Abstract
Introduction:
Traditionally, Panchagavya Ghrita (PG) has been used for the management of epilepsy, anxiety, fever and jaundice. It consists of five components of cow products namely, cow milk, clarified butter from cow milk, cow urine, curd from cow milk, and cow dung juice.
Aim:
To evaluate the effect of PG in maximal electroshock (MES) induced seizures model and its pharmacodynamic and pharmacokinetic interaction with phenytoin (PHT) and carbamazepine (CBZ) in rats.
Materials and Methods:
Male Wistar rats were administered PG 500, 1000, 2000, and 4000 mg/kg orally for 7 days and seizures were induced by MES. For interaction studies, PG (4000 mg/kg) was administered along with a sub-therapeutic dose of PHT (20 mg/kg, p.o.) and CBZ (10 mg/kg, p.o.). Behavioral parameters were assessed. Oxidative stress markers and serum levels of PHT and CBZ were estimated.
Results:
Tonic hind limb extension, cognitive impairment, and oxidative stress produced by MES were reversed by PG (4000 mg/kg). Co-administration of PG (4000 mg/kg) with a sub-therapeutic dose of PHT and CBZ potentiated antiepileptic effect and ameliorated cognitive impairment as well as oxidative stress. Although, there was a slight increase in serum levels of PHT and CBZ on co-administration with PG, it was statistically insignificant.
Conclusion:
Co-administration of PG with low doses of PHT and CBZ caused complete seizure protection. This suggests the potential of PG as an adjunct in epilepsy with improved efficacy and tolerability.
Keywords: Carbamazepine, epilepsy, oxidative stress, Panchagavya Ghrita, phenytoin
Introduction
Panchagavya Ghrita (PG) is an Ayurvedic medicine consisting of five components, namely, cow milk, clarified butter from cow milk, cow urine, curd from cow milk, and cow dung juice. It has been recommended for treatment of Apasmara (epilepsy), Jvara (fever), and Kamala (jaundice) in Charaka Samhita.[1] It is also known to have hepatoprotective[2] and immunostimulant[3] properties. Cow urine has been demonstrated to possess antioxidant property.[4,5] Ayurveda mentions milk, ghee, and curd as good diet constituents. α-Lactalbumin (milk protein) is found to be effective in experimental models of seizure and epileptogenesis.[6] PG alone as well as a constituent of many formulations has been shown to possess neuroprotective and anticonvulsant activities in rats.[7,8,9,10]
Antiepileptic drugs (AEDs) such as phenytoin (PHT), carbamazepine (CBZ), and sodium valproate are the mainstay of treatment in epilepsy and are associated with numerous side effects. Despite the availability of newer AEDs and alternative treatment options such as surgery, etc., the complete control of seizures is not seen in about 30% of the patients.[11] Although monotherapy is the first line treatment option, polytherapy is widely practiced for refractory epilepsy with the hope of achieving full control of seizures.[12] Side effects associated with monotherapy and polytherapy of AEDs are the major limitations.[13]
Ayurvedic medicines with known anticonvulsant efficacy could be used as an adjuvant to conventional AEDs, to lower the doses as well as to reduce the side effects.[14] Patients with epilepsy often concomitantly take the herbal medicines with modern AEDs without informing their physician. In previous studies, the interaction of herbal medicines with potential anticonvulsant activity has been evaluated with currently available AEDs with the intention to reduce the doses and side effects of AEDs.[15,16,17] Considering the limitations of currently available AEDs and advantages of Ayurvedic medicines, the present study was planned to evaluate the anticonvulsant activity of PG and its protective effect against seizures associated cognitive impairment and oxidative stress in maximal electroshock (MES) induced seizures model in rats.
Materials and Methods
Animals
Experiments were performed in male Wistar rats weighing 200–250 g. The animals were obtained from the Central Animal Facility of All India Institute of Medical Sciences, New Delhi, India. The rats were maintained under standard laboratory conditions with natural dark and light cycle. They were allowed free access to standard dry rat diet (M/s Ashirwad Industry, Chandigarh, India) and tap water. Animals were acclimatized for 7 days prior to experimentation. However, the rats were deprived of food 12 h before the behavioral testing, as this is known to enhance their motivation to perform the test. The experimental protocols and procedures were reviewed and approved by the Institutional Animal Ethics Committee (no. 568/IAEC/2010).
Materials
PG was obtained from Arya Vaidya Sala, Kottakkal, Kerala, India (Batch no. 140563, date of manufacture - May 2011, date of expiry - May 2013, manufacturing licence no. 1/25D/76). Each 10 g constituted Ghritam (cow ghee) - 10.549 ml; Gomaya Swarasa (fresh cow dung - 10.549 ml; Gomutram (cow urine) - 10.549 ml; Dadhi (cow curd) - 10.549 ml; and Kshiram (cow milk) - 10.549 ml. PG was prepared and standardized as per the methods described in Ayurvedic Formulary of India (AFI).[18] PG was melted by heating in a water bath (35–37°C) until it melts to a solution before administration to rats. The dose of PG was extrapolated from the human dose prescribed in AFI, which states 12 g daily. In different studies, PG has been used in a wide range of doses (500–4000 mg/kg) for experimental seizures as well as a hepatoprotective effect.[2,8,10] In the present study, 500 mg/kg dose of PG was selected to evaluate its effect on MES induced seizures. Since, no protective effect was observed with this dose, higher doses were also used to study its dose-dependent effect. Sesame oil was used as a vehicle[10] in vehicle control group.
PHT (Abbott, India) and CBZ (Novartis) were purchased from a local pharmacy. 5'5-Dithiobis (2-nitrobenzoic acid) (DTNB) and reduced glutathione (GSH) were purchased from Sigma Chemical Co., USA. All other reagents were of analytical grade and were obtained from Qualigens, India. PHT and CBZ were given in therapeutic and sub-therapeutic doses to evaluate any pharmacodynamic and pharmacokinetic interaction with PG. They were suspended in normal saline. Drugs/vehicles were given orally by gavage in a volume not >1 ml/100 g body weight. All the solutions were prepared afresh during experimentation.
Experimental protocol
Animals were randomly divided into twelve groups each containing six animals. Group I served as a normal control in which no active treatment was given. Group II was administered vehicle (sesame oil, 2 ml/kg) orally for 7 days. Groups III to VI were given PG orally in doses of 500, 1000, 2000, and 4000 mg/kg for 7 days, respectively. On day 7, seizures were induced by MES 120 min after last dose of PG/vehicle. For evaluation of pharmacodynamic interactions, therapeutic (40 mg/kg) and sub-therapeutic (20 mg/kg) doses of PHT were administered orally in a single dose on day 7 to groups VII and VIII. Group IX was given PG (4000 mg/kg) for 7 days and PHT (20 mg/kg) on day 7, MES was given 120 min after the last dose of PG and PHT. Groups X and XI received therapeutic (20 mg/kg) and sub-therapeutic (10 mg/kg) doses of CBZ orally on day 7. In group XII, PG (4000 mg/kg) was administered for 7 days and CBZ (10 mg/kg) orally on day 7. Seizures were induced by MES after 30 and 120 min administration of CBZ and PG, respectively.
Experimental induction of seizures
Electroconvulsions were produced by a suprathreshold fixed current sinus wave stimulus (current intensity - 70 mA, duration - 0.2 s, frequency - 299 Hz) delivered through ear clip electrodes using an ECT unit (Ugo Basile, Italy) as described earlier.[19] The animals were observed for the occurrence of tonic hind limb extension (THLE), that is, the hind limbs of animals outstretched 180° to the plane of the body axis.
Behavioral tests
Behavioral parameters were performed before and after induction of seizures.
Elevated plus maze test
Acquisition and retention of memory processes were assessed using elevated plus maze as described earlier.[15] The initial transfer latency was measured on day 1 and retention transfer latency was recorded 24 h later.
Passive avoidance test
Passive avoidance test was performed according to the method previously described for testing memory retention in rats.[19] The initial latency was measured on day 1 and retention latency was recorded 24 h later. The step through passive avoidance task gives information about the ability of the animals to acquire the task (learning) and to recall the task (retrieval). Therefore, it may be regarded as a measure of long-term memory.
Oxidative stress parameters
At the end of the study period, animals were sacrificed by decapitation under ether anesthesia. The brains were quickly removed, cleaned with chilled saline and stored at −80°C until biochemical analysis was done. The whole brain tissue samples homogenate (10% w/v) was prepared in ice-cold 0.1 M phosphate buffer (pH 7.4). Aliquots were prepared to estimate lipid peroxidation and reduced GSH.
Measurement of lipid peroxidation
Lipid peroxidation was measured through malondialdehyde (MDA) levels.[20] Acetic acid 1.5 ml (20%) pH 3.5, 1.5 ml of thiobarbituric acid (0.8%), and 0.2 ml of sodium dodecyl sulfate (8.1%) were added to 0.1 ml of processed tissue homogenate. The mixture was heated at 100°C for 60 min. After cooling under tap water, 5 ml of n-butanol: Pyridine (15:1, v/v) and 1 ml of distilled water were added. The mixture was then shaken vigorously and centrifuged at 4000 rpm for 10 min. The organic layer was separated from aqueous and absorbance of the organic layer was measured at 532 nm using a spectrophotometer.
Estimation of glutathione
GSH was estimated by the method described by Ellman.[21] To precipitate out the proteins, brain homogenate was centrifuged with 5% trichloro-acetic acid. The supernatant was separated; 2 ml of phosphate buffer (pH 8.4), 0.5 ml of Ellman's reagent that is DTNB 5,5'-dithiobis-(2-nitrobenzoic acid)] and 0.4 ml of distilled water were added to 0.1 ml of this supernatant. After vortexing, the absorbance was read at 412 nm within 15 min in a spectrophotometer.
Pharmacokinetic study
The animals were randomly divided into eight groups (n = 6). The AEDs/PG was administered for 7 days. Group I was administered PHT (40 mg/kg, p.o), whereas group II received PHT (40 mg/kg, p.o) with PG (4000 mg/kg, p.o). Similarly, group III was administered PHT (20 mg/kg) and group IV was given PHT (20 mg/kg, p.o) along with PG (4000 mg/kg, p.o). Group V received CBZ (20 mg/kg, p.o) alone and group VI was administered CBZ (20 mg/kg, p.o) along with PG (4000 mg/kg, p.o). Group VII was given CBZ 10 mg/kg alone and group VIII was administered CBZ (10 mg/kg) along with PG (4000 mg/kg, p.o). The blood samples (1 ml) were collected from the retro-orbital plexus at Cmax (3 h for PHT and 1 h for CBZ) and 24 h after the last dose of PHT and CBZ administration.[19] Serum levels of PHT and CBZ were estimated using high-performance liquid chromatography (HPLC).
Estimation of serum levels of phenytoin and carbamazepine using high-performance liquid chromatography
The collected blood was allowed to clot at room temperature for 30 min and was then centrifuged at 3000 rpm for 10 min. The serum was separated and stored in Eppendorf tubes at −80°C until further analysis.[22] A standard curve in the concentration range of 1–100 μg/ml for PHT and CBZ was obtained. The levels of PHT and CBZ in rat serum were determined from the peak area – concentration ratio.
The analysis was performed by HPLC (Agilent 1200 series) using C-18 (250 mm × 4.6 mm) packed column as the stationary phase. The mobile phase containing 50 mM potassium di-hydrogen orthophosphate buffer, acetonitrile, and methanol in the ratio of 60:20:20 (v/v/v) was filtered through a 0.22 μm membrane filter (Millipore) and degassed prior to use. The flow rate was 1.2 ml/min. The detection was performed at the wavelength of 210 nm. Column oven temperature was maintained at 30°C. The sample injection volume was 10 μl.
Statistical analysis
The results are expressed as mean ± standard error of the mean. Statistical analysis was performed using one-way analysis of variance (ANOVA) with Bonferroni post-hoc statistical tests for behavioral and oxidative stress data. The Chi-square test was used to compare the anticonvulsant effect between the groups. Unpaired t-test was used for serum levels of AEDs. All statistical analyses were performed using statistical package for social sciences (SPSS) v. 16 (Chicago, IL, USA). P < 0.05 was considered as significant.
Results
Effect on maximal electroshock induced seizures
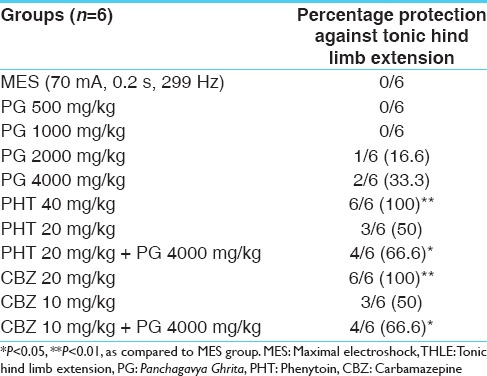
Pretreatment with PG at the dose of 2000 and 4000 mg/kg showed 16.6% and 33.3% protection, respectively, against THLE induced by MES. The therapeutic doses of PHT (40 mg/kg) and CBZ (20 mg/kg) produced significant (P < 0.01) protection against MES induced THLE. However, no significant protection was observed at sub-therapeutic doses of PHT (20 mg/kg) and CBZ (10 mg/kg) against THLE as compared to MES group. Co-administration of PG (4000 mg/kg) with the lower doses of PHT (20 mg/kg) and CBZ (10 mg/kg) showed a significant (P < 0.05) increase in percentage protection against THLE in comparison to MES group [Table 1] indicating pharmacodynamic interaction between PG and PHT/CBZ.
Table 1.
Percentage protection against MES induced THLE in rats (n=6)
Cognitive functions
Effect on elevated plus maze test
The initial transfer latency did not differ significantly, whereas there was a significant difference in retention transfer latency amongst the groups. Post-hoc analysis (F [11,60] = 16.398, P = 0.000) showed that retention transfer latency was significantly (P < 0.001) increased in the MES group as compared to normal control group, thus indicating impairment of memory in the MES group. A significant improvement in memory retention by PG was observed at the dose of 4000 mg/kg as indicated by decrease in retention transfer latency as compared to MES group which was comparable to that of the PHT treated group.
In case of pharmacodynamic study, the retention transfer latency was decreased significantly (P < 0.001) in PHT (40 mg/kg) and CBZ (20 mg/kg) groups as compared to the MES group. PHT and CBZ in the sub-therapeutic doses alone did not show any significant improvement in retention transfer latencies (P > 0.05) whereas combination of PG (4000 mg/kg) with sub-therapeutic doses of PHT and CBZ showed a significant (P < 0.001) improvement in retention transfer latencies in comparison to the MES group [Figure 1].
Figure 1.
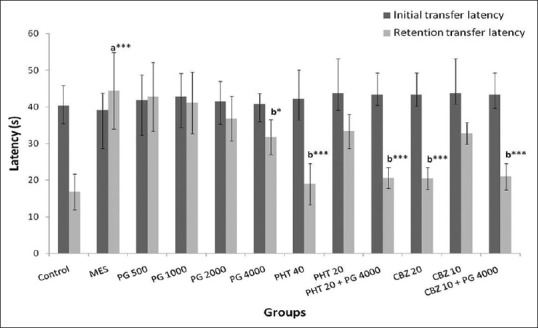
Effect of Panchagavya Ghrita (mg/kg) on initial and retention transfer latencies in the elevated plus maze test in maximal electroshock induced seizures. Data represent mean ± standard error of mean (n = 6), *P < 0.05, ***P < 0.001, a - as compared to normal control group; b - as compared to maximal electroshock group; PHT: Phenytoin, CBZ: Carbamazepine
Effect on passive avoidance test
ANOVA showed a significant difference between the retention transfer latency amongst the groups (F [11,60] = 27.72, P = 0.000). A significant decrease in retention latency (P < 0.001) in the MES group was observed as compared to normal control group whereas PG (4000 mg/kg) showed a significant (P < 0.05) increase in retention latency as compared to MES group. Similarly, retention latency was found to be increased significantly (P < 0.001) in the groups treated with PHT and CBZ at therapeutic doses. PHT at sub-therapeutic dose alone (P < 0.05) as well as in combination with PG (4000 mg/kg) showed a significant (P < 0.01) increase in retention latency as compared to the MES group. Moreover, CBZ at sub-therapeutic dose did not show a significant increase in retention latency whereas its co-administration with PG (4000 mg/kg) caused a significant (P < 0.001) increase in retention latency as compared to the MES group. PG (4000 mg/kg) in combination with sub-therapeutic doses of PHT and CBZ showed a significant increase in retention latency as compared to PG (4000 mg/kg) alone treated group (P < 0.05) as well as sub-therapeutic doses of PHT (P < 0.05) and CBZ (P < 0.01) alone treated groups [Figure 2].
Figure 2.
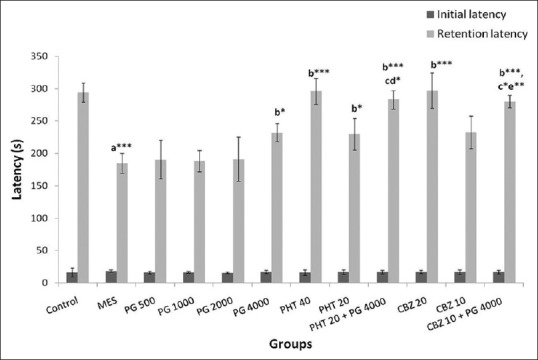
Effect of Panchagavya Ghrita on initial and retention latencies in the passive avoidance test in the maximal electroshock induced seizures. Data represent mean ± standard error of mean (n = 6) *P < 0.05, **P < 0.01, ***P < 0.001, a - as compared to normal control group; b - as compared to maximal electroshock group. c - As compared to Panchagavya Ghrita 4000 mg/kg; d - as compared to phenytoin 20 mg/kg; e - as compared to carbamazepine (10 mg/kg); PHT: Phenytoin, CBZ: Carbamazepine
Oxidative stress parameters
Effect on malondialdehyde levels in rat brain
ANOVA showed a significant difference in MDA level in rat whole brain between the groups (F [11,60] =50.93, P = 0.000). There was a significant increase (P < 0.001) in the MDA levels in MES group as compared to the normal control group. Increased MDA levels were reversed significantly (P < 0.05) in the PG (4000 mg/kg) group as compared to the MES group. The therapeutic (P < 0.001) as well as sub-therapeutic doses (P < 0.05) of PHT and CBZ showed a significant reduction in the increased MDA levels in comparison to MES group. The concomitant administration of PG (4000 mg/kg) with sub-therapeutic dose of PHT showed a significant decrease in MDA levels as compared to MES, PG (4000 mg/kg) as well as PHT (20 mg/kg) groups (P < 0.001). The decrease in MDA levels in the combination of sub-therapeutic doses of CBZ and PG (4000 mg/kg) was significant as compared to MES group (P < 0.001) as well as PG (4000 mg/kg) and CBZ alone treated groups (P < 0.01) [Figure 3].
Figure 3.
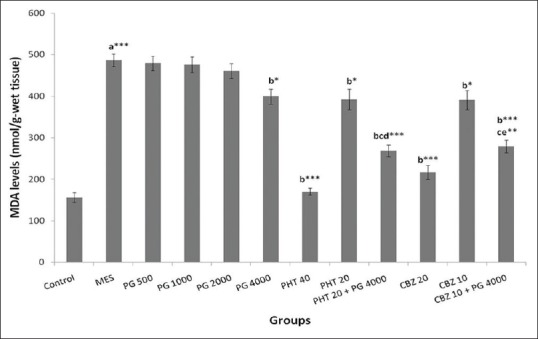
Effect of Panchagavya Ghrita on whole-brain malondialdehyde levels in the maximal electroshock induced seizures in rats. Data represent mean ± standard error of mean (n = 6), *P < 0.05, **P < 0.01, ***P < 0.001, a - as compared to normal control group; b - as compared to maximal electroshock group, c - as compared to Panchagavya Ghrita 4000 mg/kg; d - as compared to phenytoin 20 mg/kg; e - as compared to carbamazepine 10 mg/kg; PHT: Phenytoin, CBZ: Carbamazepine
Effect on glutathione levels in whole rat brain
A significant difference in GSH levels was observed amongst the groups (F [11,60] =13.71, P = 0.000). There was a significant decrease (P < 0.001) in the GSH level in MES group in comparison to the normal control group. There was a significant improvement (P < 0.05) in the decreased levels of GSH in the PG (4000 mg/kg) treated group as compared to MES group. The therapeutic and sub-therapeutic doses of PHT and CBZ showed significantly (P < 0.001 and P < 0.05, respectively) reversal of the decreased GSH level in comparison to MES group. Moreover, concomitant administration of PG (4000 mg/kg) with sub-therapeutic doses of PHT and CBZ showed a significant increase in the GSH level in comparison to MES group (P < 0.01) [Figure 4].
Figure 4.
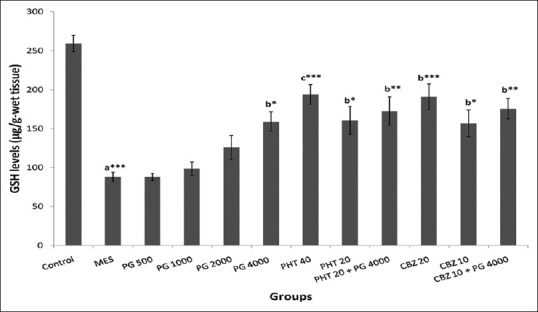
Effect of Panchagavya Ghrita on whole-brain glutathione levels in the maximal electroshock induced seizures. Data represent mean ± standard error of mean (n = 6), *P < 0.05, **P < 0.01, ***P < 0.001, a - as compared to normal control group; b - as compared to maximal electroshock group; c - as compared to Panchagavya Ghrita 4000 mg/kg; d - as compared to phenytoin 20 mg/kg; e - as compared to carbamazepine 10 mg/kg; PHT: Phenytoin; CBZ: Carbamazepine
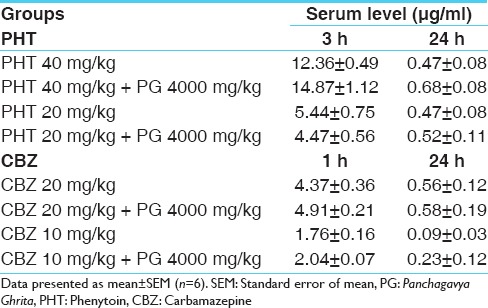
Effect on the serum levels of phenytoin and carbamazepine
Though there was slight increase in the serum levels of both PHT and CBZ in groups co-administered with PG, the levels were however well within the therapeutic range in different groups and the difference was not statistically significant as compared to the groups administered these AEDs alone [Table 2].
Table 2.
Effect of PG on serum levels (μg/ml) of PHT and CBZ
Discussion
Although AEDs are the core of epilepsy treatment; however, approximately 30% of patients with epilepsy are still refractory to seizures.[11] Therefore, there is a need for the development of AEDs with multiple mechanisms of action with no or minimal adverse effects. In the present study, the anticonvulsant effect of PG was investigated using MES induced seizure in rats. PG at the dose of 2000 and 4000 mg/kg exhibited 16.6% and 33.3% protection against THLE in MES induced seizures, respectively. This antiepileptic effect of PG is well documented in Ayurveda. The use of PG in the treatment of epilepsy is also mentioned in Charaka Samhita.[1]
Since the combination of two or more AEDs is used to control refractory seizures,[23] it is usually associated with more drug – drug interactions.[24] Moreover, the side effects of the AEDs are reported to increase with an increase in dose. Concomitant use of alternative medicines and AEDs is very common in a patient due to lack of awareness of the interaction between these drugs. The consequences of interaction may be either lack of and/or diminished efficacy of AEDs as well as unexpected side effects. Therefore, concomitant use of herbal drugs with modern medicines created an urgent need to study herb-drug interactions. In the present study, co-administration of PG (4000 mg/kg) with sub-therapeutic doses of PHT and CBZ potentiated the percentage protection against THLE from 50% (sub-therapeutic dose of AEDs alone treated groups) to 66.6% (combination of AEDs and PG treated groups). Thus, PG increased the efficacy of PHT and CBZ.
PHT and CBZ act through prolongation of Na+ channel inactivation. In a previous study, the anticonvulsant effect of PG was reported in the PTZ and MES seizure models in rats.[7] Therefore, it can be hypothesized that GABA and voltage-gated channels are involved in the anticonvulsant activity of PG. This suggests that PG may act through both the mechanisms, thus, potentiating the antiepileptic effect of PHT and CBZ.
Cognitive impairment is an important consequence of epilepsy, and furthermore, the AED treatment often contributes to the worsening of cognitive functions such as memory, attention, and learning deficits.[25] In concordance with the previous findings, results in the present study also showed MES-induced cognitive impairment.[15] Memory function in this study was assessed by the passive avoidance test and the elevated plus maze test. An increase in retention transfer latency in elevated plus maze test and a decrease in retention latency in passive avoidance test was observed in MES treated rats as compared to the normal control indicating an impairment of memory function. The observed cognitive impairment in the MES group may be attributed to the either seizures itself or seizures associated free radical cascades.[26,27,28] The therapeutic and sub-therapeutic doses of PHT and CBZ significantly ameliorated the cognitive impairment in rats as compared to MES group but the improvement in cognitive function was less with sub-therapeutic doses of PHT and CBZ treated groups. However, co-administration of PG (4000 mg/kg) along with sub-therapeutic doses of PHT and CBZ caused significant reversal of cognitive impairment in rats as compared to MES as well as sub-therapeutic doses of PHT and CBZ treated groups indicating potentiation of efficacy of PHT and CBZ by PG.
Seizure activity and chronic AEDs treatment in epileptic patients have been associated with an increased level of free radicals and reduced activity of antioxidant defense mechanisms,[29,30] which may result into recurrent seizures and cognitive deficit. GSH is thought to play a central role in defense against reactive oxygen species. It can directly detoxify reactive oxygen species and can act as a substrate for several peroxidases.[31] The results of this study showing an increased oxidative stress in the seizure groups support the findings of the previous studies. In the present study, MES induced seizures caused an imbalance between oxidant and antioxidant system resulting oxidative stress as indicated by increased MDA levels and decreased GSH levels in MES group. The imbalance between antioxidant and oxidant defensive system may be responsible for seizures as well as impairment of memory function.[32] PHT and CBZ are the conventional AEDs found to be effective against MES induced seizures. Thus, the reduced GSH levels in MES group were reversed by treatment with therapeutic doses of PHT and CBZ by protecting the animals against MES induced seizures as well as an increase in oxidative stress.
In the present study, there was a decrease in the brain MDA and increase in the brain GSH levels in groups when PG was co-administered with sub-therapeutic doses of these AEDs. PG has been known to possess antioxidant activity revealed by in vitro assays.[33] Cow products especially cow urine is rich in volatile free acids, which is a very potent antioxidant agent in turn may be responsible for the protection against seizures and seizures associated deformities.[5,34]
PG produced an anticonvulsant action against MES induced seizures in rats and potentiated the antiseizure effect of PHT and CBZ in the MES model. However, co-administration of PG with PHT and CBZ produced a slight increase in their serum levels but the increase was statistically insignificant. This suggests the probable involvement of other mechanisms in the potentiation of the antiseizure effect of the sub-therapeutic doses of these AEDs by PG, which needs to be explored in further studies.
Conclusion
Panchagavya Ghrita at the dose of 4000 mg/kg showed an anticonvulsant effect against MES induced seizures and also potentiated the anticonvulsant effect of PHT and CBZ. Moreover, the alteration in the serum levels of PHT and CBZ, when co-administered with PG, was statistically insignificant. Therefore, PG can be a potential adjunct to the conventional AEDs as it helps in increasing the efficacy, reducing the dose and decreasing the side effects of these AEDs. Further studies can be planned to study mechanistic approaches to elucidate the antiepileptic activity of PG at different dose levels in different experimental models.
Financial support and sponsorship
Council of Scientific and Industrial Research (CSIR), New Delhi, India, provided financial assistance (9/6 (431)/2011, EMR-I).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing financial assistance to carry out this research work.
References
- 1.Shastri K, Chaturvedi G, editors. Caraka Samhita of Agnivesa, Chikitsa Sthana. 18th ed. Varanasi, India: Chaukhamba Bharati Academy; 1992. pp. 332–3. [Google Scholar]
- 2.Achilya GS, Kotagale NR, Wadodkar SG, Dorle AK. Hepatoprotective activity of Panchagavya Ghrita against carbon tetrachloride induced hepatotoxicity in rats. Indian J Pharmacol. 2003;35:308–11. [Google Scholar]
- 3.Paliwal R, Sahni YP, Singh SK, Quadri MA, Kumar P. Immunomodulatory activity of Panchagavya. Int J Adv Pharm Res. 2012;3:878–84. [Google Scholar]
- 4.Wate SP, Dhanjode DP, Duragkar NJ, Tajne MR. Antioxidant potential of cow urine and its fractions: A comparative study. Int J Univers Pharm Life Sci. 2011;1:146–54. [Google Scholar]
- 5.Nagda G, Bhatt DK. Effect of treatment of cow's urine “Gomutra” and antioxidants in alleviating the lindane-induced oxidative stress in kidney of Swiss mice (Mus musculus) Mol Biol Rep. 2014;41:1967–76. doi: 10.1007/s11033-014-3044-6. [DOI] [PubMed] [Google Scholar]
- 6.Russo E, Scicchitano F, Citraro R, Aiello R, Camastra C, Mainardi P, et al. Protective activity of a-lactoalbumin (ALAC), a whey protein rich in tryptophan, in rodent models of epileptogenesis. Neuroscience. 2012;226:282–8. doi: 10.1016/j.neuroscience.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Devesh GD, John PS. Effect of Panchagavya Ghrita on some neurological parameters in albino rats. Asian J Pharm Clin Res. 2012;5:154–6. [Google Scholar]
- 8.Koneru A, Satyanarayana S, Mukkanti K, Abedulla K, Kumar P. Anticonvulsant activity of Panchagavya Ghrutham: A polyherbal Ayurvedic formulation. J Pharm Res. 2009;2:795–7. [Google Scholar]
- 9.Achliya GS, Wadodkar SG, Avinash KD. Neuropharmacological actions of Panchagavya formulation containing Emblica officinalis Gaerth and Glycyrrhiza glabra Linn in mice. Indian J Exp Biol. 2004;42:499–503. [PubMed] [Google Scholar]
- 10.Joshi R, Reeta KH, Sharma SK, Tripathi M, Gupta YK. Panchagavya ghrita, an Ayurvedic formulation attenuates seizures, cognitive impairment and oxidative stress in pentylenetetrazole induced seizures in rats. Indian J Exp Biol. 2015;53:446–51. [PubMed] [Google Scholar]
- 11.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 12.Kwan P, Brodie MJ. Combination therapy in epilepsy: When and what to use. Drugs. 2006;66:1817–29. doi: 10.2165/00003495-200666140-00004. [DOI] [PubMed] [Google Scholar]
- 13.Canevini MP, De Sarro G, Galimberti CA, Gatti G, Licchetta L, Malerba A, et al. Relationship between adverse effects of antiepileptic drugs, number of coprescribed drugs, and drug load in a large cohort of consecutive patients with drug-refractory epilepsy. Epilepsia. 2010;51:797–804. doi: 10.1111/j.1528-1167.2010.02520.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhu HL, Wan JB, Wang YT, Li BC, Xiang C, He J, et al. Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia. 2014;55:3–16. doi: 10.1111/epi.12463. [DOI] [PubMed] [Google Scholar]
- 15.Reeta KH, Mehla J, Gupta YK. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res. 2009;1301:52–60. doi: 10.1016/j.brainres.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Dandekar UP, Chandra RS, Dalvi SS, Joshi MV, Gokhale PC, Sharma AV, et al. Analysis of a clinically important interaction between phenytoin and Shankhapushpi, an Ayurvedic preparation. J Ethnopharmacol. 1992;35:285–8. doi: 10.1016/0378-8741(92)90026-n. [DOI] [PubMed] [Google Scholar]
- 17.Pattanaik S, Hota D, Prabhakar S, Kharbanda P, Pandhi P. Effect of piperine on the steady-state pharmacokinetics of phenytoin in patients with epilepsy. Phytother Res. 2006;20:683–6. doi: 10.1002/ptr.1937. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous. The Ayurvedic Formulary of India. 2nd ed. Vol. 6. Delhi: The Controller of Publications Department of ISM & H, Ministry of Health and Family Welfare, Government of India; 2003. p. 90. [Google Scholar]
- 19.Reeta KH, Mehla J, Pahuja M, Gupta YK. Pharmacokinetic and pharmacodynamic interactions of valproate, phenytoin, phenobarbitone and carbamazepine with curcumin in experimental models of epilepsy in rats. Pharmacol Biochem Behav. 2011;99:399–407. doi: 10.1016/j.pbb.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Patil KM, Bodhankar SL. Simultaneous determination of lamotrigine, phenobarbitone, carbamazepine and phenytoin in human serum by high-performance liquid chromatography. J Pharm Biomed Anal. 2005;39:181–6. doi: 10.1016/j.jpba.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 23.Stephen LJ, Brodie MJ. Seizure freedom with more than one antiepileptic drug. Seizure. 2002;11:349–51. doi: 10.1053/seiz.2002.0711. [DOI] [PubMed] [Google Scholar]
- 24.Patsalos PN, Fröscher W, Pisani F, van Rijn CM. The importance of drug interactions in epilepsy therapy. Epilepsia. 2002;43:365–85. doi: 10.1046/j.1528-1157.2002.13001.x. [DOI] [PubMed] [Google Scholar]
- 25.Aldenkamp AP. Cognitive impairment in epilepsy: State of affairs and clinical relevance. Seizure. 2006;15:219–20. [Google Scholar]
- 26.Black LC, Schefft BK, Howe SR, Szaflarski JP, Yeh HS, Privitera MD. The effect of seizures on working memory and executive functioning performance. Epilepsy Behav. 2010;17:412–9. doi: 10.1016/j.yebeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Endermann M. A time-limited residential unit for young adults with epilepsy and mild cognitive impairment: Results of a prospective pre-post-study. Seizure. 2010;19:178–84. doi: 10.1016/j.seizure.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Taylor J, Kolamunnage-Dona R, Marson AG, Smith PE, Aldenkamp AP, Baker GA, et al. Patients with epilepsy: Cognitively compromised before the start of antiepileptic drug treatment? Epilepsia. 2010;51:48–56. doi: 10.1111/j.1528-1167.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- 29.Hamed SA, Abdellah MM, El-Melegy N. Blood levels of trace elements, electrolytes, and oxidative stress/antioxidant systems in epileptic patients. J Pharmacol Sci. 2004;96:465–73. doi: 10.1254/jphs.fpj04032x. [DOI] [PubMed] [Google Scholar]
- 30.Sudha K, Rao AV, Rao A. Oxidative stress and antioxidants in epilepsy. Clin Chim Acta. 2001;303:19–24. doi: 10.1016/s0009-8981(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 31.Xu K, Stringer JL. Antioxidants and free radical scavengers do not consistently delay seizure onset in animal models of acute seizures. Epilepsy Behav. 2008;13:77–82. doi: 10.1016/j.yebeh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menon B, Ramalingam K, Kumar RV. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. Seizure. 2012;21:780–4. doi: 10.1016/j.seizure.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Athavale A, Jirankalgikar N, Nariya P, De S. Evaluation of in-vitro antioxidant activity of Panchagavya: A traditional Ayurvedic preparation. Int J Pharm Sci Res. 2012;3:2543–9. [Google Scholar]
- 34.Dutta D, Devi S, Krishnamoorthy K, Chakraborti T. Antigenotoxic/ameliorative effect of Kamdhenu Ark and redistilled Kamdhenu Ark in human polyporphonuclear leucocytes. J Ecophysiology Occup Health. 2004;4:27–36. [Google Scholar]


