Abstract
Objective:
The aim of this study was to determine whether there are any changes in the surface of bone or implant structures following the removal of a screwed dental implant.
Materials and Methods:
For this, six individual samples of acid-etched and sandblasted implants from three different manufacturers’ implant systems were used. They were screwed in a D1 bovine bone, and they were removed after primary stabilization. The bone and implant surfaces are evaluated with scanning electron microscope.
Results:
Through examination of the surfaces of the bone prior to implantation and of the used and unused implant surfaces, it was found that inhomogeneity in the implant surface can cause microcracking in the bone.
Conclusions:
This is attributed to the stress induced during the implantation of self-tapping implants and suggests that a tap drill may be required in some instances to protect the implant surface.
Keywords: Acid-etched and sandblasted implant, bone surface, implant removal
INTRODUCTION
Dental implants are at present often the preferred treatment option in instances of missing teeth as a means to protect surrounding tissues and provide proper retention. Providing oral rehabilitation with retention through implant support offers clinicians an indispensable strategy clinically in several cases.[1] Technology in this field has reached a point that has seen numerous modifications introduced to enhance implant retention and provide a suitable prosthetic loading in the shortest possible time by accelerating the rate of healing. In studies aimed at improving the osseointegration of implants, rough surfaces were found to be better than smooth surfaces with regards to enhancing the implant's mechanical attachment to the bone.[2,3] This has led to a number of different physical, chemical, and biochemical methods being developed for increasing surface roughness and improving osseointegration.[4]
Among the available chemical methods, acidification has become widely accepted and is based on etching the implant surface with strong acids. This has been reported to produce micro-holes 1.5–2 μm in size on the implant surface,[5] which assist osseointegration by increasing the available surface for the attachment of bone tissue. Physical methods include sandblasting, which involves applying abrasive ceramic particles such as alumina, titanium dioxide, and calcium phosphate (CaPO4)[2,6] via compressed air and a suitable carrier fluid. Hydroxyapatite, as well as beta tricalcium phosphate and its derivatives, can also be used as an etching material to create a retentive area on the surface and offer the advantage of being biocompatible, osteoconductive, and resorbable. In the case of titanium implants, it is typically a combination of these techniques that is most frequently used to improve its surface structure.[7,8] Crowning techniques incorporating physical and chemical methods, such as acidification-sandblasting, and biochemical methods involving hydroxyapatite have therefore proven to be the most successful.[9]
Regardless of the treatment used, it is important to ensure homogeneity over the whole surface in order to prevent varying levels of deformation in the bone-implant contact surface during implementation. Success in the postoperative period is therefore highly dependent on surface properties such as the bone type, the sharpness and design of the bur used, and the groove characteristics of the implant.[10] With this in mind, this study explores the effects of stress induced by the forces associated with D1 type implantation and observes the changes that occur in the surface of the bone and an acid-etched, sandblasted implant during its removal.
MATERIALS AND METHODS
A total of six self-tapping implants were sourced from three different manufacturers, namely: Implant A (BT Lock Dental Implants, Montecchio Maggiore, Italy), implant B (Implant Direct, CA, USA) and implant C (Implance, Trabzon, Turkey). The surfaces of these implants were roughened by acidification-sandblasting using large grit particles combined with hydroxyapatite (implant A and B) or CaPO4 (implant C). Each implant was affixed to a D1-quality bovine tibia in accordance with the manufacturer's instructions by first using a sterile-saline-cooled pilot drill, then twist drills matched to the diameter of the implant, to produce six implant sockets. While three of these sockets were used for implantation, with one implant from each manufacturer, the other three sockets served as controls. Implantation torque in all instances was within a range of 30 to 35 Ncm, and the implants were removed after primary stabilization was achieved. External parts of the bone blocks in which the sockets were located were cut by a drill at the sagittal plane under sterile saline irrigation. Once this cut reached the central region of the block (i.e., the socket area), the block surfaces were separated from each other by manually applying a mild force, with a gauge being used to prevent surface deformation.
In all, 12 samples were prepared: Screwed implants (n = 3), nonscrewed implants (n = 3), sockets that underwent screwing and implant removal (n = 3), and sockets that were prepared only with twist drills (n = 3). After drying, these were all anodized with gold in preparation for examination of their surface by scanning electron microscopy at various magnifications to assess whether any deformation or alteration of the surface was induced.
The present study was approved by the appropriate ethics review boards.
RESULTS
The most striking outcomes of the clinical assessment of implant and bone surfaces during scanning electron microscopy examination were obtained at ×20 and ×500 magnification [Figures 1–6], which revealed that the screw configuration in the bone and all three implant types are damaged by implant removal. At ×20 and ×500 magnification, comparison of the surfaces of the implants that were removed with those that were not used shows compression-type deformation in implant A [Figure 2d], patchy micro-holes on the surface of implant B [Figure 4d], and the removal of bony tissue along with the screw configuration in implant C [Figure 6c]. We can see these type of deformations on the bone surfaces which contacted with the implants A and C surfaces [Figures 2b and 6a]. At ×20 magnification, it was determined that vertical sulci present in the surface design of all the implants used caused an accumulation of bone particles [Figures 2c, 4c, and 6c]; nevertheless, no compression was observed. In the case of implant A, it was found that the surface of the unused control was not homogenous [Figure 1c] and its removal produced microfractures in the bone surface [Figure 2a]. When the screwed and removed implant surfaces structure examined under ×500 magnification, on A implant's surface sharp scratches, on B implant's surface rounded mesh type and on C implant's surface round particulate type deformation were investigated [Figures 2d, 4d, and 6d].
Figure 1.
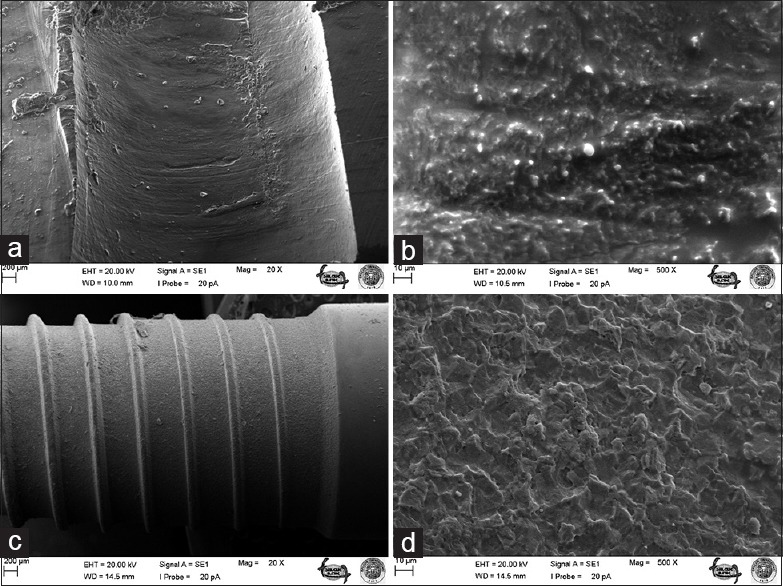
Bone surface before A implant screwing under ×20 (a) and ×500 (b) magnifications; surface of unscrewed A implant under ×20 (c) and ×500 (d) magnifications
Figure 6.
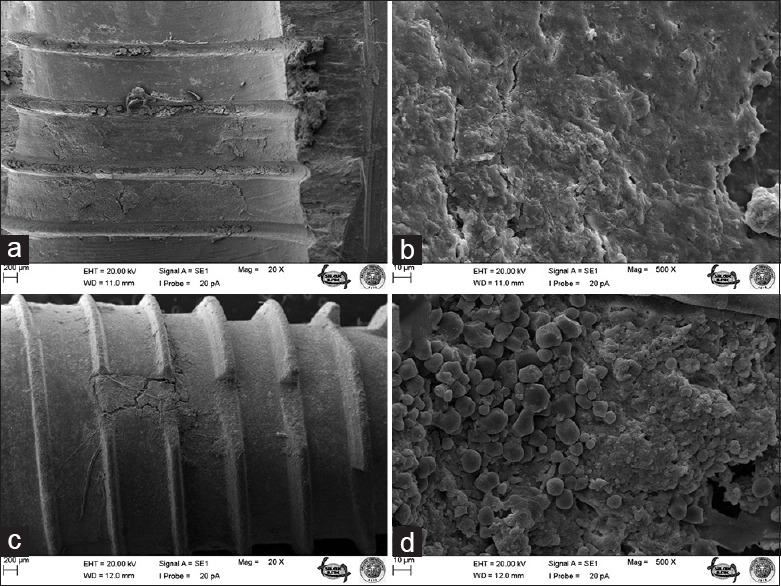
Bone surface after removal of C implant under ×20 (a) and ×500 (b) magnifications; surface of C implant after screwing and removal under ×20 (c) and ×500 (d) magnifications
Figure 2.
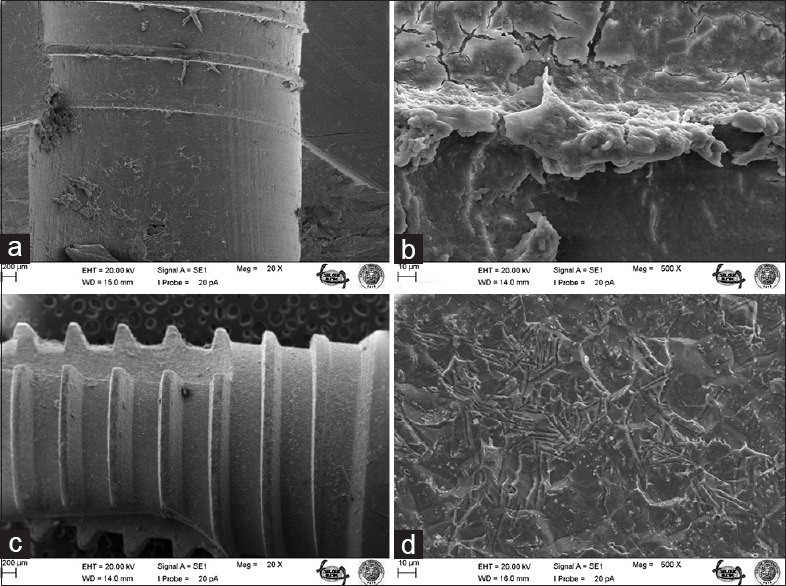
Bone surface after removal of A implant under ×20 (a) and ×500 (b) magnifications; surface of A implant after screwing and removal under ×20 (c) and ×500 (d) magnifications
Figure 4.
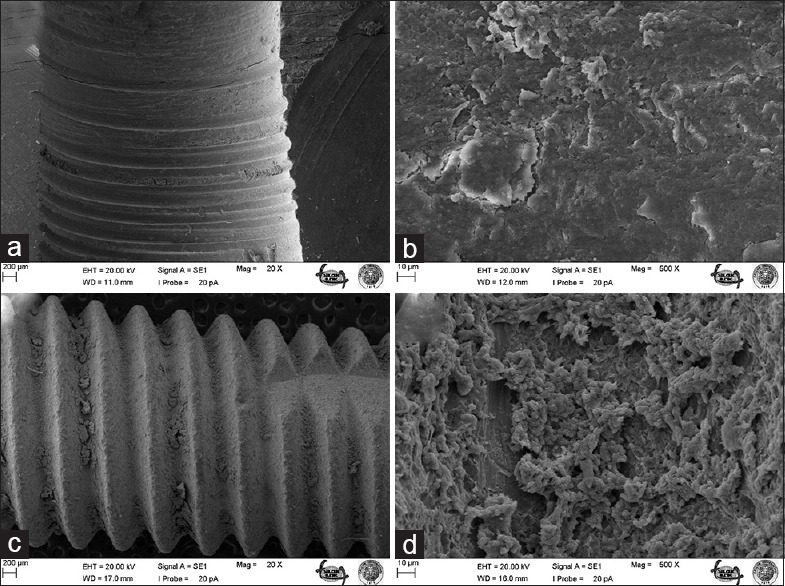
Bone surface after removal of B implant under ×20 (a) and ×500 (b) magnifications; surface of B implant after screwing and removal under ×20 (c) and ×500 (d) magnifications
Figure 5.
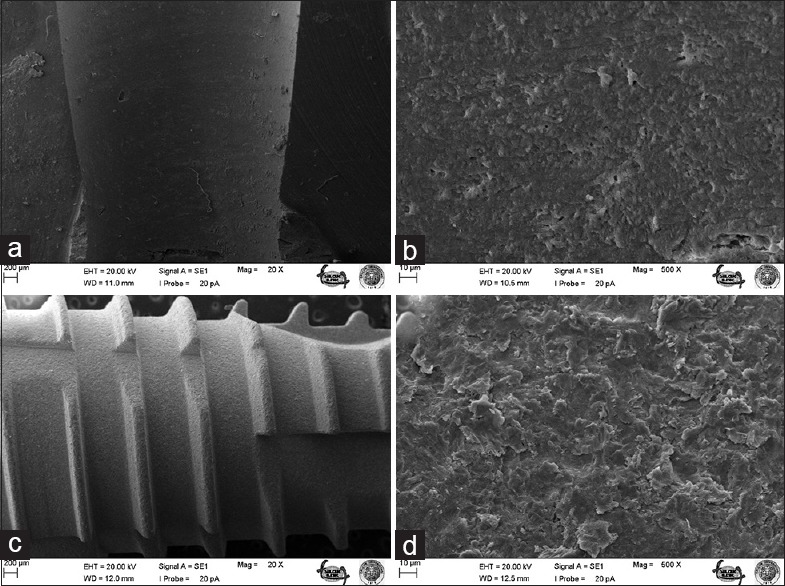
Bone surface before C implant screwing under ×20 (a) and ×500 (b) magnifications; surface of unscrewed C implant under ×20 (c) and ×500 (d) magnifications
DISCUSSION
Although implant loss can be caused by a smooth implant surface or short implant stature, the anatomic location can also play an important role.[11] Thus, although it is important to determine surgical margins by preoperative assessment, relocation may be possible by adjusting the angle and distance immediately after implant removal if anatomical threats or inconvenient neighborhoods are detected intraoperatively. In the event that such intraoperative relocation is possible, ensuring minimal impairment of the implant surface and deformation of the bone become of great importance.
The number of studies relating to the evaluation of implant surface characteristics and their modification is steadily increasing, though acidified and sandblasted surfaces still tend to be the most commonly used in clinical practice. Past studies have suggested that acid-etched and sandblasted surfaces are comparable to hydroxyapatite-coated surfaces in terms of bone contact but offer the important advantage of eliminating melting, peeling, and solvent-style deformation.[12] Despite these advantages, micro-holes were found to form on the surface of implant B [Figures 3 and 4], which suggests that acid-etched and sandblasted surfaces cannot, in fact, provide bone contact at every point.
Figure 3.
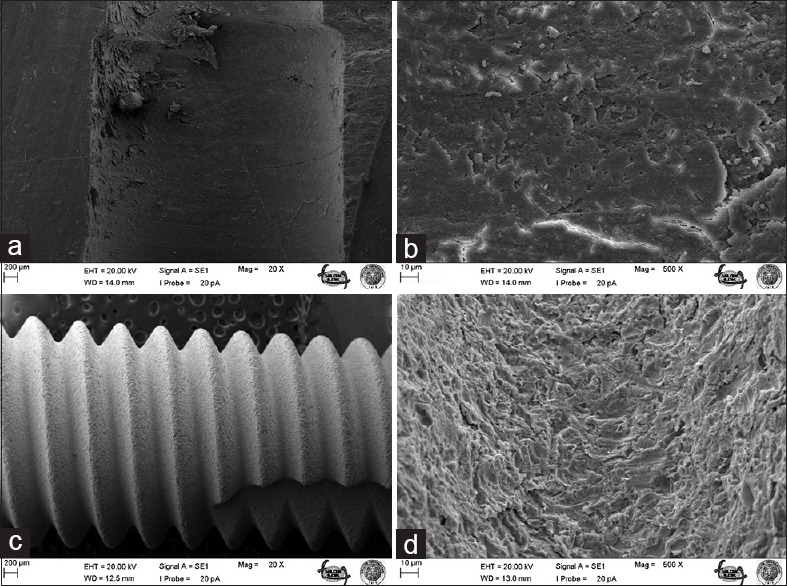
Bone surface before B implant screwing under ×20 (a) and ×500 (b) magnifications; surface of unscrewed B implant under ×20 (c) and ×500 (d) magnifications
Histological examination has revealed that a higher bone-implant contact is obtained in the case of surfaces crowned with hydroxyapatite than with those subjected to acidification and sandblasting. Nevertheless, surface deformation is encountered more frequently with crowned implants than with acidified and sandblasted surfaces. From the results of the present study, it is clear that some change has occurred in all of the implant groups and the bone surface after implantation and removal.
A coarse grit size was used in this study for sandblasting as this is known to increase bone contact;[13] and yet, no difference was observed between the implants in terms of bone contact, even though CaPO4 sandblasting was used on implant C. It is therefore believed that the use of CaPO4 sandblasting may produce a harder surface morphology than hydroxyapatite sandblasting.
Given the limitations of this study, it cannot be known for certain at what stage in the implantation and removal procedure the micro-fractures in implant A were produced; however, it is considered that they most likely occurred during the screwing process. Of the implant types tested, only implant A was to found to have an inhomogeneous surface. This is significant, as ensuring homogeneity in the surface of implants during manufacturing is important to preventing additional stress being induced in the bone during implantation. It is also worth considering that a D1-type bone was studied, with self-tapping implants clearly causing more trauma to this bone type and predisposing it to the formation of fractures. While the stress distribution in the trabecular bone occurs in a broader area during the screwing process, the stress in the cortical bone is limited to the close surrounding of the implant.[14] However, the occurrence of microfractures did not necessarily imply compression of the bone, with bone particles being observed to collect in the grooves of the implant.
CONCLUSION
As this study was focused solely on the deformation of acid-etched, sandblasted surfaces, there is still a need for follow-up work to investigate the implant surface after it is screwed into bone blocks. This could be achieved by cutting the bone at the sagittal plane, and should bring about a different point of view to that in the current literature. Nevertheless, it is apparent from this study that the use of self-tapping implants in the D1-type bone can mechanically alter the implant surface, and so a tap drill may be necessary for some circumstances to protect the surface characteristics of the implant. However, it should be noted that self-tapping implants can show different results depending on the bone type. Finally, if removal is required after implantation, then using an implant with a larger unit diameter in the second implantation would assist in primary stabilization.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Feitosa PC, de Lima AP, Silva-Concílio LR, Brandt WC, Neves AC. Stability of external and internal implant connections after a fatigue test. Eur J Dent. 2013;7:267–71. doi: 10.4103/1305-7456.115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlsson L, Röstlund T, Albrektsson B, Albrektsson T. Removal torques for polished and rough titanium implants. Int J Oral Maxillofac Implants. 1988;3:21–4. [PubMed] [Google Scholar]
- 3.Shalabi MM, Wolke JG, Jansen JA. The effects of implant surface roughness and surgical technique on implant fixation in an in vitro model. Clin Oral Implants Res. 2006;17:172–8. doi: 10.1111/j.1600-0501.2005.01202.x. [DOI] [PubMed] [Google Scholar]
- 4.Bagno A, Di Bello C. Surface treatments and roughness properties of Ti-based biomaterials. J Mater Sci Mater Med. 2004;15:935–49. doi: 10.1023/B:JMSM.0000042679.28493.7f. [DOI] [PubMed] [Google Scholar]
- 5.Massaro C, Rotolo P, De Riccardis F, Milella E, Napoli A, Wieland M, et al. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: Chemical composition. J Mater Sci Mater Med. 2002;13:535–48. doi: 10.1023/a:1015170625506. [DOI] [PubMed] [Google Scholar]
- 6.Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23:844–54. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Le Guehennec L, Goyenvalle E, Lopez-Heredia MA, Weiss P, Amouriq Y, Layrolle P. Histomorphometric analysis of the osseointegration of four different implant surfaces in the femoral epiphyses of rabbits. Clin Oral Implants Res. 2008;19:1103–10. doi: 10.1111/j.1600-0501.2008.01547.x. [DOI] [PubMed] [Google Scholar]
- 8.Braceras I, De Maeztu MA, Alava JI, Gay-Escoda C. In vivo low-density bone apposition on different implant surface materials. Int J Oral Maxillofac Surg. 2009;38:274–8. doi: 10.1016/j.ijom.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Uzun G, Keyf F. Surface characteristics of the implant systems and osseointegration. The Journal of Dental Faculty of Ataturk University. 2007;(Suppl 2):43–50. [Google Scholar]
- 10.Ercoli C, Funkenbusch PD, Lee HJ, Moss ME, Graser GN. The influence of drill wear on cutting efficiency and heat production during osteotomy preparation for dental implants: A study of drill durability. Int J Oral Maxillofac Implants. 2004;19:335–49. [PubMed] [Google Scholar]
- 11.Balshe AA, Assad DA, Eckert SE, Koka S, Weaver AL. A retrospective study of the survival of smooth- and rough-surface dental implants. Int J Oral Maxillofac Implants. 2009;24:1113–8. [PubMed] [Google Scholar]
- 12.Dávid A, Eitenmüller J, Muhr G, Pommer A, Bär HF, Ostermann PA, et al. Mechanical and histological evaluation of hydroxyapatite-coated, titanium-coated and grit-blasted surfaces under weight-bearing conditions. Arch Orthop Trauma Surg. 1995;114:112–8. doi: 10.1007/BF00422838. [DOI] [PubMed] [Google Scholar]
- 13.Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889–902. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 14.Behnaz E, Ramin M, Abbasi S, Pouya MA, Mahmood F. The effect of implant angulation and splinting on stress distribution in implant body and supporting bone: A finite element analysis. Eur J Dent. 2015;9:311–8. doi: 10.4103/1305-7456.163235. [DOI] [PMC free article] [PubMed] [Google Scholar]


