensitization across many common allergens without the need to define allergy patterns based on prior knowledge. It defines the pattern of sensitization with the estimated probability of sensitization to each allergens for all the considered allergens, which provides richer information than those defined by presence or absence of any allergen or number of allergens each individual develops sensitization to.
Although the LCA is a data-driven method, it successfully identified four sensitization patterns. Specifically, it was successful in separating children who had low risk of sensitization to those who were sensitized to multiple allergens, and in separating children who were sensitized to indoor allergens but not pollen to those who were sensitized to both indoor allergens and pollen; and among those sensitized to pollen, it further separated children into two subgroups with one group displaying higher risk of sensitization to more allergens than the other subgroup. As reflected in the two “pollen and indoor” patterns, children who were sensitized to the PR-10 tree pollen also were more likely to be sensitized to the Bet v 1-homologous allergens from plant food by means of cross-reaction.26 Similarly, children who were sensitized to Bla g 7 were also sensitized to Pen m 1 and Ani s 3, which were probable due to their cross-reactivity with Der f/p 10.27
The significant associations between belonging to the “pollen and indoor group 1” pattern and parents’ report that the child had a reported allergic reaction to food and a report of pollen causing breathing difficulty validated the definition of this pattern. We also found that dust mite allergen in bed dust was significantly associated with belonging to the “indoor” allergy pattern suggesting dust mites are common indoor allergy triggers. Although children classified to the “pollen and indoor group 1” had higher chances of urgent medical visits because of wheezing in the past 12 months and of wheezing or breathing difficulty because of animal or cigarette smoking than children in the other patterns, these factors were not significant after controlling for the parents’ reports to food and pollen allergy in the multivariable analysis.
The risk of asthma and persistent asthma was higher among children sensitized to pollens or indoor allergens than children with low risk of sensitization, especially for children in the “pollen and indoor group 1”, which was three times more prevalent in the asthmatics than non-asthmatic children. The risk of asthma-like symptom, being woken at night because of cough without a cold, had a trend to be higher in the “pollen and indoor group 1” than the other two allergenic patterns. However, the risk of wheezing with exercise was relatively small and was similar across the three allergenic patterns. Like asthma, the risk of other allergic diseases including rhinitis and eczema had a trend to be highest among children in the “pollen and indoor group 1” group. This may suggest a common mechanistic underpinning of the sensitizations relevant to allergic diseases (such as asthma, rhinitis and eczema). Unfortunately, the sample size in this study and the large overlap between these diseases do not allow us to further determine the associations between sensitization patterns and the presence of single diseases (e.g., rhinitis without asthma), but this could be an important examination in future studies.
This study contributes to further population-based examinations of the relative frequency of IgE responses to the various allergen components. For example, it was also of interest that allergens from birch and hazelnut were some of the most common with IgE detected despite urban settings of cohort. Also, among this population of children for whom cockroach exposure is common11, the frequency of IgE against Bla g 5 was almost twice that of Bla g 2, while Bla g 2 has been considered the major cockroach allergen.28 In a previous study of cockroach allergic individuals, while the frequency of positives (>0.35 IU/ml) to Bla g 2 was higher than to Bla g 5, the geometric mean concentrations of IgE were higher to Bla g 5 than to Bla g 2.29 The chip method for IgE detection may favor detection at higher concentrations, but there may also be important differences in patterns of sensitization between communities and among those with and without allergic diseases.
By design, our asthma classification based on the ISAAC questionnaires maximizes sensitivity at the cost of specificity. Our sensitivity analyses among children with frequent asthma symptoms showed a similar but stronger association with the identified sensitization patterns. Although only the 26 most common allergens were considered in this paper, we were able to identify majority of the children with allergic sensitization in our cohort, with the exception of 10 children who were sensitized to at least one of the 112 allergens but not to any of the 26 most common allergens.
We acknowledge that a limitation of this study is the relatively small number of children classified into the “pollen and indoor group 1” (n = 38) and “pollen and indoor group 2” (n = 22) patterns. Consequently, we do not have enough power to detect the significant associations between patterns of allergic sensitization and many environmental risk factors as well as to compare the effects of these two sensitization patterns on asthma and allergic diseases after adjusting for demographic and socioeconomic factors. We also acknowledge the limitations with the chip technology, with respect to the quantity of allergen, the potential inhibition of IgE binding and the use of recombinant allergens. All of these may lead to false negatives. However, a clear advantage of the chip technology is the extremely low volume of sera needed, which can be collected by finger stick (instead of venipuncture). This technology may hence provide the ability to test many allergens in pediatric (and adult) cohorts that would otherwise have been difficult. Therefore, examining these results with respect to asthma morbidity in an urban population with a high risk for asthma is relevant from a public health prospective.
In conclusion, LCA provides a useful and flexible tool in studying allergy sensitization patterns. The conventional study of asthma and allergy sensitization to particular allergens is not sufficient. The patterns of allergic sensitization identified through the LCA method can lead to better understanding of the relationship between asthma and allergic sensitization to multiple allergens. As more data on allergyic sensitization to multiple allergens become available, the LCA method will allow identifying more patterns of allergy sensitizations, examining their associations with geographic, ethnic and other factors, and studying their relationships to diseases like asthma.
Acknowledgments
Funding source: NIH NIEHS (R01 ES014400, P30 ES009089) HUD Healthy Homes Technical Studies (NYHHU 0003-11). Phadia Thermo Fisher provided the ISAC testing on the serum samples.
Abbreviation/Acronyms
- CI
confidence interval
- DOHMH
Department of Health and Mental Hygiene
- HAPN
high asthma prevalence neighborhoods
- ISAAC
International Study of Asthma and Allergy in Childhood
- LCA
latent class analysis
- LAPN
low asthma prevalence neighborhoods
- LOD
limit of detection
- NAAS
Neighborhood Asthma and Allergy Study
- NYC
New York City
- OR
odds ratio
Footnotes
Authors’ contribution: Q. C. designed and performed the statistical analysis and wrote the manuscript. X. Z. contributed to the data cleaning and statistical analysis, created the figures and tables, and was involved in drafting, revising and final approval of the manuscript. L. A. contributed to the acquisition, analyses and interpretation of data and was involved in revising and final approval of the manuscript. A. D. contributed to the acquisition, analyses and interpretation of data and was involved in revising and final approval of the manuscript. A. R. contributed to the conception and design of the study, analyses and interpretation of data and was involved in revising and final approval of the manuscript. R. M. contributed to the conception and design of the study, analyses and interpretation of data and was involved in revising and final approval of the manuscript. I. G. contributed to the conception and design of the study, analyses and interpretation of data and was involved in revising and final approval of the manuscript. M. P. contributed to the conception and design of the study, analyses and interpretation of data and was involved in drafting, revising and final approval of the manuscript.
Trial registration: Not applicable
Conflict of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Qixuan Chen, Email: qc2138@cumc.columbia.edu.
Xiaobo Zhong, Email: xz2221@cumc.columbia.edu.
Luis Acosta, Email: la181@cumc.columbia.edu.
Adnan Divjan, Email: ad708@cumc.columbia.edu.
Andrew Rundle, Email: agr3@cumc.columbia.edu.
Inge F. Goldstein, Email: ifg2@cumc.columbia.edu.
Rachel L. Miller, Email: rlm14@cumc.columbia.edu.
Matthew S. Perzanowski, Email: mp2217@cumc.columbia.edu.
References
- 1.Simpson A, Tan VY, Winn J, et al. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–6. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 2.Reinhardt R. Improving patient outcomes: state-of-the-art allergy and autoimmune diagnostic testing. MLO: medical laboratory observer. 2013;45(4):24–26. [PubMed] [Google Scholar]
- 3.Borres MP, Ebisawa M, Eigenmann PA. Use of allergen components begins a new era in pediatric allergology. Pediatr Allergy Immunol. 2011;22(5):454–61. doi: 10.1111/j.1399-3038.2011.01197.x. [DOI] [PubMed] [Google Scholar]
- 4.Patelis A, Gunnbjornsdottir M, Malinovschi A, et al. Population-based study of multiplexed IgE sensitization in relation to asthma, exhaled nitric oxide, and bronchial responsiveness. J Allergy Clin Immunol. 2012;130:397–402. e2. doi: 10.1016/j.jaci.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 5.Collins LM, Lanza ST. Latent class and latent transition analysis: with applications in the social, behavioral, and health sciences. John Wiley & Sons, Hoboken; New Jersey: 2010. [Google Scholar]
- 6.Chen Q, Just AC, Miller RL, et al. Using latent class growth analysis to identify childhood wheeze phenotypes in an urban birth cohort. Ann Allergy Asthma Immunol. 2012;108(5):311–315. e1. doi: 10.1016/j.anai.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Depner M, Fuchs O, Genuneit J, et al. Clinical and Epidemiologic Phenotypes of Childhood Asthma. Am J Respir Crit Care Med. 2014;189(2):129–138. doi: 10.1164/rccm.201307-1198OC. [DOI] [PubMed] [Google Scholar]
- 8.Weinmayr G, Keller F, Kleiner A, et al. Asthma phenotypes identified by latent class analysis in the ISAAC phase II Spain study. Clin Exp Allergy. 2013;43(2):223–32. doi: 10.1111/cea.12035. [DOI] [PubMed] [Google Scholar]
- 9.Stoltz DJ, Jackson DJ, Evans MD, et al. Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy. 2013;43(2):233–41. doi: 10.1111/cea.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asthma Facts. The City of New York Department of Health and Mental Hygiene; New York: 2003. [Google Scholar]
- 11.Olmedo O, Goldstein IF, Acosta L, et al. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. J Allergy Clin Immunol. 2011;128:284–92. e7. doi: 10.1016/j.jaci.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acosta LM, Miller RL, Goldstein IF, et al. Risks for asthma morbidity and urgent medical visits vary by community in New York City. Submitted. [Google Scholar]
- 13.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson JS, Mellins RB, Garfinkel R, et al. Asthma, body mass, gender, and Hispanic national origin among 517 preschool children in New York City. Allergy. 2008;63:87–94. doi: 10.1111/j.1398-9995.2007.01529.x. [DOI] [PubMed] [Google Scholar]
- 15.Earle CD, King EM, Tsay A, et al. High-throughput fluorescent multiplex array for indoor allergen exposure assessment. J Allergy Clin Immunol. 2007;119:428–33. doi: 10.1016/j.jaci.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Pollart SM, Smith TF, Morris EC, Gelber LE, Platts-Mills TA, Chapman MD. Environmental exposure to cockroach allergens: analysis with monoclonal antibody-based enzyme immunoassays. J Allergy Clin Immunol. 1991;87:505–10. doi: 10.1016/0091-6749(91)90009-d. [DOI] [PubMed] [Google Scholar]
- 17.Chapman MD, Filep S, Tsay A, et al. Allergen standardization: CREATE principles applied to other purified allergens. Arb Paul Ehrlich Inst Bundesinstitut Impfstoffe Biomed Arzneim Langen Hess. 2009;96:21–4. [PubMed] [Google Scholar]
- 18.Gadisseur R, Chapelle J-P, Cavalier E. A new tool in the field of in-vitro diagnosis of allergy: preliminary results in the comparison of ImmunoCAP© 250 with the ImmunoCAP© ISAC. Clin Chem Lab Med. 2011;49(2):277–280. doi: 10.1515/CCLM.2011.052. [DOI] [PubMed] [Google Scholar]
- 19.Lovasi GS, Hutson MA, Guerra M, Neckerman KM. Built environments and obesity in disadvantaged populations. Epidemiol Rev. 2009;31:7–20. doi: 10.1093/epirev/mxp005. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 21.Lazic N, Roberts G, Custovic A, et al. Multiple atopy phenotypes and their associations with asthma: similar findings from two birth cohorts. Allergy. 2013;68(6):764–770. doi: 10.1111/all.12134. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Park KH, Park JW, et al. Food allergen sensitization patterns in Korean adult food allergy patients. World Allergy Organ J. 2015;8:A7. [Google Scholar]
- 23.Majkowska-Wojciechowska B, Pełka J, Korzon L, et al. Prevalence of allergy, patterns of allergic sensitization and allergy risk factors in rural and urban children. Allergy. 2007;62(9):1044–1050. doi: 10.1111/j.1398-9995.2007.01457.x. [DOI] [PubMed] [Google Scholar]
- 24.Röckmann H, van Geel MJ, Knulst AC, Huiskes J, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Food allergen sensitization pattern in adults in relation to severity of atopic dermatitis. Clin Transl Allergy. 2014;4(1):9. doi: 10.1186/2045-7022-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soeria-Atmadja D, Onell A, Kober A, Matsson P, Gustafsson MG, Hammerling U. Multivariate statistical analysis of large-scale IgE antibody measurements reveals allergen extract relationships in sensitized individuals. J Allergy Clin Immunol. 2007;120(6):1433–40. doi: 10.1016/j.jaci.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Wensing M, Akkerdaas JH, van Leeuwen WA, Stapel SO, Brujinzeel-koomen CA, Aalberse RC, Bast BJ, Knulst AC, van Ree R. IgE to Bet v 1 and profiling: cross-reactivity patterns and clinical relevance. J Allergy Clin Immunol. 2002;110(3):435–42. doi: 10.1067/mai.2002.126380. [DOI] [PubMed] [Google Scholar]
- 27.Santiago HC, Bennuru S, Boyd A, Eberhard M, Nutman TB. Structural and immunologic crossreactivity among filarial and mite tropomyosin: implications for the hygiene hypothesis. J Allergy Clin Immunol. 2011;127(2):479–486. doi: 10.1016/j.jaci.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pomés A, Chapman MD, Vailes LD, Blundell TL, Dhanaraj V. Cockroach allergen Bla g 2: structure, function, and implications for allergic sensitization. Am J Respir Crit Care Med. 2002;165(3):391–7. doi: 10.1164/ajrccm.165.3.2104027. [DOI] [PubMed] [Google Scholar]
- 29.Satinover SM, Reefer AJ, Pomes A, Chapman MD, Platts-Mills TA, Woodfolk JA. Specific IgE and IgG antibody-binding patterns to recombinant cockroach allergens. J Allergy Clin Immunol. 2005;115(4):803–809. doi: 10.1016/j.jaci.2005.01.018. [DOI] [PubMed] [Google Scholar]