Abstract
Objective:
The aim of this study was to investigate the neutralizer effect of antioxidant agents on the bond strength of bleached enamel.
Materials and Methods:
Sixty enamel slabs were prepared from 60 freshly extracted maxillary central incisors and were divided into six groups. The negative control group received no bleaching treatment and the other groups were bleached with 35% carbamide peroxide (Opalescence Quick; Ultradent, South Jordan, USA). In Group II, composite was built immediately after bleaching and cured without any antioxidants. In Group III, bleached specimens received composite build ups delayed by 1 week. In Groups IV, V, and VI bleached specimens received applications of superoxide dismutase (SOD), sodium ascorbate (SA), and tocopherol solutions, respectively, for 10 min. Following composite bonding, the micro shear bond strength (μSBS) was measured at a speed of 1 mm/min in universal testing machine.
Statistical Analysis Used:
The μSBS values of all the groups were analyzed using the analysis of variance followed by Tukey honestly significant difference post-hoc test.
Results:
Bonding of composites to unbleached group (Group I) exhibited the highest mean SBS values and among the antioxidant-treated groups, the highest SBS values were seen with SOD (Group IV) treated samples (23.0040 ± 4.30565 MPa).
Conclusions:
Application of SA, alpha-tocopherol, and SOD can effectively reverse the bond strength with bleached enamel. SOD gave a comparatively more promising reversal of bond strength than SA and alpha-tocopherol, and deserves further studies.
Keywords: Alpha-tocopherol, shear bond strength, sodium ascorbate, superoxide dismutase
INTRODUCTION
The introduction of newer bleaching materials in the recent years has witnessed impressive esthetic outcomes clinically. However, an additional restorative treatment is often needed to attain the desired esthetics, even after bleaching.[1] The adverse effects of bleaching dental enamel are of considerable clinical relevance when bonded composite restorations ensue bleaching treatment. An important consequence of the use of carbamide peroxide is decreased bond strength of the composite resin to enamel immediately after bleaching procedure.[2,3] This decreased bond strength has been ascribed to the presence of oxygen ions that inhibit resin polymerization.[4,5,6] The reduced shear bond strength (SBS) might also be attributable to alterations in the microstructure of bleached enamel surfaces after becoming acid-etched, including reduced micro hardness, calcium loss, over etching, and loss of enamel prisms.[7] A delay by around 1–3 weeks in composite restorations has been suggested to overcome this. However, application of antioxidants such as sodium ascorbate (SA) and alpha-tocopherol can be implemented to avoid this delay.[8] Bulut et al.[9,10] in 2005 and 2006 studied the efficacy of delayed bonding and antioxidant application and reported that both approaches were effective in returning the compromised bond strength back to the control levels. Antioxidants neutralize free radicals by donating one of their electrons, ending the electron stealing reaction, and restoring the altered redox potential of the bleached surface.[8] Therefore, restorative procedures can be carried out without delay after bleaching, which shortens the overall time needed for esthetic procedures.
SA has been used in various concentrations and durations to improve composite bond strength after bleaching. Ten minute application of SA in solution form has yielded favorable results.[8] Super oxide dismutase is an antioxidant as well as a free radical scavenger enzyme. It catalyzes the dismutation of super oxide radicals to hydrogen peroxide and molecular oxygen and plays a crucial role in the protection of cells against the harmful effects of oxygen radicals.[11] Vitamin E (alpha-tocopherol), a natural and powerful antioxidant achieves reversal of bond strength by scavenging free radicals and molecular oxygen, a mode of action very similar to ascorbic acid. The beneficial effect of alpha-tocopherol is attributed to its antioxidant and alcohol solvent effect. It has recently been suggested for improving composite bonding after bleaching.[12]
SBS tests have been widely preferred to determine the bonding ability of composite because of their technical simplicity in contrast to tensile bond strength test. More recently, a new test method using specimens with reduced dimensions has been advocated by some authors to evaluate the SBS, as a substitute for lower trial shear for the so-called microbond “or” micro SBS (µSBS) test. The aim of the study is to determine the bonding between carbamide peroxide bleached enamel and composite by µSBS testing and to evaluate the reversal of bond strength after bleaching using various antioxidants (10% SA, super oxide dismutase, and alpha-tocopherol).
MATERIALS AND METHODS
The study was approved by the Institutional Ethical Committee. Sixty human single-rooted maxillary central incisors freshly extracted due to periodontal reasons, which were free of caries, crack, fractures, or other defects, were collected. Teeth were cleaned with ultrasonic scaler and stored in distilled water until usage.
Specimen preparation
The crowns of the teeth were separated at the cementoenamel junction using a diamond disk. Sixty enamel slabs measuring 3 mm × 3 mm × 2 mm were prepared from the crowns of the teeth using a two-sided diamond disk (Brasseler Savannah, USA) under water spray. Then, the enamel specimens were mounted in self-curing acrylic resin and polished using Soflex disks (3M ESPE, MN, USA) under water spray to produce flat surfaces. The specimens were then randomly divided into six groups of 10 specimens each.
Ten specimens were randomly selected for the negative control group. They were stored in distilled water at 37°C for 3 weeks and received no bleaching treatment (Group I-control group); the water was changed every day.
All the other groups were subjected to bleaching treatment which were exposed to a predetermined volume (0.01 mL) of the bleaching agent containing 35% carbamide peroxide (Opalescence Quick; Ultradent, South Jordan, USA) and applied over the prepared specimens using microbrushes.
In Group II, the surface of enamel specimens was exposed to a predetermined volume (0.01 mL) of the bleaching agent containing 35% carbamide peroxide (Opalescence Quick; Ultradent, South Jordan, USA) for 30 min. During the intervening period after rinsing the bleaching agent under running water for 1 min, the specimens were kept in distilled water at 37°C. Immediately after bleaching, etching, bonding, and curing with resin composite (Filtek P-60, 3M/ESPE) were done, without any antioxidant application.
In Group III, the surface of enamel specimens was exposed to a predetermined volume (0.01 mL) of the bleaching agent containing 35% carbamide peroxide (Opalescence Quick; Ultradent, South Jordan, USA) for 30 min. During the intervening period after rinsing the bleaching agent under running water for 1 min, the specimens were kept in distilled water at 37°C. Etching bonding and curing with resin composite (Filtek P-60, 3M/ESPE) were performed after 1 week, without antioxidant application.
In Group IV, subsequent to the application of 35% carbamide peroxide similar to Group II, the surface of the enamel specimens was exposed to superoxide dismutase (SOD) (Sigma Aldrich, USA) for 10 min. The aqueous solution of SOD was obtained in sodium sulfate solution. Composite build up was done and cured immediately.
In Group V, subsequent to the application of 35% carbamide peroxide, the surface of the enamel specimens was exposed to 10% SA for 10 min. During this exposure, the solution on the enamel surface was agitated every 10 min using a sterile brush. After the application of SA, the enamel surfaces were thoroughly rinsed under running water for 30 s and then kept in distilled water at 37°C. The 10% SA solution was prepared by dissolving 10 g of SA powder (TAICA Lab, Pondicherry, India) in 100 ml of distilled water in a standard flask. Composite was built and cured immediately.
In Group VI, subsequent to the application of 35% carbamide peroxide, the surface of the enamel specimens was exposed to tocopherol solution for 10 min. The solution was prepared by dissolving 10 g of alpha-tocopherol gel (TAICA Lab, Pondicherry, India) in 100 ml of ethyl alcohol in a standard flask. Composite resin was built and cured immediately.
Standardization of bonding surface and composite placement
Prefabricated polytetrafluoroethylene (PTFE) sheet mold with an iris of 0.7 mm internal diameter and 1 mm thickness was placed on the prepared buccal surfaces in all the specimens with the help of double side adhesive sticker to get a standardized bonding surface of 0.7 mm diameter on enamel before bonding procedure. Composite (Filtek P-60, 3M/ESPE) build-up of 0.7 mm diameter and 1 mm height was done in all the groups as follows.
Etching of the prepared specimens was done with 34% phosphoric acid (Dentsply Caulk, Dentsply Int. Inc., Milford, DE, USA) for 15 s, rinsed for 30 s, and then air dried for 20 s. Magic Bond (Vigodent, Rio de Janeiro, RJ, Brazil) resin adhesive was applied in a thin layer over the etched enamel using microbrushes and gently air spread. Magic bond adhesive is composed of hydrophobic monomers (BisGMA and methacrylic acid ester), without the presence of a solvent. The light cured composite resin (Z250-3M, St Paul, MN, USA) was packed into the opening of the PTFE sheet mold of dimension 0.7 mm in diameter and 1 mm in height using a Teflon-coated instrument and light cured for 40 s. Following curing, the mold was removed and additional curing was done for 40 s from opposite sides. This insures optimal resin polymerization and homogeneous stress distribution to the tooth resin bonding interface when performing the shear test. The resulting cylindrical test specimens are of the dimension of 0.7 mm diameter and 1 mm in height. The specimens were accordingly identified and stored in distilled water at 37°C for 24 h prior to testing.
Shear bond strength evaluation
Each specimen with acrylic mold was attached to jig of universal testing machine. A thin orthodontic ligature wire of 0.2 mm diameter was embedded in 1 cm × 1 cm acrylic resin block and suspended from the holding jig and looped around the composite cylinder and gently held flush against the enamel composite interface and tested. The wire loop and the center of load cell were aligned along a straight line to ensure correct direction of application of the shear force. Shear force was applied in a universal testing machine (Instron 3369, UKAS Corporation, University Ave, Norwood, MA 02062-2643, USA) operated at a cross head speed of 1 mm/min2.
Statistical analysis
Statistical analysis for the calculated means and standard deviation of the µSBS values of the six groups was done using SPSS version 20 (IBM, Corp, USA) using the one-way analysis of variance (ANOVA) test. Tukey honestly significant difference post-hoc test was done to ascertain any significant differences between groups (P < 0.05).
RESULTS
The bond strength values among the various groups differed significantly from each other. It was observed that about 95% of failures were adhesive in nature. The control group (Group I) exhibited the highest mean bond strength value (23.004 MPa), closely followed by the SOD group (Group IV) (22.104 MPa). The least values were exhibited by the immediate bonding group (Group II) [Table 1]. The mean µSBS values are represented in the bar graph in Figure 1.
Table 1.
Values of mean and standard deviation of micro shear bond strength of the control and test groups
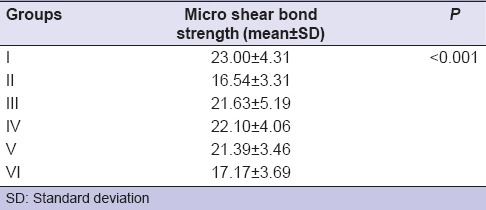
Figure 1.
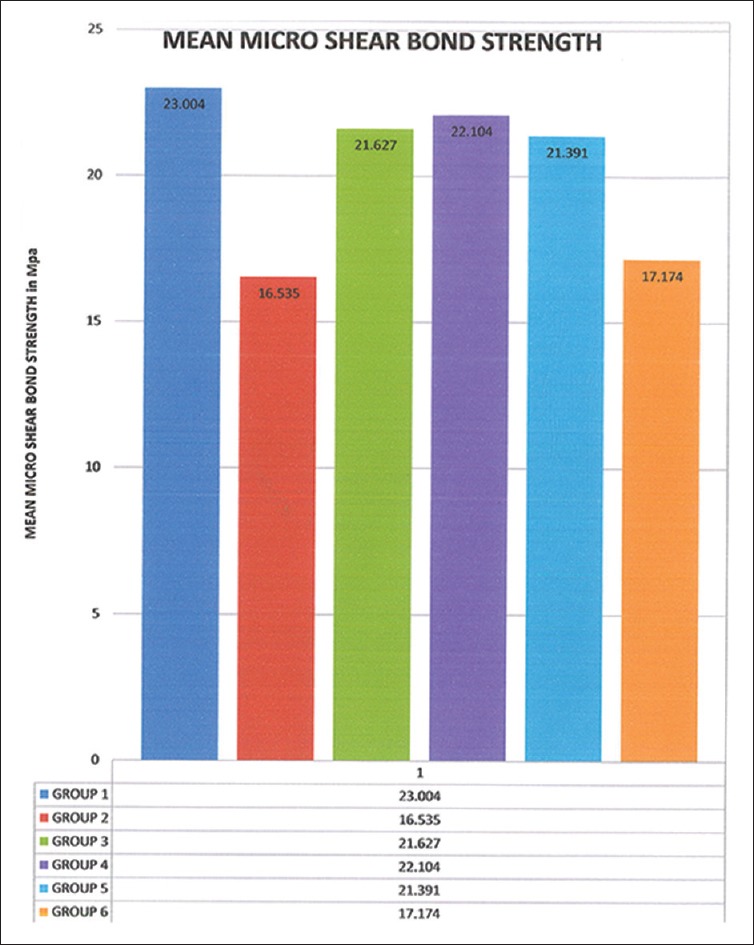
Bar graph showing the mean micro shear bond strengths of various groups (MPa) (original)
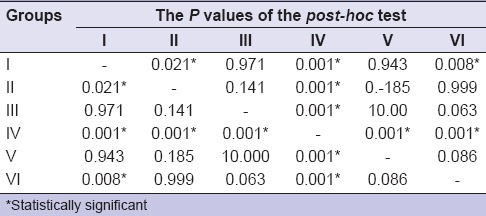
One-way ANOVA test [Table 1] revealed that mean µSBS value of Group IV differed significantly from the mean µSBS values of other test groups. Direct comparisons between the groups revealed statistically significant discrepancies in the bond strength values among the six groups and that the SBS value of Group IV was significantly higher than all the other test groups. The µSBS value among Groups I, II, III, IV, V, and VI was statistically significant at P < 0.001 [Table 2].
Table 2.
One-way analysis of variance comparison within and between the groups
DISCUSSION
A significant decrease in the bond strength of composite resins has been reported after using carbamide peroxide when compared to unbleached enamel.[13,14] The results of our study were also consistent with previous studies in terms of bond strength of bleached enamel as seen in Group II. Cavalli et al.[15] reported that various concentrations of carbamide peroxide (i.e., 10%, 16%, and 20%) affect the bond strength of composite to enamel. Akin et al.[16] assessed the effect of bleaching agents on the SBS of orthodontic brackets and concluded that 38% hydrogen peroxide adversely affected the bond strength of the orthodontic brackets. The lower bond strengths of bleached enamel and dentin are due to the imbalance in the redox potential caused by the bleaching agents.[17,18,19,20,21] Some authors postulate that the oxygen endures in the dental structure after bleaching and can impede with the polymerization of adhesive monomers.[20,22] Factors such as loss of calcium, decrease in micro hardness, and alterations in the organic part of the substrate might be responsible for a decrease in enamel bond strengths.[23] The residual oxygen from the bleaching agent interferes with resin polymerization.[6] Free radicals contain unshared electrons which are highly energetic and react rapidly with oxygen to form reactive oxygen species (ROS). Previously various antioxidants have been tried for reversal of bond strength including ascorbic acid, SA, alpha-tocopherol, proanthocyanidins, pinebark extract, and catalsases. Recently, Han et al. evaluated the effects of antioxidants after bleaching and its influence on the microleakage of composite restorations and concluded that catalase was more effective in reducing microleakage associated with reduced bond strength.[24] In this study, we used various antioxidants for the evaluation of reversal of bond strength of enamel. Vitamin E, Vitamin C, and SOD are natural antioxidants which can deactivate free radicals.
SA is a cost-effective water soluble and commonly available antioxidant material. Many authors have evaluated its effect on the bleached teeth when composite resins have been used as bonding adhesives.[3,12,25] In 2002, Lai et al.[21] asserted that when an antioxidant such as SA was applied for 3 h to enamel after bleaching with carbamide per-oxide, the composite SBS was improved.[9,21] Kunt et al.[26] and Lai et al.[21] suggested that SA allows free radical polymerization of the adhesive resin to proceed without premature termination by restoring the altered redox potential of the oxidized bonding substrate and hence reverses the compromised bonding. In 2011, Lima et al.[27] showed that even short durations of 10% SA application (i.e. 1 min) could still obviate the detrimental effect of bleaching on SBS. According to Suneetha et al. in 2014,[28] 10% SA solution was effective in the reversal of SBS immediately after bleaching. Subramonian et al.[29] in 2015 showed that application of 10% SA immediately after bleaching could neutralize the residual oxygen and could reverse the reduced bond strength, but scanning electron microscope images have demonstrated that application of ascorbic acid resulted in super etching of the already bleached enamel surface.[30]
SODs are a class of metal co-factored enzymes discovered by Irwin Fridovich and Joe McCord that detoxify these free radicals by catalyzing the dismutation of superoxide into oxygen and hydrogen peroxide. SOD reduces and reverts superoxide-induced cell damage in the body. Free radicals cause wrinkles and precancerous cell changes in the body which is neutralized by the antioxidant and anti-inflammatory actions of SOD.[11] SOD is of two types: Copper/zinc (Cu/Zn) SOD and manganese (Mn) SOD. Cu/Zn SOD defends the cytoplasm of the cells, and Mn SOD shields the mitochondria of the cells from free radical damage.[11] In this study, we used (Mn) SOD as an antioxidant for reversal of bond strength.
“Vitamin E” is the collective name for a group of potent, lipid soluble, chain-breaking antioxidants. The antioxidant activity of Vitamin E could be structurally attributed to tocopherols (α, β, γ, and δ) and four tocotrienols (α, β, γ, and δ). Alpha-tocopherol is most profusely found in nature and is responsible for the reversal of Vitamin E deficiency symptoms in humans.[31] The ROS formed as a result of fat oxidation is stopped by Vitamin E. Vitamin E acts on free radicals, which are by-products of normal metabolism and pollutants ingested by the body. These harmful chemicals damage the cells of the body and may contribute to the development of cardiovascular disease and cancer. Ten percentage alpha-tocopherol has shown high efficacy in SBS reversal of enamel and dentin submitted to a home-use bleaching treatment.[12] In 2009, Sasaki et al.[12] compared the efficacy of two different antioxidizing agents in increasing the SBS of bleached enamel and dentin, reporting that 10% alpha-tocopherol was successful, whereas 10% SA was not. Hence, in this study, we used SA, Vitamin E, and a new antioxidant SOD for the reversal of bond strength of bleached enamel as a novel approach.
The micro shear bond test has been developed by Shimada et al.[32] Advantages of micro shear bond test is that the bond test areas can be much better controlled by the use of known diameter micro bore tubing. The method Shimada employed is the application of a shear force using thin stainless steel orthodontic wire. µSBS using 0.2 mm[33] wire loop method was performed in this study because it is easier, very versatile test to assess the strength of the bond between aligned tissue and other dental substance. El Zohairy et al.[34] demonstrated that µSBS test appears to be more accurate in differentiating among the stronger adhesives than microtensile strength testing. Thus, in this study, we used the wire loop method for the assessment of µSBS.
Türkün and Kaya[3] investigated the effect of different concentrations (10%, 16%, and 20%) of carbamide peroxide on SBS of resin composite to bleached bovine enamel. They showed that all three concentrations of carbamide peroxide reduced the SBS, but higher concentrations produced greater reductions. Hence, we used 35% of carbamide peroxide which is a higher concentration used for in office bleaching. All the antioxidant solutions were applied for 10 min in this study. During the treatment period, the antioxidants SOD, alpha-tocopherol, and SA solutions were continuously refreshed, and enamel surface was agitated with sterile brush.
Results of this study showed that Group IV (SOD) and Group V (SA) have highest bond strength followed by Group III (Bond strength after 7 days) and Group VI (Vitamin E). These findings are in accordance with the previous study of Türkün and Kaya.[3] Vitamin C also reversed the bond strength in this study which is in accordance with the previous studies.[7,11,20,21] Vitamin E was used as a gel in this study. It also resulted in the bond strength reversal but less than in Group V (10% SA). This may be due to the nonaqueous nature of this antioxidant.
While SA and alpha-tocopherol are dietary antioxidants, SOD is a biological enzyme which acts at the very starting point of the free radical generation that is superoxide ion. At this level, the SOD nullifies the superoxide ion by converting it into hydrogen peroxide.[35] Also, SA and alpha-tocopherol have only a secondary antioxidant action wherein they undergo an alteration in their chemistry themselves thereby requiring the intervention of the SOD enzyme to revert them back to reduced forms.[36] This proves SOD to be a biologically more potent and efficient antioxidant enzyme which only catalyzes the redox reaction without participating in the reaction itself, a striking property possessed by all enzymes. This property might be attributed to the higher SBS values obtained with the SOD group.
CONCLUSION
There is no study in the literature until date that has employed SOD as an antioxidant for the reversal of bond strength of bleached enamel. In the present study, SOD gave a comparatively more promising reversal of bond strength than Vitamin C and Vitamin E. SOD is a nontoxic antioxidant which has been used for the 1st time in an in vitro application for the reversal of bond strength. It provides newer avenues for advanced clinical research and exploring its further applications. However, further clinical studies are needed to confirm these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors acknowledge Micro-Tek (P) Ltd., Chennai, for the utilization of universal testing machine facilities to evaluate the microtensile bond strength.
REFERENCES
- 1.Josey AL, Meyers IA, Romaniuk K, Symons AL. The effect of a vital bleaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil. 1996;23:244–50. doi: 10.1111/j.1365-2842.1996.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 2.Torres CR, Koga AF, Borges AB. The effects of anti-oxidant agents as neutralizers of bleaching agents on enamel bond strength. Braz J Oral Sci. 2006;5:971–6. [Google Scholar]
- 3.Türkün M, Kaya AD. Effect of 10% sodium ascorbate on the shear bond strength of composite resin to bleached bovine enamel. J Oral Rehabil. 2004;31:1184–91. doi: 10.1111/j.1365-2842.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 4.Breschi L, Cadenaro M, Antoniolli F, Visintini E, Toledano M, Di Lenarda R. Extent of polymerization of dental bonding systems on bleached enamel. Am J Dent. 2007;20:275–80. [PubMed] [Google Scholar]
- 5.Hegedüs C, Bistey T, Flóra-Nagy E, Keszthelyi G, Jenei A. An atomic force microscopy study on the effect of bleaching agents on enamel surface. J Dent. 1999;27:509–15. doi: 10.1016/s0300-5712(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 6.Kaya AD, Türkün M, Arici M. Reversal of compromised bonding in bleached enamel using antioxidant gel. Oper Dent. 2008;33:441–7. doi: 10.2341/07-115. [DOI] [PubMed] [Google Scholar]
- 7.Türkkahraman H, Adanir N, Güngör AY. Bleaching and desensitizer application effects on shear bond strengths of orthodontic brackets. Angle Orthod. 2007;77:489–93. doi: 10.2319/0003-3219(2007)077[0489:BADAEO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Thapa A, Vivekananda PA, Thomas MS. Evaluation and comparison of bond strength to 10% carbamide peroxide bleached enamel following the application of 10% and 25% sodium ascorbate and alpha-tocopherol solutions: An in vitro study. J Conserv Dent. 2013;16:111–5. doi: 10.4103/0972-0707.108184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulut H, Kaya AD, Turkun M. Tensile bond strength of brackets after antioxidant treatment on bleached teeth. Eur J Orthod. 2005;27:466–71. doi: 10.1093/ejo/cji044. [DOI] [PubMed] [Google Scholar]
- 10.Bulut H, Turkun M, Kaya AD. Effect of an antioxidizing agent on the shear bond strength of brackets bonded to bleached human enamel. Am J Orthod Dentofacial Orthop. 2006;129:266–72. doi: 10.1016/j.ajodo.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Petrulea M, Muresan A, Duncea I. Oxidative stress and antioxidant status in hypo- and hyperthyroidism, Egypt. In: El-Missiry MA, editor. Biochemistry, Genetics and Molecular Biology “Antioxidant Enzyme”. Ch. 8. Rijeka, Croatia: InTech; 2012. pp. 197–236. [Google Scholar]
- 12.Sasaki RT, Flório FM, Basting RT. Effect of 10% sodium ascorbate and 10% alpha-tocopherol in different formulations on the shear bond strength of enamel and dentin submitted to a home-use bleaching treatment. Oper Dent. 2009;34:746–52. [PubMed] [Google Scholar]
- 13.Titley KC, Torneck CD, Ruse ND, Krmec D. Adhesion of a resin composite to bleached and unbleached human enamel. J Endod. 1993;19:112–5. doi: 10.1016/S0099-2399(06)80504-2. [DOI] [PubMed] [Google Scholar]
- 14.McGuckin RS, Thurmond BA, Osovitz S. Enamel shear bond strengths after vital bleaching. Am J Dent. 1992;5:216–22. [PubMed] [Google Scholar]
- 15.Cavalli V, Reis AF, Giannini M, Ambrosano GM. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent. 2001;26:597–602. [PubMed] [Google Scholar]
- 16.Akin M, Aksakalli S, Basciftci FA, Demir A. The effect of tooth bleaching on the shear bond strength of orthodontic brackets using self-etching primer systems. Eur J Dent. 2013;7:55–60. [PMC free article] [PubMed] [Google Scholar]
- 17.Rose RC, Bode AM. Biology of free radical scavengers: An evaluation of ascorbate. FASEB J. 1993;7:1135–42. [PubMed] [Google Scholar]
- 18.Rotstein I. Role of catalase in the elimination of residual hydrogen peroxide following tooth bleaching. J Endod. 1993;19:567–9. doi: 10.1016/S0099-2399(06)81288-4. [DOI] [PubMed] [Google Scholar]
- 19.Barghi N, Godwin JM. Reducing the adverse effect of bleaching on composite-enamel bond. J Esthet Dent. 1994;6:157–61. doi: 10.1111/j.1708-8240.1994.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 20.Lai SC, Mak YF, Cheung GS, Osorio R, Toledano M, Carvalho RM, et al. Reversal of compromised bonding to oxidized etched dentin. J Dent Res. 2001;80:1919–24. doi: 10.1177/00220345010800101101. [DOI] [PubMed] [Google Scholar]
- 21.Lai SC, Tay FR, Cheung GS, Mak YF, Carvalho RM, Wei SH, et al. Reversal of compromised bonding in bleached enamel. J Dent Res. 2002;81:477–81. doi: 10.1177/154405910208100709. [DOI] [PubMed] [Google Scholar]
- 22.Dishman MV, Covey DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater. 1994;10:33–6. doi: 10.1016/0109-5641(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 23.Perdigão J, Francci C, Swift EJ, Jr, Ambrose WW, Lopes M. Ultra-morphological study of the interaction of dental adhesives with carbamide peroxide-bleached enamel. Am J Dent. 1998;11:291–301. [PubMed] [Google Scholar]
- 24.Han Y, Mo S, Jiang L, Zhu Y. Effects of antioxidants on the microleakage of composite resin restorations after external tooth bleaching. Eur J Dent. 2014;8:147–53. doi: 10.4103/1305-7456.130581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gökçe B, Cömlekoglu ME, Ozpinar B, Türkün M, Kaya AD. Effect of antioxidant treatment on bond strength of a luting resin to bleached enamel. J Dent. 2008;36:780–5. doi: 10.1016/j.jdent.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Kunt GE, Yilmaz N, Sen S, Dede DÖ. Effect of antioxidant treatment on the shear bond strength of composite resin to bleached enamel. Acta Odontol Scand. 2011;69:287–91. doi: 10.3109/00016357.2011.568958. [DOI] [PubMed] [Google Scholar]
- 27.Lima AF, Fonseca FM, Freitas MS, Palialol AR, Aguiar FH, Marchi GM. Effect of bleaching treatment and reduced application time of an antioxidant on bond strength to bleached enamel and subjacent dentin. J Adhes Dent. 2011;13:537–42. doi: 10.3290/j.jad.a19813. [DOI] [PubMed] [Google Scholar]
- 28.Suneetha R, Pavithra S, Thomas J, Nanga GS, Shiromany A, Shivrayan A. An in vitro comparative study of shear bond strength of composite resin to bleached enamel using synthetic and herbal antioxidants. J Int Oral Health. 2014;6:77–81. [PMC free article] [PubMed] [Google Scholar]
- 29.Subramonian R, Mathai V, Christaine Angelo JB, Ravi J. Effect of three different antioxidants on the shear bond strength of composite resin to bleached enamel: An in vitro study. J Conserv Dent. 2015;18:144–8. doi: 10.4103/0972-0707.153076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muraguchi K, Shigenobu S, Suzuki S, Tanaka T. Improvement of bonding to bleached bovine tooth surfaces by ascorbic acid treatment. Dent Mater J. 2007;26:875–81. doi: 10.4012/dmj.26.875. [DOI] [PubMed] [Google Scholar]
- 31.Brigelius-Flohé R, Traber MG. Vitamin E: Function and metabolism. FASEB J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 32.Shimada Y, Iwamoto N, Kawashima M, Burrow MF, Tagami J. Shear bond strength of current adhesive systems to enamel, dentin and dentin-enamel junction region. Oper Dent. 2003;28:585–90. [PubMed] [Google Scholar]
- 33.Foong J, Lee K, Nguyen C, Tang G, Austin D, Ch’ng C, et al. Comparison of microshear bond strengths of four self-etching bonding systems to enamel using two test methods. Aust Dent J. 2006;51:252–7. doi: 10.1111/j.1834-7819.2006.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 34.El Zohairy AA, Saber MH, Abdalla AI, Feilzer AJ. Efficacy of microtensile versus microshear bond testing for evaluation of bond strength of dental adhesive systems to enamel. Dent Mater. 2010;26:848–54. doi: 10.1016/j.dental.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Menvielle-Bourg FJ. Superoxide dismutase (SOD), a powerful antioxidant is now available orally. Phytothérapie. 2005;3:1–4. [Google Scholar]
- 36.Hacisevkđ A. An overview of ascorbic acid biochemistry. J Fac Pharm Ankara. 2009;38:233–55. [Google Scholar]


