Abstract
Objective:
The present comparative study was aimed to determine the effectiveness of Th US and TENS in the management of myofascial pain in TMD patients.
Materials and Methods:
The present randomized comparative study was on 90 patients who were further assigned in three different groups each having 30 patients; Group I was healthy control patients, Group II was receiving Th US therapy, and Group III was receiving TENS therapy. All the 90 patients were further evaluated for maximum inter incisor subjective evaluation regarding muscle pain, impediment to daily life, massage impression on visual analog scale (VAS) scale, and intensity and duration used in Th US massage.
Results:
The masseter muscle thickness in control group was 12.00 (standard deviation [SD] ±1.1) mm when compared with TMD patient of 13.00 (SD ± 1.1) mm before treatment. Statistical significant findings on VAS score of muscle pain, impediment to daily life, and massage impression were observed in Th US. After treatment, the anechoic areas disappeared or were reduced in Th US group by 95.6% and in TENS by 74.4%.
Conclusion:
Th US appeared to be subjectively better which was related to VAS score of massage impression, muscle pain, and impediment to daily life after treatment as well as sonographically related to existence of anechoic areas.
Keywords: Masseter muscle, temporomandibular joint disorders, therapeutics, transcutaneous electrical nerve stimulation, ultrasonography
INTRODUCTION
The etiology of temporomandibular disorders (TMDs) is multifactorial.[1] Different managements have been advocated which have proven to be effective for TMDs.[2,3,4,5,6,7] Transcutaneous electrical nerve stimulation (TENS) therapy is a well-known physical therapy useful for the relief of pain by use of controlled, low voltage electrical pulses applied to the nervous system.[8,9] It is safe, noninvasive, inexpensive, and an effective method of providing analgesia, with reduced potential adverse reactions related to other methods.[10,11]
Therapeutic ultrasound (Th US) is a noninvasive therapeutic method which includes vibrations above 16,000 vibrations/s or 16 Hz (range audible to the human ear). The frequency used is between 1.0 and 3.0 MHz.[12,13] It is known to accelerate healing, decrease joint stiffness, alleviate pain, increase the extendibility of collagen fibers, and reduce muscle spasm.[14,15]
Diagnostic ultrasound depicts the morphology of the muscles situated superficial to the bone, structures in the head and neck, and it is a reliable procedure.[16] It offers potential advantage over computed tomography as it can be performed noninvasively, repeatedly, and easily even at bed side and also demonstrates the internal muscle structure.[17] The literature elicits lack of studies correlating the efficacy of TENS therapy and ultrasound massage with pre- and post-treatment changes in the muscles. Therefore, a need was felt to conduct a study to assess the sonographic features of masseter muscle as an index for evaluating the efficacy between TENS therapy and Th US massage therapy in treating myofascial pain and also to observe pre- and post-treatment sonographic features, which could provide the primary line of management in TMDs.
The present study was conducted to determine the efficiency of TENS and Th US in the management of myofascial pain in TMD patients.
MATERIALS AND METHODS
This randomized controlled study was carried out on 90 patients of either sex between 20 years and 60 years. Patients were assigned in three groups.
Groups
Ninety patients who further assigned in three different groups each having 30 patients; Group I was healthy control patients, Group II was receiving Th US therapy, and Group III was receiving TENS therapy. Both sides of the masseter muscle were scanned perpendicular to the anterior border of the muscle and the surface of the mandibular ramus 2–5 cm above the inferior border of the mandible with the minimum pressure at which the thickest muscle image could be obtained. For muscle pain, visual analog scale (VAS) using a score from 0 (no pain at all) to 100 (the worst pain imaginable) was used. Patients in the study group were assigned randomly to the therapy allocated and received treatment for 12 weeks (3 times every 2 weeks) or until the VAS value for muscle pain was <10.
Diagnosis of TMD was made based upon research diagnostic criteria (RDC) for clinical TMDs conditions. Group I a and b (RDC/TMD Group I a and b)[18] and Clinical Diagnostic Criteria for TMDs (Myalgia Type I and II, myofascial pain dysfunction).[19] An informed consent and ethical clearance were obtained from ethical committee.
Detailed specific examinations for signs and symptoms of TMDs were carried out as suggested by Widmer et al.[20] Orthopantomogram (OPG) and transcranial radiographs of both sides (both open and closed mouth) were made to rule out any temporomandibular joint (TMJ) disorders. All the relevant data were entered in the proforma.
All the patients were evaluated for the following parameters before, during, and after every treatment session - maximum mouth opening (i.e., maximum inter-incisal distance) without pain (in mm), subjective evaluation regarding muscle pain on VAS scale such as pain at rest, pain with mandibular motion, and pain on chewing, subjective evaluation regarding impediment to daily life on VAS scale such as speaking, chewing, and eating, impression of TENS therapy and ultrasonic massage (example – comfort, warmth, and ease of mouth opening), parameters used in TENS therapy, and ultrasonic massage (intensity/frequency and duration).
For muscle pain, VAS using a score from 0 (no pain at all) to 100 (the worst pain imaginable) was used. For impediments to the activities of daily life, score from 0 “no impediments at all” to 100 “the worst impediments imaginable” was used. For impression of the massage, scores from 0 (uncomfortable, no warmth, or difficulty of mouth opening) to 100 (comfortable, warmth, or ease of mouth opening) were used.
Diagnostic ultrasound was performed using a SIEMENS ACUSONX 300 (2010) equipped with 8 MHz. Each patient was seated in an upright position. The patient underwent sonography at the rest state without chewing and clenching. Both sides of the masseter muscle were scanned perpendicular to the anterior border of the muscle and the surface of the mandibular ramus 2–5 cm above the inferior border of the mandible with the minimum pressure at which the thickest muscle image could be obtained. Sonograms obtained with a focal range between 0.5 cm and 2.0 cm and with an image depth of 6 cm, echo gain and dynamic range were 26 dB and 69 dB, respectively, and the masseter muscle thickness and anechoic areas were evaluated. Each value was assessed and interpreted by 3 observers.
The patients in the study group were assigned randomly to the therapy allocated and received treatment for 12 weeks (3 times every 2 weeks) or until the VAS value for muscle pain was <10. Treatment was continued, if the patient was not relieved with the symptoms or until the VAS value for muscle pain was <10. A pre- and post-treatment diagnostic ultrasound was done and the thickness of the anechoic areas of masseter muscle was evaluated.
Subjects who were included in the study were of dull regional pain in the face persisting for more than 1 month, two or more muscles of mastication tender to palpation, and recognizable pain which increases by palpation.
Objective evidence of TMJ pathology or dysfunction, other orofacial pain conditions (atypical facial pain and atypical odontalgia), other TMD treatments within the last 3 months, neurologic or psychiatric disorders, a history of pain medication abuse or current abuse, anterior disc displacement without reduction (RDC/TMD Group II b and II c), pure arthrogenic pain (RDC/TMD Group III a), pain attributable to recent trauma, dental surgery, metabolic disorders, vascular disease, neoplasia, psychiatric disorders, undiagnosed dental pain, and patients who have been treated with TENS or ultrasound therapy previously without any improvement in the conditions were excluded from the study.
Statistical analysis
Comparative analysis was carried out using “paired t-test” for intragroup and “unpaired t-test” for intergroup comparison. Comparisons of number of anechoic areas were carried out using Chi-square test. The data were analyzed using SPSS software (version 14.0) and descriptive statistics was obtained.
RESULTS
In Group I, the mean age of the subjects was 34.93 years (standard deviation [SD] ±12.57), Group II was 32 years (±10.174), and Group III was 29.73 years (±8.804) and the range was 20–60 years [Table 1]. In Group I, out of 30 patients, there were 18 female (60%) and 12 male (40%), in Group II, 23 female (76.7%) and 7 male (23.30%), and Group III, 24 female (80%) and 6 male (20%) [Table 2].
Table 1.
Age-wise distribution of patients
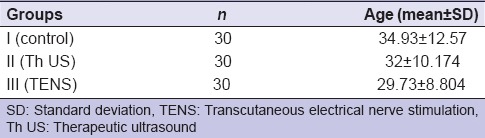
Table 2.
Sex-wise distribution of patients
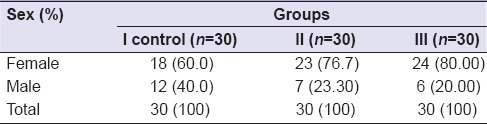
In control group, the mean ± SD of masseter muscle thickness was 12.00 (SD ± 1.1) mm and in study group (temporomandibular group) pretreatment was 13.00 (SD ± 1.1) mm and was found statistically significant (P = 0.001, i.e., <0.005) [Table 3].
Table 3.
Masseter muscle thickness in control and temporomandibular disorders group

The mean and ± SD of intensity used was 0.73 (SD ± 0.084) W/cm2 and the duration of the treatment was 9.57 weeks (SD ± 3.036) W/cm2 [Table 4].
Table 4.
Massage regimen (intensity and duration)

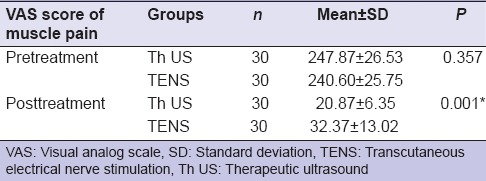
The mean and ± SD of the VAS scores of muscle pain (total VAS score of muscle pain at rest, with mandibular motion, and while chewing) in pretreatment Th US and TENS group were 247.87 (SD ± 26.53) and 240.60 (SD ± 25.75), respectively, and in posttreatment were 20.87 (SD ± 6.35) and 32.37 (SD ± 13.02), respectively. There was no statistically significant difference between two groups before treatment (P < 0.05, i.e., 0.357); however, it was statistically significant after treatment (P = 0.001) [Table 5].
Table 5.
Comparison of visual analog scale score of muscle pain pre- and post-treatment in both groups
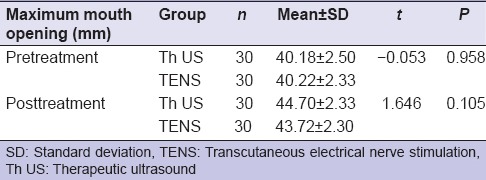
The mean ± SD of maximum mouth opening in both groups individually pretreatment and after treatment was statistically significant (P < 0.05), but when compared between two groups (Th US and TENS) before and posttreatment found no significant statistically (P > 0.05) [Table 6].
Table 6.
Comparison between pre- and post-treatment of maximum mouth openings in both group
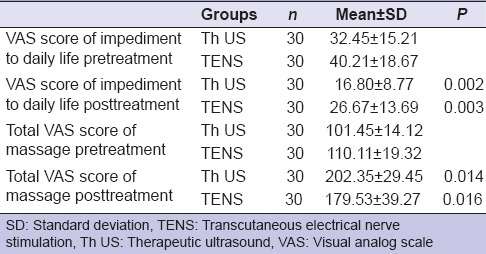
The mean ± SD of the VAS scores of impediment to daily life pretreatment in Th US and TENS was 32.45 (SD ± 15.21) and 40.21 (SD ± 18.67), respectively, after treatment in Th US and TENS group was 16.80 (SD ± 8.77) and 26.67 (SD ± 13.69), respectively. The result shows that there was statistically significant difference between both the groups (P < 0.05) and found better day-to-day life impediment in Th US group.
The mean ± SD of the VAS score of massage impression in Th US and TENS group pretreatment was 101.45 (SD ± 14.12) and 110.11 (SD ± 19.32), respectively, after treatment in Th US and TENS group was 202.35 (SD ± 29.45) and 179.53 (SD ± 39.27), respectively. However, patients who received Th US therapy experienced better massage impression compared to those received TENS therapy and found statistically significant difference between the two groups (P < 0.05) [Table 7].
Table 7.
Visual analog scale score of impediment to daily life and massage impression in both groups
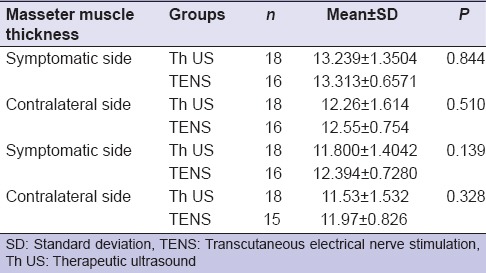
In unilateral group, no significant difference (P > 0.05) was found on symptomatic side and contralateral side when compared between two groups (Th US and TENS) pre- and post-treatment [Table 8].
Table 8.
Comparison of masseter muscle thickness pre- and post-treatment in both groups (unilateral group)
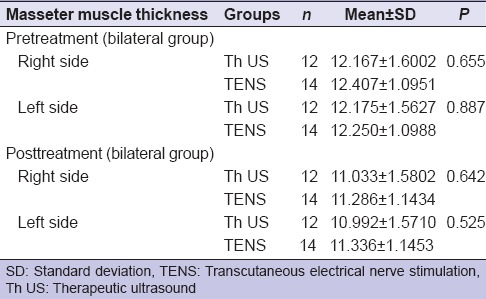
In bilateral group, no significant difference (P > 0.05) was found between both groups (Th US and TENS) pre- and post-treatment on the right and left side of the masseter muscle thickness [Table 9].
Table 9.
Comparison of masseter muscle thickness pre- and post-treatment in both groups (bilateral group)
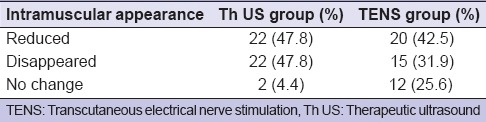
After Th US massage treatment, the anechoic areas disappeared or reduced in size in 44 muscles out of 46 muscles (95.6%) whereas in TENS therapy, the anechoic areas disappeared or reduced in size in 35 muscles out of 47 muscles (74.4%) and found Th US was more effective as compared to TENS therapy [Tables 10 and 11].
Table 10.
Intramuscular sonographic appearance of masseter muscle pretreatment in both groups

Table 11.
Intramuscular sonographic appearance of masseter muscle posttreatment in both groups
DISCUSSION
Treatment plan for TMD should not only be determined by the disorder but more importantly by the individual's needs. Different therapeutic modalities result in similar improvements in pain and dysfunction, caution is urged with regard to the use of invasive and other irreversible treatments, particularly in the initial management of TMD patients.[21] Physical therapy helps to relieve musculoskeletal pain and to restore normal function.[4,5,22] Massage is a potential mechanical stimulus and an effective trigger for the pain gate process. It can reduce a naturally occurring discomfort, causes much greater release of opiates, and helps achieve more profound pain suppression.[3]
The age distribution in the present study is consistent with other studies[4,23,24,25] where common age of occurrence was second to the fourth decades of life. Our results were in accordance with studies by Dworkin et al.,[26] Isacsson et al.,[27] and Jensen et al.[28] who reported a female predominance, whereas contrary to this, the observations of Beaton et al.[29] observed lack of any significant gender differences in their study.
The mean ± SD of masseter muscle thickness in control group was 12.00 (±1.1) mm and in TMD group before treatment was 13.00 (±1.1) mm and was found to be statistically significant (P = 0.001). Sonography was used for the measurement of masseter muscle thickness[16,30,31,32] and was confirmed to be a reliable procedure. Similar studies had been carried out by Pereira et al.[32] Kiliaridis and Kälebo[33] concluded that ultrasonography was an accurate method, with a low error (0.49 mm) for measuring the thickness of masseter muscle. Ariji et al.[17] investigated the ultrasonographic features of 32 patients with inflammatory change in the masseteric region and concluded that ultrasound is an accurate modality for measuring the thickness of masseter muscle and may provide information useful in diagnosis and treatment, especially in follow-up examination. Kubota et al.[30] investigated the thickness of the masseter muscle related to the maxillofacial morphology, including the thickness of alveolar process in the mandibular incisor region, and the thickness of the mandibular symphysis suggesting that masticatory functions influence the morphology of the mandible. Reimers et al.[34] investigated the sonographic features of inflammatory myopathies with reference to histologic findings and confirmed that the thickness of edematous muscle was greater than that of nonedematous muscle.
An increase in masseter muscle thickness has been reported in patients with TMD,[3,16,32] although the cause of thickening is not well known. In general, two possible causes are considered for increased thickness. When a muscle contracts, there occur sliding of the muscle fiber filament and an increase in the fiber diameter. Another cause would be an edematous change in the muscle.[16] TMD patients usually suffer from muscle pain for a relatively long time before visiting a dental clinic or hospital. They may hold a long-term low-level contraction, which is suggested to be caused by psychologic stress or prolonged work. Taken together, increased thickness in TMD patients is suggested to be related to muscle edema rather than muscle fiber enlargement by initial filament sliding.[3]
The mean and ± SD of the VAS scores of muscle pain before treatment found no statistically significant difference between two groups (P < 0.05); however, it was statistically significant after treatment (P < 0.05).
Similar results have been reported by El Fatih et al.[35] that the ultrasound group showed a higher success rate with 93.3% pain improvement whereas the TENS showed only a success of 53.3%. Contrary to this, the observation of Madani and Mirmortazavi[36] showed that anterior positioning splint therapy appears to be the best treatment method for reduction of pain and joint sounds in patients with TMD, compared with the other two methods (TENS and Th US therapy) studied.
In a study by Moger et al.[21] who observed that the pain reduction in the active treatment group was more than in placebo group, the difference between the groups was not statistically significant (P < 0.05). Rodrigues et al.[37] observed a significant reduction in pain intensity (P < 0.05) before and after TENS application.
Pain relief in Th US is related to washout of pain mediators by increased blood flow, changes in nerve conduction, or alterations in cell membrane permeability that decreases inflammation. This reduction in muscle pain can be attributed to the effect of TENS therapy on the electrical stimuli, pressure and touch impulses which arrive faster at the levels of the spinal cord in the substantia gelatinosa of the dorsal horn and in the higher levels of the central nervous system than the pain impulses and “close the gate” for pain impulses, resulting in a suppression of pain signals, and TENS also causes activation of endogenous analgesic systems involving opiate-like peptides, such as endorphins, thereby increasing their plasma levels. They are known to have far more analgesic efficacy than morphine.[11]
The mean ± SD of the VAS scores of impediment to daily life after treatment shows that there was statistically significant difference between both the groups (P < 0.05) and found better day-to-day life impediment in Th US group. However, patients who received Th US therapy experienced better massage impression compared to those received TENS therapy and found statistically significant difference between the two groups (P < 0.05).
A study by Grieder et al.[15] suggested that the ultrasonic therapy was not alone effective in relieving symptoms; however, it is more effective when used as an adjunct to the accepted modalities of therapy. Esposito et al.[14] concluded that ultrasound is most successful in alleviating muscle symptoms and less effective in reducing symptoms associated with the disk. Esenyel et al.[38] concluded that the effectiveness of ultrasound therapy is comparable to trigger point injections but is considered to be noninvasive treatment of choice. Majlesi and Unalan[39] observed high-power ultrasound applied to the trigger points before stretching the muscle were more effective (P < 0.05) than conventional ultrasound. The main reason is mainly due to thermal effects of Th US.[40]
The masseter muscle thickness significantly decreased after treatment on symptomatic sides in both the groups with no significant difference found when compared between two modalities of treatment (Th US and TENS) before and after treatment (P > 0.05). The decrease in thickness suggested that the imbalance in the masticatory muscles has improved by massage treatment of Th US. Mild rhythmic muscle movements by TENS causes increased blood and lymph circulation which results in reduced interstitial edema and accumulation of noxious tissue metabolites, thereby improving the physiological state of muscle which, in turn, leads to significant reduction in muscle spasm and pain.
All these results can be due to the combined effects of TENS therapy which includes neurologic, pharmacologic, physiologic, and psychologic effects.[11]
The anechoic areas were found to disappear or reduced in 95.6% Th US group and 74.4% in TENS group after treatment. Ariji et al.[3] investigated the sonographic features of the masseter muscle as indices for judging the efficacy of massage treatment with an oral rehabilitation robot and reported that the thickness was significantly decreased after treatment (P < 0.05), which was in accordance to our study. They concluded that the presence of anechoic area can be attributed to the thickening of the masseter muscle and the disappearance or reduction is mainly due to its action on arterial and venous blood flow, the blood clotting process, and the properties of the connective tissue and muscle. It also improves lymph drainage, restores the normal osmotic pressure of the interstitial fluid, thus reducing muscle edema.[41] The internal sonographic features of the muscles would be an indicator of the pathologic state of the muscles.[17]
A study carried out by Ariji et al.[16] evaluated the intramuscular echogenic band and assessed the muscle appearance into various types and found that significant difference in distribution between healthy and TMD groups existed.
Few patients in our study showed no change in anechoic area, but there was a considerable reduction on VAS score of muscle pain, impediment to daily life, and massage impression. This could be hypothesized by the fact that the muscle consists of transverse and short bands as suggested by Ariji et al.[16] which might response to therapy in a different manner. Thus, a detailed and in-depth analysis of the study of arrangement of internal bands in the muscle should be done.
Wessberg et al.[11] evaluated the efficacy of TENS therapy, splint, and occlusal adjustments in myofascial pain dysfunction syndrome (MPDS) patients and found 95% success rate immediately after posttreatment and 86% success rate after 1 year with TENS therapy combined with splint and occlusal therapy. Similar study reported by Møystad et al.[10] concluded that TENS is a simple, noninvasive treatment method that can be recommended to patients with rheumatic disease in periods of pain in the stomatognathic system. A study conducted by Moger et al.[21] concluded that the TENS therapy appears to be useful in relieving pain, especially muscular and chronic pain and along with TENS therapy, placebo should also be considered as a potent and independent therapeutic modality in its own right. The observation of Rajpurohit et al.[42] showed that MENS could be used as an effective pain-relieving adjunct to TENS.
CONCLUSION
Diagnostic ultrasonography was found to be an effective means of diagnosing TMDs by using masseter muscle thickness and intramuscular sonographic appearances. Both Th US and TENS group found significantly decreased thickness in the masseter muscle after treatment on symptomatic sides and no statistically significant difference between the two groups, and improvement in intramuscular sonographic appearance (reduced or disappeared anechoic areas) after treatment was more in Th US (95.6%) as compared to TENS therapy (74.4%).
Both therapies were effective in the reduction of muscle pain after treatment, but Th US therapy showed better results with a statistically significant difference when compared with TENS therapy. The subjective improvement in the form of impediment to daily life and massage impression was effective in both therapies, but Th US appeared to be a better modality with statistically significant difference between these two therapies. There was statistically highly significant improvement in the mouth opening in both when compared between Th US and TENS groups that it showed no statistically significant difference. Thus, results from our study justify the use of Th US therapy as well as TENS therapy in the management of TMD. However, Th US therapy was subjectively better on the basis of VAS score of muscle pain, VAS score of impediment to daily life, VAS score of massage impression, and on the basis of intramuscular sonographic appearance of the masseter muscle.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Grossi DB, Chaves TC. Physiotherapeutic treatment for temporomandibular disorders (TMD) Braz J Oral Sci. 2004;3:492–7. [Google Scholar]
- 2.Al-Ani Z, Gray RJ, Davies SJ, Sloan P, Glenny AM. Stabilization splint therapy for the treatment of temporomandibular myofascial pain: A systematic review. J Dent Educ. 2005;69:1242–50. [PubMed] [Google Scholar]
- 3.Ariji Y, Katsumata A, Hiraiwa Y, Izumi M, Sakuma S, Shimizu M, et al. Masseter muscle sonographic features as indices for evaluating efficacy of massage treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:517–26. doi: 10.1016/j.tripleo.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Gray RJ, Quayle AA, Hall CA, Schofield MA. Physiotherapy in the treatment of temporomandibular joint disorders: A comparative study of four treatment methods. Br Dent J. 1994;176:257–61. doi: 10.1038/sj.bdj.4808429. [DOI] [PubMed] [Google Scholar]
- 5.De Laat A, Stappaerts K, Papy S. Counseling and physical therapy as treatment for myofascial pain of the masticatory system. J Orofac Pain. 2003;17:42–9. [PubMed] [Google Scholar]
- 6.Alvarez-Arenal A, Junquera LM, Fernandez JP, Gonzalez I, Olay S. Effect of occlusal splint and transcutaneous electric nerve stimulation on the signs and symptoms of temporomandibular disorders in patients with bruxism. J Oral Rehabil. 2002;29:858–63. doi: 10.1046/j.1365-2842.2002.00923.x. [DOI] [PubMed] [Google Scholar]
- 7.Kato MT, Kogawa EM, Santos CN, Conti PC. TENS and low-level laser therapy in the management of temporomandibular disorders. J Appl Oral Sci. 2006;14:130–5. doi: 10.1590/S1678-77572006000200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esposito CJ, Shay JS, Morgan B. Electronic dental anesthesia: A pilot study. Quintessence Int. 1993;24:167–70. [PubMed] [Google Scholar]
- 9.New Delhi: International electro medical co.Delhi; PHYSIO TENS 4000; operating manual for physio four channel tens digital. [Google Scholar]
- 10.Møystad A, Krogstad BS, Larheim TA. Transcutaneous nerve stimulation in a group of patients with rheumatic disease involving the temporomandibular joint. J Prosthet Dent. 1990;64:596–600. doi: 10.1016/0022-3913(90)90135-y. [DOI] [PubMed] [Google Scholar]
- 11.Wessberg GA, Carroll WL, Dinham R, Wolford LM. Transcutaneous electrical stimulation as an adjunct in the management of myofascial pain-dysfunction syndrome. J Prosthet Dent. 1981;45:307–14. doi: 10.1016/0022-3913(81)90396-6. [DOI] [PubMed] [Google Scholar]
- 12.Speed CA. Therapeutic ultrasound in soft tissue lesions. Rheumatology (Oxford) 2001;40:1331–6. doi: 10.1093/rheumatology/40.12.1331. [DOI] [PubMed] [Google Scholar]
- 13.Rai S, Kaur M, Goel S, Panjwani S, Singh S. Prospective utility of therapeutic ultrasound in dentistry-review with recent comprehensive update. Adv Biomed Res. 2012;1:47. doi: 10.4103/2277-9175.100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito CJ, Veal SJ, Farman AG. Alleviation of myofascial pain with ultrasonic therapy. J Prosthet Dent. 1984;51:106–8. doi: 10.1016/s0022-3913(84)80115-8. [DOI] [PubMed] [Google Scholar]
- 15.Grieder A, Vinton PW, Cinotti WR, Kangur TT. An evaluation of ultrasonic therapy for temporomandibular joint dysfunction. Oral Surg Oral Med Oral Pathol. 1971;31:25–31. doi: 10.1016/0030-4220(71)90029-6. [DOI] [PubMed] [Google Scholar]
- 16.Ariji Y, Sakuma S, Izumi M, Sasaki J, Kurita K, Ogi N, et al. Ultrasonographic features of the masseter muscle in female patients with temporomandibular disorder associated with myofascial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:337–41. doi: 10.1016/j.tripleo.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 17.Ariji E, Ariji Y, Yoshiura K, Kimura S, Horinouchi Y, Kanda S. Ultrasonographic evaluation of inflammatory changes in the masseter muscle. Oral Surg Oral Med Oral Pathol. 1994;78:797–801. doi: 10.1016/0030-4220(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 18.Le Resche L, Michael R, Korff V. Research diagnostic criteria. Craniomandib Disord. 1992;6:327–34. [Google Scholar]
- 19.Truelove EL, Sommers EE, LeResche L, Dworkin SF, Von Korff M. Clinical diagnostic criteria for TMD. New classification permits multiple diagnoses. J Am Dent Assoc. 1992;123:47–54. doi: 10.14219/jada.archive.1992.0094. [DOI] [PubMed] [Google Scholar]
- 20.Widmer CG, Huggins KH, Fricton J. Examination and history data collection. Craniomandib Disord. 1992;6:335–55. [Google Scholar]
- 21.Moger G, Shashikanth MC, Sunil MK, Shambulingappa P. Transcutaneous electrical nerve stimulation therapy in temporomandibular disorder: A clinical study. J Indian Acad Oral Med Radiol. 2011;23:46–50. [Google Scholar]
- 22.Michelotti A, Steenks MH, Farella M, Parisini F, Cimino R, Martina R. The additional value of a home physical therapy regimen versus patient education only for the treatment of myofascial pain of the jaw muscles: Short-term results of a randomized clinical trial. J Oral Facial Pain. 2004;18:114–25. [PubMed] [Google Scholar]
- 23.Okeson JP. Management of Temporomandibular Disorders and Occlusion. 5th ed. St Louis: CV Mosby; 2003. [Google Scholar]
- 24.Durham J. Temporomandibular disorders (TMD): An overview. Oral Surg. 2008;1:60–8. [Google Scholar]
- 25.Byahatti SM. Evolution, epidemiology and etiology of temporomandibular joint disorders. J Indian Acad Oral Med Radiol. 2010;22:13–8. [Google Scholar]
- 26.Dworkin SF, Huggins KH, LeResche L, Von Korff M, Howard J, Truelove E, et al. Epidemiology of signs and symptoms in temporomandibular disorders: Clinical signs in cases and controls. J Am Dent Assoc. 1990;120:273–81. doi: 10.14219/jada.archive.1990.0043. [DOI] [PubMed] [Google Scholar]
- 27.Isacsson G, Linde C, Isberg A. Subjective symptoms in patients with temporomandibular joint disk displacement versus patients with myogenic craniomandibular disorders. J Prosthet Dent. 1989;61:70–7. doi: 10.1016/0022-3913(89)90112-1. [DOI] [PubMed] [Google Scholar]
- 28.Jensen R, Rasmussen BK, Pedersen B, Lous I, Olesen J. Prevalence of oromandibular dysfunction in a general population. J Orofac Pain. 1993;7:175–82. [PubMed] [Google Scholar]
- 29.Beaton RD, Egan KJ, Nakagawa-Kogan H, Morrison KN. Self-reported symptoms of stress with temporomandibular disorders: Comparisons to healthy men and women. J Prosthet Dent. 1991;65:289–93. doi: 10.1016/0022-3913(91)90177-x. [DOI] [PubMed] [Google Scholar]
- 30.Kubota M, Nakano H, Sanjo I, Satoh K, Sanjo T, Kamegai T, et al. Maxillofacial morphology and masseter muscle thickness in adults. Eur J Orthod. 1998;20:535–42. doi: 10.1093/ejo/20.5.535. [DOI] [PubMed] [Google Scholar]
- 31.Emshoff R, Bertram S, Brandlmaier I, Scheiderbauer G, Rudisch A, Bodner G. Ultrasonographic assessment of local cross-sectional dimensions of masseter muscle sites: A reproducible technique? J Oral Rehabil. 2002;29:1059–62. doi: 10.1046/j.1365-2842.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- 32.Pereira LJ, Gavião MB, Bonjardim LR, Castelo PM, Andrade Ada S. Ultrasonography and electromyography of masticatory muscles in a group of adolescents with signs and symptoms of TMD. J Clin Pediatr Dent. 2006;30:314–9. doi: 10.17796/jcpd.30.4.w2t51jh08762648g. [DOI] [PubMed] [Google Scholar]
- 33.Kiliaridis S, Kälebo P. Masseter muscle thickness measured by ultrasonography and its relation to facial morphology. J Dent Res. 1991;70:1262–5. doi: 10.1177/00220345910700090601. [DOI] [PubMed] [Google Scholar]
- 34.Reimers CD, Fleckenstein JL, Witt TN, Müller-Felber W, Pongratz DE. Muscular ultrasound in idiopathic inflammatory myopathies of adults. J Neurol Sci. 1993;116:82–92. doi: 10.1016/0022-510x(93)90093-e. [DOI] [PubMed] [Google Scholar]
- 35.Fatih I, Amin E, Ali I A, Laithi ES. Efficacy of physiotherapy and intraoral splint in the management of temporomandibular disorders. Saudi Dent J. 2004;16:16–20. [Google Scholar]
- 36.Madani AS, Mirmortazavi A. Comparison of three treatment options for painful temporomandibular joint clicking. J Oral Sci. 2011;53:349–54. doi: 10.2334/josnusd.53.349. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues D, Oliveira AS, Berzin F. Effect of TENS on the activation pattern of the masticatory muscles in TMD Patients. Braz J Oral Sci. 2004;3:510–5. doi: 10.1590/s1806-83242004000400003. [DOI] [PubMed] [Google Scholar]
- 38.Esenyel M, Caglar N, Aldemir T. Treatment of myofascial pain. Am J Phys Med Rehabil. 2000;79:48–52. doi: 10.1097/00002060-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Majlesi J, Unalan H. High-power pain threshold ultrasound technique in the treatment of active myofascial trigger points: A randomized, double-blind, case-control study. Arch Phys Med Rehabil. 2004;85:833–6. doi: 10.1016/j.apmr.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 40.ter Haar G. Therapeutic ultrasound. Eur J Ultrasound. 1999;9:3–9. doi: 10.1016/s0929-8266(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 41.McNeill C. History and evolution of TMD concepts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:51–60. doi: 10.1016/s1079-2104(97)90091-3. [DOI] [PubMed] [Google Scholar]
- 42.Rajpurohit B, Khatri SM, Metgud D, Bagewadi A. Effectiveness of transcutaneous electrical nerve stimulation and microcurrent electrical nerve stimulation in bruxism associated with masticatory muscle pain – A comparative study. Indian J Dent Res. 2010;21:104–6. doi: 10.4103/0970-9290.62816. [DOI] [PubMed] [Google Scholar]


