Abstract
Objective:
To evaluate the efficacy of coronally advanced flap (CAF) procedure under microsurgical approach for the management of Miller's Class I and II gingival recession defects with the use of either platelet-rich fibrin (PRF) or amnion membrane (AM) in comparison to CAF alone.
Materials and Methods:
A total of 45 sites with Miller's Class I or II gingival recession defect were randomly distributed for: Experimental Group I (CAF with PRF) sites (n = 15) which were treated with the microsurgical approach using CAF along with PRF; experimental Group II (CAF with AM) sites (n = 15) were treated with the microsurgical approach using CAF along with AM; control Group III (CAF alone) sites (n = 15) were treated with the microsurgical approach using CAF alone. Vertical gingival recession (VGR), horizontal gingival recession (HGR), gingival thickness (GT) (using transgingival probing [TGP] and ultrasonography [USG]) and patients’ response and acceptance were documented at baseline, 3 months and 6 months after surgical interventions.
Results:
CAF alone and in combination with PRF or AM, were effective techniques for root coverage with average VGR values of 1.47 ± 0.92 mm (56%), 0.67 ± 1.23 mm (36%) and 0.60 ± 1.06 mm (33%) in Group I (CAF with PRF), Group II (CAF with AM), and Group III (CAF alone), respectively. Complete coverage (100%) was obtained in 33.3% sites of Group I (CAF with PRF), 26.6% sites of Group II (CAF with AM) and 13.3% in Group III (CAF alone). Patients’ response and acceptance for surgical treatment modality in terms of patient esthetic score and decrease in hypersensitivity score was highest for Group I (CAF with PRF), whereas patient comfort score was highest for Group II (CAF with AM). At 6 months follow-up, significant increase in GT measurements (using TGP and USG) in Group I (CAF with PRF), whereas, nonsignificant increase for Group II (CAF with AM) and no change or decrease for Group III (CAF alone) as compared to baseline was observed.
Conclusion:
The present study observed enhancement in root coverage when PRF or AM are used in conjunction with CAF as compared to CAF alone. These results are based on 6-month follow-up. Therefore, the long-term evaluation may be necessary to appreciate the clinical effect of autologous PRF and AM.
Keywords: Amnion, gingival recession, gingival thickness, ultrasonography, wound healing
INTRODUCTION
Over the years, the gingival recession has been treated by some surgical techniques. In 1926, Norberg introduced the coronally repositioned periosteal flap operation,[1] and in 1993 Prato et al.[2] devised the term coronally advanced flap (CAF). CAF is frequently combined with various regenerative materials and biologic factors aiming to attain both regeneration of functional attachment apparatus and root coverage.
Recently, platelet-rich fibrin (PRF) has gained prominence status for obtaining periodontal regeneration predictably.[3] PRF obtained using Chaoukran's protocol, is a second-generation platelet concentrate.[4] It is considered as an autologous leukocyte and PRF biomaterial,[5,6,7] that consists of an intimate assemblage of cytokines, structural glycoproteins, and glycanic chains, enmeshed within a slowly polymerized network of fibrin. As an adjunct to CAF, the beneficial effects of PRF in gingival recession defect coverage has been elucidated with promising results.[8]
Within few days after fertilization, the human placenta starts developing. It is crucial to the survival and development of the fetus during the gestation. It is approximately 10–15 µm in thickness and consists of two fetal sheaths; the outer chorion and the inner amnion membrane (AM).[9] Amniotic fluid and fetus are enclosed in AM. Because of its high flexibility, it is easily separated from the chorion. AM has the unique feature in that it lacks antigenicity with potent antibacterial and anti-inflammatory properties. Allograft AM, obtained from fetal tissue encompasses growth factors that support the formation of granulation tissues by stimulating neo-vascularization and fibroblast growth and forms an initial physiologic seal with host tissue impeding microbial adulteration. Furthermore, it contains cells within the tissue that exhibit stem cells physiognomies for enhancing the clinical consequences. Besides providing a matrix for cellular migration and proliferation it also enhances the wound healing process. AM is now under the research for the management of gingival recession and revealed promising results for the same.[10] Gurinsky,[11] in a case series, reported promising results with processed dehydrated allograft amnion membrane in the management of gingival recession.
In addition to acceptable results of root coverage, the current objective of periodontal plastic surgery is to cultivate less or minimally invasive surgical techniques that favors prompt wound healing, fewer postoperative discomfort, and greater satisfaction of patient. The surgical operating microscope has been used to attain these objectives because it offers good illumination and magnification of the operative field, along with more precise and minimal trauma to involved tissue, thus permitting accurate co-adaptation of wound edges and healing by primary intention.[12] More recently, use of ophthalmic knives allow the periodontist to make precise, minimally invasive incisions while leaving a sharp wound edge. Published data reported enhanced clinical outcome in the management of gingival recessions with the use of the microsurgical approach;[13] however, clinical and laboratory training is required for effective application of surgical operating microscope with less surgical trauma and postoperative pain.[12]
The term “gingival biotype” has been used to designate the gingival thickness (GT) in the faciopalatal dimension,[14] and correct diagnosis of the type of “gingival biotype” is of the paramount importance for concocting an appropriate treatment plan and achieving esthetic expectable result.[15] Methods employed in evaluating GT are invasive methods, e.g., using injection needle, probe, histologic sections, or cephalometric radiographs and noninvasive methods, e.g., ultrasonic devices.[16]
Thus, the present study was conducted to compare the baseline, 3-month and 6-month follow-up outcomes following CAF procedures under the microsurgical approach in the management of Miller's Class I and II gingival recession defects with the use of either PRF or amnion membrane (AM) in comparison to CAF alone.
MATERIALS AND METHODS
The present controlled clinical study was conducted in Department of Periodontology, from December 2012 to November 2014. The Institutional Human Ethics Committee approved the design of the study. Based on initial screening, a total of thirty systemically healthy, currently nonsmoker (self-reported) patients (8 females and 22 males) were recruited among the patient pool visiting the department with the chief complain of dentinal hypersensitivity or nonesthetic elongation of crown. All the participants provided written informed consent according to Helsinki's Declaration after giving detailed information about the study protocol. Initial screening includes recording of demography and anamnesis followed by clinical and radiographic examination for selecting the patients based on following inclusion criteria.
Inclusion criteria
(1) Both males as well as females with age more than 18 years without any active and severe periodontal diseases. (2) Having at least one tooth in maxillary anterior teeth region with Miller's Class I or II buccal/labial gingival recession defect measuring ≥1 mm after phase I therapy. (3) Patients were having good systemic health and free from any condition that contraindicate periodontal surgery. (4) Patients must not be using antibiotics, corticosteroids, chemotherapeutics, immune modulators, or any other medications that may modify oral tissue retort during the last 6 months. (5) Selected teeth should be free of endodontic treatment, buccal, or inter-proximal restorations. (6) Patients in which no any periodontal therapy in the preceding 6 months of initial examination.
Presurgical procedure
All the selected patients were well-versed about the purpose and characteristics of the study. Patients were educated about the etiology of the gingival recession, and detailed oral hygiene maintenance directives, as well as their significance in obtaining good clinical results, were explained. All patients received complete scaling and root planing, and if indicated, the occlusal adjustment was also performed.
After phase, I therapy completion, 23 patients (18 male and 5 female) finally participated and agreed to complete the study. Among drop out of 7 patients, two were diabetics who were identified later on after phase I therapy during blood investigation, remaining five patients were satisfied after initial therapy and were reluctant to undergo any surgical procedure. A total of 45 sites with Miller's Class I or Class II recession defect were enrolled for the treatment. An equal number (n = 15) of sites (with Miller's Class I or Class II recession defect) were randomly distributed for two experimental and control groups [Figure 1].
Figure 1.
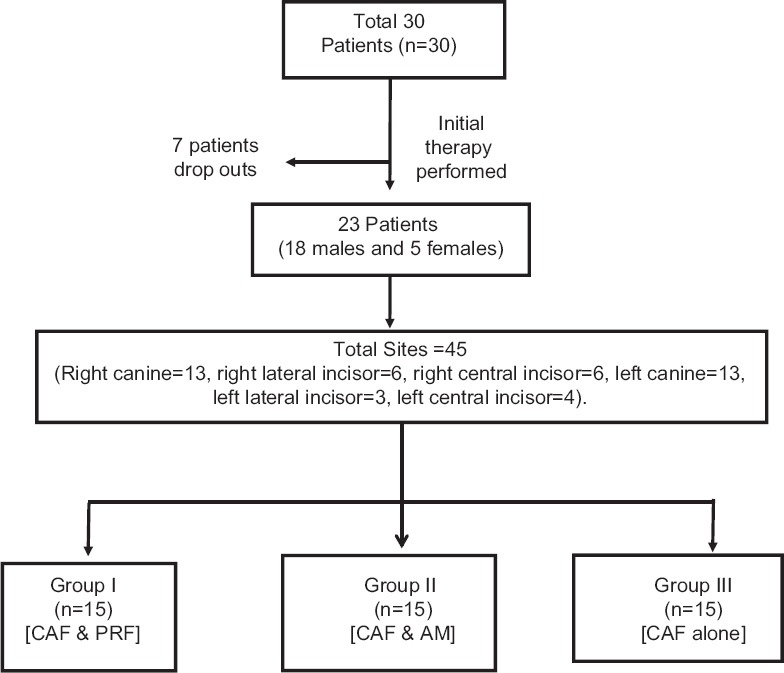
Flow chart showing study design
Selected sites were randomly treated with coronally positioned flap as advocated by Lins et al.,[17] using microsurgical protocols alone or with experimental materials as given below: Experimental Group I (CAF with PRF) sites were treated with the microsurgical approach using CAF along with PRF; experimental Group II (CAF with AM) sites were treated with the microsurgical approach using CAF along with amnion membrane (AM); control Group III (CAF alone) sites were treated with the microsurgical approach using CAF alone.
Amnion membrane
Amnion allograft membrane for the study was procured from Tissue Bank, Tata Memorial Hospital, Mumbai, India.
Platelet-rich fibrin
Autologous PRF for the study was obtained from patient blood prior to surgery as suggested by Choukroun et al.,[4] by means of a table top centrifuge (REMI, Laboratories, India).[18] PRF acquired in the middle clot was cautiously collected by removing red blood cell clot using sterilized scissor [Figure 2]. The PRF thus obtained was squeezed with sterilized and moist gauge piece to form PRF membrane. The centrifuge machine was placed closed to the operatory, and all efforts were made to minimize the time between the preparation of PRF and its placement in the defect so as to retain maximum regenerative potential.
Figure 2.
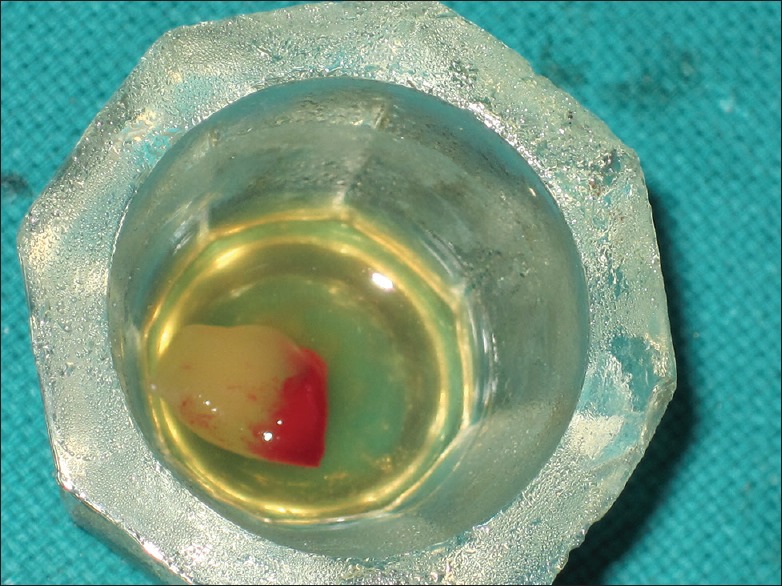
Platelet-rich fibrin clot
Surgical therapy
Baseline clinical parameters were documented on the day of the surgical appointment. Immediately prior to surgery, selected recession defects were randomly (with chit method) allocated to one of the three different treatment modalities. Preprocedural oral antisepsis was acquired using 0.2% chlorhexidine digluconate solution rinse. All surgical procedures were performed under magnification at ×5 using Surgical Operating Microscope (3D Medical system Co., USA). All the incisions were given with microsurgical (Ophthalmic disposable) blade, and all the surgical procedures (experimental and control) were performed by the same investigator [Figure 3].
Figure 3.
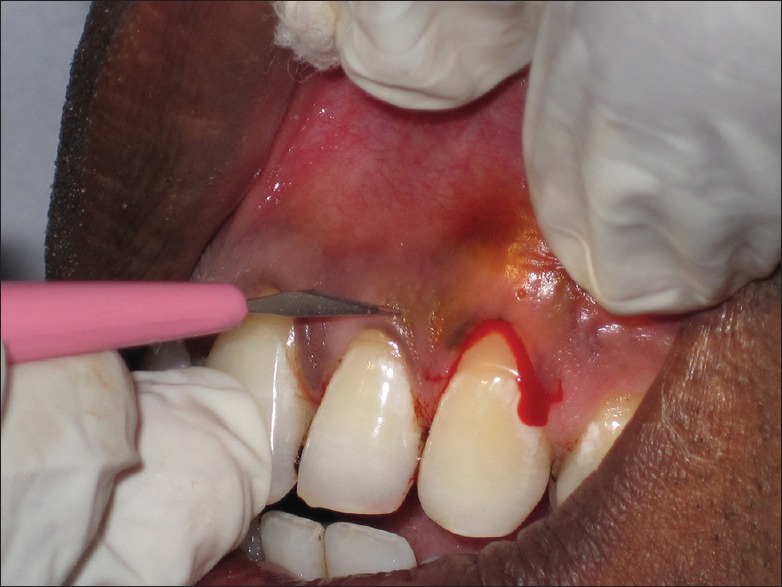
Incisions given with microsurgical (ophthalmic disposable) blade
Adequate anesthesia was obtained with 2% lignocaine hydrochloric acid with adrenaline (1:200,000) using block or infiltration technique. Prior to giving incisions, in case of experimental Group I (CAF with PRF), patient's blood samples were taken out by an assistant for preparing PRF as explained above. Two straight horizontal incisions without involving the gingival margin (GM) of the adjacent teeth were made in the interdental papillae at the height of the cementoenamel junction (CEJ) which were followed by an intrasulcular/crevicular incision on the buccal aspect on experimental tooth site. The split-full-split flap was performed using initial blunt followed by sharp dissection such that the obtained flap can be coronally repositioned without tension [Figure 4]. The residual interdental papillae were de-epithelialized to form a bed of connective tissue. With Gracey curettes, exposed root surfaces were gently scaled and planed, and followed by thorough saline irrigation.[17]
Figure 4.
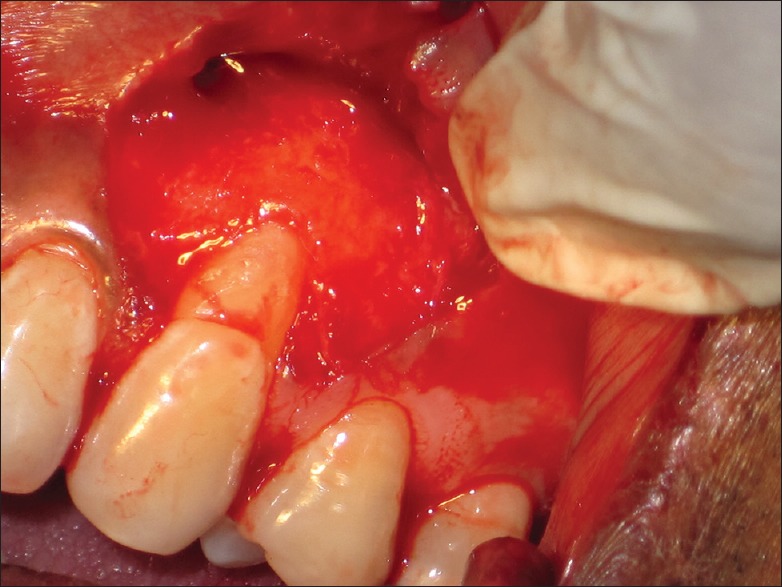
Split-full-split flap reflection
For the experimental Group I (CAF with PRF), the surgical site was flushed with previously prepared PRF fluid. PRF membrane was then positioned on the recession defect at the height of the cementoenamel junction (CEJ) [Figure 5]. For experimental Group II (CAF with AM), amnion membrane was cut to the desired size and positioned on the recession defect at the height of the CEJ [Figure 6]. For both the experimental groups, experimental membranes (PRF and AM) were extended to the minimum of 2 mm apical to the crestal area. In case of control Group III (CAF alone), no membrane was used to cover the recession defects.
Figure 5.
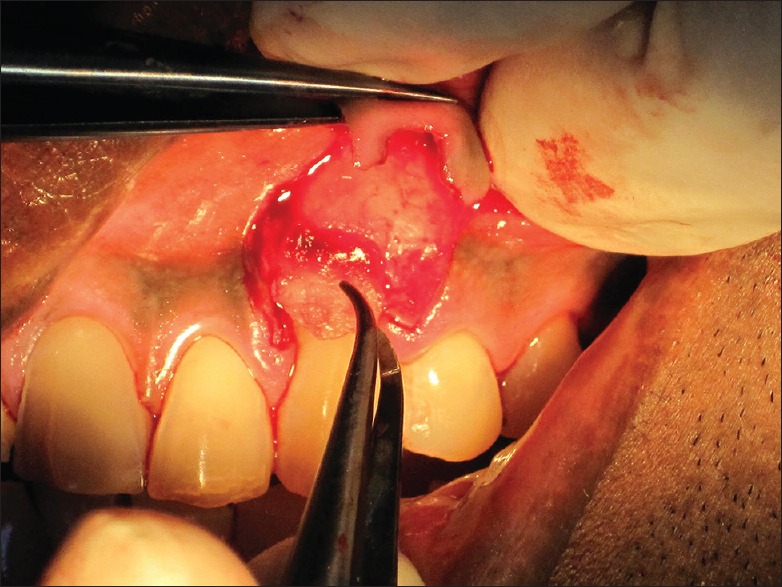
Platelet-rich fibrin membrane positioned on the recession defect up to the height of the cementoenamel junction
Figure 6.
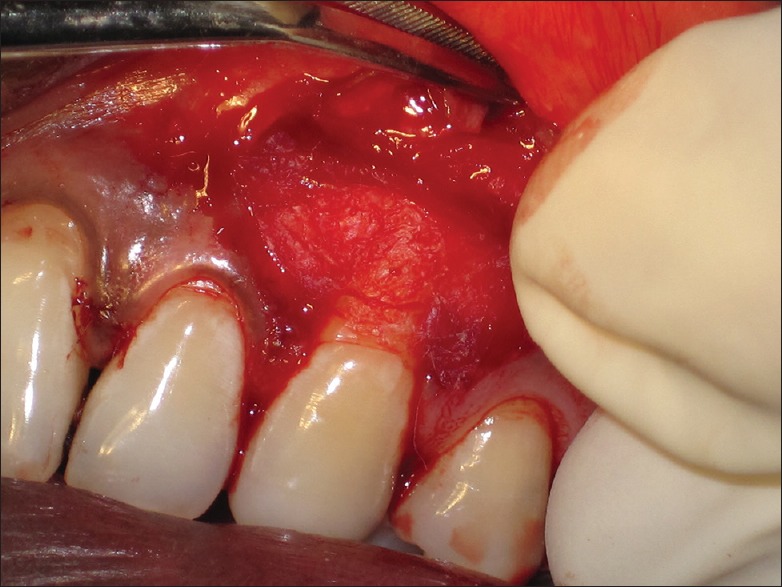
Amnion membrane cut to desired size and positioned on the recession defect at the height of the cementoenamel junction
To fully cover the membrane, raised mucoperiosteal flap was coronally positioned and sutured by using 6-0 black silk suture (Mersilk, Ethicon®). The margin of the gingival flap was repositioned on the enamel in the both experimental and control sites and was held in that position with horizontal sling suture. Interrupted sutures were placed to close the vertical releasing incisions with the same suture material [Figure 7]. Light-cured periodontal dressing (Barricaid, Dentsply, USA) was applied at the surgical site [Figure 8]. Written postsurgical instructions were given. Systemic antibiotics and anti-inflammatory drug (amoxicillin 500 mg t.d.s. and brufen (400 mg b.i.d.) for 5 days postsurgically were prescribed to all the patients. During this period, 0.2% chlorhexidine solution rinse twice a day was prescribed as means of chemical plaque control.
Figure 7.
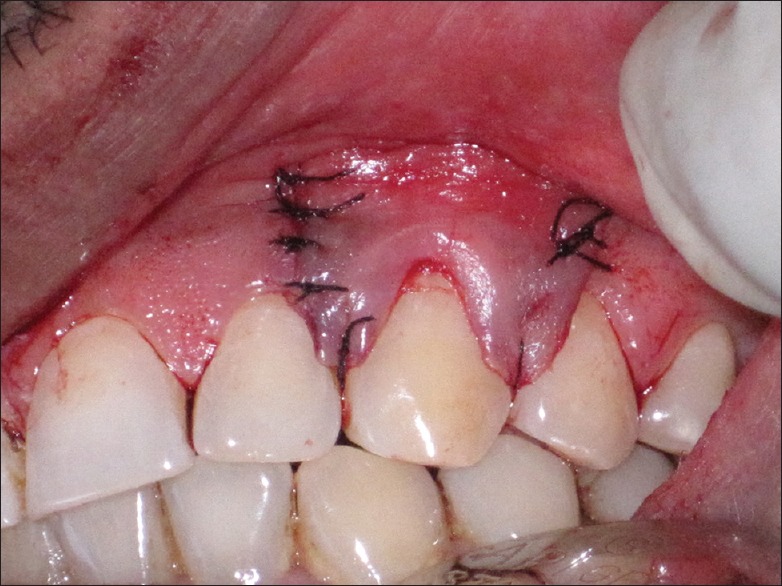
Interrupted sutures placed to close the vertical releasing incisions
Figure 8.
Light-cured periodontal dressing (Barricaid, Dentsply, USA) was applied at the surgical site
Postsurgical protocol
An inquiry regarding postsurgical problems was made within 24 h after the surgical intervention. Periodontal dressing and sutures were removed 10 days after surgery, and the operated site was irrigated using normal saline. The patients were recalled after 3 months and 6 months follow-up. Oral hygiene maintenance guidelines were strengthened, and supragingival scaling was performed if required at each appointment. Clinical parameters and patient satisfaction score were recorded on recall appointments (described later).
Parameters recorded
All the clinical parameters data were documented at baseline, 3-month, and 6-month postoperatively. To homogenize the reproducibility of clinical data, customized acrylic stent was fabricated. The customized acrylic stent was fabricated as mentioned by Lekovic et al.[19] The groove was made for reproducibility of probe position for presurgical and postsurgical measurements so that it could be at the same position and angulations. The apical end of this groove has been taken as fixed reference point. To reduce the individual variability, all the clinical measurements were recorded by single investigator throughout the study. Prior to initiating surgery, intra-examiner standardization was assessed by executing test-retest exercises for clinical data recordings in ten patients. The reproducibility expressed in terms of kappa statistics and the results obtained were near almost perfect agreement (0.81–0.99).[20]
Following parameters were recorded:
Plaque index (PI) and gingival index (GI) at selected teeth.[21,22]
Vertical component of the gingival recession (VGR)
It is the distance from to CEJ to GM recorded using UNC 15 probe [Green line-CD in diagram, Figure 9];
Figure 9.
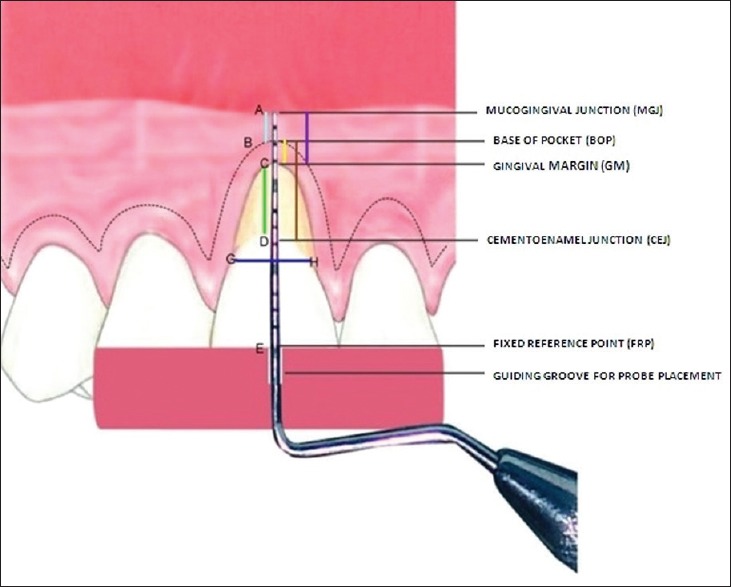
Diagrammatic representation of clinical parameters
Horizontal component of the gingival recession (HGR) at cementoenamel junction
It was measured using a divider. Distance between the points placed on CEJ, at the mesial-most and distal-most end of the selected tooth was recorded and then transferred same on a calibrated scale [Blue line-GH in the diagram, Figure 9].
Probing pocket depth (PPD)
It is defined the distance from GM to the base of pocket (BOP) and was recorded using UNC 15 probe [Yellow line-BC in diagram, Figure 9].
Clinical attachment level (CAL)
It is the distance from CEJ to BOP [Brown line BD in diagram, Figure 9].
Width of attached (AG)
It is the distance from the external part of BOP to mucogingival junction (MGJ). MGJ was detected by using the rolling technique [Sky blue line-AB in diagram, Figure 9].
Width of keratinized gingiva
It is the distance of keratinized gingiva (KG) detected using Lugol's Iodine solution that approximately includes the distance from GM to MGJ [Violet line-AC in diagram, Figure 9]. This solution stains the glycogen content of the tissue and glycogen is more in alveolar mucosa than in attached gingiva (AG) because glycogen is utilized for the process of keratinization. High glycogen content in alveolar mucosa gives a positive iodine reaction; the MGJ can thus also be visualized using Lugol's iodine solution [Figure 10];
Figure 10.
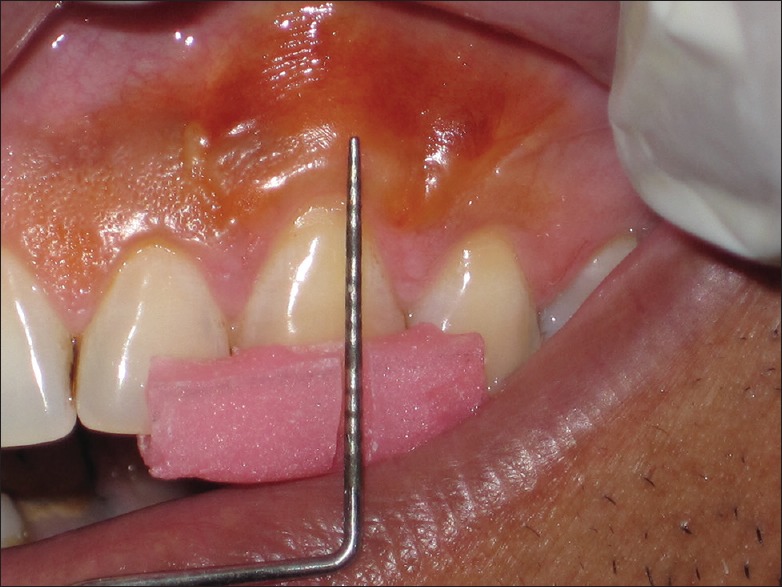
Mucogingival junction visualized by using Lugol's iodine solution
Gingival thickness using transgingival probing (GT-TGP)
It was recorded as mentioned by Vandana and Savitha,[16] using transgingival probing (TGP) method. A UNC-15 probe and an electronic digital Vernier caliper were used to measure the GT-TGP. The gingival thickness (GT) was assessed 20 min after the anesthetic injection. However, in contrast to Vandana and Savitha,[16] dimensions obtained were not rounded off to the nearest millimeter [Figure 11a and b].
Figure 11.
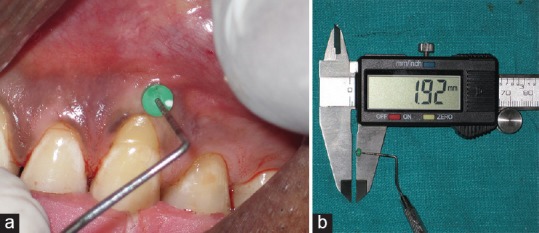
Clinical measurement of gingival thickness using (a) transgingival probing and (b) electronic digital vernier caliper
Gingival thickness measurement using ultrasonography (GT-USG)
The thickness of gingiva was also measured with the help of ultrasonography (GT-USG) at the same area by Ultrasonologist. The ultrasound B-scan (Acuson X 300 of Siemens Medical System USA), including a transducer probe, a scan display, and digital display was used. The frequency was 10 MHz. Each examination was performed with the subject sitting in an upright position and the mouth closed. The area of interest was scanned by an extra-oral probe. In the oral cavity, water was used as sound coupling medium between the probe and selected area for examination. The transducer probe was adjusted to the gingival surface such that it coincides with the bleeding point produced during TGP method.[23] Measurements recorded up to the nearest 0.1 mm, were made straight on the display screen at the time of scanning. All the sonograms at baseline as well as follow-up were performed by a same experienced radiologist at baseline, 3-month and 6-month using same ultrasound machine for the Group I [Figure 12a–c], Group II [Figure 13a–c], and for Group III [Figure 14a–c].
Figure 12.
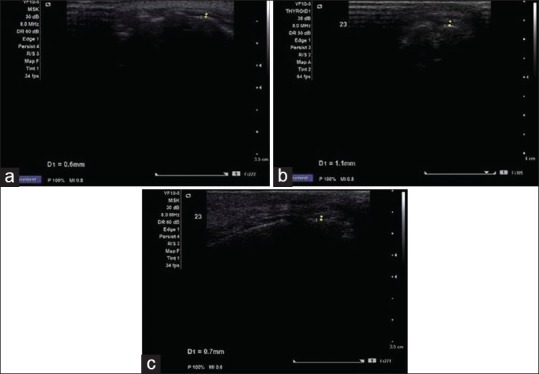
Sonograms at (a) baseline, (b) 3 months and at (c) 6 months using same ultrasound machine for Group II
Figure 13.
Sonograms at (a) baseline, (b) 3 months and at (c) 6 months using same ultrasound machine for Group I
Figure 14.
Sonograms at (a) baseline, (b) 3 months and at (c) 6 months using same ultrasound machine for Group III
Patient satisfaction analysis
Patient satisfaction regarding comfort, hypersensitivity, and esthetic appearance was analyzed subjectively based on visual analog scale,[24] at baseline, 3 months, and 6 months. To evaluate “patient comfort,” patients were asked for the pain, edema and other experiences regarding operating technique, instruments, and microscopic view, etc., to obtain patient comfort score (PCS). The perceived discomfort were graded using a visual analog scale (VAS) scale, labeled at the two end-limits with “unbearable discomfort” at one end (score zero) and “no discomfort” at the other end (score 10). At baseline, it was recorded within 24 h after treatment modality. To evaluate “esthetic appearance,” patients were asked to give score between “score 0” for unpleasing appearance (poor esthetics) to “score 10” for pleasing appearance (excellent esthetics) to obtain patient esthetic score (PES) in respect to color, appearance, and form of the selected site. “Hypersensitivity” score was recorded after blasting air (60 psi, 22°C) derived from a dental syringe that was heading for the root surface for 1 s. The syringe was held at 90° angle, 2–3 mm from the root surface. Neighboring teeth were shielded during testing with the dentist's gloved fingers; then the patient was enquired again to the score the discomfort level. The perceived discomforts were graded using a VAS scale, labeled at the two extreme limits with ’’no pain’’ at the one end (score zero) and with “unbearable pain” at the other end (score 10).[25]
Statistical analysis
The data recorded were evaluated using Statistical Package for Social Sciences (SPSS) version 15.0 (SPSS Inc., Chicago, IL, USA). The values were represented in number (%) and mean ± standard deviation. As the sample size was small, baseline data were subjected to Kolmogorov–Smirnov test for testing the normality. For majority of the variables, distribution was asymmetric; hence a nonparametric evaluation plan was adopted. Intergroup comparisons were made using the Mann–Whitney U-test, and intragroup-group change was studied using the Wilcoxon signed rank test. Other statistical formulas used were Chi-square test, Kruskall–Wallis H-test and Kappa statistics. The confidence level of the study was kept at 95%; thence a P < 0.05 indicated statistically significant association.
RESULTS
In the present randomized controlled clinical study, both males (78%) and females (22%) participated, though proportion of females was higher in Group II (33.33%) as compared to Group III (13.33%) and Group I (6.67%), but this difference was not found to be statistically significant (P = 0.139). Table 1 represents mean values of PI score, GI score, probing pocket depth (PPD), clinical attachment level (CAL), horizontal gingival recession (HGR), vertical gingival recession (VGR), AG, KG, GT by TGP as well as USG and PCS, PES and hypersensitivity score (HS) scores at baseline, 3 months and 6 months. Table 2 represents between group comparison and Table 3 represents within-the group comparison for various parameters for Group I (CAF with PRF), Group II (CAF with AM) and Group III (CAF alone) at baseline, 3 months, and 6 months.
Table 1.
Comparative measurements for Group I (CAF with PRF), Group II (CAF with AM) and Group III (CAF alone) at baseline, 3 months and 6 months
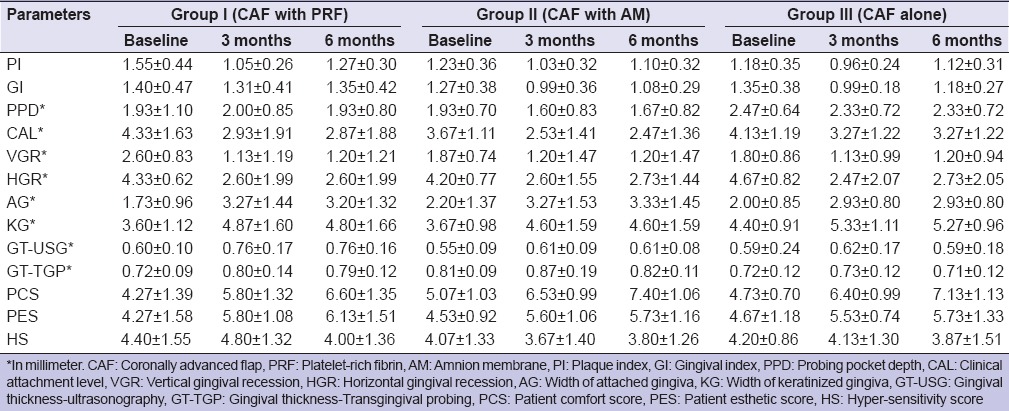
Table 2.
Between group comparison for various parameters for Group I (CAF with PRF), Group II (CAF with AM) and Group III (CAF alone) at baseline, 3 months and 6 months (Mann–Whitney U-test)
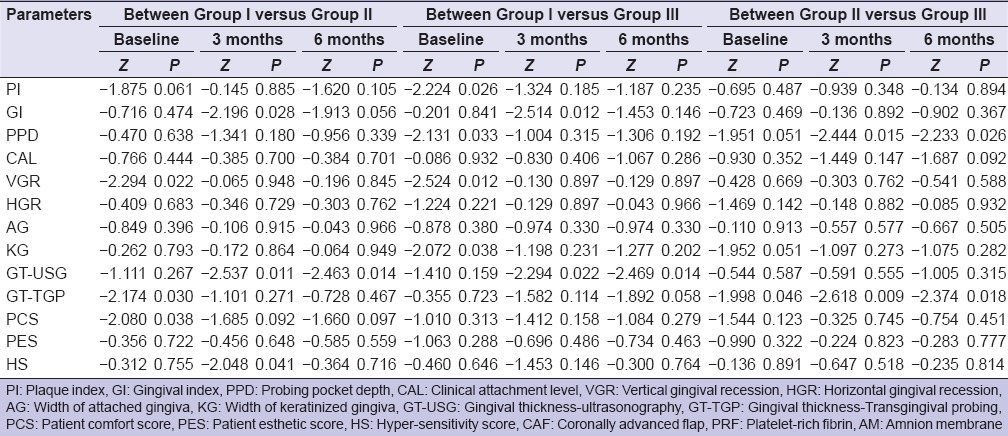
Table 3.
Intragroup comparison for mean change in various parameters for Group I (CAF with PRF), Group II (CAF with AM) and Group III (CAF alone) at baseline, 3 months and 6 months (Wilcoxon signed-rank test)
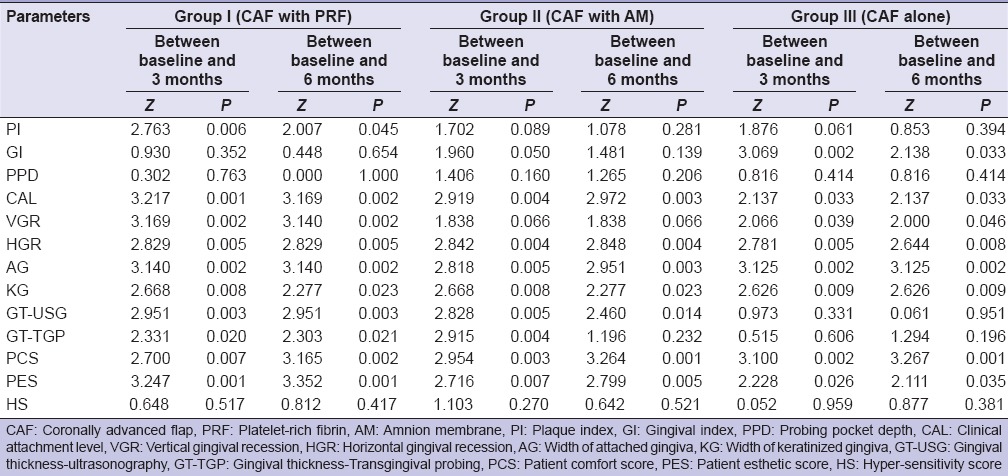
Between-group comparison of (GT-USG) by ultrasonographic method in mid-buccal region at different time intervals, it was found that at baseline GT-USG of Group I was found to be higher than that of Group II and Group III while GT-USG of Group II was lower than that of Group III, but none of the between-group differences were statistically significant. At 3 months and 6 months, GT-USG of Group I was found to be higher than that of Group II and Group III and this difference was found to be statistically significant, whereas GT-USG of Group II was found to be lower than that of Group III, but this difference was not found to be statistically significant. At baseline GT-TGP of Group II was found to be higher than Group I and Group III and difference in GT-TGP among the three groups was found to be statistically significant (P = 0.029). GT-TGP of Group II was found to be higher as compared to Group I and Group III and this difference was found to be statistically significant at 3 months but nonsignificant at 6 months.
Intergroup comparison of PCS at different time intervals in which at baseline PCS of Group II was higher as compared to Group I and Group III, but difference in PCS among the three groups was statistically significant (P = 0.188). At 3 months, PCS of Group II was higher as compared to Group I and Group III but this difference was statistically significant but became nonsignificant at 6 months.
At baseline, PES of Group III was higher as compared to Group I and Group II but the difference in PES among the three groups was not statistically significant (P = 0.781). At 3 months and 6 months, PES of Group I was higher as compared to Group II and Group III, but this difference was not found to be statistically significant.
On comparing the difference in mean HS score between any two groups, it was found that at baseline mean HS score of Group I was significantly higher than that of Group II and that of Group III, while mean HS score of Group III was found to be greater than that of Group II. None of the between-group differences was statistically significant. At 3 months and 6 months, mean HS score of Group I was significantly higher than that of Group II and Group III, but the difference was not statistically significant. Mean HS score of Group III was found to be superior to that of Group II, but this difference was not statistically significant. At 6 months, HS of Group II was found to be higher than that of Group III but this difference was statistically significant.
DISCUSSION
The present study was conducted with the primary objective to evaluate the effectiveness of PRF and amnion membrane (AM) in conjunction with CAF in the management of gingival recession (Miller's Class I and Class II) recession in maxillary anterior using microsurgical approach. The secondary objectives were to evaluate the patient satisfaction with both the experimental groups and control alone; and to evaluate the change in GT measurements both experimental and control groups. Both TGP and USG were compared for gingival biotype assessment before and after different treatment modalities.
All 23 patients completed the study uneventfully, therefore representing the nonimmunogenic nature of both the experimental materials (PRF and AM) and aptness of the surgical technique.[5,10] All the patients were maintaining their oral hygiene as per instructions given throughout the study. Compliance with initial phase and continuous reinforcement of the importance of meticulous oral hygiene resulted in minimal plaque scores. This resulted in the reduction of gingival inflammation and absence of bleeding on probing at all surgical sites.[13]
At the follow-up of 3-month, there was the nonsignificant increase in PPD values for the Group I (CAF with PRF) followed by nonsignificant reduction to baseline values in PPD values at 6 months as compared to baseline. Similar to our study, Eren and Atilla,[26] Aroca et al.[27] and Jankovic et al.,[8] also reported nonsignificant changes in PPD values at 6 months in PRF treated sites. For Group II (CAF with AM) and Group III (CAF alone), there was the nonsignificant reduction in PPD values at 3 months and at 6 months follow-up, as compared to baseline. Wallace,[28] reported PPD reduction 0.8 mm at 4 months using placental derived membrane. Paolantonio,[29] corroborated nonsignificant change after root coverage procedure to the compatible condition of gingival health after initial therapy and patient selection, thus reported the expectation of nonsignificant improvement in PPD values.
Our results of CAL gain with CAF with PRF were comparative to results observed by Jankovic et al.,[8] Uraz et al.[30] and Padma et al.,[3] whereas, Aleksic et al.[31] reported no statistically significant changes in PPD and CAL in CAF with PRF group. Wallace[28] in a case report observed CAL gain of 2.4 mm at 4 months using placental derived membrane. For Group III (CAF alone) there was nonsignificant CAL gain at 3 months (0.87 ± 1.41 mm) and 6 months (0.87 ± 1.41 mm) follow-up as compared to baseline. In a recent study, Moka et al.,[32] reported mean PPD reduction and CAL gain in CAF alone treated gingival recession defect of 0.35 mm and 2.60 mm, respectively, and the difference was statistically significant (P < 0.05). Improvement in CAL is because of recession coverage that results from the coronal shift of attachment apparatus during CAF procedures.[33] Although, this is not substantiated in the histological study, however, based on clinical examinations, improvement in CAL can be explained.
Our result showed statistically more significant recession coverage (VGR) with average values of 1.47 ± 0.92 mm (56%) in Group I (CAF with PRF); followed by Group II (CAF with AM) with mean value of 0.67 ± 1.23 mm (36%) which were higher than Group III (CAF alone) where mean value of 0.60 ± 1.06 mm (33%) was observed after 6 months postintervention. As compared to Aroca et al.[27] and Jankovic et al.[8] who reported mean root coverage of 80.7% and 88.7%, respectively, the present study observed mean root coverage of 56% in PRF treated sites. To the best of our knowledge, limited randomized clinical trial have reported efficacy of amnion membrane (AM) in gingival recession defect. Case reports by Gurinsky[11] and Wallace[28] observed root coverage of 97% (3.2 ± 1.73 mm) and 57%, respectively, in the amniotic membrane (AM) treated sites.
Complete coverage (100%) was obtained in 33.3% sites of Group I (CAF with PRF), 26.6% sites of Group II (CAF with AM). In contrast to Aroca et al.,[27] who reported complete root coverage in 19% patients of CAF with PRF group as compared to CAF alone group where 100% root coverage was obtained in 52.3% patients, our results reported superior root coverage in PRF treated site as compared to control group. Many case reports reported complete root coverage of almost 90% in PRF treated sites.[26,34,35] In a case report of bilateral gingival recession in a patient, complete root coverage (100%) was observed in both PRF as well as amniotic membrane treated gingival recession defects 7 months after the surgery.[36]
At 3 and 6 months follow-up, there was significant reduction in HGR for Group I (CAF with PRF), Group II (CAF with AM) and Group III (CAF alone) as compared to baseline (P < 0.05). Eren and Atilla,[26] also observed greater reduction in recession width in control group as compared to PRF treated sites (3.42 mm vs. 1.77 mm, respectively) after 6 months; however, at the end of 1-year they reported complete coverage of recession width in both test and control sites. Aroca et al.[27] and Uraz et al.[30] also observed the significant reduction in recession width in PRF treated sites. For the control (Group III) we observed the change in HGR value of 1.93 ± 2.15 mm as compared to the decrease of 1.75 ± 0.705 mm observed by Nanavati et al.[37] There was the greater mean reduction in HGR values in Group III (CAF alone) followed by Group I (CAF with PRF) and Group II (CAF with AM) both at the end of 3 months. However, there was increase in HGR values both in Group II (CAF with AM) and Group III (CAF alone) values in contrast to Group I (CAF with PRF), where no reduction in HGR values was observed between 3 and 6 months. Change in HGR values in Group II (CAF with AM) and Group III (CAF alone) might have been influenced by inflammation between 3 and 6 months, in contrast to Group I (CAF with PRF), where biochemical components of PRF may have an early synergistic effects on healing process.[5]
At 3 and 6 months follow-up, there was significant increase in AG values for all the groups, and the mean increased in width of AG was greater for Group I (CAF with PRF) followed by Group II (CAF with AM) and least for Group III (CAF alone). In PRF treated sites, Jankovic et al.[8] and Uraz et al.[30] reported the mean increase in keratinized tissue width of 0.88 mm and 1.00 mm respectively. Eren and Atilla[26] reported mean increase in KG of 1.2 mm and 1.10 mm in PRF treated and CAF alone recession sites at 6 months, as compared to 1.27 ± 1.67 mm and 0.87 ± 0.92 mm respectively obtained in our study. Wallace[28] reported keratinized tissue width increase of 0.2 mm at 4 months, in contrast to 0.93 ± 1.03 mm obtained in the present study at 6 months in AM treated sites.
Recent reports have emphasized that gingival tissue thickness is essential for complete root coverage and stability of the clinical outcome. The fibrin meshwork structure, when used in combination with CAF, could be responsible for obtaining increased GT in PRF treated sites. Further, chair-side availability of compatible table top centrifuge enhances both biological and clinical outcomes of PRF.[38,39]
In contrast to mean mid-buccal GT-USG values as documented in literature, in maxillary anterior region of about 0.85 ± 0.09 mm by USG,[23,40] and between 0.87 ± 0.07 mm and 1.60 ± 0.43 mm by TGP method,[16,23,41] we obtained lower average GT-USG and GT-TGP values at baseline in all groups. Although, every care was taken while recording GT-TGP measurements, however, probe angulations, the precision of manual probe markings and reproducibility of the sites may be the source of error in TGP. USG method was found to be unswerving and noninvasive method of GT measurement, as the incongruity between the measurements was nominal at the mid-buccal site, when the GT fluctuated between 0 and 0.5 mm.[41] It has been reported that as compared to TGP, GT measurement using USG seems to be more accurate, rapid and atraumatic;[23,40] and in contrast to invasive TGP, with USG, every minuscule part of precious soft tissue may be preserved even better.[23]
To evaluate patient response and acceptance for surgical treatment modality of gingival recession, patients were analyzed using PCS, PES and HS based on VAS as explained in material and method. At baseline PCS values were highest for Group II (CAF with AM), followed by Group III (CAF alone) and Group I (CAF with PRF). At the end of 6 months follow-up, there was significant increase in PCS for all the groups, but it was highest for Group II (CAF with AM) representing more patient comfort in terms of postsurgical pain and inflammation in AM treated sites. At the end of 6 months follow-up, there was significant increase in PES for all the groups, but it was highest for Group I (CAF with PRF) as compared to, almost similar PES for Group II (CAF with AM) and Group III (CAF alone). Although there is no comparative analysis, many studies have reported better healing parameters and significant recession coverage that improves patient esthetic appearance in PRF treated gingival defects that were similar to the results of the present study.[8,10,31]
At baseline, HS values were highest for the Group I (CAF with PRF), followed by Group III (CAF alone) and Group II (CAF with AM). In a systematic review by Douglas de Oliveira et al.,[42] reported that there is insufficient evidence to conclude that surgical root coverage procedure predictably reduces cervical dentinal hypersensitivity, however, present study observed nonsignificant decrease in HS values in all the groups.
In the present study, none of the treatment modalities achieved complete root coverage in more than 50% of sites. The present study observed enhancement in root coverage when PRF or AM are used in conjunction with CAF as compared to CAF alone. However, results obtained are not as prominent as reported in the already published literature. These results may be attributed to lower mean values of GT (<1 mm) in all the groups at baseline. Gingival biotype that is thick has a tendency toward maintaining a more stable soft tissue in various periodontal surgical procedures.[43,44] Low sample size, short-term study with fair oral hygiene instead of meticulous plaque control amongst subjects and lack of histological evaluation were the other limitations. These results are based on single-centered small sample size study; henceforth long-term, multi-centered randomized, controlled clinical trials are further required.
CONCLUSION
From the results of the present study, it may be inferred, that PRF should be considered as better material for root coverage and increasing GT as compared to amnion membrane (AM) and CAF alone. However, for patient acceptance in terms of healing, AM was observed to be better as compared to PRF and CAF alone. Further, PRF is autologous, simple to procure, cost effective, nonimmunogenic biomaterial with excellent handling properties, whereas amnion membrane (AM), besides being allograft material, cannot be prepared or procured in routine clinical setup. Although microsurgical technique results in better postoperative outcome however, training and extra space in clinical set up for surgical operating microscope may preclude its use in routine practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank Saraswati Dental College, Lucknow, India for providing infrastructural support for the conduct of the study.
REFERENCES
- 1.Avinash K, Selvan T. Coronally advanced flap in the treatment of recession coverage. Int J Dent Case Rep. 2014;4:1–10. [Google Scholar]
- 2.Prato GP, Clauser C, Cortellini P. Guided tissue regeneration and a free gingival graft for the management of buccal recession: A case report. Int J Periodontics Restorative Dent. 1993;13:486–93. [PubMed] [Google Scholar]
- 3.Padma R, Shilpa A, Kumar PA, Nagasri M, Kumar C, Sreedhar A. A split mouth randomized controlled study to evaluate the adjunctive effect of platelet-rich fibrin to coronally advanced flap in Miller's class-I and II recession defects. J Indian Soc Periodontol. 2013;17:631–6. doi: 10.4103/0972-124X.119281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin PRF: A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e51–5. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: From pure platelet-rich plasma P-PRP to leucocyte- and platelet-rich fibrin L-PRF. Trends Biotechnol. 2009;27:158–67. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Jankovic S, Aleksic Z, Klokkevold P, Lekovic V, Dimitrijevic B, Kenney EB, et al. Use of platelet-rich fibrin membrane following treatment of gingival recession: A randomized clinical trial. Int J Periodontics Restorative Dent. 2012;32:e41–50. [PubMed] [Google Scholar]
- 9.Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26:300–11. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 10.Chopra A, Thomas BS. Amniotic membrane – A novel material for regeneration and repair. J Biomim Biomater Tissue Eng. 2013;18:106. [Google Scholar]
- 11.Gurinsky B. A novel dehydrated amnion allograft for use in the treatment of gingival recession: An observational case series. J Implant Adv Clin Dent. 2009;1:124–30. [Google Scholar]
- 12.Bittencourt S, Del Peloso Ribeiro E, Sallum EA, Nociti FH, Jr, Casati MZ. Surgical microscope may enhance root coverage with subepithelial connective tissue graft: A randomized-controlled clinical trial. J Periodontol. 2012;83:721–30. doi: 10.1902/jop.2011.110202. [DOI] [PubMed] [Google Scholar]
- 13.Burkhardt R, Lang NP. Coverage of localized gingival recessions: Comparison of micro- and macrosurgical techniques. J Clin Periodontol. 2005;32:287–93. doi: 10.1111/j.1600-051X.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 14.De Rouck T, Eghbali R, Collys K, De Bruyn H, Cosyn J. The gingival biotype revisited: Transparency of the periodontal probe through the gingival margin as a method to discriminate thin from thick gingiva. J Clin Periodontol. 2009;36:428–33. doi: 10.1111/j.1600-051X.2009.01398.x. [DOI] [PubMed] [Google Scholar]
- 15.Kan JY, Morimoto T, Rungcharassaeng K, Roe P, Smith DH. Gingival biotype assessment in the esthetic zone: Visual versus direct measurement. Int J Periodontics Restorative Dent. 2010;30:237–43. [PubMed] [Google Scholar]
- 16.Vandana KL, Savitha B. Thickness of gingiva in association with age, gender and dental arch location. J Clin Periodontol. 2005;32:828–30. doi: 10.1111/j.1600-051X.2005.00757.x. [DOI] [PubMed] [Google Scholar]
- 17.Lins LH, de Lima AF, Sallum AW. Root coverage: Comparison of coronally positioned flap with and without titanium-reinforced barrier membrane. J Periodontol. 2003;74:168–74. doi: 10.1902/jop.2003.74.2.168. [DOI] [PubMed] [Google Scholar]
- 18.Bains R, Bains VK, Loomba K, Verma K, Nasir A. Management of pulpal floor perforation and grade II Furcation involvement using mineral trioxide aggregate and platelet rich fibrin: A clinical report. Contemp Clin Dent. 2012;3(Suppl 2):S223–7. doi: 10.4103/0976-237X.101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Kenney EB. Comparison of platelet-rich plasma, bovine porous bone mineral, and guided tissue regeneration versus platelet-rich plasma and bovine porous bone mineral in the treatment of intrabony defects: A reentry study. J Periodontol. 2002;73:198–205. doi: 10.1902/jop.2002.73.2.198. [DOI] [PubMed] [Google Scholar]
- 20.Viera AJ, Garrett JM. Understanding interobserver agreement: The kappa statistic. Fam Med. 2005;37:360–3. [PubMed] [Google Scholar]
- 21.Silness J, Loe H. Periodontal disease in pregnancy. Acta Odontal Scand. 1964;22:747–59. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 22.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 23.Issrani R, Chavva S, Prabhu N, Keluskar V, Jirge V, Kumbujkar V, et al. Transgingival probing and ultrasonographic methods for determination of gingival thickness – A comparative study. Adv Hum Biol. 2013;3:43–51. [Google Scholar]
- 24.Gould D, Kelly D, Goldstone L, Gammon J. Examining the validity of pressure ulcer risk assessment scales: Developing and using illustrated patient simulations to collect the data. J Clin Nurs. 2001;10:697–706. doi: 10.1046/j.1365-2702.2001.00525.x. [DOI] [PubMed] [Google Scholar]
- 25.Tammaro S, Wennström JL, Bergenholtz G. Root-dentin sensitivity following non-surgical periodontal treatment. J Clin Periodontol. 2000;27:690–7. doi: 10.1034/j.1600-051x.2000.027009690.x. [DOI] [PubMed] [Google Scholar]
- 26.Eren G, Atilla G. Platelet rich fibrin in the treatment of bilateral gingival recession. Clinic Adv Periodontics. 2012;2:154–60. [Google Scholar]
- 27.Aroca S, Keglevich T, Barbieri B, Gera I, Etienne D. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: A 6-month study. J Periodontol. 2009;80:244–52. doi: 10.1902/jop.2009.080253. [DOI] [PubMed] [Google Scholar]
- 28.Wallace SC. Root coverage grafting comparing placental derived membrane to acellular dermis matrix: A case series. Dentistry. 2012;2:137. [Google Scholar]
- 29.Paolantonio M. Treatment of gingival recessions by combined periodontal regenerative techniques, guided tissue regeneration, and subpedicle connective tissue grafts. A comparative clinical study. J Periodontol. 2002;73:53–62. doi: 10.1902/jop.2002.73.1.53. [DOI] [PubMed] [Google Scholar]
- 30.Uraz A, Sezgin Y, Yalim M, Taner L, Cetiner D. Comparative evaluation of platelet-rich fibrin membrane and connective tissue graft in the treatment of multiple adjacent recession defects: A clinical study. J Dent Sci. 2013;20:1–10. [Google Scholar]
- 31.Aleksic Z, Jankovic S, Dimitrijevic B, Divnic-Resnik T, Milinkovic I, Lekovic V. The use of platelet-rich fibrin membrane in gingival recession treatment. Srp Arh Celok Lek. 2010;138:11–8. doi: 10.2298/sarh1002011a. [DOI] [PubMed] [Google Scholar]
- 32.Moka LR, Boyapati R, Srinivas M, Swamy N, Swarna C, Putcha M. Comparison of coronally advanced and semilunar coronally repositioned flap for the treatment of gingival recession. J Clin Diagn Res. 2014;8:ZC04–8. doi: 10.7860/JCDR/2014/8928.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akkaya M, Böke F. Shallow localized gingival recession defects treated with modified coronally repositioned flap technique: A case series. Eur J Dent. 2013;7:368–72. doi: 10.4103/1305-7456.115425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar AP, Fernandes B, Surya C. Platelet rich fibrin: A promising approach for root coverage. J Interdiscip Dent. 2011;1:115–8. [Google Scholar]
- 35.Jadhav T, Thomas BS. Platelet rich fibrin membrane for recession coverage. E J Dent. 2012;2:223–7. [Google Scholar]
- 36.Shetty SS, Chatterjee A, Bose S. Bilateral multiple recession coverage with platelet-rich fibrin in comparison with amniotic membrane. J Indian Soc Periodontol. 2014;18:102–6. doi: 10.4103/0972-124X.128261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nanavati B, Bhavsar NV, Jaydeepchandra M. Coronally Positioned Flap for Root Coverage: Comparison between smokers and nonsmokers. J Int Oral Health. 2013;5:21–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Mathur A, Bains VK, Gupta V, Jhingran R, Singh GP. Evaluation of intrabony defects treated with platelet-rich fibrin or autogenous bone graft: A comparative analysis. Eur J Dent. 2015;9:100–8. doi: 10.4103/1305-7456.149653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta V, Bains VK, Singh GP, Mathur A, Bains R. Regenerative potential of platelet rich fibrin in dentistry: Literature review. Asian J Oral Health Allied Sci. 2011;1:23–8. [Google Scholar]
- 40.Savitha B, Vandana KL. Comparative assesment of gingival thickness using transgingival probing and ultrasonographic method. Indian J Dent Res. 2005;16:135–9. doi: 10.4103/0970-9290.29908. [DOI] [PubMed] [Google Scholar]
- 41.Kolte R, Kolte A, Mahajan A. Assessment of gingival thickness with regards to age, gender and arch location. J Indian Soc Periodontol. 2014;18:478–81. doi: 10.4103/0972-124X.138699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglas de Oliveira DW, Oliveira-Ferreira F, Flecha OD, Gonçalves PF. Is surgical root coverage effective for the treatment of cervical dentin hypersensitivity? A systematic review. J Periodontol. 2013;84:295–306. doi: 10.1902/jop.2012.120143. [DOI] [PubMed] [Google Scholar]
- 43.Eger T, Müller HP, Heinecke A. Ultrasonic determination of gingival thickness. Subject variation and influence of tooth type and clinical features. J Clin Periodontol. 1996;23:839–45. doi: 10.1111/j.1600-051x.1996.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 44.Bains VK, Gupta V, Srivastava R, Agarwal SK. Accretion of gingival height by gingival thickness augmentation: A clinical report. Asian J Oral Health Allied Sci. 2013;3:25–31. [Google Scholar]


